Abstract
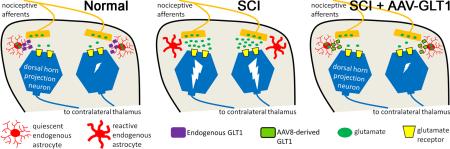
Development of neuropathic pain occurs in a major portion of traumatic spinal cord injury (SCI) patients, resulting in debilitating and often long-term physical and psychological burdens. Following SCI, chronic dysregulation of extracellular glutamate homeostasis has been shown to play a key role in persistent central hyperexcitability of superficial dorsal horn neurons that mediate pain neurotransmission, leading to various forms of neuropathic pain. Astrocytes express the major CNS glutamate transporter, GLT1, which is responsible for the vast majority of functional glutamate uptake, particularly in the spinal cord. In our unilateral cervical contusion model of mouse SCI that is associated with ipsilateral forepaw heat hypersensititvity (a form of chronic at-level neuropathic pain-related behavior), we previously reported significant and long-lasting reductions in GLT1 expression and functional GLT1-mediated glutamate uptake in cervical spinal cord dorsal horn. To therapeutically address GLT1 dysfunction following cervical contusion SCI, we injected an adeno-associated virus type 8 (AAV8)-Gfa2 vector into the superficial dorsal horn to increase GLT1 expression selectively in astrocytes. Compared to both contusion-only animals and injured mice that received AAV8-eGFP control injection, AAV8-GLT1 delivery increased GLT1 protein expression in astrocytes of the injured cervical spinal cord dorsal horn, resulting in a significant and persistent reversal of already-established heat hypersensitivity. Furthermore, AAV8-GLT1 injection significantly reduced expression of the transcription factor and marker of persistently increased neuronal activation, ΔFosB, in superficial dorsal horn neurons. These results demonstrate that focal restoration of GLT1 expression in the superficial dorsal horn is a promising target for treating chronic neuropathic pain following SCI.
Keywords: contusion, neuropathic pain, astrocyte, glutamate transporter, adeno-associated virus
Introduction
A significant portion of traumatic spinal cord injury (SCI) patients experience one or more forms of neuropathic pain, often in a chronic manner. These include enhanced responsiveness to noxious stimuli (hypersensitivity), painful sensation in response to formally innocuous stimuli (allodynia), and often spontaneous pain (Hulsebosch et al. 2009).
Hyperexcitability of dorsal horn pain projection neurons (“central sensitization”) is a major substrate for neuropathic pain (Gwak and Hulsebosch 2011b). This includes decreased threshold for activation (i.e. action potential generation), increased spontaneous activity, expansion of peripheral receptive field, and occurrence of after-discharges in dorsal horn spinothalamic pain projection neurons (Latremoliere and Woolf 2009). In particular, alterations in properties and proportion of wide-dynamic range pain neurons (in both superficial and deeper laminae) occur following SCI. Furthermore, many of these changes in dorsal horn pain neuron properties are persistent, likely accounting for the chronic nature of neuropathic pain after SCI (Crown et al. 2006; Gwak and Hulsebosch 2009; Hensley et al. 2000; Svensson and Yaksh 2002; Tournier et al. 1997).
Dysregulation of extracellular glutamate homeostasis contributes to dorsal horn neuron hyperexcitability after SCI. Glutamate is the primary neurotransmitter released from primary afferent terminals onto second-order dorsal horn pain projection neurons and is thought to play a major mechanistic role in central sensitization (Tao et al. 2005). Increased activation of glutamate receptors (particularly NMDA) can be mediated by dorsal horn alterations such as increased levels of extracellular glutamate and changes in expression, localization and function (via phosphorylation, for example) of glutamate receptor subunits in these neurons (Bleakman et al. 2006). Additional mechanisms that may account for glutamate-mediated central changes in nociceptive transmission in dorsal horn following SCI include: (1) increased activation of astrocytes and microglia by glutamate (Bradesi 2010; Cao and Zhang 2008; Hansson 2006; Ren 2010), (2) decreased inhibitory tone due to glutamate-mediated excitotoxic loss of GABAergic interneurons (Gwak and Hulsebosch 2011a), and (3) reduced recycling of glutamine from astrocytes back to inhibitory neurons for GABA synthesis because of decreased glutamate uptake and subsequent conversion of glutamate to glutamine in reactive astrocytes (Ortinski et al. 2010).
