Abstract
Production of mitochondrial reactive oxygen species and integrity of mitochondrial DNA (mtDNA) are crucial in breast cancer progression and metastasis. Therefore, we evaluated the role of mtDNA damage in breast cancer by genetically modulating the DNA repair enzyme 8-oxoguanine DNA glycosylase (OGG1) in the PyMT transgenic mouse model of mammary tumorigenesis. We generated mice lacking OGG1 (KO), mice overexpressing human OGG1 subunit 1α in mitochondria (Tg), and mice simultaneously lacking OGG1 and overexpressing human OGG1 subunit 1α in mitochondria (KO/Tg). We found that Tg and KO/Tg mice developed significantly smaller tumors than KO and wildtype mice after 16 weeks. Histological analysis revealed a roughly two-fold decrease in the incidence of lung metastases in Tg mice (33.3%) compared to wildtype mice (62.5%). Furthermore, lungs from Tg mice exhibited nearly a 15-fold decrease in the average number of metastatic foci compared to WT mice (p≤0.05). Primary tumors isolated from Tg mice also demonstrated reduced total and mitochondrial oxidative stress, diminished mtDNA damage, and increased mitochondrial function. Targeting hOGG1 to the mitochondria protected cells from mtDNA damage resulting in downregulation of HIF-1α and attenuated phosphorylation of Akt. Collectively, we demonstrate proof of concept that mtDNA damage results in breast cancer progression and metastasis in vivo. Moreover, our findings offer new therapeutic strategies for modulating the levels of mtDNA repair enzymes to delay or stall metastatic progression.
Keywords: breast cancer, metastases, mitochondria, mitochondrial DNA damage, mitochondrial DNA repair, oxidative stress, ROS, mitochondrial dysfunction
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death among women worldwide. Despite improvements in diagnosis, treatment, and longevity, the effect on mortality has been modest. A lack of understanding about the natural history of the disease is a major contributory factor to this limitation. On a molecular level, it is still not fully understood which of the changes in breast tumors lead to invasion and metastasis. Among the potential causes are mitochondrial dysfunction, mitochondrial reactive oxygen species (ROS, mtROS) generation and mitochondrial DNA (mtDNA) damage. Recently, mtROS production has been proposed to play an important role in breast cancer progression in both in vitro (1) and in vivo models (1, 2). Also, it has been shown that mitochondrial polymorphism and mtDNA variations modify breast cancer risk in patients (3). mtDNA variations may cause inefficient oxidative phosphorylation and affect the efficiency of electron transport chains leading to the accumulation of ROS, mtDNA damage, and increased cancer risk. Studies using cytoplasmic hybrid technology have shown that mutations in mtDNA were associated with overproduction of ROS and can regulate tumor cell metastasis (4). Taken together, these recent findings suggest that integrity of mtDNA is crucial in cancer progression and metastasis.
The molecular mechanisms of ROS-driven tumor progression are not completely understood, but it has been shown that increased ROS generation upregulates the expression and activities of the pathways involving Akt, MAPK, hypoxia-inducible factor 1 (HIF-1α), and remodeling of the extracellular matrix by metalloproteinases (MMPs) which ultimately lead to tumor progression and metastasis (rev in 5, 6). On the other hand, ROS-induced damage to mtDNA in cancer cells causes alterations in mtDNA transcription either through base mispairing, which results in defective transcripts, or decreased transcription due to polymerase blocking. Since mammalian mtDNA encodes 13 polypeptides of the electron transport chain, 2 rRNAs and 22 tRNAs, alterations in mitochondrial transcription could change electron transport complexes to cause decreased ATP production and also, lead to defective electron transfer, which would cause additional ROS production, thus establishing a “vicious cycle” between mtDNA damage and ROS generation, since any damage to the respiratory chain may enhance ROS production and thus heighten the oxidative stress to all other mitochondrial components, including mtDNA. This increased oxidative stress will exacerbate mitochondrial dysfunction which ultimately leads to activation of signaling pathways leading to cancer progression.
