Abstract
Grade-3 follicular lymphoma (FL) has aggressive clinical behavior. To evaluate the optimal first transplantation approach in relapsed/refractory grade-3 FL patients, we compared the long-term outcomes after allogeneic (allo-) vs. autologous hematopoietic cell transplantation (auto-HCT) in the rituximab-era. A total of 197 patients undergoing first RIC allo-HCT or first auto-HCT during 2000-2012 were included. Rituximab-naïve patients were excluded. Allo-HCT recipients were younger; more heavily pretreated, and had a longer interval between diagnosis and HCT. The 5-year probabilities of non-relapse mortality (NRM), relapse/progression, progression-free survival (PFS) and overall survival (OS) for auto-HCT vs. allo-HCT groups were 4% vs. 27% (p<0.001); 61% vs. 20% (p<0.001); 36% vs. 51% (p=0.07) and 59% vs. 54% (p=0.7), respectively. On multivariate analysis auto-HCT was associated with reduced risk of NRM (RR=0.20; p=0.001). Within the first 11months post-HCT auto- and allo-HCT had similar risks of relapse/progression and PFS. Beyond 11months, auto-HCT was associated with higher risk of relapse/progression (RR=21.3; p=0.003) and inferior PFS (RR=3.2; p=0.005). In the first 24 months post-HCT, auto-HCT was associated with improved OS (RR=0.42; p=0.005), but in long-time survivors (beyond 24 months) it was associated with inferior OS (RR=3.6; p=0.04). RIC allo-HCT as the first transplant approach can provide improved PFS and OS, in long-term survivors.
Keywords: grade 3 follicular lymphoma, reduced intensity allo-HCT, auto-HCT, long-time survival
Introduction
Follicular lymphoma (FL) is the most common subtype of indolent non-Hodgkin lymphoma (NHL), and accounts for 20-30% of all NHL. The WHO classification uses a three-grade system (grades 1-3) in FL, based on the number of centroblasts in 10 neoplastic follicles, expressed per high-power microscopic field.1 According to this, grade 3 of FL is defined as a tumor with a follicular growth pattern harboring more than 15 centroblasts per high power field by histological examination. This histopathological subtype represents approximately 10-20% of all FL cases. Due to the relative paucity of this lymphoma subtype, the management of grade 3 FL remains an area of controversy.2-6 Patients with grade 3 FL are often excluded from clinical trials7 or their outcome are not reported separately in FL studies.8 In the absence of large prospective studies limited to grade 3 FL, the management of these patients typically follows the diffuse large B-cell lymphoma (DLBCL) guidelines.9,10
While both autologous (auto-) and allogeneic hematopoietic cell transplantation (allo-HCT) can provide disease control in relapse/refractory FL11,12, no study to our knowledge, has assessed the relative efficacy of these two transplantation modalities specifically in grade 3 FL patients. Outcome studies from the pre rituximab-era that lumped grade 3 FL patients with either more indolent grade 1-2 histologies13,14, or along with more aggressive DLBCL15 suggest that post HCT outcomes of grade 3 patients are distinct from both indolent and aggressive B-cell lymphoproliferative disorders. These observations underscore the need for specifically reappraising the role of auto- and allo-HCT in relapse/refractory grade 3 FL, in the era of chemoimmunotherapies. We report here, outcomes of patients with grade 3 FL who underwent either a first auto- or first allo-HCT in the rituximab era.
Materials and Methods
Data sources
More than 450 transplantation centers worldwide participating in the CIBMTR contribute data on HCTs to a statistical center at the Medical College of Wisconsin. Participating centers are required to report all HCTs consecutively, with compliance monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians' reviews of submitted data, and on-site audits of participating centers ensure data quality. Observational studies by the CIBMTR are performed in compliance with federal regulations with ongoing review by the institutional review board of the Medical College of Wisconsin. All patients provided an informed consent according to the declaration of Helsinki.
Patients
Patients with a histologically proven diagnosis of relapsed/refractory grade 3 follicular lymphoma (FL), undergoing a first auto-HCT or a first reduced-intensity conditioning/non-myeloablative (RIC/NMA) allo-HCT, reported to the CIBMTR between 2000 and 2012 years, were eligible for inclusion in this study. RIC/NMA allo-HCT recipients with a history of prior auto-HCT were not included, as the primary objective of the study was to assess outcomes of auto- vs. allo-HCT in grade 3 FL, when either modality is used as the first transplantation approach. Donor-source for the allo-HCT cohort was restricted to either HLA-identical siblings or at least a 7/8 (antigen or allele-level) matched unrelated donors (URD). Pediatric patients (<18 years), recipients of alternative donor HCT (e.g. umbilical cord blood, haploidentical, mismatched URD), and patients receiving ex vivo graft manipulation (T-cell depleted or CD34 selection) were not included in the analysis. In addition FL patients undergoing histological transformation to DLBCL and those not receiving rituximab-containing therapies before HCT were excluded from this study.