Glutamate is efficiently cleared from the synapse by plasma membrane glutamate transporters. Astrocytes express the major glutamate transporter, GLT1, which is responsible for the vast majority of glutamate uptake in the CNS, particularly in spinal cord (Anderson and Swanson 2000; Maragakis and Rothstein 2006). Some studies have focused on therapeutically targeting glutamate receptor over-activation in SCI models (Papastefanaki and Matsas 2015). However, regulation of extracellular glutamate homeostasis by GLT1 following SCI has received far less attention, particularly in the context of modulating excitability of dorsal horn neurons involved in pain neurotransmission. After SCI, astrocyte loss and/or altered GLT1 physiology result in dysregulation of extracellular glutamate homeostasis, as well as further glial activation and consequent compromise in GLT1 function. A number of groups have reported long-term decreases in GLT1 expression in various SCI animal models (Olsen et al. 2010; Vera-Portocarrero et al. 2002). We have also observed significant GLT1 down-regulation after thoracic (Lepore et al. 2011a; Lepore et al. 2011b) and cervical (Li et al. 2014b) SCI, including in superficial dorsal horn in both mouse and rat models of cervical contusion that are associated with persistent neuropathic pain-related behavior (Putatunda et al. 2014; Watson et al. 2014).
Importantly, studies need to model clinically-relevant aspects of human SCI. Injury to cervical spinal cord represents greater than half of all human SCI cases, in addition to often resulting in the most severe physical and psychological debilitation (Lane et al. 2008). Furthermore, contusion/compression-type trauma predominates in human SCI (McDonald and Becker 2003). Despite this make-up of the clinical population, the vast majority of animal studies (particularly those testing therapies for neuropathic pain) have not employed cervical contusion paradigms. Using targeted intraspinal delivery of an adeno-associated virus type 8 (AAV8)-Gfa2 vector (Li et al. 2014b), we tested effects of increasing GLT1 expression selectively in superficial dorsal horn astrocytes on both neuropathic pain-related behavior and persistently increased neuronal activation in a cervical contusion model of mouse SCI (Nicaise et al. 2012; Watson et al. 2014).
Materials and Methods
Contusion SCI
All procedures were approved by the Thomas Jefferson University IACUC. Thirty male C57BL/6 mice (25–30g; Jackson Laboratory) were used. Briefly, spinal cord was exposed by unilateral laminectomy at C5, and unilateral contusion was induced on the right side of the spinal cord with the Infinite Horizon impactor (Precision Systems and Instrumentation) using a 0.7 mm tip, 40 kilodynes of force and two seconds of dwell time (Watson et al. 2014).
Virus injection
AAV8-eGFP and AAV8-GLT1 were generated and injected as previously described (Li et al. 2014b). Following unilateral laminectomy, injections containing 1.5 μl of virus (titer of 1013 genomic particles/ml) were administered into superficial dorsal horn at C6 and C7 at 0.2–0.3mm below the dorsal surface using a Hamilton gas-tight syringe, 33-gauge 45° beveled needle and UMP3 micropump (World Precision International).
Hargreaves thermal testing
Hargreaves Apparatus (UgoBasile) testing was used to assess thermal sensitivity in the forepaw ipsilateral to contusion (Hargreaves et al. 1988). Forepaw withdrawal latency was measured in response to thermal application to forepaw plantar surface. Withdrawal consisted of a quick limb movement away from the infrared stimulus and was sometimes accompanied by licking and/or guarding of the forepaw. However, we did not observe such supraspinally-mediated responses in all instances. In our experience, this is common in mice with SCI-induced neuropathic pain-related behaviors. Therefore, we did not record or quantify whether such responses occurred while conducting the Hargreaves testing. Three trials were conducted for each forepaw during each testing session, with an inter-trial interval of 120 seconds. The tester was blinded to the injection condition for both the SCI groups and the laminectomy/uninjured groups.
Grip strength testing
Grip strength was measured using the DFIS-2 Series Digital Force Gauge (Columbus Instruments) by allowing the mouse to grasp a thin bar attached to the gauge, followed by pulling the animal away from the gauge until forelimb or hindlimb released the bar. Force measurements were averaged from three trials (Nicaise et al. 2012).
Tissue processing
Animals were sacrificed 5 weeks following injury or laminectomy-only by transcardial perfusion with 4% paraformaldehyde. Animals receiving injury alone (but no AAV injections) were sacrificed 3 weeks following contusion. The cervical/rostral thoracic spinal cord was sectioned transversely at 30 μm using a cryostat.
Lesion size analysis and motor neuron counts
Slides were stained with 0.5% cresyl violet acetate/Eriochrome cyanine. Lesion area was determined by tracing both total area of hemi-spinal cord and lesion area ipsilateral to contusion (Nicaise et al. 2012). Lesion epicenter was defined as the section with the largest percent lesioned tissue (relative to total tissue area in the same section). Lesion volume was determined using the Cavalieri estimator. Ventral horn motor neurons with a clearly identifiable nucleus and a cell soma greater than 200μm2 were counted. Blinded procedures were used in conducting these histological analyses.