Our previous data showed that the mitochondrial targeted DNA repair enzyme, human 8-oxoguanine (8-OxoG) DNA glycosylase/AP lyase (hOGG1) enhanced mitochondrial DNA repair, increased mitochondrial function and protected different cell types from oxidant-mediated death (7-9). Additionally, we and others have shown that targeting of hOGG1 to mitochondria decreased mtROS production, thus, supporting the vicious cycle hypothesis (8-10). The goal of this study is to investigate whether mtDNA damage controls mitochondrial dysfunction, increases mtROS production and enhances breast cancer progression in a well established genetic model of breast cancer, PyMT mouse.
Materials and Methods
Animals
Double transgenic female mice were generated by crossing FVB/N-Tg(MMTV-PyMT)634Mul/J male mice (The Jackson Laboratory), which are well-characterized and a widely used preclinical transgenic model of metastatic breast cancer (11) with the [OGG1-/- (KO)], mitochondrial targeted [(hOGG1), Tg], KO/Tg and wild type (WT) females on a congenic C57/BL6 background (9, 12, 13). Genotyping of mice was performed using genomic DNA derived from tails and PCR with a set of primers described for PyMT animals (Jackson Lab website) and, for OGG1 models, using previously described PCR primers (12, 13). Female double transgenic mice were maintained for 16 weeks. All procedures used in this study were approved by the Institutional Animal Care and Use Committees of the University of South Alabama and fully complied with the guidelines from the National Institute of Health.
Gross evaluation of tumor and histological analysis
Starting at 16 weeks, mice were sacrificed, and all mammary tumors were carefully excised and weighed. Mammary tumors and lungs sections were cut into smaller portions, and fixed with 10% neutral buffered formalin for at least 24 hours before embedding in paraffin. Sections were cut at 5 microns, stained with hematoxylin and eosin (H&E), and evaluated for invasion and lung metastases in a blind fashion by an experienced histopathologist (Dr. Andrea Kahn, Pathology Department, University of South Alabama). Analyses and descriptions were in accordance with the guidelines put forth by the mouse mammary gland pathology consensus meeting in Annapolis (14) to render a diagnosis of the tumors. The grade for invasion (scored from 1 to 3) was estimated when areas of invasion other than the more expansile center of the lesion were identifiable.
Isolation of cellular fractions and 8-oxoG activity assay
Mammary tumors were dissected and homogenized in liquid nitrogen. Mitochondrial and nuclear fractions were isolated and the purity of the preparation was checked as we previously described (10). The OGG1 activity was performed as previously described with minor modifications (7, 8).
Mitochondrial DNA damage analysis and copy number
MtDNA damage and copy number was assessed as described previously with some modifications (15). Briefly, mammary tumors were homogenized in liquid nitrogen and total DNA was isolated using a Qiagen DNAeasy kit for the DNA isolation (Qiagen, Valencia, CA). DNA was digested with EcoRI, precisely quantified and quantitative alkaline Southern blot analysis was performed to evaluate changes in the density of mtDNA lesions using mouse mtDNA specific probe [cytochrome c oxidase, I subunit, (15)]. The results for mtDNA damage obtained after quantitative alkaline Southern blot analysis were normalized for mtDNA copy number and presented (as A.U.).
Mitochondrial ATP level
Mitochondria were isolated from fresh tumor tissues and mitochondrial ATP level was analyzed as we previously described (15). Values were normalized to protein content.
Lactate dehydrogenase activity assay
Tumors were extracted for protein and then, their lactate dehydrogenate (LDH) activity was measured using an LDH assay kit (BioVision). The results were normalized to the tumor weight.
Protein isolation, Western blot analysis and oxidative protein carbonylation
Total cellular or mitochondrial fractions from mammary tumors were isolated as described previously (15). The antibodies used were to H1F-1α, phospho-Akt (Ser 473), total Akt (Cell Signaling, Beverly, MA); Cox IV, an Subunit IV component (Mitosciences) and actin (Sigma). Complexes formed were detected with horseradish peroxidase conjugated anti-mouse IgG or anti-rabbit IgG antibodies (Promega, Madison, WI) using chemiluminescent reagents (SuperSignal, Pierce, Rockford, IL). An oxidative protein carbonylation (PCO) assay was performed as we described previously (15). Where indicated, the resultant band images were scanned and analyzed using Fujifilm Image Gauge Version 2.2 software. For H1F-1α protein content, data were normalized to the density of actin bands. Additionally, for PCO, a value of 1 was arbitrary assigned to the results from the PyMT/WT group and all data were normalized to the density of bands of PyMT/WT.
