Abstract
Purpose of review
Adipose tissue (AT) is a critical endocrine and immunological organ that regulates systemic energy homeostasis. During the pathogenesis of obesity, adipocyte hypertrophy is accompanied by AT inflammation, impeding insulin sensitivity and endocrine function of AT and other tissues. Adipocyte cholesterol accumulates in proportion to triglyceride (TG) as adipocytes undergo hypertrophy. Recent studies suggest that dietary cholesterol contributes to increased adipocyte cholesterol. However, how dietary cholesterol accumulates in adipocytes and its metabolic consequences are poorly understood. This review summarizes recent advances in knowledge of adipocyte cholesterol balance and highlights the emerging role of dietary cholesterol in AT cholesterol balance, inflammation, and systemic energy metabolism.
Recent findings
Perturbation of cholesterol balance in adipocytes alters intracellular cholesterol distribution and modulates adipocyte insulin and proinflammatory signaling. Adipocyte cholesterol levels are maintained by a balance between dietary cholesterol uptake from TG-enriched lipoproteins and cellular cholesterol efflux to high density lipoprotein. Recent animal studies established a critical role for dietary cholesterol in promoting AT inflammation, thereby worsening obesity-mediated metabolic complications.
Summary
Recent studies identified high dietary cholesterol as a potentiator of AT inflammation and dysfunction. Reducing excessive dietary cholesterol intake is suggested as a simple, but novel, way to attenuate obesity-associated metabolic diseases.
Keywords: Adipose tissue, dietary cholesterol, adipose inflammation, adipocyte size, obesity
Introduction
Adipose tissue (AT) is a versatile and active endocrine organ [1]. Adipocytes secrete hormones (adipokines) [2], store excess energy as triglyceride (TG), release fatty acids (FA) during fasting, contribute to systemic glucose disposal [3], and participate in HDL biogenesis [4]. During the progression of obesity, in which chronic energy intake exceeds expenditure, AT undergoes significant cellular changes, so-called “adipose tissue remodeling”, triggering hypertrophic adipocyte expansion and AT immune cell infiltration [5-7]. Enlarged adipocytes exhibit decreased insulin responsiveness, decreased glucose uptake, and increased secretion of proinflammatory adipokines [8-11]. Cholesterol accumulation and redistribution from the plasma membrane to the lipid droplet (LD) monolayer is a crucial determinant of adipocyte function during hypertrophic expansion [12-18]. Rodent models of diet-induced obesity using a high fat (HF) diet are well-established to promote adipocyte hypertrophy and AT macrophage infiltration [19, 20]; however, little is known regarding the role of dietary cholesterol in these processes. New studies, summarized below, now suggest an important role for dietary cholesterol as an independent inducer of adipocyte hypertrophy and AT inflammation.
This review consists of three parts in which we summarize the current knowledge concerning: 1) cholesterol as a determinant of adipocyte size and function, 2) regulators of adipocyte cholesterol balance, and 3) effects of dietary cholesterol on AT inflammation.
Cholesterol as a determinant of adipocyte size and function
AT is a major site for free cholesterol (FC) storage in humans. About 25% of the body pool of cholesterol is stored in adipose depots, which can increase to ~50% in obese states [21]. As adipocyte hypertrophy progresses during obesity development, cellular cholesterol content increases [12]. A distinguishing feature of adipocyte cholesterol compared to other cholesterol-laden cell types is the relative lack of esterified cholesterol; >95% of adipose cholesterol is FC [14]. Due to its polar carbon 3 hydroxyl group in the steroid backbone, FC has limited solubility (2-3%) in the core of LDs [22]. However, FC does accumulate in the LD phospholipid (PL) monolayer interface and in the plasma membrane [23]. During hypertrophic expansion, adipocytes acquire more FC and redistribute FC from the plasma membrane to the LD interface, resulting in a relative deletion of plasma membrane FC and enrichment of LD monolayer FC [12-18]. For example, the plasma membrane FC/protein ratio decreases 40-50% in larger (50 μm diameter) vs. small (40 μm diameter) adipocytes isolated from lean rats [15] and in subcutaneous adipocytes isolated from obese vs. lean rats [16]. Differentiation of 3T3-L1 preadipocytes into TG-enriched adipocytes is associated with decreased plasma membrane FC [24].