Immunohistochemistry
Immunohistochemical analysis was performed at injury epicenter and 1.05 mm caudal to epicenter. Antibodies used were rabbit ΔfosB (IHC 1:100; Santa Cruz), mouse GFAP (IHC 1:400, Sigma Aldrich), rabbit GLT1 (IHC 1:800, kindly provided by Jeffrey Rothstein's lab), rabbit Olig2 (1:200; Millipore), mouse NeuN (1:100; EMD Millipore), rabbit Tuj1 (IHC 1:100, EMD Millipore) and rabbit His (1:50; Cell Signaling Technology).
Fluorescence quantification
eGFP-transduced area of dorsal horn was calculated by outlining and measuring this area in each analyzed section using ImageJ software (National Institutes of Health). GLT1 intensity was quantified in superficial laminae (I–II) using Metamorph software (Molecule Devices). ΔfosB-positive cells were quantified by counting stained nuclei in laminae I-II.
Statistical analyses
All data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism (GraphPad Software). Hargreaves test data were analyzed using a two-way ANOVA. Histological and grip strength data were assessed with one-way ANOVA or unpaired t-test. Statistical significance was defined as p<0.05.
Results
Intraspinal AAV8-GLT1 injection increased GLT1 expression selectively in astrocytes of superficial dorsal horn following cervical contusion SCI
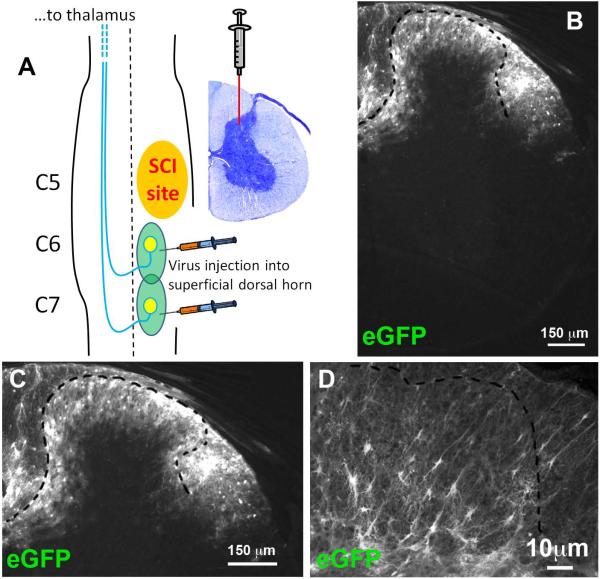
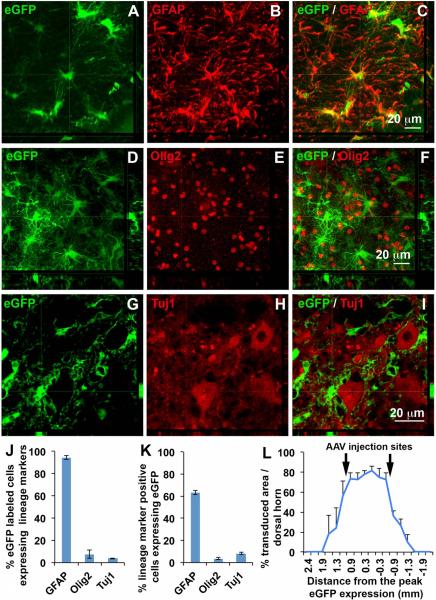
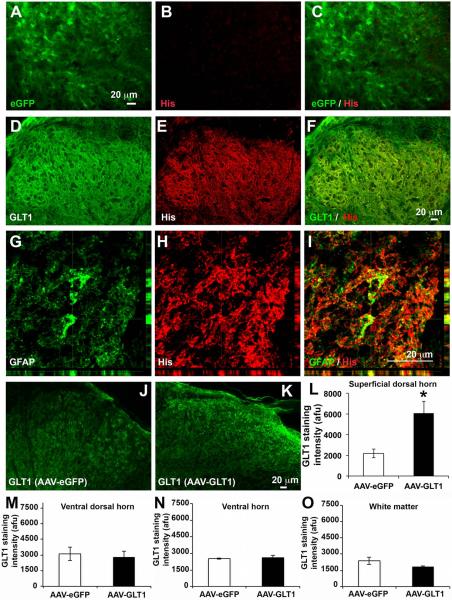
We intraspinally delivered GLT1-His fusion gene or eGFP gene using AAV8-Gfa2 viral vectors that drive expression under control of the GFAP promoter. We generated unilateral contusion at level C5, and we then injected AAV8-Gfa2-eGFP or AAV8-Gfa2-GLT1 into superficial dorsal horn at C6 and C7 1 week post-injury, a time point after heat hypersensitivity in the ipsilateral forepaw was already established (Fig. 1 A). We targeted C6 and C7 as this is the location of afferent nociceptive input from the forepaw plantar surface. We first assessed location and efficiency of viral transduction in cervical spinal cord at 5 weeks post-injection. eGFP reporter expression was highly localized to superficial dorsal gray matter (Fig. 1 B, C). A vast majority of transduced cells exhibited an astrocyte-like morphology (Fig. 1 D). AAV8-Gfa2 vector predominantly targeted GFAP+ astrocytes (Fig. 2 A–C), with little-to-no transduction of Olig2+ cells of the oligodendrocyte lineage (Fig. 2 D–F), Tuj1+ neurons (Fig. 2 G–I) or NeuN+ neurons (not shown). Quantification results (Fig. 2 J) show that most eGFP+ cells expressed GFAP (93.6 ± 1.4% of all eGFP-labeled cells), while only a small percentage of eGFP+ cells expressing Olig2 (7.4 ± 3.7 %) or Tuj1 (3.6 ± 0.3 %). AAV8 