Abstract
Rationale
Abnormalities in excitatory/inhibitory neurotransmission are hypothesized to contribute to autism spectrum disorder (ASD) etiology. BTBR, an inbred mouse strain, displays social deficits and repetitive self-grooming, offering face validity to ASD diagnostic symptoms. Reduced GABAergic neurotransmission in BTBR suggests that GABAA receptor positive allosteric modulators (PAMs) could improve ASD-relevant BTBR phenotypes. The neuroactive steroid ganaxolone acts as a PAM, displaying anticonvulsant properties in rodent epilepsy models and an anxiolytic-like profile in the elevated plus-maze.
Objectives
We evaluated ganaxolone in BTBR and C57BL/6J mice in standardized assays for sociability and repetitive behaviors. Open field and anxiety-related behaviors were tested as internal controls and for comparison with the existing neuroactive steroid literature.
Results
Ganaxolone improved aspects of social approach and reciprocal social interactions in BTBR, with no effect on repetitive self-grooming, and no detrimental effects in C57BL/6J. Ganaxolone increased overall exploratory activity in BTBR and C57BL/6J in the open field, social approach, and elevated plus-maze, introducing a confound for the interpretation of social improvements. Allopregnanolone and diazepam similarly increased total entries in the elevated plus-maze, indicating that behavioral activation may be a general property of GABAA receptor PAMs in these strains.
Conclusions
Ganaxolone shows promise for improving sociability. In addition, ganaxolone, as well as other GABAA receptor PAMs, enhanced overall BTBR activity. The translational implications of specific sociability improvements and non-specific behavioral activation by ganaxolone in the BTBR model remains to be determined. Future studies to explore whether PAMs provide a novel profile with unique benefits for ASD treatment will be worthwhile.
Keywords: autism spectrum disorder, neuroactive steroid, ganaxolone, allopregnanolone, diazepam, anxiety, sociability, social approach, repetitive behaviors, open field
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder diagnosed by (a) unusual reciprocal social interactions and impaired communication and (b) restricted and repetitive patterns of interests and behaviors (American Psychiatric Association 2013). Currently, there are no FDA-approved pharmacological treatments that ameliorate the core ASD symptoms of social impairments and repetitive behaviors. Animal models of autism-relevant behaviors provide preclinical tools for deciphering the genetic and environmental contributions to ASD etiology. In addition, mouse models of autism provide research tools to explore the efficacy and safety of potential pharmacological therapeutics for the reversal of autism-relevant behaviors.
BTBR T+Itpr3tf/J (BTBR), a mouse model of idiopathic autism, displays a lack of sociability, reduced ultrasonic vocalizations, and high levels of repetitive behaviors such as self-grooming and marble burying (Blanchard et al. 2012; Bolivar et al. 2007; Han et al. 2014; McFarlane et al. 2008; Silverman et al. 2010), as compared to other inbred strains of mice with high sociability and low repetitive behaviors, such as C57BL/6J (B6). Recently, hippocampal neurons from BTBR mice were found to have a reduced frequency of GABAA receptor-mediated spontaneous inhibitory synaptic currents (Han et al. 2014). Further, GABAergic circuits utilized in multisensory integration in the insular cortex were reported to be deficient in BTBR (Gogolla et al. 2014). Enhancement of GABAergic neurotransmission by administration of a low dose of the benzodiazepine clonazepam to BTBR reversed the deficit in inhibitory synaptic events, and improved social interaction, as measured by the 3-chambered social approach task (Blanchard et al. 2012; Han et al. 2014). These findings suggest that pharmacological agents that reverse the deficit in inhibitory neurotransmission in mouse models of ASD can improve ASD-relevant behaviors by correcting the excitatory/inhibitory imbalances in synaptic activity implicated in ASD (Rubenstein and Merzenich 2003).
Allopregnanolone, an endogenous metabolite of progesterone, and its synthetic 3β-methyl analog ganaxolone, are potent positive allosteric modulators (PAMs) of GABAA receptors, the major inhibitory neurotransmitter receptor in the central nervous system (Dubrovsky 2005; Morrow 2007; Paul and Purdy 1992; Reddy and Rogawski 2012). These compounds have dose-dependent biphasic effects on many behaviors in several animal models (Belelli and Lambert 2005; Lambert et al. 1995). Consequently, ganaxolone is attractive as a potential therapy for ASD-relevant behaviors that may result from reduced inhibitory neurotransmission. Ganaxolone is currently being investigated for its preclinical and clinical efficacy in the treatment of epilepsy (Beekman et al. 1998; Hogenkamp et al. 2014; Reddy and Rogawski 2010; 2012; Ungard et al. 2000) (ClinicalTrials.gov NCT02358538, NCT01963208, NCT00441896, NCT00465517), post-traumatic stress disorder (Pinna and Rasmusson 2014) (NCT01339689), anxiety (Hogenkamp et al. 2014), and Fragile X syndrome (Heulens et al. 2012; Kooy et al. 2013) (NCT01725152).
Here we address the hypothesis that ganaxolone will improve social behaviors and/or reduce repetitive behaviors in BTBR mice. B6 mice were used to characterize ganaxolone in a second, commonly used inbred mouse strain. Standard tests for exploratory locomotion and anxiety-related behaviors were conducted to compare the effects of ganaxolone in BTBR versus B6, and for comparison to previously published ganaxolone findings (Hogenkamp et al. 2014; Ungard et al. 2000).
METHODS
Mice
Male and female BTBR T+Itpr3tf/J (BTBR), C57BL/6J (B6) and 129S1/SvImJ (129) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at the University of California Davis School of Medicine in Sacramento, CA. An AAALAC-accredited vivarium maintained the breeding cages and subject mice in ventilated cages on a conventional lighting cycle, with temperature and humidity controls, and with food and water ad libitum. Behavioral testing was conducted in dedicated testing rooms during the light phase of the circadian cycle (07:00-19:00). Male and female mice were used in a between-subjects design, as previous studies have demonstrated no sex differences in BTBR and in B6 on assays of sociability, repetitive behaviors and open field locomotion (Silverman et al., 2010, 2012). An experimenter blind to the drug treatment of subject mice manually scored behavioral videos, which were labeled only with the coded animal number. For ganaxolone studies, behavioral assays were conducted sequentially in the following testing order beginning at 6 weeks of age: elevated plus-maze, 3-chambered social approach, spontaneous repetitive behaviors, open field, marble burying and male-female reciprocal social interactions. For elevated plus-maze testing, separate groups of experimentally naïve mice were used for the allopregnanolone (male and female BTBR and B6) and diazepam (male B6) studies. All procedures were conducted in compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the UC Davis Institutional Animal Care and Use Committee.
Drug preparation and treatment
Ganaxolone (ScinoPharm, Taiwan Limited, Tainan, Taiwan) was prepared in 30% Captisol (a polyanionic β-cyclodextrin derivative; Ligand Pharmaceuticals, La Jolla, CA) in saline (United States Pharmacopeia (USP) standard formulation) and sonicated with heat for 30 min. Allopregnanolone was prepared in 22% Captisol in USP saline. Diazepam was prepared in 1% Tween in USP saline. Doses of ganaxolone (5, 10, 20 and 30 mg/kg) or its vehicle, allopregnanolone (5 and 10 mg/kg) or its vehicle, and diazepam (1 and 2 mg/kg) or its vehicle were acutely administered intraperitoneally 30 min prior to behavioral testing using a 10 ml/kg injection volume. Separate cohorts of mice were used for each drug study. Drug treatments within a cohort of mice were counterbalanced across behavioral tasks, with a washout of at least 4 days between experiments.