Adipocyte FC content affects adipocyte function. For example, methyl-β-cyclodextrin-mediated cholesterol depletion of 3T3-L1 adipocytes results in defective glucose uptake and oxidation, diminished GLUT-4 expression, and impaired insulin signaling [15, 25]. Decreasing 3T3-L1 adipocyte FC content by incubation with methyl-β-cyclodextrin, apolipoprotein A-I (apoA-I), or HDL decreased plasma membrane lipid rafts, leading to decreased palmitate-induced inflammation, reactive oxygen species, and monocyte chemotactic factor expression [26]. In vivo, transgenic overexpression of apoA-I in mice fed a diet high in fat, sucrose, and cholesterol led to decreased AT inflammation and macrophage content compared with non-transgenic controls, presumably by reducing adipocyte FC content [26]. These combined results suggest that optimal adipocyte membrane FC is necessary to preserve normal adipocyte function.
Regulators of adipocyte cholesterol balance
Adipocytes acquire cholesterol through de novo biosynthesis and lipoprotein uptake, and eliminate excess FC by protein-mediated efflux. De novo cholesterol biosynthesis is reportedly very low in adipocytes [12, 13] and uptake of plasma low density lipoprotein (LDL) by the LDL receptor is also low relative to the liver [27]. Adipocytes also bind lipoproteins via the scavenger receptor class B, member 1 (SR-BI), low density lipoprotein receptor-related protein 1 (LRP1), and the very low density lipoprotein (VLDL) receptor, providing additional pathways for cholesterol uptake from circulating plasma lipoproteins [28-31].
A direct correlation between dietary cholesterol levels and adipocyte cholesterol content was first identifed in a rat model [13]. In general, dietary cholesterol levels appear more strongly correlated with adipocyte cholesterol content than plasma cholesterol concentrations [13, 21]. This relationship likely results from adipocyte uptake of chylomicron, chylomicron remnant, and VLDL particles that contain dietary cholesterol cargo and apolipoprotein E (apoE), a ligand for the VLDL receptor (VLDLR) and LRP receptors [29-33]. ApoE appears important in adipocyte TG accumulation, since AT from apoE knockout mice has less TG compared to wild-type mice [34, 35]. Insulin stimulates the activity of LRP on primary adipocytes, resulting in increased uptake of cholesteryl esters from apoE-enriched β-VLDL [32]. The VLDLR binds to apoE-containing TG-rich lipoproteins, enhancing lipoprotein lipase (LPL) activity and cellular lipid entry [36]. Although not expressed in adipocyte precursor cells, VLDLR expression is upregulated in differentiated adipocytes [37], allowing the simultaneous acquisition of dietary cholesterol and TG from lipoproteins. Mice lacking the VLDLR were leaner and resistant to the HF diet-mediated weight gain due to reduced LPL activity and attenuated FA uptake [38]. Nguyen et al. reported that VLDLR deficiency also significantly attenuated adipocyte inflammation mediated by a high-fat diet, confirming that VLDLR expression is crucial in regulating adipocyte size and function [39]. Intriguingly, deletion of proprotein convertase subtilisin/kexin 9 (PCSK9), which normally promotes liver LDLR degradation, increased TG accumulation in visceral fat by enhancing expression of VLDLR in adipocytes [40]. Taken together, these studies suggest that circulating TG-rich lipoproteins are the primary source of adipocyte cholesterol and that cholesterol uptake occurs in association with TG accrual through membrane-anchored lipoprotein receptors, such as VLDLR and LPR.
Excess adipocyte FC is effluxed to apoA-I and other amphipathic apolipoproteins via the ATP binding cassette transporter A1 (ABCA1), forming nascent HDL particles, or to plasma HDL particles by SR-BI and ATP binding cassette transporter G1 (ABCG1) [41]. Zhang et al first showed that adipocyte cholesterol transporters were important for adipocyte cholesterol efflux [42]. Using differentiated mouse embryonic fibroblasts from Abca1−/−, Sr-b1−/− and Abcg1−/− mice, they identified ABCA1 and SR-BI, but not ABCG1, as major cholesterol transporters for effluxing FC from adipocytes to apoAI and HDL, repectively [42].
A critical in vivo role for ABCA1 in adipocyte cholesterol efflux and cellular cholesterol homeostasis was elucidated using Abca1flox/flox mice crossed with aP2 Cre recombinase mice to generate adipocyte-specific Abca1 knockout (ASKO) mice [4]. ASKO mice generated by this strategy had almost no ABCA1 protein expression in adipocytes, but partial and variable macrophage expression. ASKO mice had a striking increase in adipocyte cholesterol content and a small (~15%), but significant, decrease in plasma HDL cholesterol concentrations, suggesting that adipocyte ABCA1 provides a finite source of the plasma HDL cholesterol pool [4]. In another study, high fat-high cholesterol (HFHC)-fed ASKO mice demonstrated increased body weight, larger fat pad mass, increased AT cholesterol content, and impaired glucose tolerance and insulin sensitivity compared to control mice [43]. However, interpretation of the systemic metabolic results from the latter study is confounded by the partial and variable ABCA1 expression in macrophages, which are the main source of AT pro-inflammatory cytokine production that reduce insulin sensitivity [44]. Future studies using adipocyte-specific ABCA1 deletion with intact macrophage ABCA1 expression will be necessary to clarify the role of ABCA1 in adipocyte function.
