Abstract
Prenatal cocaine exposure (PCE) is associated with long-term and negative effect on arousal regulation. Recent neuroimaging studies have examined brain mechanisms related to arousal dysregulation with cross-sectional experimental designs; but longitudinal changes in the brain, reflecting group differences in neurodevelopment, have never been directly examined. To directly assess the interaction of PCE and neurodevelopment, the present study used a longitudinal design to analyze functional magnetic resonance imaging (fMRI) data collected from 33 adolescents (21 with PCE and 12 non-exposed controls) while they performed the same working memory task with emotional distracters at two points in time. The mean age of participants was 14.3 years at time_1 and 16.7 years at time_2. With confounding factors statistically controlled, the fMRI data revealed significant exposure-by-time interaction in the activations of the amygdala and default mode network (DMN). For the control adolescents, brain activations associated with emotional arousal (amygdala) and cognitive effort (DMN) were both reduced at time_2 as compared to that at time_1. However, these activation reductions were not observed in the PCE group, indicating persistently high levels of emotional arousal and cognitive effort. In addition, correlations between longitudinal changes in the brain and in behavior have shown that adolescents with persistently high emotional arousal were more likely in need of high cognitive effort; and their cognitive performance was more likely to be affected by distractive challenges. The present results complement and extend previous findings from cross-sectional studies with further evidence supporting the view of PCE associated long-term teratogenic effects on arousal regulation.
Keywords: prenatal cocaine exposure, adolescent, longitudinal design, functional magnetic resonance imaging, amygdala, default mode network
1. Introduction
Prenatal cocaine exposure (PCE) has long-term effect on arousal regulation with ample evidence reported in animal (Johns, Means et al. 1992, Romano and Harvey 1998, Campbell, Bliven et al. 2000, Salas-Ramireza, Frankfurt et al. 2010) as well as in human studies of neonates (Dipietro, Suess et al. 1995, Regalado, Schechtman et al. 1995, Karmel and Gardner 1996), infants (Bendersky and Lewis 1998, Coles, Bard et al. 1999, Bard, Coles et al. 2000, Schuetze and Eiden 2006, Schuetze, Eiden et al. 2007, Eiden, McAuliffe et al. 2009, Eiden, Veira et al. 2009), young children (Bandstra, Morrow et al. 2001, Dennis, Bendersky et al. 2006, Bada, Das et al. 2007, Kable, Coles et al. 2008, Chaplin, Fahy et al. 2009), and adolescents (Li, Coles et al. 2009, Chaplin, Freiburger et al. 2010, Lester, LaGasse et al. 2010, Li, Santhanam et al. 2011, Li, Santhanam et al. 2013). Specifically, individuals with PCE often exhibit a reduced threshold in response to perceived distractions, or salient but task-irrelevant stimuli, which in turn may compromise the availability of their attention resource for on-going cognition and behavior (Mayes, Grillon et al. 1998, Mayes 2002, Harvey 2004). Arousal regulation refers to how different stimuli are gated to different cortical regions and reflects one’s capability to adjust and allocate mental resources for distinct yet interactive streams of information processing (Damasio 1995). The behavioral manifestations in children with PCE thus suggest an imbalanced gating of stimulation to limbic and cognitive regions such that allocation of processing resource is inappropriately biased towards salient but task-irrelevant information (Mayes 2002).
In examining the neurobiological mechanisms of this PCE effect, our previous neuroimaging studies have shown that adolescents with PCE have reduced capacity to suppress brain activations in the amygdala (Li, Coles et al. 2009, Li, Santhanam et al. 2013) and in the default mode network (DMN) (Li, Santhanam et al. 2011), which is assumed to be involved in mediating task-irrelevant arousal. Neuroimaging studies from other groups also reported PCE-related morphological alterations in caudate (Avants, Hurt et al. 2007, Roussotte, Soderberg et al. 2012), amygdala (Rao, Wang et al. 2007, Rando, Chaplin et al. 2013), and prefrontal cortex (Roussotte, Soderberg et al. 2012, Liu, Lester et al. 2013, Rando, Chaplin et al. 2013), as well as activation alterations in frontal and striatal regions (Sheinkopf, Lester et al. 2009). These observations also may contribute to the anatomical and functional underpinnings of arousal dysregulation. However, as a newly emerging research field of PCE, neuroimaging studies to date have only been cross-sectional in design with brain changes over time in the same individuals not directly examined.
Adolescence is the period during which developmental and social problems become acute for children of substance abusing parents (Loeber and Farrington 2000). For example, recent studies have shown in teens with PCE problems of social interaction (Greenwald, Chiodo et al. 2011) and adolescent substance use (Delaney-Black, Chiodo et al. 2011, Chaplin, Visconti et al. 2014). Although these problems may also be influenced by prenatal exposure of other drugs and environmental factors (Lambert and Bauer 2012), may mainly occur in an emotional context (Mayes 2002), or may simply reflect delayed maturation (Bennett, Birnkrant et al. 2015), these findings still suggest the importance of a deeper understanding the impact of PCE on the developing brain, particularly on the interplay of cognitive and limbic systems. In typically developing adolescents, the ability to regulate emotion and attention increases substantially (Crone and Dahl 2012) with children demonstrating decreasing emotional arousal (Guyer, Monk et al. 2008) and increasing executive functioning (Giedd 2008). However, while adolescents with PCE are known to be generally over-sensitive to salient but task-irrelevant distractions (Mayes 2002), there is still a lack of direct evidence as to whether this teratogenic effect can be offset by neurodevelopmental changes associated with maturity.
To examine these questions, the present study analyzed fMRI data acquired from the same group of adolescents, with or without PCE, when they were performing the same working memory task with emotional distracters at two different times about 2.4 years apart. These adolescents were recruited from a larger sample designed to assess developmental effect of PCE (Kable, Coles et al. 2008, Li, Coles et al. 2009, Deshpande, Li et al. 2010, Li, Santhanam et al. 2011, Li, Zhu et al. 2013, Li, Santhanam et al. 2013) and neuroimaging data was obtained twice from the same individuals. At both time points, the fMRI experiments used identical paradigm and stimuli that required participants to perform a working memory task while suppressing distraction from emotional pictures. The memory and emotion stimuli in this task were two streams of information competing for processing resources thus challenging individual’s capacity for arousal regulation.
During the time_2 test, given that (i) brains are more mature, (ii) subjects are more familiar with the task and the imaging environment, and (iii) emotional stimuli are believed to be less salient/distractive with repeated viewing, it was hypothesized that the non-exposed participants would improve their memory performance with increasing age; and their brain activations would reflect reduced emotional arousal and less cognitive effort. For the exposed participants, based on aforementioned long-term effect of arousal dysregulation, brain activation changes related to PCE were expected to reflect either a delayed development or a deviant development. If the activation changes over time follow the same trend in the two groups but are only reduced for the PCEs, a delayed development would be noted; but if the activation changes exhibit a different trend in the two groups, a deviance interpretation would be supported.