injection efficiently transduced a significant portion of all GFAP+ astrocytes in laminae I-II (63.5 ± 2.2 %), in comparison with only a small percentage of oligodendrocyte lineage cells (3.6 ± 0.8 %) and neurons (8.1 ± 1.0 %) (Fig. 2 K). Quantification of the area of eGFP reporter expression in the dorsal horn showed that transduction was robustly and continuously distributed from approximately 1 mm rostral to 1 mm caudal to each injection site (Fig. 2 L). We examined co-expression of either eGFP reporter or GLT1 with His-tag that is fused to the N-terminus of GLT1 to differentiate exogenous and endogenous GLT1 protein in injured spinal cord. There was no His expression in AAV8-eGFP injected spinal cord (Fig. 3 A–C), whereas we detected robust His expression in AAV8-GLT1 injected mice where it co-localized with GLT1 (Fig. 3 D–F). The His-tag+ area showed enhanced GLT1 immunostaining intensity compared to surrounding regions that contained only endogenous GLT1. Exogenous His-GLT1 was predominantly expressed in GFAP+ astrocytes (Fig. 3 G–I). Quantification of GLT1 immunofluorescence intensity in laminae I-II at C6 shows significantly increased GLT1 protein expression in AAV8-GLT1 mice compared to AAV8-eGFP (Fig. 3 J–L, p < 0.05). Unlike superficial dorsal horn, the ventral dorsal horn, the ventral horn and the white matter do not show increased levels of GLT1 protein expression in AAV8-GLT1 mice compared to AAV8-eGFP mice (Fig. 3 M, N, O, p = 0.73, 0.73, 0.19 respectively). These observations collectively demonstrate that intraspinal AAV8-Gfa2 delivery specifically and efficiently targeted astrocytes, as well as focally and significantly increased GLT1 expression, in the superficial dorsal horn in cervical contusion mice.
Figure 1. Intraspinal injections of AAV8-eGFP transduced superficial regions of the dorsal horn.
(A) Experimental paradigm showing unilateral C5 contusion and AAV8 injection into superficial dorsal horn at C6 and C7. (B) eGFP reporter expressing cells in dorsal horn. (C) Higher magnification image showing eGFP reporter expressing cells in dorsal horn. (D) Astrocyte-like morphology of eGFP+ cells.
Figure 2. AAV8-GLT1 selectively transduced astrocytes of superficial dorsal horn.
(A–C) eGFP expression in GFAP+ astrocytes. (D–F) Lack of co-localization of Olig2 and eGFP. (G–I) Lack of co-localization of Tuj1 and eGFP. (J) Percentage of eGFP+ cells expressing GFAP, Olig2 or Tuj1. (K) Percentage of GFAP, Olig2 or Tuj1 expressing cells that were eGFP labeled. (L) Percentage of eGFP transduced area in dorsal horn. n = 3 mice/group.
Figure 3. AAV8-GLT1 increased GLT1 expression in superficial dorsal horn.