Although in vitro studies suggest ABCG1 does not play a significant role in adipocyte cholesterol efflux [42], an in vivo study showed that whole body ABCG1 knockout mice consuming a high-fat diet weighed less than their wild-type counterparts and had smaller fat pads and reduced adipocyte size, presumably due to increased energy expenditure and body temperature and decreased food intake [45]. The high fat-fed ABCG1 knockout mice had minimal changes in systemic metabolism, including decreased non-esterified FA after a 24-hour fast and improved plasma glucose clearance. A recent study showed that ABCG1 silencing in 3T3 adipocytes resulted in decrease TG accumulation, smaller lipid droplet size, and decreased activity of lipoprotein lipase (LPL), an enzyme that hydrolyzes TG to FA for uptake by cells [46].
Paradoxically, although FC efflux from 3T3 cells was compromised with ABCG1 silencing, cellular FC was actually decreased to ~33% of that in control cells [46]. Local silencing of ABCG1 in epididymal white adipose tissue (eWAT) of HFHC-fed mice using lentiviruses expressing short hairpin RNAs resulted in decreased body weight, and smaller eWAT pads and adipocytes, with no change in food intake or physical activity relative to mice injected with control lentivirus [46]. The authors suggest that defective sphingomyelin efflux in the absence of ABCG1 expression results in increased plasma membrane sphingomyelin and lipid raft content, which in turn, inhibits LPL activity, limiting uptake of FA and TG accretion by adipocytes.
Supporting the in vitro and mouse studies are results demonstrating an ABCG1 haplotype that is associated with increased adiposity in humans [46]. However, as with ABCA1 studies summarized above, results of studies using whole body ABCG1 knockout mice or eWAT silencing of ABCG1 should be interpreted with caution, since macrophage ABCG1 expression was also diminished in both studies. Clarification of the role of adipocyte ABCG1 in obesity and metabolism must await studies using adipocyte-specific ABCG1 knockout mice.
Another recent study using knockout mice and bone marrow transplantation experiments demonstrated that adipocyte phospholipid transfer protein (PLTP) stimulates adipocyte FC efflux and contributes a small, but significant, pool of plasma HDL cholesterol [47]. These studies suggest that adipocyte PL efflux increases adipocyte FC efflux and may contribute to the overall regulation of adipocyte FC:TG ratio.
Although adipocyte expression of ABCA1 and ABCG1 and FC efflux are proportional to adipocyte size and TG content, the reason for this association is not clear. A recent study suggests that cholesterol efflux from adipocytes to apoA-I and HDL, presumably via ABCA1 and ABCG1, respectively, activates hormone-sensitive lipase-induced lipolysis of adipocyte TG, simultaneously decreasing adipocyte FC and TG [48]. This process likely maintains a similar FC:TG ratio as adipocytes become smaller, and may be a compensatory mechanism to maintain optimal adipocyte function as adipocyte size decreases or increases during TG lipolysis or accretion.
Additional questions remain regarding adipocyte FC content and adipocyte cell death [49], other dietary constituents (e.g., phospholipids, n-3 polyunsaturated fatty acids [50, 51], or vitamin D [52]) that may influence adipose tissue cholesterol balance, and unidentified membrane-bound or intracellular regulators controlling adipocyte cholesterol balance.
Effects of dietary cholesterol on AT inflammation
The general rule that large adipocytes are more inflamed than smaller ones is well-established by numerous studies showing that overnutrition instigates AT remodeling [5]. Although dietary cholesterol may be a primary source of AT cholesterol, the relationship between dietary cholesterol and adipose inflammation is not fully understood. For example, inhibition of intestinal cholesterol absorption with ezetimibe in mice did not affect AT inflammation or adipocyte size, despite less weight gain and reduced plasma serum amyloid protein concentrations compared to control mice [53]. However, another study observed improved insulin sensitivity in ezetimibe-treated mice fed a HFHC diet [54]. A confounding feature of these studies is a decrease in absorption of saturated FA, which may independently affect AT and insulin resistance outcomes [53, 54].