Behavioral assays
Elevated plus-maze anxiety-related test
Subject mice were placed in the center area of a black Plexiglas automated elevated plus-maze (Med-Associates, St. Albans City, VT), under 300 lux white light illumination, for a 5 minute test session as previously described (Flannery et al. 2015; Silverman et al. 2015).
Three-chambered social approach
Evaluation of sociability was conducted under low light conditions (40 lux) in 4 identical units of our 3-chambered social approach apparatus, beginning 30 min after drug administration, and employing two 10 min habituation sessions and a 10 min social approach session with a novel 129/ImJ partner as previously described (Brielmaier et al. 2014; Silverman et al. 2015).
Repetitive behaviors
Mice were individually placed in an empty standard mouse cage covered with a filter top lid under low light conditions (40 lux) and video recorded for 20 min for the manual scoring of repetitive self-grooming during the last 10 min of the test session.
Open field locomotion
Open field activity was evaluated to control for any direct drug effects on general activity. Exploratory locomotion was assessed in individual mice using a VersaMax Animal Activity Monitoring System (AccuScan Instruments, Columbus, OH) for a 1 hr test session under low light conditions (40 lux).
Marble burying
The number of marbles buried during a 30 min test session under low light conditions (40 lux) was scored as a corroborative measure of repetitive behavior. Individual animals were placed in a standard mouse cage filled with 2 cm of corncob bedding, in which 20 black glass marbles (14 mm in diameter; landofmarbles.com; product code: chin204) were placed in a 4×5 grid on top of the bedding. After 30 min, the number of marbles buried (>50% covered) were counted as an index of repetitive behavior at the end of the testing session.
Male-female reciprocal social interactions
Male subjects were group housed and sexually naïve at time of testing. B6 females in pro-estrus or estrus (open vagina with pink or reddish pink surrounding tissue) were used as partner mice. A 5 min testing session was conducted in a sound attenuating chamber (ENV-018V; Med Associations, St. Albans, VT), with interior walls covered with convoluted foam sheets (Uline, Pleasant Prairie, WI), and under dim, red light conditions (10 lux). Each female partner was given at least 30 min of rest time between tests. Frequency and duration of social behaviors were scored from videos using Noldus Observer software (Noldus Information Technology, Leesburg, VA).
Statistical Analysis
For social approach, a paired t-test was conducted to compare time spent in the chamber with the novel object versus time spent in the chamber with the novel mouse, within each strain and within each drug dose or vehicle treatment group, as previously described (Silverman et al. 2015). The number of seconds spent with the novel mouse is highly variable across cohorts of the same strain or genotype. In contrast, the comparison of time spent with the novel mouse versus time spent with the novel object is highly consistent across cohorts of the same strain or genotype. Therefore, an ANOVA comparison across treatment groups or across strains is inappropriate for the parameter of time with the novel object and novel mouse, thus requiring individual paired t-tests. Effect size estimates for significant pairwise comparisons were calculated using Cohen's d. Cohen’s d values greater than 0.5 were considered to be medium in strength, while values greater than 0.8 were considered to have a large effect size. Center chamber times are shown in the graphs for illustrative purposes. Time spent sniffing the novel object versus the novel mouse was analyzed by a paired t-test. Sociability was defined as significantly more time spent in the chamber with the novel mouse than in the chamber with the novel object, and/or significantly more time spent sniffing the novel mouse than sniffing the novel object. For number of entries into the 2 side chambers, data were analyzed within strain by Repeated Measures Analysis of Variance (ANOVA), using a between groups factor of dose and a within group factor of chamber. A priori Bonferroni or Dunnett’s test was used to compare each drug group to its vehicle control group. Male-female reciprocal social interactions, repetitive self-grooming, marble burying, and elevated plus-maze data were analyzed with a one-way ANOVA conducted within strain for each behavioral parameter. After a significant overall ANOVA, a Dunnett’s post hoc test was used to compare each drug dose group to its vehicle control group. Open field activity across time was analyzed with a Repeated Measures ANOVA conducted within strain, using a between groups factor of dose and a within group factor of time, to evaluate drug effects on total distance, horizontal activity, vertical activity and center distance. After a significant ANOVA, a priori Dunnett’s test was used to compare each drug group to its vehicle control group. Statistical analyses were performed using GraphPad Prism (Version 5.02) and Statistica (Version 12). Data graphs were created using GraphPad Prism.
RESULTS
Three-chambered social approach
In the social approach task, B6 mice displayed its usual species-typical normal sociability after vehicle and after each dose of ganaxolone (Veh: t(14)=3.8, p<0.002, Cohen’s d=1.87; 5 mg/kg: t(14)=2.3, p<0.04, Cohen’s d=1.17; 10 mg/kg: t(16)=8.8, p<0.0001, Cohen’s d=4.10; 20 mg/kg: t(17)=2.2, p<0.05, Cohen’s d=0.79; Figure 1a). B6 mice administered vehicle or ganaxolone also displayed significantly more sniffing of a novel mouse compared to a novel object after vehicle and after each dose of ganaxolone (Veh: t(14)=6.2, p<0.0001, Cohen’s d=2.36; 5 mg/kg: t(14)=4.6, p<0.0004, Cohen’s d=2.07; 10 mg/kg: t(16)=10.6, p<0.0001, Cohen’s d=3.47; 20 mg/kg: t(17)=5.4, p<0.0001, Cohen’s d=1.95; Figure 1c).
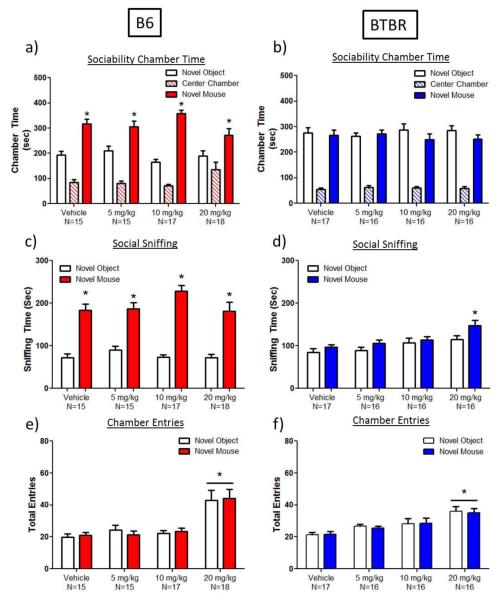
Fig. 1.