Statistical analysis
Data are expressed as means ± SE. Differences between groups were assessed using one-way ANOVA (GraphPadPrism) followed by Bonferroni analysis where appropriate. Statistical significance was determined at the 0.05 level.
Results and Discussion
Targeting of hOGG1 into mitochondria in both PyMT/Tg and PyMT/KO/Tg mice suppressed tumor growth and metastases
We transgenically expressed hOGG1 in the mitochondria and generated PyMT/Tg and PyMT/KO/Tg mice. Starting at 16 weeks, mice were sacrificed, and all mammary tumors were carefully excised and weighed. As shown in Table 1, the appearance of palpable tumors in the PyMT/KO mice was about 2 weeks earlier and in PyMT/KO/TG was delayed for 2 weeks compared to the PyMT/WT group.
Table 1.
Characteristics of mice
| PyMT mice genotype | Primary tumor incidence | Mean age in week to first palpable tumor | Metastatic tumor incidence |
|---|---|---|---|
| KO | 100% | 9.35± 0.7* a | 82%* a |
| WT | 100% | 11.86 ± 0.1 | 62.5% |
| WT/Tg | 100% | 12.89± 0.26 | 33.3%* |
| KO/Tg | 92.3% | 13.64± 0.3* | 36.4%* |
n=13-16, significance at
p≤0.05 vs WT group
vs all other groups, one way ANOVA.
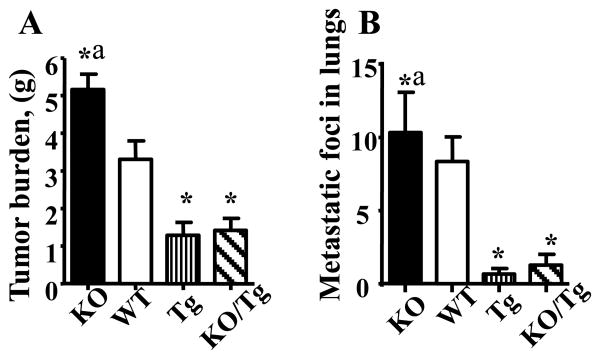
Representative images of 12-16 week-old PyMT female mice of different OGG1 genotypes are shown in the Supplementary Fig. S1. Note the increased number of large and bulky tumors in the PyMT/KO mouse compared to all other genotypes. Previously, two reports demonstrated that PyMT mice on the C57BL/6 background have a longer mammary tumor latency (92 days) compared to PyMT on the FVB background (53 days) while the incidence of lung metastasis was only slightly reduced (2, 16). Our results are in line with these reports since the first tumor appearance were delayed in all genetic models (C57BL/6 background) compared to PyMT on FVB background (2, 16). Interestingly, the KO/Tg group displayed a lower (92.3%) primary tumor incidence as compared to the 100% in all other groups. By recording the total tumor wet weight per mouse, it was observed that both PyMT/Tg and PyMT/KO/Tg mice developed significantly smaller tumors compared with PyMT/WT. This effect was especially pronounced with PyMT/KO mice (Fig.1A). Histopathology analysis showed that the majority of the tumors had a similar overall pattern of advanced invasive carcinoma, but there were a few (derivatives from PyMT/WT) with a more distinct morphology with squamous differentiation.
Figure 1.
Protection against mtDNA damage by overexpression of hOGG1 reduced tumor weight (A) and lung metastases (B) in PyMT mice. n=13-16, *p < 0.05 vs WT, ap < 0.05 vs all other groups, one way ANOVA.
Mitochondrial expression of hOGG1 in both PyMT/Tg and PyMT/KO/Tg mice prevented lung's metastases
As shown in Table 1, we found about a two fold difference (p≤0.05) in the metastatic tumor incidence in lungs between PyMT/Tg (33.3%) and PyMT/WT mice (62.5%). Remarkably, the lungs of PyMT/Tg mice showed about a 15 fold decrease in average number of metastatic foci compared to those of PyMT/WT mice (p≤0.05. Fig.1B, Supplemental Fig.S2.). Therefore, based on these data, we conclude that mtDNA regulates both progression and the proliferation of tumor foci at a distant site of metastasis.