2. Methods
2.1. Participants
Thirty-three adolescents (21 with PCE and 12 non-exposed) were included in the analysis for the present study. They were a sub-sample from our previous fMRI study of PCE (N=56) (Li, Coles et al. 2009). In that study (when time_1 imaging data acquisition occurred), PCE associated arousal dysregulation was examined with cross-sectional group comparisons. In this study, which includes imaging data acquisition at time_2, we scanned the same group of participants again with identical experiment settings for longitudinal comparison. However, the present sample size was reduced from N=56 to N=33 due to attrition at time_2 (11 were not located, 3 were not scanned for imaging-related concerns such as installation of teeth braces, 3 had excessive head motion, 2 had scheduling issues, 2 quit imaging before completion, 1 dropped for scanner malfunction, and 1 refused to re-participate). The present 33 adolescents are individuals with fMRI data available at both time points. They were not significantly (p>0.3) different from the time_1 sample of N=55 on any one of the following variables: birth weight, head circumference, gestational age, Apgar scores, birthmother's age, amount of prenatal drug and alcohol use, child's age at time_1 imaging, family monthly income level, verbal performance, and full scale IQ.
In the present sample, adolescents were on average 14.3 (SD=1.9) years of age at time_1 and 16.7 (SD=2.1) at time_2. The participants, predominantly African-American with low income and born during 1989–1994, were recruited from birth cohorts originally identified as part of two longitudinal studies of PCE effects on development (Coles, Platzman et al. 1992, Brown, Bakeman et al. 1998). Their detailed demographic characteristics are shown in Table 1. Sources of information used to determine maternal cocaine and other substance use in pregnancy included medical records, maternal self-report following delivery (Coles, Platzman et al. 1992), the Addiction Severity Index carried out at 6 weeks postpartum (McLellan, Luborsky et al. 1980) and postpartum maternal and infant urine screens. These measures also provided information about maternal use of cigarettes, alcohol and other drugs.
Table 1.
Characteristics of teens.
| Variable | Control (n = 12)a | PCE (n = 21)a | p-valueb |
|---|---|---|---|
| Age - Time_1 (M,SD) | 14.4 (2.2) | 14.3 (1.8) | 0.86 |
| Age - Time_2 (M,SD) | 16.8 (2.3) | 16.6 (2.0) | 0.87 |
| Duration Between Time_1 and Time_2 (Month,SD) | 25.4 (3.3) | 27.2 (4.2) | 0.22 |
| Gender, No. (%) | 0.51 | ||
| Male | 6 (50.0) | 13 (61.9) | |
| Female | 6 (50.0) | 8 (38.1) | |
| Ethnic Background, No. (%) | 0.44 | ||
| African-American | 12 (100.0) | 20 (95.2) | |
| White | 0 (0.0) | 1 (4.8) | |
| Total monthly household income, $(M,SD) (n = 32) | 2132 (1421) | 1116 (834) | 0.016 |
| Handedness, No. (%) | 0.91 | ||
| Right | 11 (91.7) | 19 (90.5) | |
| Left | 1 (8.3) | 2 (9.5) | |
| Birth Weight | 2853 (770) | 2750 (662) | 0.69 |
| Full scale IQ – WASI | 89.3 (6.7) | 86.8 (12.1) | 0.45 |
| Verbal IQ – WASI | 89.3 (7.7) | 87.0 (11.9) | 0.54 |
| Performance IQ – WASI | 91.8 (9.9) | 89.1 (12.5) | 0.53 |
If data are not available for all participants, the n used for the analysis is stated next to the variable name.
Independent sample t-tests were completed for the continuous variables; chi square analyses were completed for categorical variables.
PCE group status was determined by maternal self-report and/or positive urine screens at recruitment postpartum. Positive maternal urine screens at labor and delivery and during pregnancy noted in the medical record were also accepted as evidence of use. Mothers were excluded if they used other drugs with teratogenic properties (e.g. Antabuse, seizure medications, warfarin, and insulin), benzodiazepines, antipsychotic drugs, or any addictive substances other than cocaine and marijuana. Mothers had to be 19 years of age or older, English speaking and free of major medical conditions. Additionally, their infants needed to be singletons or firstborns of multiple births and either healthy full term or preterm without major medical complications. Preterms who were less than 30 weeks of gestational age were excluded. Preterm infants and their mothers were subsequently dropped from the recruiting cohort if the infants developed the following complications during their neonatal course: received oxygen for more than 28 days, developed major infection, had seizures, were diagnosed with intraventricular hemorrhage grade III or IV, or with perventricular leukomalacia, were diagnosed with genetic disorders or major malformations, were HIV infected, or had major surgery. Additional information on maternal background characteristics and other substance use in pregnancy is provided in Table 2. More information regarding the determination of substance use, participants inclusionary criteria, and classification of participants into experimental groups have been described extensively in previous reports (Coles, Platzman et al. 1992, Brown, Bakeman et al. 1998).
Table 2.
Maternal characteristics at child’s birth.
| Variable | Control (n = 12)a | PCE (n = 21)a | p-valueb |
|---|---|---|---|
| Age (M,SD) | 27.0 (5.9) | 28.3(4.4) | 0.47 |
| Marital status, No. (%) | 0.25 | ||
| Married | 2 (16.7) | 1 (4.8) | |
| Single, divorced, separated, widowed | 10 (83.3) | 20 (95.2) | |
| Other substance use in pregnancy | |||
| Tobacco – cigarettes/week (n = 32) | 11.7 (40.4) | 62.5 (49.9) | 0.006 |
| Alcohol – oz. absolute alcohol/week (n = 32) | 0.0 (0.0) | 1.0 (2.2) | 0.050 |
| Marijuana – Joints/week | 0.0 (0.0) | 1.9 (3.5) | 0.019 |
If data are not available for all participants, the n used for the analysis is stated next to the variable name.
Independent sample t-tests were completed for the continuous variables; chi square analyses were completed for categorical variables.
Use of alcohol and drugs by adolescent participants was measured both through self-report and by the use of urine drug screens for amphetamines, barbiturates, benzodiazepines, marijuana, cocaine, opiates, and phencyclidine. Self-report measures at time_1 included modified versions of the Adolescent Alcohol Involvement Scale (Mayer and Filstead 1979) and the Adolescent Drug Involvement Scale (Moberg and Hahn 1991). At time_2, self-reported measures included the TimeLine Followback Questionnaire, for alcohol (Sobell and Sobell 1992) and the Drug Grid (Coles, Platzman et al. 1992).
At time_1, one individual in each group reported drinking alcohol as much as once a month, and 3 others (PCE: 2; control: 1) reported drinking once a year. Only one of these individuals (PCE) reported drinking at time_2, although an additional 4 controls and 3 PCE participants reported occasional alcohol use at this time (“several times a month” or less). At time_1, 5 teens with PCE reported having tried marijuana and 2 admitted using it up to twice a month. At time_2, 4 teens (PCE: 3; control: 1) reported daily marijuana use, and 2 PCE teens reported smoking marijuana “several times a month”. Group differences for most of these self-reports were not significant (p>0.3) except for marijuana use at time_1, which was marginal in significance (p=0.086). Aside from these reports, other drug use was denied.
Results of urine drug screens were generally consistent with the teens’ self-report. These data were based on toxicology samples taken on the same day of the MRI scanning. We have toxicology data from 32 participants at time_1 and all 33 participants at time_2. Of the 224 (32 participants × 7 drugs) measures completed at time_1, only 2 were positive with 1 for amphetamines and 1 for marijuana; both these participants were from the PCE group. Of the 231 measures completed at time_2, only 9 were positive. Eight of these positive screens were from the PCE group with 1 participant being positive for amphetamines, 6 for marijuana, and 1 of these 6 also positive for cocaine. The other 1 positive screen was for marijuana from a control group participant. No group differences in urine drug screens were noted (p=0.18 for marijuana at time_2; p>0.6 for all other measures).