A recent study in patients with hypercholesterolemia demonstrated that combination ezetimibe-simvastatin treatment decreased plasma leptin, visfatin, tumor necrosis factor α, and FA concentrations, and increased adiponectin levels compared to patients treated with simvastatin alone [55]. Furthermore, the effect of the combined therapy on markers of systemic inflammation was stronger in insulin-resistant than in insulin-sensitive individuals. These results are compatible with the hypothesis that blocking cholesterol absorption reduces AT inflammation.
A seminal study by Subramanian et al demonstrated that mice fed dietary cholesterol (0.15% w/w) had increased HF diet-induced weight gain, adipocyte size, macrophage infiltration, and proinflammatory cytokine expression compared with mice fed an isocaloric HF diet without added cholesterol, establishing a positive association between dietary cholesterol and AT inflammation [19]. Dietary cholesterol was also associated with attenuated insulin sensitivity and increased atherosclerosis [19].
However, rodents and humans have distinct differences in cholesterol metabolism [27]. To overcome this limitation, Chung et al designed a study to determine the role of dietary cholesterol in adipocyte hypertrophy and inflammation in nonhuman primates (African green monkeys) [56], which are phylogenetically more similar to humans than rodents. Monkeys were fed one of three isocaloric diets containing 0.002, 0.2 or 0.4 mg cholesterol/kcal for 10 weeks. Increasing dietary cholesterol resulted in increased adipocyte size and adipose tissue FC accumulation, macrophage content, and proinflammatory gene expression in visceral, but not subcutaneous, fat. In visceral fat, increased dietary cholesterol intake was also associated with decreased expression of genes involved in cholesterol biosynthesis and lipoprotein uptake and increased expression of proteins involved in cholesterol efflux. Interestingly, these changes occurred without significant changes in body weight among diet groups, differing from results in high fat-high cholesterol diet-fed mice [19]. Why visceral fat had a different response to increased dietary cholesterol versus subcutaneous fat is unknown and will require further investigation.
Although the metabolic consequences of high-cholesterol diets in mice and nonhuman primates were not identical, the fundamental concept that dietary cholesterol promotes proinflammatory remodeling of adipose tissue (i.e., adipocyte hypertrophy and macrophage infiltration) was firmly established by these studies.
Another intriguing question is whether dietary cholesterol affects WAT browning, characterized by the emergence of brown-like adipocytes (beige) within WAT that dissipate energy via mitochondrial uncoupling of oxidative phosphorylation and ATP production. Currently, no studies have tested the role of dietary cholesterol on WAT browning. However, recent studies have shown that M2 macrophage polarization promotes WAT browning [57, 58], whereas obesity-mediated toll-like receptor 4 activation of M1 macrophages abrogates WAT browning [59]. Given recent evidence that high dietary cholesterol intake promotes proinflammatory WAT remodeling, it is likely that dietary cholesterol intake is negatively associated with WAT browning, although this idea has not been formally tested.
Conclusion
FC is a metabolically dead-end molecule – it cannot be degraded by cells and is toxic at high concentrations. Hence, most cells restrict FC accumulation and toxicity by esterifying FC to inert cholesteryl esters. However, unlike other cells, adipocytes can accumulate high levels of FC as cell size increases with TG storage. Adipocyte FC content, a critical determinant of adipocyte size and function, is maintained by a balance between dietary cholesterol uptake through circulating lipoproteins and FC efflux to apolipoproteins and HDL. Recent data support the concept that increased dietary cholesterol promotes adipose tissue remodeling and inflammation (Figure 1). Whether these initial findings in rodents and nonhuman primates will be confirmed in humans must await future studies. Based on available data in animal models, AT cholesterol content appears to be a novel and viable target to attenuate AT inflammation and attendant metabolic complications. A recent human study supports the notion that inhibiting intestinal absorption may attenuate AT inflammation in the absence of body weight loss [55]. Thus, recommending a prudent intake of dietary cholesterol potentially could be a simple strategy to lessen adipocyte hypertrophy and dysfunction, even without weight loss.
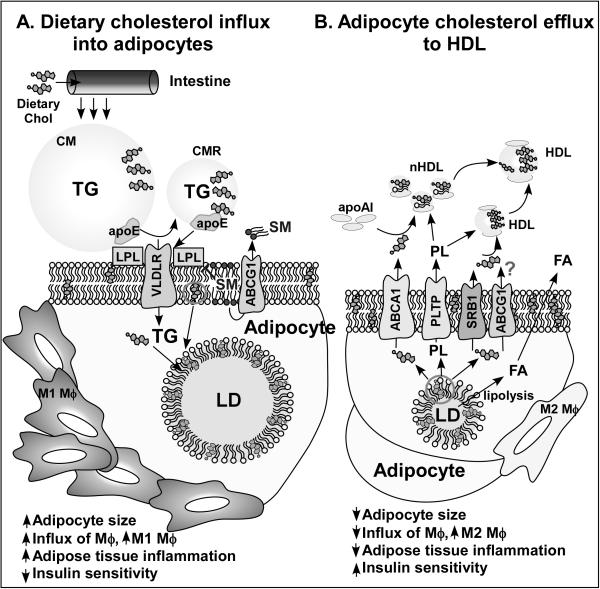
Figure 1. Hypothetical model for regulation of adipose inflammation by dietary cholesterol.