Definitions
The intensity of allo-HCT conditioning regimens was categorized RIC/NMA using established consensus criteria.16 Previously established criteria17 for evaluation the degree of HLA matching were used for URD. Complete remission (CR) to last therapy line before HCT on CIBMTR forms is defined as complete resolution of all known disease on radiographic (CAT-scan) assessments, while partial remission (PR) is defined as ≥50% reduction in the greatest diameter of all sites of known disease and no new sites of disease. Resistant disease is defined as <50% reduction in the diameter of all disease sites, or development of new disease sites. Rituximab resistance was defined as (a) failure to achieve at least a PR to a rituximab-containing therapy line or (b) relapse/progression during or within six months of finishing a rituximab-based therapy.18
Study Endpoints
Primary outcomes were non-relapse mortality (NRM), progression/relapse, progression-free survival (PFS) and overall survival (OS). NRM was defined as death without evidence of lymphoma progression/relapse; relapse was considered a competing risk. Progression/relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For PFS, a patient was considered a treatment failure at the time of progression/relapse or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up. The OS was defined as the interval from the date of transplantation to the date of death or last follow-up. Acute GvHD was defined and graded based on the pattern and severity of organ involvement using established criteria.19 Chronic GvHD was defined as the development of any evidence of chronic GvHD based on clinical criteria.20 Neutrophil recovery was defined as the first of 3 successive days with absolute neutrophil count (ANC) ≥500/μL after post-transplantation nadir. Platelet recovery was considered to have occurred on the first of three consecutive days with platelet count 20,000/μL or higher, in the absence of platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk.
Statistical analysis
Adjusted probabilities of PFS and OS were calculated as described previously.21 Adjusted cumulative incidences of NRM, lymphoma progression/relapse, hematopoietic recovery and second malignancies were calculated to accommodate for competing risks.22 Patient-, disease- and transplant- related factors were compared between auto-HCT and allo-HCT groups using the Chi-square test for categorical variables and the Wilcoxon sample test for continuous variables. Associations among patient-, disease, and transplantation-related variables and outcomes of interest were evaluated using Cox proportional hazards regression. Backward elimination was used to identify covariates that influenced outcomes. Covariates with a p<0.05 were considered significant. The proportional hazards assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Covariates violating the proportional hazards assumption were added as time-dependent covariates in the Cox regression model. Interactions between the main effect and significant covariates were examined. Results are expressed as relative risk (RR). All analyses were performed using SAS 9.3. The variables considered in multivariate analysis are shown in Table 1S of supplemental appendix.
Results
Patients
A total of 197 patients with relapsed or refractory grade 3 FL undergoing a first auto-HCT (n=136) or a first RIC allo-HCT (n=61) between 2000 and 2012 years, were identified who fulfilled the inclusion criteria. Patient-, disease- and transplant-related characteristics are detailed in Table 1. Patients receiving allografts were younger, more heavily pretreated, and had a longer interval between diagnosis and HCT. More auto-HCT recipients had a history of previous doxorubicin-based chemotherapies. The frequency of chemotherapy- and rituximab-resistance before HCT did not differ between both groups. There was no significant difference in the duration of remission following first-line therapy between the two transplant groups. BEAM (carmustine, etoposide, cytarabine, melphalan) and CBV (cyclophosphamide, carmustine and etoposide) were the most common conditioning regimens for auto-HCT. Fludarabine in combination with an alkylating agent was the most common RIC approach in the allo-HCT cohort. Eight auto-HCT patients and 13 allo-HCT patients received total body irradiation (TBI)-based conditioning regimens. Five patients each in allo-HCT group received antithymocyte globulin and alemtuzumab with conditioning.
Table 1. Characteristics of patients who underwent auto- or allo-HCT for non- transformed relapsed/refractory follicular lymphoma grade 3 from 2000-2012 reported to the CIBMTR.
| Variable | Allo-HCT | Auto-HCT | P-value |
|---|---|---|---|
| Number of patients | 61 | 136 | |
| Age at transplant, years | 0.006 | ||
| Median | 53 (36-64) | 57 (27-76) | |
| Male gender | 41 (67) | 79 (58) | 0.22 |
| Karnofsky Performance Score | 0.21 | ||
| <90% | 18 (30) | 39 (29) | |
| 90-100% | 42 (69) | 86 (63) | |
| Missing | 1 (2) | 11 (8) | |
| Race | 0.81 | ||
| Caucasian/White | 54 (89) | 121 (89) | |
| Black | 2 (3) | 8 (6) | |
| Others | 5 (8) | 7 (5) | |
| Disease stage at diagnosis | 0.11 | ||
| I-II | 8 (13) | 25 (18) | |
| III-IV | 52 (85) | 105 (77) | |
| Unknown | 1 (2) | 6 (4) | |
| Time from diagnosis to transplant, months | 32 (5-159) | 24 (6-224) | 0.02 |