(A–C) Lack of His-tag expression in AAV8-eGFP injected dorsal horn. (D–F) Co-localization of His-tag with GLT1 protein in dorsal horn. (G–I) Expression of exogenous His-tag+ GLT1 in GFAP+ astrocytes. Increased GLT1 expression in laminae I-II of AAV8-GLT1 (K) compared to AAV8-eGFP (J) mice. Quantification of GLT1 immunostaining intensity in: superficial dorsal horn (L), n = 6/group; in ventral portion of the dorsal horn (M) n = 3/group; in ventral dorsal horn (N), n =3/group; in white matter (O), n = 3/group
GLT1 overexpression in superficial dorsal horn astrocytes attenuated already-established neuropathic pain-related behavior following cervical contusion SCI
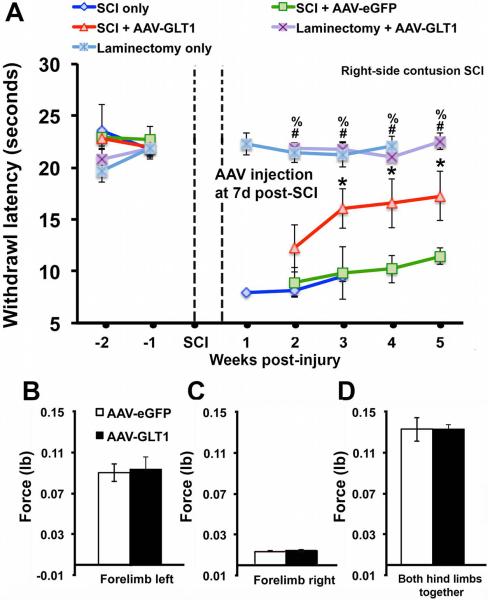
We tested whether GLT1 overexpression exerted beneficial effects on persistent heat hypersensitivity by conducting weekly Hargreaves heat testing starting two weeks pre-injury and continuing until 5 weeks post-injury (4 weeks post-virus delivery). It is important to note this hypersensitivity behavior may not be associated with affective pain. AAV8-GLT1 significantly attenuated heat hypersensitivity (measured as latency to withdraw) in the ipsilateral forepaw starting at two weeks post-injection compared to both injured mice that received AAV8-eGFP and injured mice that received no virus (Fig. 4A). Importantly, heat hypersensitivity was already established in the injury-only control group by the time of virus injection, demonstrating that AAV8-GLT1 delivery at 1 week post-contusion was able to significantly reverse the neuropathic pain-related behavior phenotype. In addition, the attenuation of heat hypersensitivity was maintained until at least 5 weeks after contusion (the latest time point examined). The initial decrease in withdrawal latency was similar between the two AAV8 groups at 1 week post-virus injection, and the effects of AAV8-GLT1 did not occur until 2 weeks post-injection. In two additional control groups, we found that laminectomy-only and laminectomy-only followed by AAV8-GLT1 injection at 1 week post-laminectomy resulted in no detectable differences in withdrawal latency compared to pre-surgery baseline. These findings demonstrate that hypersensitivity was only induced by contusion SCI and that the beneficial effect of AAV8-GLT1 in injured mice was not due to hypoalgesia. Lastly, AAV8-GLT1 only partially attenuated heat hypersensitivity as a statistically-significant difference between this group and the two laminectomy-only control groups was maintained until sacrifice. We also measured both forelimb and hindlimb grip strength at 5 weeks post-injury in contusion mice that received AAV8-eGFP or AAV8-GLT1. We observed no significant differences for ipsilateral forepaw, contralateral forepaw or both hindpaws between the two groups (Fig. 4B–D), suggesting that effects on thermal sensory behavior were not due to changes in motor function.
Figure 4. GLT1 overexpression attenuated already-established neuropathic pain-related behavior following cervical contusion.
(A) Contused mice receiving AAV8-GLT1 showed an attenuated decrease in ipsilateral forepaw withdrawal latency compared to injured animals that received AAV8-eGFP or injury only. Mice receiving laminectomy-only or laminectomy-only followed by AAV8-eGFP injection showed no change in withdrawal latency compared to pre-surgery baseline. * SCI+AAV8-GLT1 vs. SCI+AAV8-eGFP. # SCI+AAV8-GLT1 vs. Laminectomy-only. % SCI+AAV8-GLT1 vs. Laminectomy+AAV8-GLT1. (B–D) There were no significant differences in grip strength for ipsilateral forepaw, contralateral forepaw or both hindpaws between injured AAV8-eGFP and AAV8-GLT1 mice. SCI+AAV8-eGFP, n = 9; SCI+AAV8-GLT1, n = 9; SCI-only, n = 4; Laminectomy-only, n = 4; Laminectomy+AAV8-GLT1, n = 4.