Analysis of OGG1 activity in mammary tumors from double transgenic PyMT mice
OGG1 activity was examined in mammary tumors isolated from nuclear and mitochondrial fractions from all four groups of mice. First, mitochondrial and nuclear fractions were isolated and the purity of the preparation was checked by the enrichment of mitochondrial or nuclear specific proteins as determined by Western blot (Supplemental Fig.S3A and C). As expected, there was no notable difference in OGG1 activity in nuclear fractions isolated from mammary tumors in the PyMT WT, Tg or KO/ Tg groups (Supplemental Fig.S3B). There was minimal OGG1 activity in nuclear fractions from PyMT/KO group (Supplemental Fig.S3B), which, most likely, indicates the involvement of some alternative nuclear enzyme/s in the repair of 8-OxoG in the nuclear fractions of KO mice. On the contrary, as shown in Supplemental Fig.S2D, OGG1 activity was elevated in mitochondria isolated from tumors from both Tg and KO/Tg animals, whereas OGG1 activity was lower in KO mice compared to WT mice.
Tumors isolated from PyMT/Tg mice showed reduced total and mitochondrial oxidative stress, diminished mtDNA damage, increased mitochondrial function and reduced LDH activity
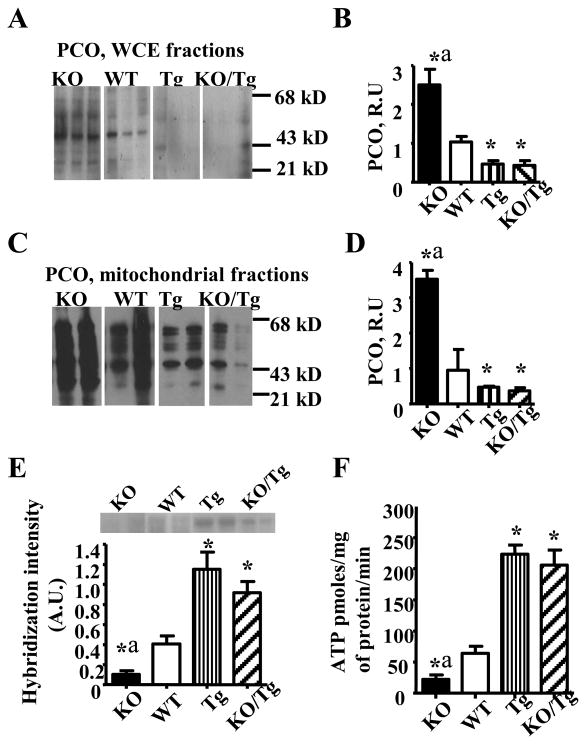
First, we evaluated both total and mitochondrial oxidative stress using a common marker of oxidative stress in proteins, PCO. Whole cell extracts (WCE, Fig.2A and B) and mitochondrial fractions (Fig.2C and D) from PyMT/Tg and KO/Tg tumors showed reduced levels of PCO compared to WT or KO groups, indicating decreased oxidative stress. Next, we evaluated mtDNA damage and copy number in mammary tumors isolated from all four groups of mice. mtDNA damage was assessed as the diminished intensity in the 14 kb major restriction band, indicating that DNA breaks have caused smaller size fragments, which migrate further down in the gel and, thus, reduced the number of full size restriction fragments (Fig.2E, top). Tumors from both PyMT/Tg and KO/Tg groups showed significantly greater full size fragment band intensity indicating that they have reduced mtDNA damage. Consistent with the reduction in mtDNA damage and reduction of the oxidative stress marker, we found that tumors overexpressing mitochondrial hOGG1 have significantly enhanced mitochondrial levels of ATP compared to both KO and WT mice (Fig.2F). Additionally, LDH activity was significantly increased in PyMT/KO tumors while tumors isolated from PyMT/Tg mice have reduced LDH activity (Supplementary Fig. S4).
Figure 2.
Mitochondrial overexpression of hOGG1 reduced total (A, B) and mitochondrial oxidative stress (C, D), mtDNA damage (E) and increased mitochondrial function (F) in mammary tumors in PyMT mice. (n= 6-7). *p < 0.05 vs WT, ap < 0.05 vs all other groups, one way ANOVA.