Because the task paradigm involves emotionally negative pictures, which could affect subjects with different traumatic histories differently, children's reported social history (Platzman, Coles et al. 2001) was examined for evidence of stable custody arrangements and a history of physical or sexual abuse. Of 7 items related to stability and trauma (years at present address, changes in house hold composition in the last year, stability in custody, protective services involvement, reported abuse/neglect, school discipline problems and legal problems), only 1 item, changes in custody was higher in the PCE group. For this factor, 7 adolescents with PCE had changed caregiver versus 0 in the control group (Fisher’s Exact Text, 1-sided, p=0.035).
All participating families consented for this study according to a protocol approved by Emory University’s Institutional Review Board. Adolescents provided written assent and adults, including both caregivers and 18+ year olds, informed consent, to participate.
2.2. Task paradigm
To examine PCE associated arousal dysregulation, the experimental task and paradigm were designed for probing interactions of cognitive and emotional processes (Drevets and Raichle 1998, Yamasaki, LaBar et al. 2002, Northoff, Heinzel et al. 2004, Dolcos and McCarthy 2006, Li, Coles et al. 2009). Specifically, with emotionally neutral and negative pictures embedded in a presentation stream of letters for an N-back working memory task (Smith and Jonides 1998, Owen, McMillan et al. 2005)(Fig.1), the paradigm was a 2×2 factorial design involving 4 experimental conditions: 0-back memory with neutral picture distraction (Neu0), 1-back memory with neutral distraction (Neu1), 0-back memory with negative distraction (Neg0), and 1-back memory with negative distraction (Neg1). This task would elicit both emotional (negative vs. neutral) and cognitive (1-back vs. 0-back) brain activations thus enabling examinations of individual’s capability in allocating mental resources for different processing.
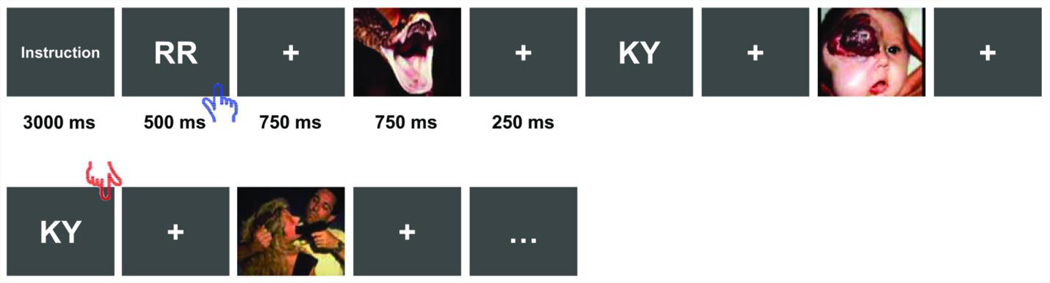
Figure 1.
Schematic diagram of the experimental paradigm. Each task block began with an instruction asking subjects to either perform the 0-back or 1-back working memory task. The letter pairs were interleaved by fixation crosses and distracter pictures (duration labeled). These pictures were either neutral or negative (only negative pictures shown here) within each fMRI block. The blue/red hands indicate the display on which a button response is required for the 0-back/1-back task. This figure is reproduced from our previous publication (Li, Coles et al. 2009) with permission obtained from the publisher.
In the task, participants were instructed to ignore the distracting pictures and to perform the N-back working memory task solely based on the presented letters. In the 0-back condition (low memory demand), they were asked to press a button whenever the displaying letter pair was “RR”; in the 1-back condition (high memory demand), they were to press the button whenever the displaying letter pair exactly matched the last one displayed. The distracting pictures were selected from the international affective picture system (Lang, Bradley et al. 1997) with adult normative ratings of the arousal/valence scores being 5.7/2.9 (SD=0.8/0.8) for the negative and 3.2/5.2 (SD=0.8/0.6) for the neutral pictures. Task block of the 4 experimental conditions (Neu0, Neu1, Neg0, and Neg1) was each repeated 6 times and pseudo-randomly distributed in 2 fMRI scan runs. In each task block, the instruction lasted for 3000ms, followed by trials each having the following structure: an uppercase letter pair presented for 500ms, a 750ms fixation cross, a 750ms presentation of the distracter picture, and finally another 250ms fixation cross (Fig.1). There were 12 trials in each block and 12 blocks in each fMRI scan run.
2.3. Imaging data acquisition
Functional MRI data were acquired from each participant using a Siemens 3T MR scanner (Trio, Siemens Medical Solutions, Malvern, PA) with a 12 channel head coil and exactly the same imaging parameters at both time_1 and time_2. Specifically, a T2*-weighted echo-planar imaging sequence was used with TR=3000ms, TE=30ms, flip angle=90°, FOV=192×192cm2, volume measurements=120, slices/thickness/gap=30/3mm/0mm, and matrix=64×64. For anatomical reference and brain normalization, T1-weighted 3D structural images were also acquired with an isotropic spatial resolution of 1mm3.
2.4. Behavioral data analysis
For each of the 4 experimental conditions, index of accuracy (IDA) and reaction time (RT) were measured from each participant (Li, Coles et al. 2009). The IDA considered both response sensitivity and specificity and was calculated as
The RT was calculated only from correct responses. Both IDA and RT were analyzed with a repeated-measure ANOVA involving 3 within-subject factors of EMOTION (neutral vs. negative stimuli), MEMORY (0-back vs. 1-back memory demands), and TIME (1st vs. 2nd visits) plus 1 between-subject factor of GROUP (PCE vs. non-exposed adolescents)*.
2.5. Imaging data analysis
The present fMRI data were analyzed with AFNI (http://afni.nimh.nih.gov) within the framework of general linear modeling. Specifically, after the pre-processing pipeline of slice timing correction, volume registration, anatomy-to-EPI alignment (Saad, Glen et al. 2009), ICA-based artifact reduction (Tohka, Foerde et al. 2008), and spatial smoothing (FWHM=5mm, AFNI’s 3dBlurToFWHM), individual’s 4D BOLD signals were submitted to a multiple regression analysis for regression coefficients (beta values) of the 4 experimental conditions to be estimated. The regressors involved were 6 parameters of head motion (nuisance regressors) and temporal profiles of task responses (regressors of interest) generated by convolving the boxcar stimulus functions with a standard impulse response function (Cohen 1997). The obtained regression coefficients were then normalized to the base-line or constant fit of BOLD signal at individual level and transformed into the Talairach space (Talairach and Tournoux 1988). For comparing across experimental conditions (EMOTION and MEMORY), across GROUP, and across TIME, these spatially-transformed maps of regression coefficients were subsequently submitted to a voxel-wise ANOVA (Chen, Adleman et al. 2014). Finally, for controlling false positives caused by multiple comparison, a cluster threshold was applied, which was determined by a Monte Carlo simulation (AFNI’s 3dClustSim).