A. Dietary cholesterol enters the blood circulation in chylomicron particles. CM triglyceride is hydrolyzed to free fatty acids by lipoprotein lipase, resulting in CM remnant particles, FA uptake, and re-esterification into TG that is stored in the adipocyte lipid droplet. CMR, which are enriched with dietary cholesterol, bind to VLDL receptors expressed on the adipocyte plasma membrane through apolipoprotein E and are internalized, leading to a simultaneous increase in TG and free cholesterol content as adipocytes hypertrophy. During adipocyte hypertrophy, FC redistributes from the plasma membrane to the LD monolayer, resulting in a relative depletion of plasma membrane FC, which leads to adipocyte dysfunction, increased adipose tissue inflammation, and decreased insulin sensitivity. Optimal plasma membrane sphingomyelin content, maintained by ABCG1, appears necessary to maintain lipoprotein lipase activity for CM TG hydrolysis and adipocyte FA uptake. B. When adipocyte size decreases during LD TG hydrolysis to FA, adipocyte FC content is decreased via FC efflux to apoA-I, mediated by ABCA1, and to HDL, mediated by SRBI and probably by ABCG1, helping maintain a relativity constant FC:TG ratio for optimal adipocyte function. Thus, as adipocyte size and TG content is reduced, FC from the lipid droplet redistributes to the plasma membrane, restoring insulin sensitivity and reducing adipose tissue inflammation. Adipocyte phospholipid transfer protein activity may also be necessary for optimal FC efflux and nascent HDL particle formation. ABCA1, ATP binding cassette transporter A1; CM, chylomicron; CMR, chylomicron remnants; FA, free fatty acid; FC, free cholesterol; HDL, high-density lipoprotein; LD, lipid droplet; LPL, lipoprotein lipase; mϕ, macrophage; nHDL, nascent HDL; PLTP, phospholipid transfer protein; SM, sphingomyelin; SRBI, scavenger receptor class B, member 1; TG, triglyceride; VLDLR, very low-density lipoprotein receptor.
Key Points.
During progression of obesity, adipocyte size increases with the accumulation of TG and FC, and adipose tissue inflammation and adipocyte dysfunctional ensues.
Although excess caloric consumption partially mediates obesity, the role of individual dietary constituents, particularly cholesterol, in development of obesity is poorly understood.
Emerging evidence supports a critical role for adipocyte FC in controlling adipocyte size and function, and several key proteins (VLDLR, ABCA1, ABCG1, PLTP, and SR-BI) have recently been identified as important in maintaining adipocyte FC homeostasis.
Recent studies demonstrates that high dietary cholesterol consumption leads to increased adipose tissue inflammation and dysfunction in mice and nonhuman primates, and that inhibition of cholesterol absorption with ezetimibe results in reduced plasma biomarkers of adipose tissue inflammation in hypercholesterolemic subjects. These combined results suggest that reducing high dietary cholesterol intake may be an efficient way to reduce adipose tissue inflammation and dysfunction.
Acknowledgments
The authors thank Karen Klein (Biomedical Research Services and Administration at Wake Forest School of Medicine) for editing the manuscript.
Financial support and sponsorship
This work was supported by National Institutes of Health Grants R01 HL119962 (JSP) and American Heart Association Scientist Development Grant 13SDG14410043 (SC).