Lack of specific effects of ganaxolone on 3-chambered social approach in adult C57BL/6J (B6) and BTBR T+Itpr3tf/J (BTBR) mice. a) B6 mice displayed normal sociability after vehicle or ganaxolone administration, as measured by more time spent in the chamber with the novel mouse than in the chamber with the novel object. b) BTBR mice displayed their characteristic lack of sociability, spending approximately equal amounts of time in the chamber with the novel mouse and in the chamber with the novel object after vehicle or ganaxolone. c) B6 mice administered vehicle or ganaxolone displayed significant sociability, spending more time sniffing the novel mouse than the novel object. d) BTBR mice administered vehicle or ganaxolone doses of 5 and 10 mg/kg exhibited a characteristic lack of sociability on the sniffing parameter, displaying approximately equal amounts of sniffing directed at the novel mouse and the novel object. In contrast, after treatment with the high dose of ganaxolone (20 mg/kg), BTBR displayed significantly more sniffing of the novel mouse versus the novel object as measured by observer-scored directed sniffing. e) B6 mice administered 20 mg/kg ganaxolone exhibited significantly higher entries into both side chambers during sociability testing. f) Similarly, BTBR mice administered 20 mg/kg ganaxolone displayed significantly higher entries into both side chambers during sociability testing. Higher entries, indicating more overall exploration, may confound the interpretation of specific social improvements at the 20 mg/kg dose of ganaxolone. a-d) *p<0.05, within drug treatment group (novel mouse compared to novel object). e-f) The black line indicates a main effect of drug, with no drug × chamber interaction. * p<0.0003, as compared to vehicle
BTBR mice displayed its usual lack of sociability, showing no significant difference between time spent in the chamber with the novel mouse compared to time spent in the chamber with the novel object after vehicle or ganaxolone administration (Figure 1b). BTBR mice administered vehicle or ganaxolone at the lower doses, 5 and 10 mg/kg, also exhibited a characteristic lack of sociability (i.e., no significant difference between time spent sniffing the novel mouse versus the novel object) (Figure 1d). In contrast, after the 20 mg/kg dose of ganaxolone, BTBR mice displayed sociability, i.e. spent significantly more time sniffing the novel mouse versus the novel object, spending 50% more time with the novel mouse than vehicle-treated BTBR mice (t(15)=2.7, p<0.02, Cohen’s d=0.75).
Ganaxolone increased the number of entries B6 mice made into the chambers containing the novel object and novel mouse during social approach testing (main effect of dose: F(3,61)=8.6, p<0.0001; Figure 1e), significant at the highest dose tested, 20 mg/kg ganaxolone (112% increase) (Dunnett’s test, p<0.0002). Ganaxolone similarly increased the number of entries BTBR mice made into the side chambers with the novel object and novel (main effect of dose: F(3,61)=6.0, p<0.002; Figure 1f), significant at the highest dose, 20 mg/kg ganaxolone (65% increase) (Dunnett’s test, p<0.0003).
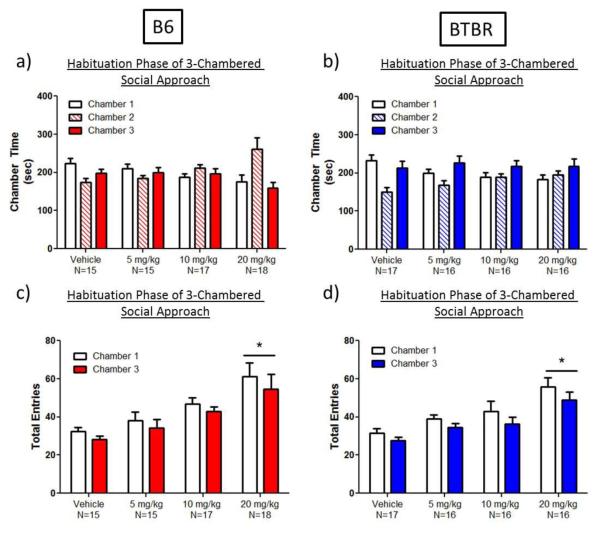
During the habituation session preceding social approach testing, ganaxolone had no effect on chamber time (Figure 2a-b). There was no significant interaction between drug treatment and chamber entries in either strain (Figure 2c-d). Ganaxolone (20 mg/kg) increased the number of entries made into the 2 side chambers by both B6 (91% increase; main effect of dose: F(3,61)=6.5, p<0.0008; Dunnett’s test, p<0.0004; Figure 2c) and BTBR (77% increase; main effect of dose: F(3,61)=8.2, p<0.0002; Dunnett’s test, p<0.0001; Figure 2d), consistent with increased exploratory activity. While a slight side bias was seen, with more chamber entries made into Chamber 1 compared to Chamber 3 in B6 (main effect of chamber: F(1,61)=11.4, p<0.002) and in BTBR (main effect of chamber: F(1,61)=16.3, p<0.0002), the effect was small, and the novel object and novel mouse were counterbalanced during the sociability phase to avoid any potential influences on sociability due to side bias.
Fig. 2.
Ganaxolone increased exploratory locomotion during the habituation phase in the 3-chambered apparatus in adult C57BL/6J (B6) and BTBR mice. a-b) Ganaxolone administration did not significantly affect chamber time in B6 or BTBR mice. c-d) B6 and BTBR mice administered 20 mg/kg ganaxolone exhibited increased entries into both side chambers during chamber habituation. The black line indicates a main effect of drug, with no drug × chamber interaction. * p<0.0001, as compared to vehicle
Male-female reciprocal social interactions
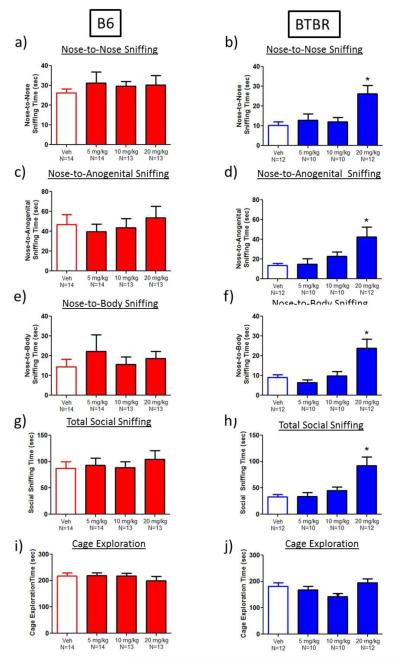
Ganaxolone had no effect on the duration of total social sniffing or on specific types of social sniffing (e.g., nose-to-nose, nose-to-anogenital, and nose-to-body) in male B6 mice interacting with an estrous female (Figure 3a, 3c, 3e and 3g). In BTBR, ganaxolone at 20 mg/kg increased nose-to-nose sniffing behavior by 158% compared to vehicle (main effect of dose: (F(3,43)=6.1, p<0.002, Dunnett’s test, p<0.05, Figure 3b). Similarly, 20 mg/kg ganaxolone increased the duration of nose-to-anogenital (209% increase) and nose-to-body sniffing (163% increase), and total social sniffing (181% increase) in BTBR (nose-to-anogenital: main effect of dose: F(3,43)=4.6, p<0.008; nose-to-body: main effect of dose: F(3,43)=8.0, p<0.0003; total social sniffing: main effect of dose: F(3,43)=7.9, p<0.0003; Dunnett’s tests, p<0.05; Figure 3d, 3f, 3h). Ganaxolone treatment had no significant effect on total arena exploration in male B6 or BTBR mice (Figure 3i-j).
Fig. 3.