2.6. Confounding factors control
Confounding effect controls in studies of PCE usually consider factors associated with prenatal multi-drug exposure and compromised socioeconomic status (Ackerman, Riggins et al. 2010, Lambert and Bauer 2012, Buckingham-Howes, Berger et al. 2013). To control for these effects, 5 confounding factors were included as nuisance covariates in statistical models whenever a GROUP effect was involved in the analysis. These 5 factors are prenatal exposures of (i) tobacco (cigarette/week), (ii) alcohol (ounce/week), and (iii) marijuana (joints/week), as well as (iv) total monthly household income ($) and (v) changes of caregiver (yes or no). Due to the limited sample size that a neuroimaging study of PCE can typically achieve (Derauf, Kekatpure et al. 2009, Ackerman, Riggins et al. 2010, Buckingham-Howes, Berger et al. 2013), it was statistically inefficient to consider all the confounding covariates simultaneously. Therefore, with a practical rule of thumb that number of covariates should be less than [(0.1 × sample size) - (number of groups - 1)] (http://en.wikiversity.org/wiki/Advanced_ANOVA/ANCOVA), the 5 confounding factors were statistically controlled one at a time. However, if a group difference was significant in all 5 analyses when each covariate was individually controlled, statistics with all covariates simultaneously controlled were also reported.
Because self-reports and urine drug screens all indicated limited alcohol and drug use with only the current marijuana exposure approached a marginal level of group difference, statistical controls of adolescent’s current substance use only included 1 variable, urine marijuana screen (positive=1 and negative=0). This factor was not modeled as a nuisance covariate in ANOVA because the using status changed with time. Instead, the effect was removed individually with regression analysis on all the behavioral and imaging measurements before ANOVA.
3. Results
3.1. Behavioral Results
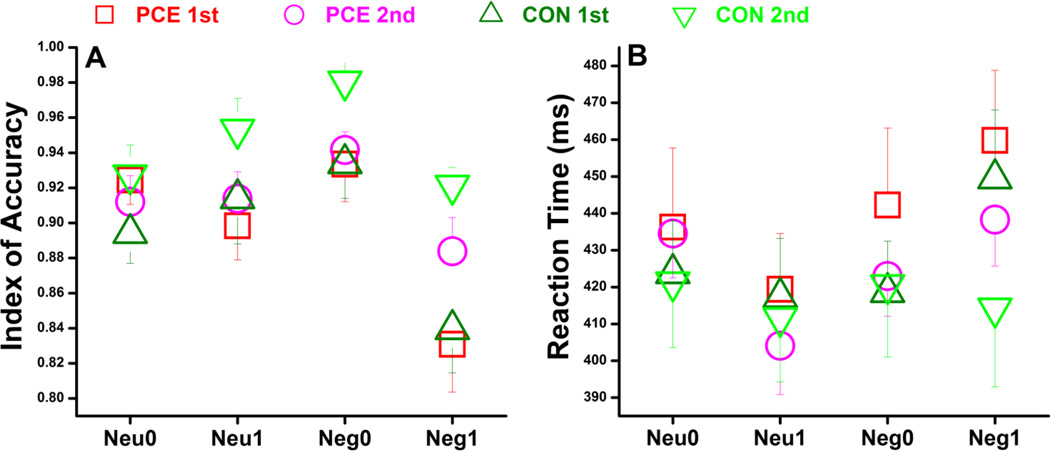
Although the present task and stimuli were deliberately designed to minimize behavioral group differences for minimizing the possibility of ascribing brain activation differences to task difficulty (Li, Coles et al. 2009), the non-exposed participants generally performed better than the exposed (Fig.2)† but this general GROUP effect was not significant for either IDA (F1,31=0.37, p=0.55) or RT (F1,31=0.29, p=0.59). Compared with time_1, both groups generally increased memory accuracy and reduced reaction time at time_2. This TIME effect was significant for IDA (F1, 31=9.6, p=0.004, ɳ2=0.24) but not for RT (F1, 31=1.2, p=0.28). In examining factor interactions associated with either TIME or GROUP, only one significant interaction of EMOTION × MEMORY × GROUP × TIME for RT (F1, 31=4.6, p=0.039, ɳ2=0.13) was noted. This result shows that at time_1, negative distractions made the memory task more difficult for both groups (increasing RT difference of 1-back vs. 0-back by 37ms for the control and 34ms for the PCE group); whereas at time_2, while negative distractions still made the memory task more difficult for the exposed adolescents (increasing RT difference by 46ms), this emotional interference almost disappeared for the non-exposed controls (increasing RT difference by only 3ms). When considering caregiver changes, family income, and prenatal multidrug exposure in the statistical model, this group difference generally held marginal when these confounding factors were controlled one at a time (controlling caregiver: F1,30=3.8, p=0.061, ɳ2=0.11; income: F1,30=3.0, p=0.095, ɳ2=0.090; tobacco: F1,30=3.2, p=0.086, ɳ2=0.095; alcohol: F1,30=7.6, p=0.010, ɳ2=0.20; and marijuana: F1,30=3.5, p=0.070, ɳ2=0.11).
Figure 2.
Comparisons of index of accuracy (A) and reaction time (B) across subject groups (PCE vs. CON), testing visits (1st vs. 2nd), and experimental conditions (Neu0, Neu1, Neg0, Neg1). For clear visualization, the error bars (standard error) are only shown either in the positive or negative directions but not in both.
Note again that the present task paradigm and stimuli were deliberately designed to minimize group differences on behavioral measurements (Li, Coles et al. 2009); so similar group performances or marginal group differences should not be considered as experimental evidence for negligible PCE effect on behavior. On the contrary, even though the RT interaction of EMOTION × MEMORY was deliberately made equal between groups at time_1 (37ms vs. 34ms), PCE effect emerges ~2.4 years later at time_2 (3ms vs. 46ms).
3.2. Functional MRI Results
With an activation threshold of p<0.05/voxel plus a 999mm3 cluster (p<0.05 corrected), the voxel-wise ANOVA revealed two significant results: (i) one cluster of right amygdala (1080mm3, Talairach coordinates with RAI orientation: −24.9 4.7 −10.1, Fmax=8.5, p=0.007) showing an effect of EMOTION × GROUP × TIME, and (ii) two clusters of DMN (4779mm3, 5.3 49.0 23.6, Fmax=14, p<0.001; 2511mm3, −0.6 −53.5 17.6, Fmax=16, p<0.001) showing an effect of MEMORY × GROUP × TIME. These effects are visualized in Fig.3 and Fig.4 with the EMOTION effect calculated as the activation (beta value) contrast of [(Neg0+Neg1)-(Neu0+Neu1)] and the MEMORY effect as the contrast of [(Neu1+Neg1)-(Neu0+Neg0)].
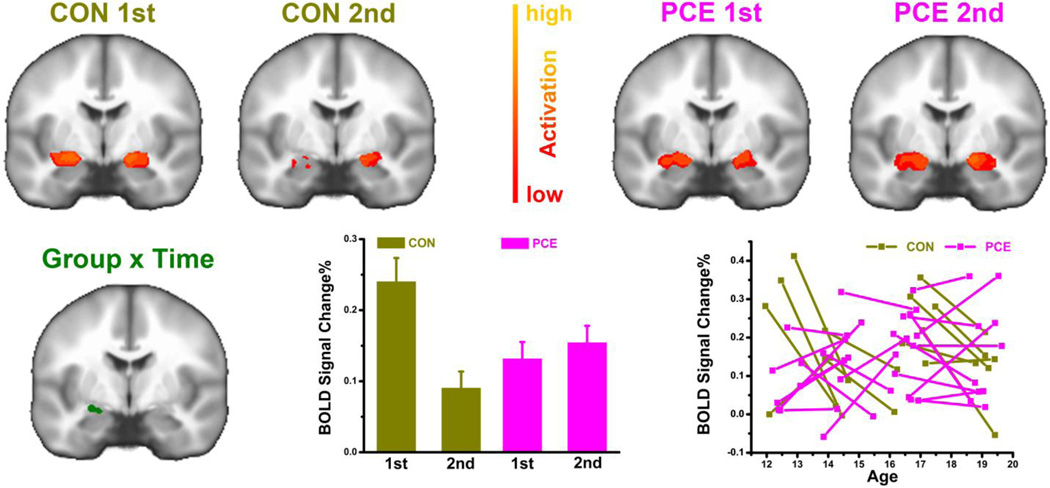
Figure 3.