| B Symptoms at diagnosis | 24 (39) | 36 (26) | 0.14 |
| Elevated LDH concentration at transplant | 17 (28) | 53 (39) | 0.23 |
| Unknown | 7 (11) | 18 (13) | |
| Bulky disease at diagnosis | 7 (11) | 16 (12) | 0.99 |
| Bone marrow involved with FL at HCT | 6 (10) | 3 (2) | 0.02 |
| Missing | 52 (85) | 115 (85) | |
| Extranodal involvement at transplant | 10 (16) | 23 (17) | 0.86 |
| Missing | 1 (2) | 4 (3) | |
| Rituximab-resistant | 30 (49) | 84 (62) | 0.12 |
| Not evaluable | 3 (5) | 10 (7) | |
| Median/Mean chemotherapy lines (range) | 3/3.3 (1-5) | 3/2.9 (1-5) | 0.01 |
| Duration of first-line therapy response | 0.31 | ||
| <1 year | 22 (36) | 44 (32) | |
| ≥1 year | 34 (56) | 87 (64) | |
| Missing | 5 (8) | 5 (4) | |
| History of radiation therapy before HCT | 10 (16) | 26 (19) | 0.65 |
| History of doxorubicin-based therapies | 49 (80) | 125 (92) | 0.02 |
| Disease response at transplant | 0.07 | ||
| CR | 22 (36) | 43 (32) | |
| PR | 27 (44) | 80 (59) | |
| Chemoresistant/untreated relapse | 12 (20) | 13 (10) | |
| Type of donor | N/A | N/A | |
| HLA-identical sibling | 36 (59) | ||
| Unrelated well-matched (8/8 allele level) | 23 (38) | ||
| Unrelated partially matched (7/8 antigen level or 7/8 allele level) | 2 (3) | ||
| TBI-based conditioning | 9 (15) | 8 (6) | 0.04 |
| Conditioning regimens (AlloHCT)*** | N/A | N/A | |
| Fludarabine/Busulfan ±TBI | 7 (11) | ||
| Fludarabine/Melphalan ±TBI | 14 (23) | ||
| BEAM and similar | 4 (7) | ||
| 2Gy TBI ± Fludarabine | 6 (10) | ||
| Fludarabine/Cyclophosphamide | 21 (34) | ||
| CBV | 3 (5) | ||
| Othersa | 6 (10) | ||
| Conditioning regimens (AutoHCT) | N/A | N/A | |
| TBI-based**** | 8 (6) | ||
| BEAM and similar | 99 (73) | ||
| CBV or similar | 20 (15) | ||
| BuMEL/BuCy | 5 (4) | ||
| Others* | 4 (3) | ||
| Graft type | |||
| Bone marrow | 3 (5) | 1 (1) | 0.05 |
| Peripheral blood | 58 (95) | 135 (99) | |
| GVHD prophylaxis | N/A | N/A | |
| Tacrolimus-based | 33 (54) | ||
| Cyclosporine-based | 24 (39) | ||
| Others** | 4 (7) | ||
| ATG or alemtuzumab used | 10 (16) | N/A | N/A |
| Median follow up of survivors, months | 57 (5-132) | 59 (3-145) | |
Abbreviations: BEAM=carmustine, etoposide, cytarabine, melphalan; CBV = cyclophosphamide, BCNU and etoposide; CR = complete remission; GVHD = graft versus host disease; N/A=not applicable.
TBI+pantostatin (n=1), melphalan+cyclophosphamide (n=1), busulfan alone (n=1), busulfan+cyclophosphamide (n=1), busulfan+cyclophosphamide+etoposide (n=1), TBI+busulfan+cytarabine (n=1).
Carboplatin+mitoxanthron+thiotepa (n=1), carboplatin+thiotepa+etoposide (n=1), melphalan alone (n=1) and nitro alone (n=1).
Other GVHD prophylaxis: not specified (n=4).
TBI doses for allo-HCT patients 400cGy=1, 600cGy=1, 200cGy=7, missing=4
TBI doses for auto-HCT patients 1000cGy=1, 1200cGy=6, 1320=1
Rates of engraftment and GvHD
Neutrophil and platelet engraftment was similar in the auto- and allo-HCT groups (Table 2). The cumulative incidence of acute GvHD (grade II-IV) at day +100 was 25% (95%CI: 15-36%). The cumulative incidence of chronic GvHD at 5 years post-transplant was 55% (95%CI: 40-71%). In the allo-HCT group, GvHD was the most frequent cause of death, as opposed to relapsed FL in the auto-HCT cohort (Table 2S in Supplemental Appendix).
Table 2.
Univariate analysis for patients with grade 3 follicular lymphoma.
| Outcomes | Allo-HCT (N = 61) | Auto-HCT (N = 136) | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| N | Prob (95% CI) | N | Prob (95% CI) | ||
| ANC recovery >0.5 × 109/L | |||||
| 28-day | 100 (87-100)% | 100 (93-100) | 0.99 | ||
| 100-day | 100 (87-100)% | 100 (93-100)% | 0.99 | ||
| Platelet recovery ≥ 20 × 109 | 42 | 68 | |||
| 28-day | 83 (67-92)% | 82 (71-90)% | 0.90 | ||
| 100-day | 88 (72-95)% | 96 (86-99)% | 0.22 | ||
| Acute GVHD (II-IV) | 61 | N/A | |||
| 100-day | 25 (15-36)% | ||||
| Chronic GVHD | 60 | N/A | |||
| 1-year | 47 (34-59)% | ||||
| 3-year | 53 (38-68)% | ||||
| 5-year | 55 (40-71)% | ||||
| New malignancy | 59 | 128 | |||
| 1-year | 3 (0-8)% | 1 (0-4)% | 0.52 | ||
| 3-year | 6 (2-15)% | 6 (3-12)% | 0.992 | ||
| 5-year | 8 (2-15)% | 9 (4-16)% | 0.533 | ||
| Adjusted probabilities | |||||
| NRM | 61 | 135 | |||
| 1-year | 16 (7-25)% | 0 (0-0)% | <0.001 | ||
| 3-year | 21 (11-31)% | 2 (0-4)% | <0.001 | ||
| 5-year | 27 (17-38)% | 4 (0-8)% | <0.001 | ||
| Relapse/Progression | 61 | 135 | |||
| 1-year | 20 (10-29)% | 36 (28-44)% | 0.010 | ||
| 3-year | 20 (10-29)% | 56 (48-64)% | <0.001 | ||
| 5-year | 20 (10-29)% | 61 (53-69)% | <0.001 | ||
| PFS | 61 | 135 | |||
| 1-year | 63 (52-75)% | 64 (56-72)% | 0.883 | ||
| 3-year | 58 (46-70)% | 42 (34-51)% | 0.04 | ||
| 5-year | 51 (37-64)% | 36 (27-44)% | 0.066 | ||
| Overall survival | 61 | 136 | |||
| 1-year | 70 (58-81)% | 87 (81-93)% | 0.79 | ||
| 3-year | 61 (48-73)% | 70 (62-78)% | 0.22 | ||
| 5-year | 54 (40-67)% | 59 (50-68)% | 0.70 | ||
Abbreviations: ANC = neutrophil recovery; NRM= non-relapse mortality; PFS = progression-free survival; PROB = probability; CI = confidence interval; NA = not applicable; NE = not evaluable
Probabilities of neutrophil and platelet recovery, platelet recovery, acute GVHD, chronic GVHD, treatment-related mortality and progression/relapse were calculated using the cumulative incidence estimate. Progression-free survival and overall survival was calculated using the Kaplan-Meier product limit estimate.