Male-female reciprocal social interaction parameters were increased in BTBR after ganaxolone treatment. a) Ganaxolone did not affect the reciprocal social interaction parameter of nose-to-nose sniffing in B6 mice. b) Ganaxolone (20 mg/kg) increased nose-to-nose sniffing in BTBR male mice. c) Ganaxolone did not affect nose-to-anogenital sniffing by B6 male mice. d) Ganaxolone (20 mg/kg) increased BTBR nose-to-anogenital sniffing of female stimulus mice. e) Ganaxolone did not affect nose-to-body sniffing by B6 male mice. f) Ganaxolone (20 mg/kg) increased BTBR nose-to-body sniffing of female stimulus mice. g) Ganaxolone did not affect total social sniffing by B6 male. h) Ganaxolone (20 mg/kg) increased BTBR total social sniffing of female stimulus mice. i) Ganaxolone did not alter the time engaged in exploring the arena during the reciprocal interaction session in B6 or BTBR mice. j) Ganaxolone had no significant effect on total cage exploration in male B6 or BTBR mice. *p<0.05, as compared to vehicle
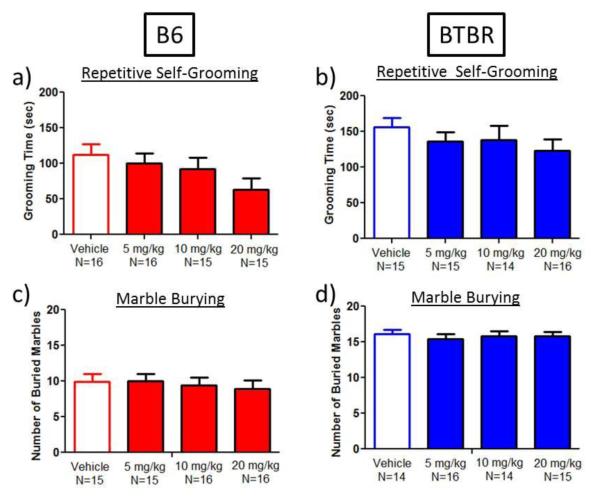
Repetitive self-grooming and marble burying
Ganaxolone had no significant effect on self-grooming (Figure 4a-b) or on marble burying (Figure 4c-d) in either B6 or BTBR mice.
Fig. 4.
Repetitive behaviors were unaffected by ganaxolone treatment. a-b) Ganaxolone did not significantly reduce repetitive self-grooming scores in either B6 or BTBR mice. c-d) Ganaxolone did not change the number of buried marbles in B6 or BTBR mice
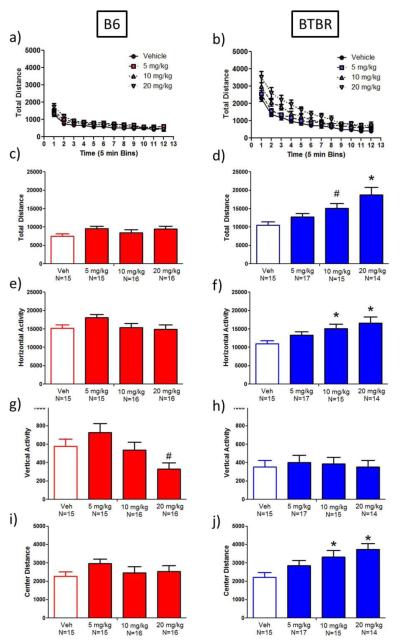
Open Field
Ganaxolone significantly increased total distance travelled in BTBR (main effect of dose: F(3,57)=6.4, p<0.001; Dunnett’s test, p<0.001 and 0.06, for 20 mg/kg (78% at 20 mg/kg compared to vehicle), and 10 mg/kg, respectively; Figure 5a-d) but not in B6. This effect varied across time in both strains (dose × time interaction: B6: F(33,638)=1.7, p<0.013; BTBR: F(33,627)=3.4, p<0.0001). During the 1 hr test session, the total distance traversed by B6 and BTBR mice declined over time, indicating that both strains habituated normally to the novel open field chamber, independent of dose (main effect of time: B6: F(11,638)= 81.6, p<0.0001; Figure 5a; BTBR: F(11,627)=215.3, p<0.0001; Figure 5b).
Fig. 5.
Open field activity was increased by ganaxolone in BTBR but not B6 mice. a-d) Ganaxolone administration did not affect total distance travelled or horizontal activity in B6 mice. Ganaxolone increased total distance travelled (20 mg/kg) and horizontal activity (10 and 20 mg/kg) in BTBR mice. g-h) On the vertical activity parameters, ganaxolone (20 mg/kg) marginally decreased vertical activity in B6 mice. Ganaxolone did not significantly alter vertical activity in BTBR mice. i-j) Ganaxolone had no effect on center distance travelled in B6 mice. Ganaxolone (10 and 20 mg/kg) increased center distance travelled in BTBR mice. *p<0.05, #p<0.09, as compared to vehicle
Ganaxolone significantly increased horizontal activity in BTBR mice at 10 (38% increase) and 20 mg/kg (51% increase) (main effect of dose: F(3,57)=4.1, p<0.02; Dunnett’s test, p<0.05; Figure 5f), but not B6 mice (Figure 5e). Horizontal activity in B6 and BTBR mice varied by ganaxolone dose (dose × time interaction: B6: F(33,638)=1.5, p<0.05; BTBR: F(33,627)=1.5, p<0.0001). Horizontal activity significantly decreased over time in both strains (main effect of time: B6: F(11,638)=76.6, p<0.0001; BTBR: F(11,627)=173.6, p<0.0001). Although ganaxolone reduced vertical activity in B6 mice, marginally significant at the 20 mg/kg dose (43% decrease; main effect of dose: F(3,58)=4.0, p<0.02; Dunnett’s test, p<0.09; Figure 5g), ganaxolone did not alter BTBR vertical activity (Figure 5h). Vertical activity in B6 and BTBR mice decreased over time (B6: main effect of time: F(11,638)=2.9, p<0.001; BTBR: main effect of time: F(11,627)=14.5, p<0.0001).
Ganaxolone significantly increased center distance in BTBR mice at 10 (49% increase) and 20 mg/kg (68% increase) compared to vehicle-treated BTBR mice (main effect of dose: F(3,57)=4.3, p<0.009; Dunnett’s test, p<0.05; Figure 5j), but not in B6 mice (Figure 5i). In BTBR mice, center distance varied by dose (dose × time interaction: F(33,627)=1.5, p<0.04). Center distance was reduced over time in both strains (main effect of time: B6: F(11,638)=30.9, p<0.0001; BTBR: F(11,627)=94.4, p<0.0001).
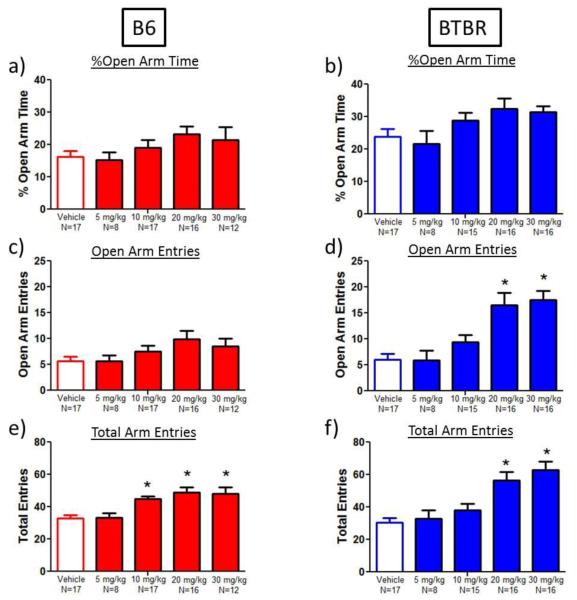
Elevated plus-maze
Since neuroactive steroids have been reported to exert anxiolytic-like effects in mice, ganaxolone was evaluated in B6 and BTBR mice in the elevated plus-maze, an anxiety-related task that is sensitive to anxiolytic drugs (Crawley 1985; File et al. 2004; Lister 1987). An anxiolytic-like trend was seen for ganaxolone in B6 mice on the elevated plus-maze (Figure 6) on open arm entries (main effect of dose: F(4,69)=2.1, p<0.09; Figure 6c). In BTBR mice, ganaxolone increased the percent time spent on the open arms (main effect of dose: F(4,71)=2.6, p<0.04; Figure 6b) and the number of open arm entries in BTBR mice (main effect of dose: F(4,71)=10.4, p<0.0001; Figure 6d). BTBR mice administered 20 (176% increase) or 30 mg/kg (192% increase) of ganaxolone made significantly more open arm entries compared to vehicle-treated BTBR mice (Dunnett’s test, p<0.05).