Comparison of emotional amygdala activations across subject groups (PCE vs. CON) and testing visits (1st vs. 2nd). The activation is defined as fMRI signal contrast of “Negative > Neutral” shown in a coronal slice of y=7mm. For the right amygdala with a significant effect of GROUP × TIME, emotional activation levels (BOLD signal change%) are plotted both aggregately (bottom middle) with data averaged within group/test and individually (bottom right) with each subject as a line connecting time_1 and time_2.
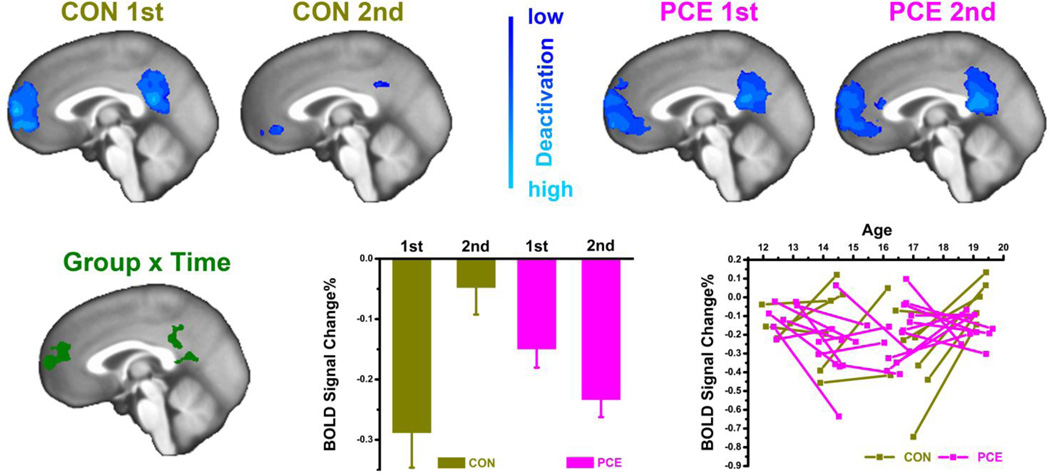
Figure 4.
Comparisons of DMN deactivations across subject groups (PCE vs. CON) and testing visits (1st vs. 2nd). The deactivation is defined as fMRI signal contrast of “1-back < 0-back” shown in a sagittal slice of x=−1mm. For the anterior and posterior cingulate regions with a significant effect of GROUP × TIME, deactivation levels (BOLD signal change%) are plotted both aggregately (bottom middle) with data averaged within group/test and individually (bottom right) with each subject as a line connecting time_1 and time_2.
The negative distracters activated bilateral amygdala of the control group at time_1; however, this activation in the controls was reduced at time_2 (Fig.3 top left). In contrast, the amygdala activation was not reduced but even increased a little for the exposed group at time_2 (Fig.3 top right). This GROUP × TIME interaction of the EMOTION effect was found significant in the region of right amygdala shown in the bottom row of Fig.3. For this region, the bar and line graphs depict activations elicited by the negative emotion at the group and individual levels, respectively. With the activation values averaged across all the voxels in this region of right amygdala, this interaction of EMOTION × GROUP × TIME was significant when controlling confounding factors of caregiver changes (F1,30=17, p<0.001, ɳ2=0.36), guardian’s total income (F1,30=16, p<0.001, ɳ2=0.35), tobacco exposure (F1,30=9.2, p=0.005, ɳ2=0.24), alcohol exposure (F1,30=14, p=0.001, ɳ2=0.31), marijuana exposure (F1,30=14, p=0.001, ɳ2=0.32), or even when the income and prenatal multidrug exposures were all controlled simultaneously (F1,26=5.9, p=0.022, ɳ2=0.19).
Similar to the EMOTION effect observed in the right amygdala, MEMORY effect in the DMN is graphically compared between groups and time in Fig.4. At time_1, both groups suppressed their DMN activation with an increased memory demand; however, this suppression was much attenuated at time_2 for the non-exposed youths but was even strengthened for the adolescents with PCE. This GROUP × TIME interaction was significant in DMN regions of medial prefrontal cortex and posterior cingulate cortex shown in the bottom row of Fig.4. For the mean activation values of these regions, the interaction of MEMORY × GROUP × TIME was significant with potential confounding factors of caregiver changes, guardian’s total income, and prenatal multidrug exposures controlled one at a time (controlling caregiver: F1,30=20, p<0.001, ɳ2=0.40; income: F1,30=16, p<0.001, ɳ2=0.35; tobacco: F1,30=8.5, p=0.007, ɳ2=0.22; alcohol: F1,30=16, p<0.001, ɳ2=0.35; and marijuana: F1,30=17, p<0.001, ɳ2=0.37), or also when they were all controlled simultaneously (F1,26=3.9, p=0.049, ɳ2=0.14).
3.3. Correlations between regional activations and behavior
The aforementioned data indicate that (i) emotional interference persisted over time in the PCE but decreased in the control group (behavioral data of RT); (ii) emotional responses in the brain persisted over time in the PCE but decreased in the control group (fMRI data of amygdala); and (iii) efforts to suppress task-irrelevant activity persisted over time in the PCE but decreased in the control group (fMRI data of DMN). With these results, a natural hypothesis arises that there could be correlations between these over-time changes of brain response and behavior. To test this hypothesis, the experimental measurements corresponding to (i), (ii), and (iii) were subsequently submitted to a bivariate correlation analysis. Specifically, the emotional interference in behavior was calculated as RT interactions of [(Neg1-Neg0)-(Neu1-Neu0)], the emotional response in amygdala was calculated as activation contrasts of [(Neg0+Neg1)-(Neu0+Neu1)], and the DMN suppression was calculated as activation contrasts of [(Neu1+Neg1)-(Neu0+Neg0)].