Log-rank test
Non-relapse mortality
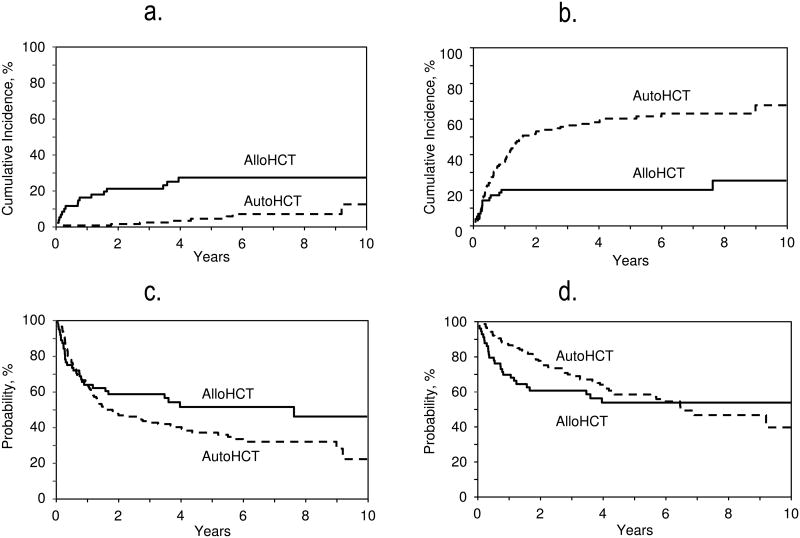
Seventeen patients in the allo-HCT group and 7 in the auto-HCT group experienced NRM (Table 2S in the Supplemental Appendix). The adjusted probability of NRM at 5 years was significantly higher in the allo-HCT group (27% vs. 4%; p<0.001) (Table 2; Figure 1a). On multivariate analysis, auto-HCT (relative risk [RR]=0.20, 95%CI: 0.08-0.50; p=0.0006) and TBI-free conditioning regimens (RR=0.33, p=0.02) were associated with a lower risk of NRM, while a low Karnofsky performance score (KPS) <90 (RR=3.12; p=0.01) was associated with a higher NRM risk (Table 3).
Figure 1.
Adjusted probabilities for NRM (5 years; auto-HCT, n=135; allo-HCT, n=61; p<0.001) [Figure 1a]. Adjusted probabilities for relapse (5 years; n=135; allo-HCT, n=61; p<0.001) [Figure 1b]. Adjusted probabilities for PFS (5 years; n=135; allo-HCT, n=61; p=0.07) [Figure 1c] Adjusted probabilities for OS (5 years; n=136; allo-HCT, n=61; p=0.7) [Figure 1d]
Table 3.
Multivariate analysis for grade 3 follicular lymphoma patients.