GLT1 overexpression in superficial dorsal horn astrocytes reduced chronic dorsal horn neuron activation following unilateral cervical contusion SCI
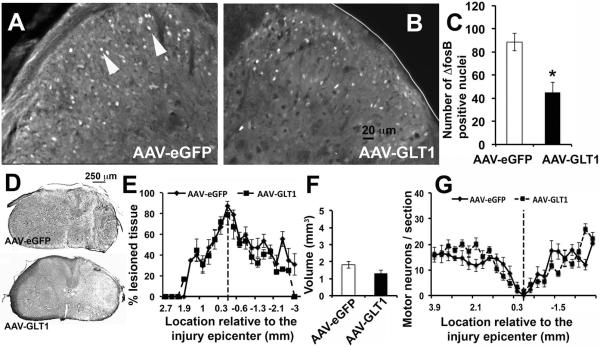
We previously reported chronic increases in numbers of superficial dorsal horn neurons expressing the transcription factor, ΔFosB, following unilateral cervical contusion compared to uninjured laminectomy-only control mice and rats (Putatunda et al. 2014; Watson et al. 2014). ΔFosB is (1) a truncated splice version of the immediate early gene c-fos, (2) a molecular biomarker of chronic neuronal activation (Robison and Nestler 2011), and (3) is thought to transcriptionally mediate some of the long-term changes that result from chronic activation of pain transmission neurons (McClung et al. 2004). For example, ΔFosB regulates expression of key genes involved in plasticity associated with persistent neuropathic pain-related behavior, including CDK5 and the GluA2 and GluN1 subunits of AMPARs and NMDARs, respectively (Nestler 2008). Compared to AAV8-eGFP, AAV8-GLT1 significantly reduced numbers of ΔFosB+ neurons in ipsilateral laminae I-II of C6 at 5 weeks post-injury (Fig. 5A–C), demonstrating that focal GLT1 overexpression reduced persistently increased neuronal activation in superficial dorsal horn. GLT1 function can also exert effects on important SCI outcomes such as excitoxicity, particular when early events post-injury are targeted. To examine the possibility that effects on neuropathic pain-related behavior were indirectly due to neuroprotection rather than modulation of dorsal horn neuron excitability, we assessed both lesion size and neuronal sparing at 5 weeks post-contusion. There were no differences between AAV8-eGFP and AAV8-GLT1 groups in lesion size at multiple rostral-caudal distances from the lesion epicenter (Fig. 5D–E), maximum extent of lesion area at epicenter (78.5 ± 9.3% for AAV8-GLT1; 87.1 ± 4.4% for AAV8-eGFP; p = 0.37), overall lesion volume (Fig. 5F), or numbers of spared motor neurons (Fig. 5G). These results show that the beneficial effect of GLT1 overexpression correlated with reduced numbers of ΔFosB+ neurons in dorsal horn, while other histological measures remained unaffected.
Figure 5. GLT1 overexpression reduced chronic dorsal horn neuron activation following cervical contusion.
(A–C) Reduced numbers of ∆FosB+ neurons (A, arrowheads) in ipsilateral laminae I-II in AAV8-GLT1 mice compared to AAV8-eGFP (p = 0.02). Cresyl violet staining illustrates epicenter in AAV8-eGFP (D, top) and AAV8-GLT1 (D, bottom) groups. Lesion size (E), lesion volume (F) and motor neuron counts (G) were not different between groups. n = 6–8/group.
Discussion
We show that focally elevating GLT1 expression selectively in astrocytes of superficial dorsal horn results in significant reversal of at-level neuropathic pain-related behavior that has already developed, as well as reduction in persistently increased activation of dorsal horn neurons. These findings have important implications for therapeutically targeting chronic neuropathic pain after SCI.
We waited to deliver AAV8-GLT1 vector until after heat hypersensitivity had already developed, demonstrating that restoration of GLT1 expression can reverse an established pathological pain state. Such a delayed approach could be valuable for treatment of SCI patients as therapeutic intervention for neuropathic pain-related behavior would likely not be administered prophylactically. We waited until one week post-injury to deliver virus; however, we have previously shown that it takes 7–10 days following AAV8 injection to achieve robust transduction and expression in vivo, suggesting that the window of opportunity for targeting neuropathic pain in this SCI model extends even beyond one week. This delay in expression may have also accounted for the observation that AAV8-GLT1 did not begin to significantly attenuate heat hypersensitivity until two weeks post-injection.
SCI-induced neuropathic pain is particularly difficult to treat. For example, currently available oral pharmacological interventions commonly reduce neuropathic pain intensity by only 20–30% or show no effect (Baastrup and Finnerup 2008). We observed a partial, but significant, amelioration of neuropathic pain-related behavior in mice by GLT1 overexpression. Furthermore, the long-term behavioral efficacy of AAV8-GLT1 lasted until animals were sacrificed at 5 weeks post-injury, demonstrating the persistent therapeutic effects of targeting GLT1. Importantly, we obtained these results in a patient-relevant model of cervical contusion.
Anatomically-targeted delivery to superficial dorsal horn, coupled with cell population-specific transduction of astrocytes, provides an innovative opportunity to therapeutically modulate glutamate homeostasis, while avoiding unwanted side effects associated with more diffuse delivery methods. For example, targeting glutamate receptor over-activation (i.e. AMPA and NMDA antagonists) has diffuse and non-specific effects in CNS, PNS and potentially other tissues, as well as only a short duration of intervention (Bleakman et al. 2006). While intraspinal delivery methods provide for robust modulation of GLT1 expression focally in dorsal horn, it will be important in future studies to test the effects of less-invasive pharmacological approaches that may be able to enhance GLT1 function (Olsen et al. 2010).