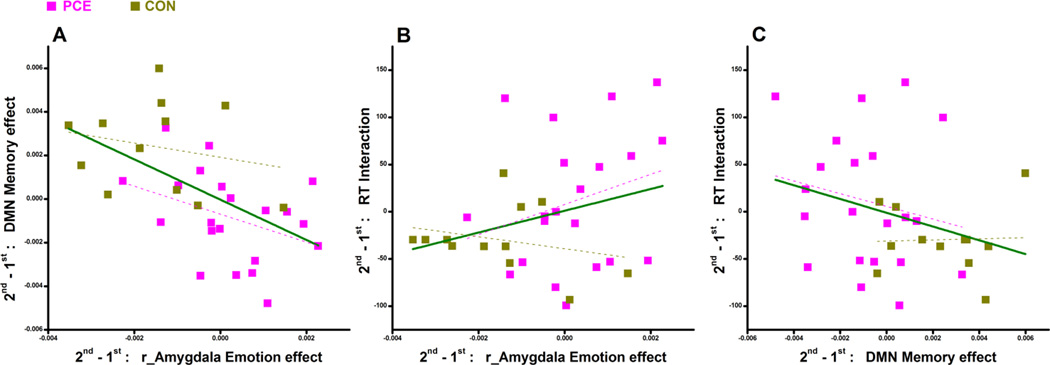
As shown in Fig.5A, there was a significant (r=−0.54, p=0.001) negative correlation between the over-time changes of amygdala activation and over-time changes of DMN suppression, suggesting that if one could not decrease his/her amygdala response (emotional arousal) at time_2, then he/she might need to suppress DMN more (more attentional effort). The group differences mentioned above also appeared again here in Fig.5A indicating that the exposed adolescents were generally still high on emotional arousal and with high attentional effort at time_2. Because higher emotional arousal means higher emotional interference on behavioral performance, one would expect a positive correlation between the over-time changes of amygdala activation and over-time changes of RT interaction. Indeed, that was the observation shown in Fig.5B although this correlation was not significant (r=0.28, p=0.12). The variable correlations shown in Fig.5A and Fig.5B have determined a negative correlation between the over-time changes of DMN suppression and the over-time changes of RT interaction; and this correlation (r=−0.30, p=0.094) is shown in Fig.5C. Of note, all the correlation statistics reported here were 2-tailed. However, as the correlation directions (being positive or negative) could be reasonably expected/hypothesized in Fig.5B and Fig.5C, 1-tailed statistics (thus increased significance) may also be appropriate.
Figure 5.
Pairwise correlations between the over-time changes (2nd - 1st) of RT interaction, right amygdala activation, and DMN deactivation. Regression lines have been provided for each group (PCE: magenta; controls: dark yellow) as well as for all participants combined (green).
4. Discussion
Prenatal cocaine exposure has been well documented to be negatively associated with brain development (Ackerman, Riggins et al. 2010, Lambert and Bauer 2012, Buckingham-Howes, Berger et al. 2013, Coyle 2013). While there is converging neurobehavioral evidence for specific PCE effects on arousal regulation and neurocognitive functioning, neuroimaging studies on neurobiological mechanisms are just emerging with implications for development and functioning in adolescence investigated even less. Attempting to gain more insight about the neurodevelopmental effects of PCE, the present study is the first to apply fMRI with a longitudinal design to this population. When exposed and non-exposed adolescents performed the same task at different ages, their brain activation changes differed across time in the amygdala and DMN regions.
Because amygdala is considered a critical region mediating negative emotion (Aggleton 1993, Phelps 2006, Holzel, Carmody et al. 2010) and the levels of negative emotion should be reduced when repetitively performing the same task (Bradley, Lang et al. 1993, Breiter, Etcoff et al. 1996, Wendt, Schmidt et al. 2012), it was not surprising that a reduced amygdala activation was observed in the control group. However, the amygdala activation was not reduced at all for the PCE group during the time_2 test. In other words, the exposed brains still tend to trigger automatic emotional responses even when the distractors are no longer novel and even when individuals are more mature and able to cognitively deem the distractors’ irrelevance. This result provides direct longitudinal evidence supporting the view that PCE is associated with a long-term and persistent compromise in the capacity to regulate emotional arousal (Kable, Coles et al. 2008, Chaplin, Fahy et al. 2009, Li, Coles et al. 2009, Chaplin, Freiburger et al. 2010, Li, Santhanam et al. 2013). The persistence of this PCE effect could be due to the altered “fetal programming” of the hypothalamic-pituitary-adrenal axis (Lester and Padbury 2009), which can put exposed adolescents at a higher risk for not only attentional and cognitive deficits but also for social behavior problems (Greenwald, Chiodo et al. 2011), for onset of stress-related diseases (Miller, Chen et al. 2007), and/or for teen substance use (Delaney-Black, Chiodo et al. 2011, Chaplin, Visconti et al. 2014).
The present task involves attention allocation between working memory stimuli and emotional distractors. Since the PCE participants were still high on emotional arousal at time_2 relative to time_1, presumably they also need a high level of attentional effort to offset this distraction to achieve a behavioral performance comparable to the non-exposed adolescents. Attentional effort can be inferred by DMN activation as it reflects intrinsic mental operations that need to be suppressed/suspended to facilitate specific and goal-oriented tasks (Gusnard and Raichle 2001, Raichle, MacLeod et al. 2001, Raichle and Snyder 2007, Anticevic, Cole et al. 2012). While the older controls have shown the typical increase of this intrinsic processing during development (Sun, Berl et al. 2013), the older PCEs exhibited a sustained suppression of this intrinsic operation. The implication for this finding in development is two-fold. On one hand, this can be a neural compensational mechanism for the maturing adolescents with PCE as they do exhibit capability of keeping goal-oriented attention focused with distraction. But on the other hand, it also suggests a limited span of attentional resource available to the exposed than to the control adolescents. In more challenging situations, either with a higher cognitive demand or increased distraction, the exposed adolescents could be more vulnerable than their non-exposed peers due to this relatively limited amount of spared mental resource. In supporting this point, Savage and colleagues actually have already shown this pattern of behavioral group differences (Savage, Brodsky et al. 2005). They compared behavioral performances between PCE and control children during a “vigilance” task, which “measures the child’s ability to focus attention”, and during a “distractibility” task, which is a “more difficult version of the vigilance task” with distracting factors. In their results, the two groups performed equally well in the “vigilance” task but the exposed group performed worse in the “distractibility” task. Similarly in the present study, although behavioral group differences were deliberately minimized and generally insignificant, adolescents with PCE tended to perform worse in the most challenging condition of “Neg1” (Fig.2).
While our previous cross-sectional studies have shown limited capacity for adolescents with PCE in coping with distractive challenges (Li, Coles et al. 2009, Li, Santhanam et al. 2011, Li, Santhanam et al. 2013), the present longitudinal data have further confirmed that this limited capacity tends to persist over time. In the current task, memory performance is challenged by emotional distractors either at a low (neutral) or high (negative) level, and this emotional challenge can be measured by the interaction effect of MEMORY × EMOTION. At time_1, the two groups both exhibited this challenge effect with the RT interaction being 37ms for the control and 34ms for the PCE adolescents. However, at time_2, the negative distractions no longer affected memory RT of the controls (interaction reduced to 3ms) but still affected memory RT of the PCEs (interaction still high at 46ms). When behavioral data are correlated with imaging measurements, individuals who were not able to cope with distractions well at time_2 also did not reduce amygdala activation and needed to suppress DMN more. In a recent review of PCE and adolescent development (Buckingham-Howes, Berger et al. 2013), the elucidation of relations between brain measurement and behavioral functioning has been identified as one of the future directions for neuroimaging studies of PCE. Besides the recent report of correlations between gray matter volume and substance use initiation (Rando, Chaplin et al. 2013), the present study revealed additional aspect of brain-behavior relationship in neurodevelopment.