Footnotes
Conflicts of interest
None
References
- 1*.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–59. doi: 10.1530/JOE-13-0339. [This is a topical review on the role of adipokines in metabolic diseases.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–e19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegelman BM, Flier JS. Adipogenesis and Obesity: Rounding Out the Big Picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 4.Chung S, Sawyer JK, Gebre AK, et al. Adipose tissue ATP binding cassette transporter A1 contributes to high-density lipoprotein biogenesis in vivo. Circulation. 2011;124:1663–1672. doi: 10.1161/CIRCULATIONAHA.111.025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of Clinical Investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. The Journal of Clinical Investigation. 1968;47:153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler G, Kiss S, Keszthelyi L, et al. Expression of tumor necrosis factor (TNF)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C-peptide level. European Journal of Endocrinology. 2003;149:129–135. doi: 10.1530/eje.0.1490129. [DOI] [PubMed] [Google Scholar]
- 10.Smith U. Effect of cell size on lipid synthesis by human adipose tissue in vitro. J Lipid Res. 1971;12:65–70. [PubMed] [Google Scholar]
- 11.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. Journal of Clinical Endocrinology & Metabolism. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 12.Kovanen PT, Nikkilä EA, Miettinen TA. Regulation of cholesterol synthesis and storage in fat cells. Journal of Lipid Research. 1975;16:211–223. [PubMed] [Google Scholar]
- 13.Angel A, Farkas J. Regulation of cholesterol storage in adipose tissue. J Lipid Res. 1974;15:491–499. [PubMed] [Google Scholar]
- 14.Schreibman PH, Dell RB. Human adipocyte cholesterol. Concentration, localization, synthesis, and turnover. The Journal of Clinical Investigation. 1975;55:986–993. doi: 10.1172/JCI108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Lay S, Krief S, Farnier C, et al. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J Biol Chem. 2001;276:16904–16910. doi: 10.1074/jbc.M010955200. [DOI] [PubMed] [Google Scholar]
- 16.Guerre-Millo M, Guesnet P, Guichard C, et al. Alteration in membrane lipid order and composition in metabolically hyperactive fatty rat adipocytes. Lipids. 1994;29:205–209. doi: 10.1007/BF02536730. [DOI] [PubMed] [Google Scholar]
- 17.Krause BR, Hartman AD. Relationship between cell size, plasma cholesterol and rat adipocyte cholesterol storage. Biochim Biophys Acta. 1976;450:197–205. doi: 10.1016/0005-2760(76)90091-6. [DOI] [PubMed] [Google Scholar]
- 18.Bjorntorp P, Sjostrom L. The composition and metabolism in vitro of adipose tissue fat cells of different sizes. Eur J Clin Invest. 1972;2:78–84. doi: 10.1111/j.1365-2362.1972.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian S, Han CY, Chiba T, et al. Dietary Cholesterol Worsens Adipose Tissue Macrophage Accumulation and Atherosclerosis in Obese LDL Receptor–Deficient Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Members WG, Roger VL, Go AS, et al. Heart Disease and Stroke Statistics—2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause BR, Hartman AD. Adipose tissue and cholesterol metabolism. Journal of Lipid Research. 1984;25:97–110. [PubMed] [Google Scholar]
- 22.Miller KW, Small DM. Triolein-cholesteryl oleate-cholesterol-lecithin emulsions: structural models of triglyceride-rich lipoproteins. Biochemistry. 1983;22:443–451. doi: 10.1021/bi00271a030. [DOI] [PubMed] [Google Scholar]
- 23.Prattes S, Horl G, Hammer A, et al. Intracellular distribution and mobilization of unesterified cholesterol in adipocytes: triglyceride droplets are surrounded by cholesterol-rich ER-like surface layer structures. Journal of Cell Science. 2000;113:2977–2989. doi: 10.1242/jcs.113.17.2977. [DOI] [PubMed] [Google Scholar]
- 24.Storch J, Shulman SL, Kleinfeld AM. Plasma membrane lipid order and composition during adipocyte differentiation of 3T3F442A cells. Studies in intact cells with 1-[4-(trimethylamino)phenyl]-6-phenylhexatriene. J Biol Chem. 1989;264:10527–10533. [PubMed] [Google Scholar]
- 25.Parpal S, Karlsson M, Thorn H, Strålfors P. Cholesterol Depletion Disrupts Caveolae and Insulin Receptor Signaling for Metabolic Control via Insulin Receptor Substrate-1, but Not for Mitogen-activated Protein Kinase Control. Journal of Biological Chemistry. 2001;276:9670–9678. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- 26.Umemoto T, Han CY, Mitra P, et al. Apolipoprotein AI and High-Density Lipoprotein Have Anti-Inflammatory Effects on Adipocytes via Cholesterol Transporters: ATP-Binding Cassette A-1, ATP-Binding Cassette G-1, and Scavenger Receptor B-1. Circulation Research. 2013;112:1345–1354. doi: 10.1161/CIRCRESAHA.111.300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 28.Kraemer FB, Laane C, Park B, Sztalryd C. Low-density lipoprotein receptors in rat adipocytes: regulation with fasting. Am J Physiol. 1994;266:E26–32. doi: 10.1152/ajpendo.1994.266.1.E26. [DOI] [PubMed] [Google Scholar]