| Variable | N | RR | 95% CI Lower Limit | 95% CI Upper Limit | P-value |
|---|---|---|---|---|---|
| Non-relapse mortality | |||||
| Main effect | |||||
| Allo-HCT | 61 | 1 | |||
| Auto-HCT | 135 | 0.202 | 0.082 | 0.501 | 0.0006 |
| KPS | |||||
| ≥90% | 127 | 1 | |||
| <90% | 57 | 3.124 | 1.328 | 7.351 | 0.0091 |
| Missing | 12 | 2.178 | 0.26 | 18.221 | 0.4727 |
| TBI | |||||
| Yes | 16 | 1 | |||
| No | 180 | 0.327 | 0.126 | 0.849 | 0.0216 |
| Progression/Relapse | |||||
| Main effect (≤ 11 months) | |||||
| Allo-HCT | 61 | 1 | |||
| Auto-HCT | 135 | 1.615 | 0.863 | 3.025 | 0.1341 |
| Main effect (> 11 months) | |||||
| Allo-HCT | 36 | 1 | |||
| Auto-HCT | 87 | 21.329 | 2.896 | 157.075 | 0.0027 |
| Sex | |||||
| Male | 119 | 1 | |||
| Female | 77 | 0.632 | 0.408 | 0.979 | 0.04 |
| Chemosensitivity at HCT | |||||
| CR | 65 | 1 | |||
| PR | 106 | 1.322 | 0.817 | 2.14 | 0.2559 |
| Chemoresistant/untreated | 25 | 3.13 | 1.653 | 5.925 | 0.0005 |
| Prior radiotherapy | |||||
| Yes | 36 | 1 | |||
| No | 160 | 0.584 | 0.359 | 0.951 | 0.0307 |
| Progression-free survival | |||||
| Main effect | |||||
| Allo-HCT | 61 | 1 | |||
| Auto-HCT | 135 | 0.869 | 0.521 | 1.448 | 0.5892 |
| Main effect (> 11 months) | |||||
| Allo-HCT | 36 | 1 | |||
| Auto-HCT | 87 | 3.238 | 1.437 | 7.295 | 0.0046 |
| Chemosensitivity at HCT | |||||
| CR | 65 | 1 | |||
| PR | 106 | 1.4 | 0.915 | 2.144 | 0.1214 |
| Chemoresistant/untreated | 25 | 2.835 | 1.606 | 5.004 | 0.0003 |
| Overall survival | |||||
| Main effect (≤24 months) | |||||
| Allo-HCT | 61 | 1 | |||
| Auto-HCT | 136 | 0.427 | 0.235 | 0.776 | 0.0052 |
| Main effect (>24 months) | |||||
| Allo-HCT | 32 | 1 | |||
| Auto-HCT | 95 | 3.587 | 1.053 | 12.217 | 0.041 |
| Age | |||||
| < 40 | 11 | 1 | |||
| 40-49 | 45 | 3.173 | 0.724 | 13.917 | 0.1258 |
| 50-59 | 80 | 2.601 | 0.609 | 11.114 | 0.1972 |
| ≥60 | 61 | 5.374 | 1.253 | 23.052 | 0.0236 |
| Chemosensitivity at HCT | |||||
| CR | 65 | 1 | |||
| PR | 107 | 1.271 | 0.762 | 2.122 | 0.3584 |
| Chemoresistant/untreated | 25 | 2.674 | 1.388 | 5.152 | 0.0033 |
| TBI | |||||
| Yes | 17 | 1 | |||
| No | 180 | 0.443 | 0.22 | 0.895 | 0.0232 |
Abbreviations: N = number of patints; RR=relative risk; KPS= Karnosfky performance status; TBI=total body irradiation; CR= complete remission; PR=partial remission; CI = confidence interval.
Disease progression/relapse
The adjusted probability of disease progression/relapse at 5 years was significantly higher in the auto-HCT group as compared to the allo-HCT group (61% vs. 20%; p<0.001) (Table 2, Figure 1b). In multivariate models, the main effect (auto-vs. allo-HCT) displayed a time-varying effect on the risk of lymphoma progression/relapse. During the first 11 months after HCT no significant difference was seen between the two groups in terms of risk of progression/relapse (RR=1.62, 95%CI: 0.86-3.03; p=0.13). Beyond 11 months, auto-HCT was associated with a significantly higher risk of progression/relapse (RR=21.33, 95%CI: 2.90-157.08; p=0.003). Other factors associated with reduced risk of progression/relapse included female sex (RR=0.63, 95% CI: 0.41-0.98, p=0.04) and no history of radiation therapy prior to HCT (RR=0.58; 95% CI: 0.36-0.95, p=0.03). Patients with chemorefractory disease experienced increased risk of progression/relpase (RR=3.13; 95% CI: 1.65-5.93, p=0.0005).
One patient in the allo-HCT group received post HCT maintenance with rituximab, while 11 patients in the auto-HCT group received rituximab maintenance and 1 patient received interferon maintenance. Sixteen (12%) auto-HCT patients after experience disease progression or relapse underwent subsequent allo-HCT.
Progression-free survival
The adjusted probability of 5-year PFS did not differ significantly following allo-HCT as compared to auto-HCT (51% vs. 36%; p=0.07) (Table 2, Figure 1c). On multivariate analysis, the main effect (auto-HCT vs. allo-HCT) displayed a time-varying effect on the risk of treatment failure. During the first 11 months post-HCT, no significant difference was seen between the two groups in terms of risk of treatment failure (RR=0.87, 95%CI: 0.52-1.45; p=0.59). But beyond 11 months, auto-HCT was associated with a significantly higher risk of treatment failure (i.e. inferior PFS) (RR=3.24, 95%CI: 1.44-7.30; p=0.005). The chemosensitive disease was predictive of improved PFS in the whole cohort (Table 3).
Overall survival
The median follow-up in the allo- and auto-HCT groups was 57 months (range 5-132) and 59 months (range 3-145), respectively. The 5-year adjusted probability of OS for the auto-HCT and allo-HCT groups was 59% and 54%, respectively (p=0.70) (Table 2; Figure 1d). On multivariate analysis, within the first 24 months after transplantation, auto-HCT was associated with a reduced risk of mortality (i.e. improved OS) (RR=0.43; 95%CI: 0.24-0.78; p=0.005). In contrast, beyond 24 months, auto-HCT was associated with a higher risk for mortality (i.e. inferior OS) (RR=3.59; 95%CI: 1.05-12.22; p=0.04). Other factors positively impacting on OS in the whole cohort were younger age (<60 years) and chemosensitive disease before HCT (Table 3).