The mean ages of 14.3 and 16.7 at the two tests do not imply that the observed effects are specific to brain maturation between these ages. As shown in Table 1, Fig.3, and Fig.4, the ages of both groups actually were widely distributed ranging from ~12 at time_1 to ~19 at time_2, covering almost the entire temporal duration of adolescence. Therefore, the present data may represent a general effect of PCE over a wide range of ages. The compromised emotional arousal is likely to be an issue for exposed adolescents regardless of specific age, and this issue is likely to persist with increasing age. However, the present task-state fMRI can only measure a mixed effect of neurodevelopment and task repetition. While the effect of GROUP × TIME does reflect PCE associated arousal dysregulation in both situations, the within-subject factor of TIME does not disentangle how much of the observed effects can be attributed to neurodevelopment and how much to task repetition. Instead of the 2-level factor of TIME, we have also attempted at a longitudinal modeling (using AFNI’s 3dLME) with a continuous factor of chronological AGE, but this analysis exhibited no significant result. The lack of an AGE-modeling result could be due to the limited sample size across a relatively wide age range.
The present analysis did not show significant effects of GROUP or GROUP × TIME in dorsal-lateral and ventral prefrontal regions, in which we previously identified PCE effect with a larger sample size in cross-sectional studies at time_1 (Li, Coles et al. 2009, Li, Santhanam et al. 2013). It may indicate that the GROUP differences seen at time_1 are changed at time_2, but this change is not big enough to reveal a significant GROUP×TIME interaction. Besides effect size, sample size is certainly the other factor determining the significance. Due to increased expense and complexity (than neurobehavioral studies), limited sample size is typical in neuroimaging studies in this population (Derauf, Kekatpure et al. 2009, Ackerman, Riggins et al. 2010, Buckingham-Howes, Berger et al. 2013). However, while this longitudinal sample is relatively small, the present findings offset this shortcoming by directly revealing longitudinal changes, by attributing behavioral manifestations to specific regional brain alterations, and by confirming cross-sectionally identified vulnerability in amygdala and DMN (Li, Coles et al. 2009, Li, Santhanam et al. 2011). The small sample size in controls is also offset by the consistent activation patterns with previous studies, where reduced emotional arousal and enhanced attentional capacity were reported in typical development of adolescents (Giedd 2008, Guyer, Monk et al. 2008).
Related to sample size, limitations of the present study also include the simplified control of social environmental variables. Though we generally refer the observed group differences as PCE effect, it is possible that we are actually reporting a mixed effect of the teratogenic exposure and associated environmental influences. With limited statistical power, caregiver changes and family income (poverty) were the only 2 social environmental risk factors controlled, but many other risk factors (e.g. insensitive parenting) were still left unconsidered. Though impairments of attention and self-regulation were consistently reported in large-sample neurobehavioral studies with environmental variables better controlled (Ackerman, Riggins et al. 2010), additional clarification would benefit as well from collaborative large-sample neuroimaging like the effort made in similar population of prenatal alcohol exposure (Mattson, Foroud et al. 2010).
5.5. Conclusion
Prenatal cocaine exposure is associated with persistently high levels of emotional arousal and interference in neurodevelopment of adolescents. Correlating with behavioral measurements, longitudinal neuroimaging observations complement and extend neurobehavioral and cross-sectional evidences supporting the view that PCE may pose a long-term teratogenic effect on arousal regulation.
Supplementary Material
Highlights.
Thirty-three adolescents were scanned twice at the mean ages of 14.3 and 16.7.
FMRI reveals developmental vulnerability in amygdala and default mode network.
Longitudinal changes of brain activation correlate with behavioral performance.
Prenatal cocaine exposure has long-term effect on arousal regulation in adolescent.
Acknowledgements
This study was supported by Georgia research alliance, NIH grants RO1 DA17795 and RO1 DA033393, Natural Science Foundation of SZU (No.201564).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Capitalized words EMOTION, MEMORY, TIME, and GROUP are specifically used to refer ANOVA factors in this entire manuscript
Within-subject behavioral changes across different ages are shown in Supplementary figure 1.
References
- Ackerman JP, Riggins T, Black MM. A Review of the Effects of Prenatal Cocaine Exposure Among School-Aged Children. Pediatrics. 2010;125(3):554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends in Neurosciences. 1993;16(8):328–333. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Hurt H, Giannetta JM, Epstein CL, Shera DM, Rao H, Wang J, Gee JC. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatric Neurology. 2007;37(4):275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Wright LL, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–e359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicology and Teratology. 2001;23:545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch ME. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation in 8-week-old infants. Developmental Psychobiology. 2000;36:194–212. [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Developmental Psychology. 1998;34(3):555–564. [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Birnkrant JM, Carmody DP, Lewis M. Effects of prenatal cocaine exposure on pubertal development. Neurotoxicol Teratol. 2015;47:146–153. doi: 10.1016/j.ntt.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: habituation in humans. Behav Neurosci. 1993;107(6):970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brown JV, Bakeman R, Coles CD, Sexson WR, Demi AS. Maternal drug use during pregnancy: are preterm and full-term infants affected differently? Developmental Psychology. 1998;34(3):540–554. doi: 10.1037//0012-1649.34.3.540. [DOI] [PubMed] [Google Scholar]
- Buckingham-Howes S, Berger SS, Scaletti LA, Black MM. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131(6):e1917–e1936. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JO, Bliven TD, Silveri MM, Snyder KJ, Spear LP. Effects of prenatal cocaine on behavioral adaptation to chronic stress in adult rats. Neurotoxicology and Teratology. 2000;22:845–850. doi: 10.1016/s0892-0362(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Fahy T, Sinha R, Mayes LC. Emotional arousal in cocaine exposed toddlers: prediction of behavior problems. Neurotoxicology and Teratology. 2009;31:275–282. doi: 10.1016/j.ntt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Freiburger MB, Mayes LC, Sinha R. Prenatal cocaine exposure, gender, and adolescent stress response: a prospective longitudinal study. Neurotoxicology and Teratology. 2010;32(6):595–604. doi: 10.1016/j.ntt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Visconti KJ, Molfese PJ, Susman EJ, Klein LC, Sinha R, Mayes LC. Prenatal cocaine exposure differentially affects stress responses in girls and boys: associations with future substance use. Development and Psychopathology. 2014 doi: 10.1017/S0954579414000716. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Coles CD, Bard KA, Platzman KA, Lynch ME. Attentional response at eight weeks in prenatally drug-exposed and preterm infants. Neurotoxicology and Teratology. 1999;21(5):527–537. doi: 10.1016/s0892-0362(99)00023-9. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Smith I, James ME, Falek A. Effects of cocaine and alcohol use in pregnancy on neonatal growth and neurobehavioral status. Neurotoxicology and Teratology. 1992;14:23–33. doi: 10.1016/0892-0362(92)90025-6. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Brain structural alterations induced by fetal exposure to cocaine persist into adolescence and affect behavior. JAMA Psychiatry. 2013;70(10):1113–1114. doi: 10.1001/jamapsychiatry.2013.1949. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Damasio AR. On some functions of the human prefrontal cortex. Ann N Y Acad Sci. 1995;769(1):241–252. doi: 10.1111/j.1749-6632.1995.tb38142.x. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Partridge RT, Ager J, Sokol RJ. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicology and Teratology. 2011;33(110–119) doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Developmental Psychobiology. 2006;42(4):688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, Kekatpure M, Neyzi N, Lester B, Kosofsky B. Neuroimaging of children following prenatal drug exposure. Seminars in Cell and Developmental Biology. 2009;20:441–451. doi: 10.1016/j.semcdb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Hu X. Recursive cluster elimination based support vector machine for disease state prediction using resting state functional and effective brain connectivity. PLoS One. 2010;5(12):e14277. doi: 10.1371/journal.pone.0014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro JA, Suess PE, Wheeler JS, Smouse PH, Newlin DB. Reactivity and regulation in cocaine-exposed neonates. Infant Behavior and Development. 1995;18:407–414. [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12(3):353–385. [Google Scholar]