Protection from mtDNA damage by targeting of hOGG1 into mitochondria results in downregulation of H1F-1α and attenuated phosphorylation of Akt
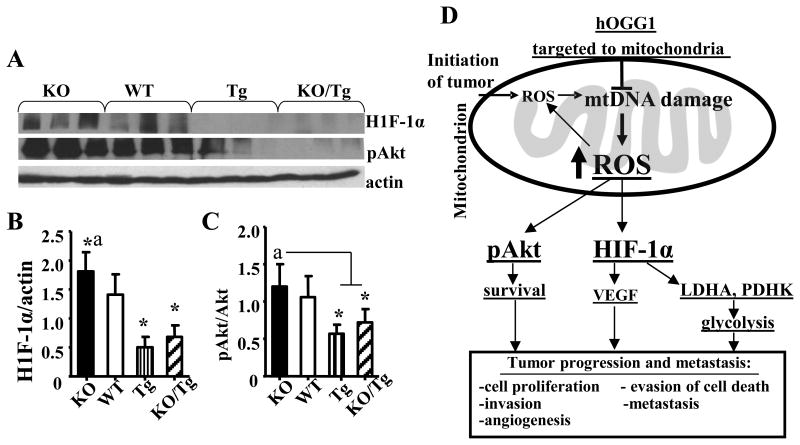
Next, we compared the effect of mtDNA damage on the expression of H1F-1α and phosphorylation of Akt (Ser473) in mammary tumors by Western blot (Fig. 3A). As shown in Fig. 3A, B and C tumors isolated from PyMT/Tg and KO/TG mice have reduced levels of both H1F-1α and pAkt (Ser473) compared to PyMT/WT group.
Figure 3.
Targeting of hOGG1 to mitochondria in mammary tumors of PyMT mice reduced expression of H1F-1α and phosphorylation of Akt. A, Whole cell lysates were isolated from mammary tumors and analyzed by Western blot analysis with the indicated antibodies. Representative blots are shown. B, Values from densitometry for H1F-1α were normalized to the actin data and are the means ± SE. (n= 6-7). *p < 0.05 vs WT, ap < 0.05 vs all other groups, one way ANOVA. C, The values from densitometry from (p-Akt) Western blots were normalized to the level of total Akt, and expressed as fold of difference. Data and are the means ± SE. (n= 6-7). *p < 0.05 vs WT, ap < 0.05 vs all other groups, one way ANOVA. D, Schematic diagram depicting the vicious cycle of events between mtDNA damage, ROS and breast cancer progression and metastasis.
Our study provides a proof-of-concept that mtDNA damage controls mitochondrial function, mtROS production and thus, leads to breast cancer progression and metastases in a mouse genetic model. Until recently, mtDNA has received little attention in cancer research as prior studies have largely focused on the nuclear genome. Because most chemical carcinogens damage preferentially mtDNA rather than nuclear DNA, mtDNA is considered to be their major cellular target. Our findings are in line with a recent study which showed that targeting of mitochondrial catalase decreased mitochondrial oxidative stress and suppressed invasive breast cancer in PyMT mice (2).
Most importantly, we would like to emphasize that we believe that mtDNA damage, a prerequisite for somatic mtDNA mutations, can be detected much earlier than mtDNA mutation and thus is a better marker of cancer progression, metastasis and treatment than mtDNA mutations. Also, as opposed to mtDNA mutations, which can not be reversed, mtDNA damage can be repaired, and thus reversed using DNA repair enzymes, thus providing important clinical and translational significance for this potential therapeutic strategy. Interestingly, a very recent study showed that the absence of OGG1 is associated with an aggressive breast cancer phenotype in patients (17), which supports the clinical relevance of this study. However, results about the role of the hOGG1 and, particularly, mt-hOGG1 involvement in development of cancer are highly controversial (18, 19). We have used the 1-α isoform (transcript variant 1a) of OGG1 as it displays DNA glycosylase activity in both the nucleus and mitochondria, as opposed to the mitochondria localized β-OGG1(transcript variant 2a), which has been reported to lack such activity (20). Thus, our results which confirmed that targeting of hOGG, isoform 1-α into mitochondria protectes against mtDNA damage and thus suppresses mtROS production, protectes against mitochondrial dysfunction, and thereby reduces ROS-dependant tumor progression and metastasis, are of significant importance in relationship to cancer biology. Moreover, we have shown increased HIF-1α and increased LDH activity in PyMT/KO tumors. Based on our results and previous reports we propose the working model shown in Fig. 3D. The increased ROS leads to stabilization and activation of HIF-1α, which in turn activates its target genes (e.g. LDH, et al) subsequently leading to glycolysis (Warburg effect, Fig.3D).