Fig. 6.
Complex effects of ganaxolone on the elevated plus-maze. To compare the actions of ganaxolone on social and repetitive behaviors to previously reported effects on anxiety-related behaviors, ganaxolone was evaluated in B6 and BTBR mice in the elevated plus-maze. a-b) Ganaxolone did not significantly affect percent open arm time in B6 or BTBR mice. c-d) High doses of ganaxolone tended to increase open arm entries in B6 and significantly increased open arm entries in BTBR (20 and 30 mg/kg) mice. e-f) Ganaxolone administration (10-30 mg/kg) increased total entries in B6 and BTBR mice. Higher total arm entries, indicating more overall exploration, is consistent with findings in the social approach chamber (Fig. 1 and 2), and may confound the interpretation of specific anxiolytic-like effects of ganaxolone. *p<0.05, as compared to vehicle
However, it is important to note that ganaxolone significantly increased the number of total arm entries at the 20 mg/kg and 30 mg/kg doses in both B6 and BTBR mice (B6: 49% and 46% increase; main effect of dose: F(4,69)=7.4, p<0.0001; BTBR: 84% and 106% increase; F(4,71)=10.3), p<0.0001; Dunnett’s test, p<0.05; Figures 6e, 6f). Higher total arm entries indicates higher general exploration, consistent with our findings in the open field and social approach tests, thus introducing an artifact in the interpretation of an anxiolytic-like effect of ganaxolone at 20 mg/kg.
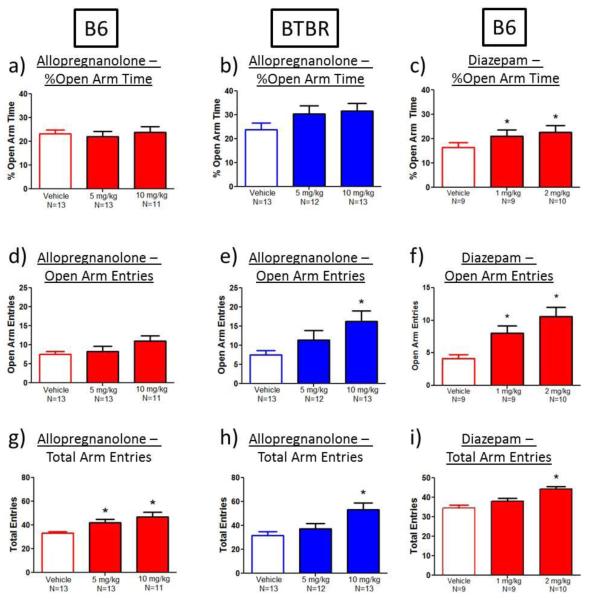
We conducted parallel elevated plus-maze experiments with another neuroactive steroid, allopregnanolone, shown in Figure 7, with the expectation that allopregnanolone would serve as a positive control. Allopregnanolone 10 mg/kg increased open arm entries in BTBR by 118% compared to vehicle (main effect of dose: F(2,37)=3.9, p<0.03; Dunnett’s test, p<0.05) and significantly increased total arm entries in both strains (main effect of dose: B6: 27% and 41% increase; F(2,36)=6.9, p<0.003; Dunnett’s test, p<0.05 for 5 and 10 mg/kg; BTBR: 68% increase; F(2,37)=6.0, p<0.006; Dunnett’s test, p<0.05 for 10 mg/kg; Figure 7g, 7h). Increased number of total arm entries indicates higher levels of locomotor activity, again introducing a possible artifact in the interpretation of specific anxiety-related actions.
Fig. 7.
A standard benzodiazepine anxiolytic, diazepam, produced a selective anxiolytic-like effect on the elevated plus-maze, while the neurosteroid, allopregnanolone, did not. a-b) Allopregnanolone administration did not significantly increase percent open arm time in B6 or BTBR mice. c) Diazepam administration (1 and 2 mg/kg) significantly increased percent open arm time in B6 mice. d-e) Allopregnanolone administration did not significantly increase open arm entries in B6 mice, but did increase open arm entries in BTBR mice (10 mg/kg). f) Diazepam administration (1 and 2 mg/kg) significantly increased open arm entries in B6 mice. g-h) Allopregnanolone administration (5-10 mg/kg) increased total entries in B6 and BTBR mice. i) A standard dose of diazepam (1 mg/kg) which showed anxiolytic-like effects on open arm time and open arm entries, did not affect total number of arm entries, although a high dose of diazepam (2 mg/kg) increased total arm entries in B6 mice. *p<0.05, as compared to vehicle
As a further internal control to confirm that elevated plus-maze methods and procedures were performed appropriately, we conducted parallel treatments with diazepam, a standard benzodiazepine with a well-established anxiolytic-like profile, in B6 mice on the elevated plus-maze (Cole and Rodgers 1995; Crawley 1985; Griebel et al. 2000). Diazepam significantly increased the percent time spent on the open arms (main effect of dose: F(2,27)=5.9, p<0.008; Figure 7c) and the number of open arm entries (main effect of dose: F(2,27)=8.3, p<0.002; Figure 7f), with significant effects at the 1 (78% and 95% increase, respectively) and 2 mg/kg (88% and 158% increase, respectively) doses (Dunnett’s test, p<0.05). At 1 mg/kg, diazepam produced a clear anxiolytic-like effect, with no increase in the control measures of total arm entries. However, at the relatively high 2 mg/kg dose, diazepam significantly increased total arm entries by 28% (main effect of dose: F(2,27)=13.6, p<0.0001; Dunnett’s test, p<0.05; Figure 7i), indicating the same potential confound due to increased general activity that we detected after neuroactive steroid treatment in B6 mice.
DISCUSSION
Although ASD is diagnosed during childhood in approximately 1% of the population (Elsabbagh et al. 2012; Kim et al. 2011), there are currently no FDA-approved pharmacological interventions that improve the diagnostic symptoms of social impairments and repetitive behaviors. Therefore, there is a great unmet need for medical treatments for the core symptoms of autism (Helton et al. 1996). Mouse models can serve as preclinical tools to evaluate the safety and efficacy of proposed targeted drug treatments for ASD-relevant behaviors. The BTBR inbred strain has been extensively characterized for its autism-relevant behaviors, including lack of sociability demonstrated in many different social assays across several laboratories, high levels of repetitive self-grooming, reduced vocalizations during social interactions, and cognitive impairments on some tasks (McFarlane et al. 2008; Pobbe et al. 2010; Scattoni et al. 2011; Silverman et al. 2015; Yang et al. 2011b). In addition, pharmacological modulation of excitatory or inhibitory neurotransmission in BTBR mice has resulted in reversals of its characteristic low sociability, high repetitive behaviors and cognitive impairments (Han et al. 2014; Silverman et al. 2013; Silverman et al. 2015; Silverman et al. 2012). These studies suggest that pharmacological manipulations of excitatory/inhibitory imbalance may reveal promising targets for clinical drug therapies for ASD.