Secondary malignancies
The 5 year cumulative incidence rates of second malignancies did not differ significantly between both cohorts (allo-HCT=8%, auto-HCT=9%; p=0.53) (Table 2). The details of the second malignancies are summarized in Table 3S of the supplemental appendix. Non-melanoma skin cancers were the most frequent second malignancy type in the allo-HCT group. Secondary hematological malignancies were only seen in the auto-HCT cohort (n=4). Four patients (accounting for 7% of mortality post auto-HCT) in the auto-group but none of the patients from allo-HCT group died because of a secondary malignancy (Table 2S).
Discussion
The relative benefits of auto- vs. allo-HCT in the biologically distinct subgroup of grade 3 FL patients have not been analyzed separately, to date.11,12,23 In the current CIBMTR analysis we aimed to assess the role of auto- vs. allo-HCT as the first transplantation strategy in rituximab-treated cohort of grade 3 FL, and made several observations: First, allo-HCT remains associated with higher NRM. Second, disease relapse beyond one year post allo-HCT was rare, while late relapses were frequent following auto-HCT. Third risk of second hematological malignancies was confined to the auto-HCT cohort. Fourth, allo-HCT was associated with improved late survival outcomes.
The management of grade 3 FL is controversial. Some investigators treat grade 3a histology as FL, while others approach it as DLBCL. Despite the unique biology of grade 3b histology6, the distinction between FL grade 3a and 3b, to date has not been shown to have clinical significance, conclusively. The National Comprehensive Cancer Network recommends treating grade 3 FL according DLBCL guidelines, without making a distinction between grades 3a vs. 3b.9 These guidelines are reflective of available literature where except one notable exception24, the majority of published experience suggests neither any difference in outcomes of grade 3a vs. 3b subtypes nor any added benefit of anthracycline-based therapies in grade 3b patients.25-28 CIBMTR forms prior to 2013 did not collect information regarding grade 3a vs. 3b histology and the current analysis cannot to address whether this distinction has any bearing on HCT outcomes. In addition, while patients with transformed DLBCL were excluded, assignment to grade 3 histology in CIBMTR registry is according to data reported by transplant centers, without a central histopathological review. While acknowledging this limitation, it is also important to highlight that CIBMTR systematically conducts onsite data audits at individual transplant centers to ensure quality and accuracy of data reported the registry.
The frequent inclusion of grade 3 FL with the more indolent or aggressive lymphomas undergoing auto-13,14 or allo-HCT15 has meant that the relative benefits of these two transplantation modalities in this unique subset of FL remains unknown. In the pre-rituximab era, Pham et al.13 observed no significant impact of grade 3 histology on survival outcomes of FL patients (grade 3, n=38, 17% of the whole cohort) after auto-HCT. In contrast, Vose et al.14 found grade 3 histology to be associated with increased risk of treatment failure (RR 1.97, p=0.004), progression (RR 2.14, p=0.006) and worse survival outcomes after auto-HCT. In the context of allo-HCT, CIBMTR15 reported on 533 patients with refractory aggressive lymphomas, including 80 patients with (15%) grade 3 FL, undergoing allo-HCT. Interestingly, the authors found a positive prognostic impact of grade 3 histology on survival outcomes (RR 0.53, p<0.001) compared to DLBCL, underscoring the differential post transplantation course of grade 3 FL, compared to more aggressive histologies.
A graft-versus-Lymphoma (GvL) effect had been suggested for patients with indolent lymphomas based on plateau in post HCT PFS29-33 and on observed responses following withdrawal of immunosuppression and donor lymphocyte infusions.31,33 In our current study, disease relapses 1-year after allo-HCT are virtually absent (Table 2), compared to continued risk of relapse following auto-HCT, potentially reflecting clinically relevant GvL effects in grade 3 FL. With the limitations of our registry analysis and sample size in mind, after adjusting for significant covariates, multivariate models in our study suggest that, while in the early post HCT period both transplantation approaches provide comparable survival outcomes, long-term survivors (beyond 2 years) of allo-HCT may enjoy a late PFS and OS benefit. In addition, multivariate models in the current study also identify several clinically relevant modifiable and non-modifiable factors impacting transplantation outcomes. Poor performance score and advancing recipient age were predictors of NRM and OS (Table 3), underscoring the importance of careful patient selection in determining HCT outcomes. Despite, restricting the current study to RIC allografts, TBI in conditioning was associated with higher NRM and overall mortality, suggesting that even within the context lower-intensity transplants, the type and composition of preparative regimen remains a modifiable factor capable of impacting transplantation outcomes.
We also carefully evaluated the rates of second malignancies after both transplant approaches. Histopathological reports of all second cancers were obtained from the transplantation centers and reviewed for accuracy. In the published literature, second malignancies are observed in 5-20% of lymphoma patients after auto-11,34-36and in 2-6% of patients after allo-HCT.37,38 In the current analysis we found no significant difference in the cumulative incidence of all second malignancies between auto- and allo-HCT cohorts (Table 2); however, second hematological malignancies were confined to the auto-HCT group (Table 3S). The most frequent second cancers in allo-group were non-melanoma skin cancers, a potential curable malignancy in majority of cases. Second cancers accounted for 7% of the mortality following autografting, and none of the patients undergoing allo-HCT died because of a second cancer.