There is a growing appreciation for the role played by astrocytes in pathological pain (Carlton et al. 2009; Gwak et al. 2012; Nesic et al. 2005), particularly in hyperexcitability of nociceptive circuitry (Scholz and Woolf 2007). The anatomical mechanisms of hyperalgesia, allodynia and spontaneous pain differ; nevertheless, significant and chronic astrocyte activation occurs in all of these pain states, regardless of whether there is central SCI or peripheral nerve damage (Hansson 2006). After SCI, elevation in the levels of factors such as glutamate, CGRP and substance P can activate astrocytes, resulting in astrocytic release of ATP, NO and pro-inflammatory cytokines that facilitate nociceptive neurotransmission by increasing excitability of dorsal horn pain neurons. These molecules can also activate neighboring astrocytes to maintain and anatomically spread pain to uninjured areas (Hulsebosch 2008). Astrocyte activation can also compromise key physiological functions such as glutamate uptake (Lepore et al. 2011a), resulting in further dysregulation of extracellular glutamate homeostasis. Dysregulation of glutamate signaling can itself promote dorsal horn neuron hyperexcitability and can also lead to additional astrocyte stimulation, both locally and in more distant locations Furthermore, this gliopathic response can persist, possibly maintaining a chronic neuropathic state following SCI (Bleakman et al. 2006; Cao and Zhang 2008; Gwak and Hulsebosch 2011a; Ortinski et al. 2010; Tao et al. 2005). We show that mechanistically addressing one aspect of astrocyte dysfunction (i.e. GLT1 function) can significantly and persistently reduce neuropathic pain after SCI. In models of chronic neuropathic pain such as peripheral nerve injury, maintaining GLT1 expression via drugs such as propentofylline can also block neuronal hyperexcitability and development of pathological pain (Gwak et al. 2008; Gwak and Hulsebosch 2009; Tawfik et al. 2006; Tawfik et al. 2008). Similar approaches have also been successful in SCI models; however, the cellular mechanism and the anatomical site(s) of action of these pharmacological interventions are not clear, unlike with the targeted approach we employed in this study.
As mentioned above, chronic hyperexcitability of second order dorsal horn neurons is a major mechanism associated with the pathogenesis of neuropathic pain following SCI (Gwak and Hulsebosch, 2011b). Genes of the Fos family, including ΔFosB, are expressed immediately following neuronal stimulation. ΔFosB lacks the C-terminal fragment compared to the full length FosB and has an unusually long half-life. Following neuronal stimulation, ΔFosB is expressed at a relatively low level. However, its levels accumulate following repeated neuronal stimulation due to the stability of the protein. Therefore, it serves as a reliable marker for chronic neuronal activation (McClung et al., 2004 and Nestler, 2008). Excitingly, we found that there were significantly reduced numbers of ΔFosB+ neurons in the superficial dorsal horn at 5 weeks post-injury in AAV8-GLT1 animals as compared to the control AAV-eGFP injected animals. We restricted our ΔFosB analysis to the ipsilesional superficial dorsal horn of the cervical spinal cord as this is the location of AAV-GLT1 delivery. Given the important role played by other areas in nociceptive transmission, including supraspinal structures such as the thalamus (Hulsebosch et al. 2009), it will be interesting in future work to assess the effects of restoring GLT1 function in the dorsal horn on neuronal excitability in these other key locations.
In our previous work, when we selectively increased GLT1 expression in endogenous reactive astrocytes in the unilateral cervical contusion model using our AAV8-GLT1 vector, we paradoxically found that secondary degeneration of respiratory motor neurons and diaphragm denervation were worsened (Li et al. 2014b). This effect was due to compromise in the protective glial scar-forming properties of endogenous astrocytes, which resulted in unexpected lesion expansion. However, we only found this effect with pre-treatment prior to injury (which boosted GLT1 levels by the time of injury), but not when we injected virus with a delay post-injury. These findings demonstrate that increasing GLT1 in endogenous astrocytes is problematic only for very early events post-SCI and only related to secondary degeneration. Consistent with this, lesion size and motor neuron loss were not affected in the present study when AAV8-GLT1 was injected at 7 days post-contusion. In our previous work using astrocyte progenitor transplantation (Li et al. 2014a), we also found that delivery of an exogenous source of astrocytes that expresses high levels of GLT1 (in the exact same cervical contusion model) results in significant preservation of motor neurons and diaphragm function. This finding, as well as other studies that tested pharmacologically elevating (Olsen et al. 2010) or genetically reducing (Lepore et al. 2011a) GLT1 in SCI, demonstrates that targeting GLT1 is a promising neuroprotective strategy. The current data now also show the power of modulating GLT1 levels for addressing neuronal hyperexcitability and consequent neuropathic pain-related behavior after SCI.
Main points.
We injected AAV8-GLT1 into a mouse model of cervical contusion SCI.
AAV8-GLT1 increased GLT1 expression selectively in astrocytes, reduced activation of superficial dorsal horn neurons, and reversed already-established heat hypersensitivity.