- 29.Desai KS, Steiner G, Takeuchi I, Hollenberg CH. Very low density lipoprotein binding to adipocytes. Biochim Biophys Acta. 1980;620:341–351. doi: 10.1016/0005-2760(80)90125-3. [DOI] [PubMed] [Google Scholar]
- 30.Barbaras R, Grimaldi P, Negrel R, Ailhaud G. Binding of lipoproteins and regulation of cholesterol synthesis in cultured mouse adipose cells. Biochim Biophys Acta. 1985;845:492–501. doi: 10.1016/0167-4889(85)90215-0. [DOI] [PubMed] [Google Scholar]
- 31.Fazio S, Linton MF. Unique pathway for cholesterol uptake in fat cells. Arterioscler Thromb Vasc Biol. 2004;24:1538–1539. doi: 10.1161/01.ATV.0000140821.25572.1b. [DOI] [PubMed] [Google Scholar]
- 32.Descamps O, Bilheimer D, Herz J. Insulin stimulates receptor-mediated uptake of apoE-enriched lipoproteins and activated alpha 2-macroglobulin in adipocytes. Journal of Biological Chemistry. 1993;268:974–981. [PubMed] [Google Scholar]
- 33.Vassiliou G, McPherson R. A novel efflux-recapture process underlies the mechanism of high-density lipoprotein cholesteryl ester-selective uptake mediated by the low-density lipoprotein receptor-related protein. Arterioscler Thromb Vasc Biol. 2004;24:1669–1675. doi: 10.1161/01.ATV.0000134295.09932.60. [DOI] [PubMed] [Google Scholar]
- 34.Huang ZH, Reardon CA, Mazzone T. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 2006;55:3394–3402. doi: 10.2337/db06-0354. [DOI] [PubMed] [Google Scholar]
- 35*.Lasrich D, Bartelt A, Grewal T, Heeren J. Apolipoprotein E promotes lipid accumulation and differentiation in human adipocytes. Experimental cell research. 2015;337:94–102. doi: 10.1016/j.yexcr.2015.07.015. [This study demonstrated that silencing of apoE in human adipocytes impairs TG accumulation and expression of genes involved in initiation of adipocyte differentiation.] [DOI] [PubMed] [Google Scholar]
- 36.Takahashi S, Suzuki J, Kohno M, et al. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- 37.Tao H, Hajri T. Very low density lipoprotein receptor promotes adipocyte differentiation and mediates the proadipogenic effect of peroxisome proliferator-activated receptor gamma agonists. Biochem Pharmacol. 2011;82:1950–1962. doi: 10.1016/j.bcp.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Yagyu H, Lutz EP, Kako Y, et al. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem. 2002;277:10037–10043. doi: 10.1074/jbc.M109966200. [DOI] [PubMed] [Google Scholar]
- 39**.Nguyen A, Tao H, Metrione M, Hajri T. Very Low Density Lipoprotein Receptor (VLDLR) Expression Is a Determinant Factor in Adipose Tissue Inflammation and Adipocyte-Macrophage Interaction. J Biol Chem. 2014;289:1688–1703. doi: 10.1074/jbc.M113.515320. [This report shows that VLDLR expression in adipocytes and macrophages is an important contributor of TG accumulation, inflammation, and ER stress in high fat diet-induced obesity in mice. The authors found a synergetic interaction between adipocyte and macrophage inflammatory signaling that exacerbates overall adipose tissue inflammation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roubtsova A, Munkonda MN, Awan Z, et al. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler Thromb Vasc Biol. 2011;31:785–791. doi: 10.1161/ATVBAHA.110.220988. [DOI] [PubMed] [Google Scholar]
- 41.Rosenson RS, Brewer HB, Davidson WS, et al. Cholesterol Efflux and Atheroprotection. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, McGillicuddy FC, Hinkle CC, et al. Adipocyte modulation of high-density lipoprotein cholesterol. Circulation. 2010;121:1347–1355. doi: 10.1161/CIRCULATIONAHA.109.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.de Haan W, Bhattacharjee A, Ruddle P, et al. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. J Lipid Res. 2014;55:516–523. doi: 10.1194/jlr.M045294. [This study shows that genetic deletion of adipocyte and macrophage ABCA1 in high fat diet-fed mice results in increased weight gain and fat mass and defective glucose tolerance and systemic insulin sensitivity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odegaard JI, Ganeshan K, Chawla A. Adipose tissue macrophages: Amicus adipem? Cell Metab. 2013;18:767–768. doi: 10.1016/j.cmet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchmann J, Meyer C, Neschen S, et al. Ablation of the cholesterol transporter adenosine triphosphate-binding cassette transporter G1 reduces adipose cell size and protects against diet-induced obesity. Endocrinology. 2007;148:1561–1573. doi: 10.1210/en.2006-1244. [DOI] [PubMed] [Google Scholar]