The application of the findings from the current analysis into clinical practice warrants consideration of certain key facts. The current registry study does not represent a decision analysis designed to guide selection of one transplant modality over another, at a certain post in a grade 3 FL patient's disease course. The choice of either transplantation modality for any given patient in our data set reflects a complex interplay of several factors including; transplant center's practice/preference, patient age, donor availability and presence of medical comorbidities, among others. With these limitations in mind, the current study provides, hitherto unpublished data to aid clinical decision making. Collectively our data indicate that in relapsed/refractory grade 3 FL, either auto- or allo-HCT when applied as the first transplantation modality can provide durable disease control. Hence the choice of first transplantation modality, in current era should take into account several practical considerations. The potential benefits of auto-HCT including disease control with relatively low morbidity and NRM, should be carefully weighed against the continued risk of disease relapse and higher risk of second hematological malignancies following this procedure. On the other hand, early NRM and quality-of-life considerations (often secondary to GvHD) remain a limitation of allo-HCT. Having said that, disease relapses 1-year after allo-HCT in our study were rare (Figure 1b), and patients who are able to survive the initial procedure-related toxicities, appear to have improved long term survival outcomes. Based on our data derived from chemoimmunotherapy treated grade 3 FL, selecting auto-HCT as first transplant modality in less fit, or elderly FL patients, or those without eligible donors, relatively earlier in the disease course appears reasonable. In carefully selected individuals, allo-HCT potentially later in the disease course can provide (at least) comparable survival outcomes, with substantially reduced risk of therapy failure.
In conclusion, our study shows that in grade 3 FL patients treated with contemporary rituximab-based chemoimmunotherapies, RIC allo-HCT when compared to auto-HCT is associated with higher NRM, but significantly reduced risk of disease relapse and potentially improved survival outcomes in a subset of long-term survivors.
Supplementary Material
Acknowledgments
We would like to acknowledge the following working committee members for their contributions to the manuscript: Mahmoud D. Aljurf, Siddhartha Ganguly, Mark S. Hertzberg, Leona A. Holmberg, Andreas Klein, Taiga Nishihori, Richard F. Olsson, and Nishitha M. Reddy. Also, Maggie Simaytis for administrative assistance.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; *Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Presented in part as an oral presentation at the annual meetings of the American Society of Clinical Oncology, Chicago, IL, May 31, 2015.
Disclosure of conflict of interest: There are no relevant conflicts of interest to disclose.
Supplementary information is available at BMT's website.
References
- 1.Nathwani BN, Harris NL, Weisenberger DD, et al. Follicular lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours Pathology & Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 162–167. [Google Scholar]
- 2.Bilalovic N, Blystad AK, Golouh R, Nesland JM, Selak I, Trinh D, et al. Expression of bcl-6 and CD10 protein is associated with longer overall survival and time to treatment failure in follicular lymphoma. Am J Clin Pathol. 2004;121(1):34–42. doi: 10.1309/TNKL-7GDC-66R9-WPV5. [DOI] [PubMed] [Google Scholar]
- 3.Pruneri G, Valentini S, Fabris S, Del Curto B, Laszlo D, Bertolini F, et al. Cyclin D3 immunoreactivity in follicular lymphoma is independent of the t(6;14)(p21.1;q32.3) translocation or cyclin D3 gene amplification and is correlated with histologic grade and Ki-67 labeling index. Int J Cancer. 2004;112(1):71–77. doi: 10.1002/ijc.20354. [DOI] [PubMed] [Google Scholar]
- 4.Guo Y, Karube K, Kawano R, Kawano R, Yamaguchi T, Suzumiya J, et al. Low-grade follicular lymphoma with t(14;18) presents a homogeneous disease entity otherwise the rest comprises minor groups of heterogeneous disease entities with Bcl2 amplification, Bcl6 translocation or other gene aberrances. Leukemia. 2005;19(6):1058–1063. doi: 10.1038/sj.leu.2403738. [DOI] [PubMed] [Google Scholar]
- 5.Naresh KN. MUM1 expression dichotomises follicular lymphoma into predominantly, MUM1-negative low-grade and MUM1-positive high-grade subtypes. Haematologica. 2007;92(2):267–268. doi: 10.3324/haematol.10682. [DOI] [PubMed] [Google Scholar]
- 6.Horn H, Schmelter C, Leich E, Salaverria I, Katzenberger T, Ott MM, et al. Follicular lymphoma grade 3B is a distinct neoplasm according to cytogenetic and immunohistochemical profiles. Haematologica. 2011;96(9):1327–1334. doi: 10.3324/haematol.2011.042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 8.Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105(4):1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 9.Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Non-Hodgkin's lymphomas, version 2.2014. J Natl Compr Canc Netw. 2014;12(6):916–946. doi: 10.6004/jnccn.2014.0086. [DOI] [PubMed] [Google Scholar]
- 10.Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Ladetto M, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii76–82. doi: 10.1093/annonc/mdr388. [DOI] [PubMed] [Google Scholar]