Acknowledgements
This work was supported by NINDS grant R01NS079702 to A.C.L.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–75. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol Motil. 2010;22:499–511. doi: 10.1111/j.1365-2982.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32:972–83. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147:265–76. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–22. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011a;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury. Curr Pain Headache Rep. 2011b;15:215–22. doi: 10.1007/s11916-011-0186-2. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234:362–72. doi: 10.1016/j.expneurol.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol (Oxf) 2006;187:321–7. doi: 10.1111/j.1748-1716.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–13. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci. 2008;31:538–47. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, O'Donnell J, Bonner JF, Paul C, Miller ME, Rauck B, Kushner RA, Rothstein JD, Fischer I, Maragakis NJ. Spatial and temporal changes in promoter activity of the astrocyte glutamate transporter GLT1 following traumatic spinal cord injury. J Neurosci Res. 2011a;89:1001–17. doi: 10.1002/jnr.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, O'Donnell J, Kim AS, Yang EJ, Tuteja A, Haidet-Phillips A, O'Banion CP, Maragakis NJ. Reduction in expression of the astrocyte glutamate transporter, GLT1, worsens functional and histological outcomes following traumatic spinal cord injury. Glia. 2011b;59:1996–2005. doi: 10.1002/glia.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Javed E, Hala TJ, Sannie D, Regan KA, Maragakis NJ, Wright MC, Poulsen DJ, Lepore AC. Transplantation of glial progenitors that overexpress glutamate transporter GLT1 preserves diaphragm function following cervical SCI. Molecular Therapy. 2014a doi: 10.1038/mt.2014.236. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Nicaise C, Sannie D, Hala TJ, Javed E, Parker JL, Putatunda R, Regan KA, Suain V, Brion JP, et al. Overexpression of the astrocyte glutamate transporter GLT1 exacerbates phrenic motor neuron degeneration, diaphragm compromise, and forelimb motor dysfunction following cervical contusion spinal cord injury. J Neurosci. 2014b;34:7622–38. doi: 10.1523/JNEUROSCI.4690-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–89. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–54. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Becker D. Spinal cord injury: promising interventions and realistic goals. Am J Phys Med Rehabil. 2003;82:S38–49. doi: 10.1097/01.PHM.0000086994.53716.17. [DOI] [PubMed] [Google Scholar]
- Nesic O, Lee J, Johnson KM, Ye Z, Xu GY, Unabia GC, Wood TG, McAdoo DJ, Westlund KN, Hulsebosch CE, et al. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem. 2005;95:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–55. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Putatunda R, Hala TJ, Regan KA, Frank DM, Brion JP, Leroy K, Pochet R, Wright MC, Lepore AC. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J Neurotrauma. 2012;29:2748–60. doi: 10.1089/neu.2012.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Campbell SC, McFerrin MB, Floyd CL, Sontheimer H. Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17 beta-oestradiol treatment. Brain. 2010;133:1013–25. doi: 10.1093/brain/awq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–91. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia. 2015;63:1101–25. doi: 10.1002/glia.22809. [DOI] [PubMed] [Google Scholar]
- Putatunda R, Hala TJ, Chin J, Lepore AC. Chronic at-level thermal hyperalgesia following rat cervical contusion spinal cord injury is accompanied by neuronal and astrocyte activation and loss of the astrocyte glutamate transporter, GLT1, in superficial dorsal horn. Brain Res. 2014;18:64–79. doi: 10.1016/j.brainres.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K. Emerging role of astroglia in pain hypersensitivity. Jpn Dent Sci Rev. 2010;46:86. doi: 10.1016/j.jdsr.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–37. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Yaksh TL. The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553–83. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- Tao YX, Gu J, Stephens RL., Jr Role of spinal cord glutamate transporter during normal sensory transmission and pathological pain states. Mol Pain. 2005;1:30. doi: 10.1186/1744-8069-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik VL, Lacroix-Fralish ML, Bercury KK, Nutile-McMenemy N, Harris BT, Deleo JA. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: heterogeneity of the quiescent phenotype. Glia. 2006;54:193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Regan MR, Haenggeli C, Lacroix-Fralish ML, Nutile-McMenemy N, Perez N, Rothstein JD, DeLeo JA. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience. 2008;152:1086–92. doi: 10.1016/j.neuroscience.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C, Thomas G, Pierre J, Jacquemin C, Pierre M, Saunier B. Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinase) Eur J Biochem. 1997;244:587–95. doi: 10.1111/j.1432-1033.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Mills CD, Ye Z, Fullwood SD, McAdoo DJ, Hulsebosch CE, Westlund KN. Rapid changes in expression of glutamate transporters after spinal cord injury. Brain Res. 2002;927:104–10. doi: 10.1016/s0006-8993(01)03329-7. [DOI] [PubMed] [Google Scholar]
- Watson JL, Hala TJ, Putatunda R, Sannie D, Lepore AC. Persistent at-level thermal hyperalgesia and tactile allodynia accompany chronic neuronal and astrocyte activation in superficial dorsal horn following mouse cervical contusion spinal cord injury. PLos One. 2014 doi: 10.1371/journal.pone.0109099. [DOI] [PMC free article] [PubMed] [Google Scholar]