Several synthetic neuroactive steroids act as positive allosteric modulators (PAMs) of the GABAA receptor (Carter et al. 1997; Hosie et al. 2006; Lambert et al. 2003; Wang 2011). Ganaxolone and the endogenous neurosteroid allopregnanolone reduced seizures in animal models of epilepsy (Beekman et al. 1998; Carter et al. 1997; Gasior et al. 2000; Heulens et al. 2012; Reddy 2013; Reddy et al. 2004; Wieland et al. 1997), similar to other GABA enhancing compounds (Bertram and Lothman 1990; Distler et al. 2013; Oakley et al. 2013; Rundfeldt et al. 1995; Treiman 2001). We reasoned that neuroactive steroids that act as PAMs of GABAergic neurotransmission may be beneficial in treating neurodevelopmental disorders in which GABA receptors, GABAergic interneurons or other aspects of GABA-mediated inhibition are reduced. For example, risk genes implicated in ASD include mutations in the 15q11-13 chromosomal region, which encodes the three GABAA receptor subunit genes GABRB3, GABRA5, and GABRG3 (Buxbaum et al. 2002; Hogart et al. 2009; Hogart et al. 2007). Furthermore, ganaxolone has been proposed as a treatment for Fragile X syndrome (Heulens et al. 2012) (NCT01725152), a neurodevelopmental disorder in which approximately 30-60% of cases meet the diagnostic criteria for ASD (Abbeduto et al. 2014; Bailey et al. 1998; Hagerman et al. 1986; Harris et al. 2008; McDuffie et al. 2014; Rogers et al. 2001; Thurman et al. 2015).
To investigate the therapeutic potential of neuroactive steroids in treating ASD diagnostic symptoms, we employed the BTBR inbred strain mouse model of autism, which displays highly robust, well-replicated social deficits and repetitive behaviors (Blanchard et al. 2012; Burket et al. 2013; Gould et al. 2011; McFarlane et al. 2008; Moy et al. 2007; Pobbe et al. 2011; Yang et al. 2011a; Yang et al. 2007; Zhang et al. 2015) and reduced GABAergic neurotransmission (Gogolla et al. 2014; Han et al. 2014). In BTBR mice, the highest dose of ganaxolone selectively improved social sniffing of a novel mouse in the 3-chambered social approach test, and increased social sniffing parameters during male-female reciprocal social interaction. However, general activity was increased in BTBR at this dose during the social tasks, and in a separate open field test, and on total arm entries in the elevated plus-maze, suggesting that increased exploratory locomotion may be contributing to the effects of ganaxolone on sociability. Ganaxolone-induced increases in locomotion were also seen in B6, and have been reported for other mouse strains including Swiss Webster (Ungard et al. 2000), although hyperactivity at high doses of ganaxolone is not reported consistently across all mouse strains or studies (Hogenkamp et al. 2014; Pinna and Rasmusson 2014). Strain differences may be contributing to variable reports of ganaxolone-induced hyperactivity.
Ganaxolone did not reduce repetitive self-grooming or marble burying in BTBR. This lack of effect of a GABAA PAM was unpredicted, and contrasts with reduced repetitive behaviors in BTBR and C58/J mice by a GABAB agonist (Silverman et al., 2015), requiring further investigation. Neuroactive steroids are being investigated for anxiolytic efficacy, based on their inhibition of tonic signaling via extrasynaptic GABAA receptors in the amygdala, a region critical for emotional behaviors (Cardinal et al. 2002; Kuraoka and Nakamura 2006; Murray 2007; Phelps 2006; Romo-Parra et al. 2015). To confirm that ganaxolone produced anxiolytic actions at the doses tested in our social and repetitive behavior assays, we tested the same acute dose of 20 mg/kg in B6 and BTBR mice for its activity on the elevated plus-maze. Results indicted a trend for increased percent open arm time, indicating an anxiolytic-like effect. However, the 20 mg/kg ganaxolone dose also increased the total open arm entries, which is the control measure for general exploration in this assay. Increased general activity on the elevated plus-maze in these strains is a potential confound to the interpretation of an anxiolytic profile of ganaxolone, consistent with the same artifact of increased exploratory activity confounding the interpretation of a social improvement. It is important to note that an excellent publication demonstrated that ganaxolone produces an anxiety-like effect in mice on the elevated plus-maze in the CD-1 strain, as opposed to the B6 and BTBR strains used in the present experiments (Hogenkamp et al. 2014). Strain differences in response to ganaxolone on general activity may explain the divergent findings.
To ensure that our methods produced results consistent with the larger literature on anxiolytic drug responses to neuroactive steroids (Bitran et al. 1991; Darbra and Pallares 2012; Evans et al. 2012; Modol et al. 2011; Reddy et al. 2005; Rodgers and Johnson 1998; Wieland et al. 1995; Zimmerberg et al. 2010), we tested the endogenous neurosteroid allopregnanolone on the elevated plus-maze in B6 and BTBR mice. In B6 mice, allopregnanolone showed trends for anxiolytic activity, but also increased total entries in the elevated plus maze. In BTBR mice, the high dose of allopregnanolone that increased open arm entries also increased total arm entries. Together, these data suggest that allopregnanolone increased general activity in these two inbred strains of mice at doses that improved anxiety-like behavioral parameters, similar to ganaxolone. Previous reports of improvement of anxiety-like behavior with allopregnanolone employed different mouse strains (e.g. NIH Swiss-Webster, DBA/2, progesterone receptor knockout mice) without statistically significant effects on locomotion (Reddy et al. 2005; Rodgers and Johnson 1998; Wieland et al. 1995).
Parallel experiments with the benzodiazepine diazepam were performed to ensure that the elevated plus-maze testing parameters were sensitive to drug-induced anxiolytic effects. As expected, diazepam produced anxiolytic-like actions in B6 mice at the 1 mg/kg dose, which did not affect general activity, replicating previous reports (Garrett et al. 1998; Griebel et al. 2000). At a higher dose (2 mg/kg), diazepam improved anxiety-like parameters while also increasing locomotion. It is important to note that many diazepam studies in mice employed DBA/2, CD-1 and NIH Swiss mice strains (Cole and Rodgers 1995; Dalvi and Rodgers 1999; Griebel et al. 2000; Helton et al. 1996; Helton et al. 1998; Johnson and Rodgers 1996; Rodgers et al. 1992). Different strains are reported to show differential responses to diazepam on anxiety-related and activity-related parameters of elevated plus-maze exploration. Diazepam-induced increases in locomotion have been noted elsewhere, with other inbred strains of mice under other lighting conditions at doses below those that indicated behavioral sedation (Cole and Rodgers 1995; Rodgers et al. 1992), including B6 mice (LaBuda and Fuchs 2001; Lepicard et al. 2000). Additionally, several reports suggest that B6 mice may not be as sensitive to the anxiolytic effects of prototypical anxiolytic drugs, compared to other strains (Griebel et al. 2000; Thompson et al. 2015).
Given the recent reports of reduced inhibitory neurotransmission in BTBR, a neuroactive steroid that acts as a PAM of GABAA receptors presents an opportunity for therapeutic intervention in individuals with ASD who harbor a mutation in a gene that reduces inhibitory neurotransmission. In the present studies, the neuroactive steroid ganaxolone produced an apparent improvement in aspects of social behaviors in two assays conducted in the BTBR mouse model of autism. However, our findings appear to be confounded by increased general exploratory activity, detected in the social, anxiolytic, and open field assays in both BTBR and B6. In addition, like many benzodiazepines, neuroactive steroids may display a narrow therapeutic window in some strains and species. Greater focus on strain differences in behavioral responses to GABA modulators (Garrett et al. 1998; Griebel et al. 2000) (Crawley and Davis 1982) may improve the translational value of mouse models of neurodevelopmental disorders, and enhance the evaluation of GABAergic PAMs as a novel therapeutic strategy with unique benefits for ASD.