- 11.Montoto S, Canals C, Rohatiner AZ, Taghipour G, Sureda A, Schmitz N, et al. Long-term follow-up of high-dose treatment with autologous haematopoietic progenitor cell support in 693 patients with follicular lymphoma: an EBMT registry study. Leukaemia. 2007;21(11):2324–2331. doi: 10.1038/sj.leu.2404850. [DOI] [PubMed] [Google Scholar]
- 12.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(12):5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham RN, Gooley TA, Keeney GE, Press OW, Pagel JM, Greisman HA, et al. The impact of histologic grade on the outcome of high-dose therapy and autologous stem cell transplantation for follicular lymphoma. Bone Marrow Transplant. 2007;40(11):1039–1044. doi: 10.1038/sj.bmt.1705864. [DOI] [PubMed] [Google Scholar]
- 14.Vose JM, Bierman PJ, Loberiza FR, Lynch JC, Bociek GR, Weisenburger DD, et al. Long-term outcomes of autologous stem cell transplantation for follicular non-Hodgkin lymphoma: effect of histological grade and Follicular International Prognostic Index. Biol Blood Marrow Transplant. 2008;14(1):36–42. doi: 10.1016/j.bbmt.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Hamadani M, Saber W, Ahn KW, Carreras J, Cairo MS, Fenske TS, et al. Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol Blood Marrow Transplant. 2013;19(5):746–753. doi: 10.1016/j.bbmt.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24(2):203–216. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 20.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson SP, Canals C, Luang JJ, Tilly H, Crawley C, Cahn JY, et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: an analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplant. 2013;48(11):1409–1414. doi: 10.1038/bmt.2013.83. [DOI] [PubMed] [Google Scholar]
- 24.Wahlin BE, Yri OE, Kimby E, Holte H, Delabie J, Smeland EB, et al. Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br J Haematol. 2012;156(2):225–233. doi: 10.1111/j.1365-2141.2011.08942.x. [DOI] [PubMed] [Google Scholar]
- 25.Chau I, Jones R, Cunningham D, Wotherspoon A, Maisey N, Norman AR, et al. Outcome of follicular lymphoma grade 3: is anthracycline necessary as front-line therapy? Br J Cancer. 2003;89(1):36–42. doi: 10.1038/sj.bjc.6601006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsi ED, Mirza I, Lozanski G, Hill J, Pohlman B, Karafa MT, et al. A clinicopathologic evaluation of follicular lymphoma grade 3A versus grade 3B reveals no survival differences. Arch Pathol Lab Med. 2004;128(8):863–868. doi: 10.5858/2004-128-863-ACEOFL. [DOI] [PubMed] [Google Scholar]
- 27.Hans CP, Weisenburger DD, Vose JM, Hock LM, Lynch JC, Aoun P, et al. A significant diffuse component predicts for inferior survival in grade 3 follicular lymphoma, but cytologic subtypes do not predict survival. Blood. 2003;101(6):2363–2367. doi: 10.1182/blood-2002-07-2298. [DOI] [PubMed] [Google Scholar]
- 28.Shustik J, Quinn M, Connors JM, Gascoyne RD, Skinnider B, Sehn LH. Follicular non-Hodgkin lymphoma grades 3A and 3B have a similar outcome and appear incurable with anthracycline-based therapy. Ann Oncol. 2011;22(5):1164–1169. doi: 10.1093/annonc/mdq574. [DOI] [PubMed] [Google Scholar]
- 29.Branson K, Chopra R, Kottaridis PD, McQuaker G, Parker A, Schey S, et al. Role of non-myeloablative allogeneic stem-cell transplantation after failure of autologous transplantation in patients with lymphoproliferative malignancies. J Clin Oncol. 2002;20(19):4022–4031. doi: 10.1200/JCO.2002.11.088. [DOI] [PubMed] [Google Scholar]
- 30.Hosing C, Saliba RM, McLaughlin P, Andersson B, Rodriguez MA, Fayad L, et al. Long-term results favor allogeneic over autologous hematopoietic stem cell transplantation in patients with refractory or recurrent indolent non-Hodgkin's lymphoma. Ann Oncol. 2003;14(5):737–744. doi: 10.1093/annonc/mdg200. [DOI] [PubMed] [Google Scholar]
- 31.van Besien K, Loberiza FR, Jr, Bajorunaite R, Armitage JO, Bashey A, Burns LJ, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102(10):3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 32.van Besien KW, de Lima M, Giralt SA, Moore DF, Jr, Khouri IF, Rondon G, et al. Management of lymphoma recurrence after allogeneic transplantation: the relevance of graft-versus-lymphoma effect. Bone Marrow Transplant. 1997;19(10):977–982. doi: 10.1038/sj.bmt.1700781. [DOI] [PubMed] [Google Scholar]
- 33.Mandigers CM, Verdonck LF, Meijerink JP, Dekker AW, Schattenberg AV, Raemaekers JM. Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003;32(12):1159–1163. doi: 10.1038/sj.bmt.1704290. [DOI] [PubMed] [Google Scholar]
- 34.Lenz G, Dreyling M, Schiegnitz E, Forstpointner R, Wandt H, Freund M, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(9):2667–2674. doi: 10.1182/blood-2004-03-0982. [DOI] [PubMed] [Google Scholar]
- 35.Sebban C, Mounier N, Brousse N, Belanger C, Brice P, Haioun C, et al. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF-94 randomized study from the Groupe d'Etude des Lymphomes de l'Adulte (GELA) Blood. 2006;108(8):2540–2544. doi: 10.1182/blood-2006-03-013193. [DOI] [PubMed] [Google Scholar]
- 36.Gyan E, Foussard C, Bertrand P, Michenet P, le Gouill S, Berthou C, et al. High-dose therapy followed by autologous purged stem cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by the GOELAMS with final results after a median follow-up of 9 years. Blood. 2009;113(5):995–1001. doi: 10.1182/blood-2008-05-160200. [DOI] [PubMed] [Google Scholar]
- 37.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24(7):1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.