- 46**.Frisdal E, Le Lay S, Hooton H, et al. Adipocyte ATP-binding cassette G1 promotes triglyceride storage, fat mass growth, and human obesity. Diabetes. 2015;64:840–855. doi: 10.2337/db14-0245. [The authors demonstrate that silencing adipocyte ABCG1 decreases LPL activity and TG accumulation through decreased PPARγ activation. They also show that a single nucleotide polymorphism in ABCG1 that results in increased expression is associated with human obesity.] [DOI] [PubMed] [Google Scholar]
- 47*.Jiang H, Yazdanyar A, Lou B, et al. Adipocyte phospholipid transfer protein and lipoprotein metabolism. Arterioscler Thromb Vasc Biol. 2015;35:316–322. doi: 10.1161/ATVBAHA.114.303764. [This study shows that adipocyte PLTP activity plays a small but significant contribution to plasma PLTP activity and promotes adipose tissue cholesterol efflux.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Wei H, Averill MM, McMillen TS, et al. Modulation of adipose tissue lipolysis and body weight by high-density lipoproteins in mice. Nutr Diabetes. 2014;4:e108. doi: 10.1038/nutd.2014.4. [The authors demonstrate that apoA-I and HDL stimulate adipocyte hormone-sensitive lipase lipolytic activity, presumably through regulation of adipocyte plasma membrane cholesterol content.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giordano A, Murano I, Mondini E, et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54:2423–2436. doi: 10.1194/jlr.M038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.LeMieux MJ, Kalupahana NS, Scoggin S, Moustaid-Moussa N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J Nutr. 2015;145:411–417. doi: 10.3945/jn.114.202952. [This study shows that eicosapentaenoic acid supplementation of high fat-fed mice results in decreased adipocyte hypertrophy and inflammation without affecting body weight.] [DOI] [PubMed] [Google Scholar]
- 51**.Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metabolism. 2015;22:658–668. doi: 10.1016/j.cmet.2015.07.026. [The authors demonstrate an interaction between dietary fat saturation and white adipose tissue inflammation and insulin resistance that depends on Toll-like receptor signaling and is independent of adiposity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caron-Jobin M, Morisset AS, Tremblay A, et al. Elevated serum 25(OH)D concentrations, vitamin D, and calcium intakes are associated with reduced adipocyte size in women. Obesity (Silver Spring) 2011;19:1335–1341. doi: 10.1038/oby.2011.90. [DOI] [PubMed] [Google Scholar]
- 53.Umemoto T, Subramanian S, Ding Y, et al. Inhibition of intestinal cholesterol absorption decreases atherosclerosis but not adipose tissue inflammation. J Lipid Res. 2012;53:2380–2389. doi: 10.1194/jlr.M029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labonte ED, Camarota LM, Rojas JC, et al. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/−mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G776–783. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Krysiak R, Zmuda W, Okopien B. The effect of simvastatin-ezetimibe combination therapy on adipose tissue hormones and systemic inflammation in patients with isolated hypercholesterolemia. Cardiovasc. Ther. 2014;32:40–46. doi: 10.1111/1755-5922.12057. [This study shows that hypercholesterolemic patients treated with combination ezetimibe-simvastatin compared to simvastatin only have decreased plasma biomarkers of adipose tissue inflammation. The combined therapy was more effective in insulin-resistant compared to insulin-sensitive subjects.] [DOI] [PubMed] [Google Scholar]
- 56**.Chung S, Cuffe H, Marshall SM, et al. Dietary cholesterol promotes adipocyte hypertrophy and adipose tissue inflammation in visceral, but not in subcutaneous, fat in monkeys. Arterioscler Thromb Vasc Biol. 2014;34:1880–1887. doi: 10.1161/ATVBAHA.114.303896. [This is the first study to demonstrate that increasing dietary cholesterol in nonhuman primates leads to increased adipose tissue inflammation and adipocyte hypertrophy in the absence of weight gain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee MW, Odegaard JI, Mukundan L, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu Y, Nguyen KD, Odegaard JI, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okla M, Wang W, Kang I, et al. Activation of Toll-like Receptor (TLR) 4 Attenuates Adaptive Thermogenesis via Endoplasmic Reticulum Stress. J Biol Chem. 2015 doi: 10.1074/jbc.M115.677724. [DOI] [PMC free article] [PubMed] [Google Scholar]