Acknowledgements
This work was supported by the UC Davis MIND Institute, the Autism Research Training Program (NIH/NIMH Grant T32MH073124-10, Interdisciplinary Training for Autism Researchers), and the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125). We thank Lisa Olsen in the Rogawski lab for her kind assistance in providing compounds and vehicles. In addition, we thank Dr. Jill Silverman, Dr. Mu Yang, Michael Pride and Jane Hayes, UC Davis MIND Institute investigators in the Crawley lab, for their training on specific methods used by Dr. Kazdoba in the behavioral assays and statistical analyses of the data.
Dr. Hagerman has received funding from the Department of Defense and from Marinus Pharmaceuticals to study ganaxolone in a controlled trial in Fragile X syndrome, both with and without autism. Dr. Rogawski is a consultant to Sage Therapeutics.
Footnotes
Drs. Kazdoba, Zolkowska and Crawley do not have any conflicts of interest to report.
References
- Abbeduto L, McDuffie A, Thurman AJ. The fragile X syndrome-autism comorbidity: what do we really know? Front Genet. 2014;5:355. doi: 10.3389/fgene.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th edition American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- Bailey DB, Jr., Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of autism and developmental disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Beekman M, Ungard JT, Gasior M, Carter RB, Dijkstra D, Goldberg SR, Witkin JM. Reversal of behavioral effects of pentylenetetrazol by the neuroactive steroid ganaxolone. The Journal of pharmacology and experimental therapeutics. 1998;284:868–77. [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nature reviews Neuroscience. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Lothman EW. NMDA receptor antagonists and limbic status epilepticus: a comparison with standard anticonvulsants. Epilepsy research. 1990;5:177–84. doi: 10.1016/0920-1211(90)90036-u. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain research. 1991;561:157–61. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Defensor EB, Meyza KZ, Pobbe RL, Pearson BL, Bolivar VJ, Blanchard RJ. BTBR T+tf/J mice: autism-relevant behaviors and reduced fractone-associated heparan sulfate. Neuroscience and biobehavioral reviews. 2012;36:285–96. doi: 10.1016/j.neubiorev.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behavioural brain research. 2007;176:21–6. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J, Senerth JM, Silverman JL, Matteson PG, Millonig JH, DiCicco-Bloom E, Crawley JN. Chronic desipramine treatment rescues depression-related, social and cognitive deficits in Engrailed-2 knockout mice. Genes, brain, and behavior. 2014;13:286–298. doi: 10.1111/gbb.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burket JA, Benson AD, Tang AH, Deutsch SI. D-Cycloserine improves sociability in the BTBR T+ Itpr3tf/J mouse model of autism spectrum disorders with altered Ras/Raf/ERK1/2 signaling. Brain research bulletin. 2013;96:62–70. doi: 10.1016/j.brainresbull.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr., Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Molecular psychiatry. 2002;7:311–6. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and biobehavioral reviews. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. The Journal of pharmacology and experimental therapeutics. 1997;280:1284–95. [PubMed] [Google Scholar]
- Cole JC, Rodgers RJ. Ethological comparison of the effects of diazepam and acute/chronic imipramine on the behaviour of mice in the elevated plus-maze. Pharmacology, biochemistry, and behavior. 1995;52:473–8. doi: 10.1016/0091-3057(95)00163-q. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neuroscience and biobehavioral reviews. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Davis LG. Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain research bulletin. 1982;8:609–12. doi: 10.1016/0361-9230(82)90087-9. [DOI] [PubMed] [Google Scholar]
- Dalvi A, Rodgers RJ. Behavioral effects of diazepam in the murine plus-maze: flumazenil antagonism of enhanced head dipping but not the disinhibition of open-arm avoidance. Pharmacology, biochemistry, and behavior. 1999;62:727–34. doi: 10.1016/s0091-3057(98)00220-2. [DOI] [PubMed] [Google Scholar]
- Darbra S, Pallares M. Effects of early postnatal allopregnanolone administration on elevated plus maze anxiety scores in adult male Wistar rats. Neuropsychobiology. 2012;65:20–7. doi: 10.1159/000328161. [DOI] [PubMed] [Google Scholar]
- Distler MG, Gorfinkle N, Papale LA, Wuenschell GE, Termini J, Escayg A, Winawer MR, Palmer AA. Glyoxalase 1 and its substrate methylglyoxal are novel regulators of seizure susceptibility. Epilepsia. 2013;54:649–57. doi: 10.1111/epi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29:169–92. doi: 10.1016/j.pnpbp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, Montiel-Nava C, Patel V, Paula CS, Wang C, Yasamy MT, Fombonne E. Global prevalence of autism and other pervasive developmental disorders. Autism research : official journal of the International Society for Autism Research. 2012;5:160–79. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Sun Y, McGregor A, Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315–26. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- File SE, Lippa AS, Beer B, Lippa MT. Crawley Jacqueline N, et al., editors. Animal tests of anxiety. Current protocols in neuroscience. 2004 doi: 10.1002/0471142301.ns0803s26. Chapter 8: Unit 8 3. [DOI] [PubMed] [Google Scholar]
- Flannery BM, Silverman JL, Bruun DA, Puhger KR, McCoy MR, Hammock BD, Crawley JN, Lein PJ. Behavioral assessment of NIH Swiss mice acutely intoxicated with tetramethylenedisulfotetramine. Neurotoxicology and teratology. 2015;47:36–45. doi: 10.1016/j.ntt.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett KM, Niekrasz I, Haque D, Parker KM, Seale TW. Genotypic differences between C57BL/6 and A inbred mice in anxiolytic and sedative actions of diazepam. Behavior genetics. 1998;28:125–36. doi: 10.1023/a:1021424108213. [DOI] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–96. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. Journal of neurochemistry. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–70. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Jackson AW, 3rd, Levitas A, Rimland B, Braden M. An analysis of autism in fifty males with the fragile X syndrome. American journal of medical genetics. 1986;23:359–74. doi: 10.1002/ajmg.1320230128. [DOI] [PubMed] [Google Scholar]
- Han S, Tai C, Jones CJ, Scheuer T, Catterall WA. Enhancement of inhibitory neurotransmission by GABAA receptors having alpha2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 2014;81:1282–9. doi: 10.1016/j.neuron.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman H, Hagerman RJ. Autism profiles of males with fragile X syndrome. American journal of mental retardation : AJMR. 2008;113:427–38. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton DR, Berger JE, Czachura JF, Rasmussen K, Kallman MJ. Central nervous system characterization of the new cholecystokininB antagonist LY288513. Pharmacology, biochemistry, and behavior. 1996;53:493–502. doi: 10.1016/0091-3057(95)02122-1. [DOI] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. The Journal of pharmacology and experimental therapeutics. 1998;284:651–60. [PubMed] [Google Scholar]