- Eiden RD, McAuliffe S, Kachadourian L, Coles C, Colder C, Schuetze P. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicology and Teratology. 2009;31:60–68. doi: 10.1016/j.ntt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Development. 2009;80(2):528–543. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. Journal of Adolescent Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Chiodo LM, Hannigan JH, Sokol RJ, Janisse J, Delaney-Black V. Teens with heavy prenatal cocaine exposure respond to experimental social provocation with escape not aggression. Neurotoxicology and Teratology. 2011;33:198–204. doi: 10.1016/j.ntt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA. Cocaine effects on the developing brain: current status. Neuroscience and Biobehavioral Reviews. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience. 2010;5(1):11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Anderson DR, Means LW, McMillen BA. Prenatal exposure to cocaine II: effects on open-filed activity and cognitive behavior in Sprague-Dawley rats. Neurotoxicology and Teratology. 1992;14:343–349. doi: 10.1016/0892-0362(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Lynch ME, Platzman K. Physiological responses to social and cognitive challenges in 8-year-olds with a history of prenatal cocaine exposure. Developmental Psychobiology. 2008;50(3):251–265. doi: 10.1002/dev.20285. [DOI] [PubMed] [Google Scholar]
- Karmel BZ, Gardner JM. Prenatal cocaine exposure effects on arousal-modulated attention during the neonatal period. Developmental Psychobiology. 1996;29(5):463–480. doi: 10.1002/(SICI)1098-2302(199607)29:5<463::AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Lambert BL, Bauer CR. Developmental and behavioral consequences of prenatal cocaine exposure: a review. Journal of Perinatology. 2012;32:819–828. doi: 10.1038/jp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system: technical manual and affective ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention; 1997. [Google Scholar]
- Lester BM, LaGasse LL, Shankaran S, Bada HS, Bauer CR, Lin R, Das A, Higgins R. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. Journal of Pediatrics. 2010;157(2):288–295. doi: 10.1016/j.jpeds.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Li K, Zhu D, Guo L, Li Z, Lynch ME, Coles C, Hu X, Liu T. Connectomics signatures of prenatal cocaine exposure affected adolescent brains. Human Brain Mapping. 2013;34(10):2494–2510. doi: 10.1002/hbm.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Hamann S, Peltier S, LaConte S, Hu X. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicology and Teratology. 2009;31(6):342–348. doi: 10.1016/j.ntt.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S, Hu X. Increased "default mode" activity in adolescents prenatally exposed to cocaine. Human Brain Mapping. 2011;32(5):759–770. doi: 10.1002/hbm.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S, Hu X. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiatry Research: Neuroimaging. 2013;213(1):47–55. doi: 10.1016/j.pscychresns.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lester BM, Neyzi N, Sheinkopf SJ, Gracia L, Kekatpure M, Kosofsky BE. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatrics. 2013;167(4):348–354. doi: 10.1001/jamapediatrics.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Farrington DP. Young children who commit crime: Epidemiology, developmental origins, risk factors, early interventions, and policy implications. Development and Psychopathology. 2000;12(4):737–762. doi: 10.1017/s0954579400004107. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Ramo I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP, Cifasd Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44(7–8):635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J, Filstead WJ. The Adolescent Alcohol Involvement Scale. An instrument for measuring adolescents' use and misuse of alcohol. J Stud Alcohol. 1979;40(3):291–300. doi: 10.15288/jsa.1979.40.291. [DOI] [PubMed] [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology and Teratology. 2002;24:385–395. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Grillon C, Granger R, Schottenfeld R. Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Annals of the New York Academy of Sciences. 1998;846:126–143. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moberg DP, Hahn L. The adolescent drug involvement scale. Journal of Adolescent Chemical Dependency. 1991;2(1):75–88. [Google Scholar]
- Northoff G, Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual-Leone A, Schlaug G. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Hum Brain Mapp. 2004;21(3):202–212. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Platzman KA, Coles CD, Lynch ME, Bard KA, Brown JV. Assessment of the caregiving environment and infant functioning in polydrug families: Use of a Structured Clinical Interview. Infant Mental Health Journal. 2001;22(3):351–373. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rando K, Chaplin TM, Potenza MN, Mayes L, Sinha R. Prenatal cocaine exposure and gray matter volume in adolescent boys and girls: relationship to substance use initiation. Biological Psychiatry. 2013;74(7):482–489. doi: 10.1016/j.biopsych.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120(5):1245–1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Regalado MG, Schechtman VL, Angel APD, Bean XD. Sleep disorganization in cocaineexposed neonates. Infant Behavior and Development. 1995;18(3):319–327. [Google Scholar]
- Romano AG, Harvey JA. Prenatal cocaine exposure: long-term deficits in learning and motor performance. Annals of the New York Academy of Sciences. 1998;846:89–108. [PubMed] [Google Scholar]
- Roussotte F, Soderberg L, Warner T, Narr K, Lebel C, Behnke M, Davis-Eyler F, Sowell E. Adolescents with prenatal cocaine exposure show subtle alterations in striatal surface morphology and frontal cortical volumes. Journal of Neurodevelopmental Disorders. 2012;1:22. doi: 10.1186/1866-1955-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local pearson correlation. Neuroimage. 2009;44(3):839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Ramireza KY, Frankfurt M, Alexander A, Luine VN, Friedman E. Prenatal cocaine exposure increases anxiety, impairs cognitive function and increases dendritic spine density in adult rats: influence of sex. Neuroscience. 2010;169(3):1287–1295. doi: 10.1016/j.neuroscience.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J, Brodsky Nl, Malmud E, Giannetta Jm, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. Journal of Developmental and Behavioral Pediatrics. 2005;26(1):42–47. [PubMed] [Google Scholar]
- Schuetze P, Eiden RD. The association between maternal cocaine use during pregnancy and physiological regulation in 4- to 8-week-old infants: an examination of possible mediators and moderators. Journal of Pediatric Psychology. 2006;31(1):15–26. doi: 10.1093/jpepsy/jsj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Coles CD. Prenatal cocaine and other substance exposure: effects on infant autonomic regulation at 7 months of age. Developmental Psychobiology. 2007;49(3):276–289. doi: 10.1002/dev.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, LaGasse LL, Durston S, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Developmental Neuroscience. 2009;31:159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95(20):12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. New York: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sun B, Berl MM, Burns TG, Gaillard WD, Hayes L, Adjouadi M, Jones RA. Age association of language task induced deactivation induced in a pediatric population. Neuroimage. 2013;65:23–33. doi: 10.1016/j.neuroimage.2012.09.071. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, Poldrack RA. Automatic independent component labeling for artifact removal in fMRI. Neuroimage. 2008;39:1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt J, Schmidt LE, Lotze M, Hamm AO. Mechanisms of change: effects of repetitive exposure to feared stimuli on the brain's fear network. Psychophysiology. 2012;49(10):1319–1329. doi: 10.1111/j.1469-8986.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99(17):11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.