In addition, these results have important clinical and translational significance, as most current chemo-therapeutic agents, radiation therapy and surgical procedures all increase oxidative stress, and, therefore, will induce more damage to mtDNA, increase mtROS and, thus, can drive tumor recurrence and metastasis. Thus, this study provides the basis for novel therapeutic approaches by which modulating the level of mitochondrial OGG1 may decrease cancer progression and metastasis.
Supplementary Material
Acknowledgments
Financial support: This research was supported by USA CRF Award (L.I.Rachek) and NIH R01CA149646 (M.Tan).
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1.Pelicano H, Lu W, Zhou Y, Zhang W, Chen Z, Hu Y, et al. Mitochondrial dysfunction and reactive oxygen species imbalance promote breast cancer cell motility through a CXCL14-mediated mechanism. Cancer Res. 2009;69:2375–83. doi: 10.1158/0008-5472.CAN-08-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh J, Enns L, Fatemie S, Hopkins H, Morton J, Pettan-Brewer C, et al. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer. 2011;11:191. doi: 10.1186/1471-2407-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai RK, Leal SM, Covarrubias D, Liu A, Wong LJ. Mitochondrial genetic background modifies breast cancer risk. Cancer Res. 2007;67:4687–94. doi: 10.1158/0008-5472.CAN-06-3554. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–4. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53–9. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 7.Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL. Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem. 2002;277:44932–7. doi: 10.1074/jbc.M208770200. 2002. [DOI] [PubMed] [Google Scholar]
- 8.Yuzefovych LV, Solodushko VA, Wilson GL, Rachek LI. Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology. 2012;153:92–100. doi: 10.1210/en.2011-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuzefovych LV, Schuler AM, Chen J, Alvarez D, Eide L, LeDoux SP, et al. Alteration of mitochondrial function and insulin sensitivity in primary mouse skeletal muscle cells isolated from transgenic and knockout mice: role of OGG1. Endocrinology. 2013;154(8):2640–9. doi: 10.1210/en.2013-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Xu M, Xo R, Mates A, Wilson GL, Pearsall AWt, et al. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage. 2010;18:424–432. doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Esbensen Y, Kunke D, Suganthan R, Rachek L, Bjoras M, et al. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J Neurosci. 2011;31:9746–51. doi: 10.1523/JNEUROSCI.0852-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A. 1999;96:13300–5. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–88. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 15.Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One 8. 2013:e54059. doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davie SA, Maglione JE, Manner CK, Young D, Cardiff RD, MacLeod CL, et al. Inducible nitric oxide synthase deficient mice. Transgenic Res. 2007;16(2):193–201. doi: 10.1007/s11248-006-9056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karihtala P, Kauppila S, Puistola U, Jukkola-Vuorinen A. Absence of the DNA repair enzyme human 8-oxoguanine glycosylase is associated with an aggressive breast cancer phenotype. Br J Cancer. 2012;106:344–7. doi: 10.1038/bjc.2011.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakumi K, Tominaga Y, Furuichi M, Xu P, Tsuzuki T, Sekiguchi M, et al. Ogg1 knockout-associated lung tumorigenesis and its suppression by Mth1 gene disruption. Cancer Res. 2003;63:902–5. [PubMed] [Google Scholar]
- 19.Zhang H, Mizumachi T, Carcel-Trullols J, Li L, Naito A, Spencer HJ, et al. Targeting human 8-oxoguanine DNA glycosylase (hOGG1) to mitochondria enhances cisplatin cytotoxicity in hepatoma cells. Carcinogenesis. 2007;28:1629–37. doi: 10.1093/carcin/bgm072. [DOI] [PubMed] [Google Scholar]
- 20.Hashiguchi K, Stuart JA, deSuoza-Pinto NC, Bohr VA. The C-terminal α0 helix of human Ogg1 is essential for 8-oxoguanine DNA glycosylase activity: the mitochondrial β-Ogg1 lacks this domain and does not have glycosylase activity. Nucleic Acids Res. 2004;32:5596–5608. doi: 10.1093/nar/gkh863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.