- Heulens I, D'Hulst C, Van Dam D, De Deyn PP, Kooy RF. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behavioural brain research. 2012;229:244–9. doi: 10.1016/j.bbr.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Hogart A, Leung KN, Wang NJ, Wu DJ, Driscoll J, Vallero RO, Schanen NC, LaSalle JM. Chromosome 15q11-13 duplication syndrome brain reveals epigenetic alterations in gene expression not predicted from copy number. Journal of medical genetics. 2009;46:86–93. doi: 10.1136/jmg.2008.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM. 15q11-13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Human molecular genetics. 2007;16:691–703. doi: 10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenkamp DJ, Tran MB, Yoshimura RF, Johnstone TB, Kanner R, Gee KW. Pharmacological profile of a 17beta-heteroaryl-substituted neuroactive steroid. Psychopharmacology. 2014;231:3517–24. doi: 10.1007/s00213-014-3494-5. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Johnson NJ, Rodgers RJ. Ethological analysis of cholecystokinin (CCKA and CCKB) receptor ligands in the elevated plus-maze test of anxiety in mice. Psychopharmacology. 1996;124:355–64. doi: 10.1007/BF02247441. [DOI] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, Cheon KA, Kim SJ, Kim YK, Lee H, Song DH, Grinker RR. Prevalence of autism spectrum disorders in a total population sample. The American journal of psychiatry. 2011;168:904–12. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- Kooy F, I. H, V. S, T. A, A. P, R. W, D'Hooge R, Baltschun D, Rooms L. Hippocampal defects in the Fmr1 knockout mouse. American Society of Human Genetics. 2013 [Google Scholar]
- Kuraoka K, Nakamura K. Impacts of facial identity and type of emotion on responses of amygdala neurons. Neuroreport. 2006;17:9–12. doi: 10.1097/01.wnr.0000194383.02999.c5. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. The anxiolytic effect of acute ethanol or diazepam exposure is unaltered in mu-opioid receptor knockout mice. Brain research bulletin. 2001;55:755–60. doi: 10.1016/s0361-9230(01)00569-x. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends in pharmacological sciences. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Progress in neurobiology. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacology, biochemistry, and behavior. 2000;67:739–48. doi: 10.1016/s0091-3057(00)00419-6. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, Abbeduto L. Symptoms of Autism in Males with Fragile X Syndrome: A Comparison to Nonsyndromic ASD Using Current ADI-R Scores. Journal of autism and developmental disorders. 2014 doi: 10.1007/s10803-013-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, brain, and behavior. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Modol L, Darbra S, Pallares M. Neurosteroids infusion into the CA1 hippocampal region on exploration, anxiety-like behaviour and aversive learning. Behavioural brain research. 2011;222:223–9. doi: 10.1016/j.bbr.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Morrow AL. Recent developments in the significance and therapeutic relevance of neuroactive steroids--Introduction to the special issue. Pharmacology & therapeutics. 2007;116:1–6. doi: 10.1016/j.pharmthera.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behavioural brain research. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in cognitive sciences. 2007;11:489–97. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Oakley JC, Cho AR, Cheah CS, Scheuer T, Catterall WA. Synergistic GABA-enhancing therapy against seizures in a mouse model of Dravet syndrome. The Journal of pharmacology and experimental therapeutics. 2013;345:215–24. doi: 10.1124/jpet.113.203331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6:2311–22. [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual review of psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Pinna G, Rasmusson AM. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Frontiers in cellular neuroscience. 2014;8:256. doi: 10.3389/fncel.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behavioural brain research. 2011;216:446–51. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behavioural brain research. 2010;214:443–9. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Hormones and behavior. 2013;63:254–66. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. The Journal of pharmacology and experimental therapeutics. 2004;310:230–9. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O'Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy research. 2010;89:254–60. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroids - Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Behaviorally selective effects of neuroactive steroids on plus-maze anxiety in mice. Pharmacology, biochemistry, and behavior. 1998;59:221–32. doi: 10.1016/s0091-3057(97)00339-0. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Lee C, Shepherd JK. Effects of diazepam on behavioural and antinociceptive responses to the elevated plus-maze in male mice depend upon treatment regimen and prior maze experience. Psychopharmacology. 1992;106:102–10. doi: 10.1007/BF02253596. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of developmental and behavioral pediatrics : JDBP. 2001;22:409–17. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Romo-Parra H, Blaesse P, Sosulina L, Pape HC. Neurosteroids increase tonic GABAergic inhibition in the lateral section of the central amygdala in mice. Journal of neurophysiology: jn 00045 2015. 2015 doi: 10.1152/jn.00045.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, brain, and behavior. 2003;2:255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundfeldt C, Wlaz P, Honack D, Loscher W. Anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. Comparison of diazepam, bretazenil and abecarnil. The Journal of pharmacology and experimental therapeutics. 1995;275:693–702. [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes, brain, and behavior. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Oliver CF, Karras MN, Gastrell PT, Crawley JN. AMPAKINE enhancement of social interaction in the BTBR mouse model of autism. Neuropharmacology. 2013;64:268–82. doi: 10.1016/j.neuropharm.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben H, Baker S, Crawley JN. GABA Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Science translational medicine. 2012;4:131ra51. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:976–89. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Grabowski-Boase L, Tarantino LM. Prototypical anxiolytics do not reduce anxiety-like behavior in the open field in C57BL/6J mice. Pharmacology, biochemistry, and behavior. 2015;133:7–17. doi: 10.1016/j.pbb.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Kover ST, Hagerman RJ, Abbeduto L. Autism Symptomatology in Boys with Fragile X Syndrome: A Cross Sectional Developmental Trajectories Comparison with Nonsyndromic Autism Spectrum Disorder. Journal of autism and developmental disorders. 2015 doi: 10.1007/s10803-015-2443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Ungard JT, Beekman M, Gasior M, Carter RB, Dijkstra D, Witkin JM. Modification of behavioral effects of drugs in mice by neuroactive steroids. Psychopharmacology. 2000;148:336–43. doi: 10.1007/s002130050060. [DOI] [PubMed] [Google Scholar]
- Wang M. Neurosteroids and GABA-A Receptor Function. Frontiers in endocrinology. 2011;2:44. doi: 10.3389/fendo.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Belluzzi J, Hawkinson JE, Hogenkamp D, Upasani R, Stein L, Wood PL, Gee KW, Lan NC. Anxiolytic and anticonvulsant activity of a synthetic neuroactive steroid Co 3-0593. Psychopharmacology. 1997;134:46–54. doi: 10.1007/s002130050424. [DOI] [PubMed] [Google Scholar]
- Wieland S, Belluzzi JD, Stein L, Lan NC. Comparative behavioral characterization of the neuroactive steroids 3 alpha-OH,5 alpha-pregnan-20-one and 3 alpha-OH,5 beta-pregnan-20-one in rodents. Psychopharmacology. 1995;118:65–71. doi: 10.1007/BF02245251. [DOI] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism research : official journal of the International Society for Autism Research. 2011a;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Crawley Jacqueline N, et al., editors. Automated three-chambered social approach task for mice. Current protocols in neuroscience. 2011b doi: 10.1002/0471142301.ns0826s56. Chapter 8: Unit 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2007;25:515–21. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WQ, Smolik CM, Barba-Escobedo PA, Gamez M, Sanchez JJ, Javors MA, Daws LC, Gould GG. Acute dietary tryptophan manipulation differentially alters social behavior, brain serotonin and plasma corticosterone in three inbred mouse strains. Neuropharmacology. 2015;90:1–8. doi: 10.1016/j.neuropharm.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Martinez AR, Skudder CM, Killien EY, Robinson SA, Brunelli SA. Effects of gestational allopregnanolone administration in rats bred for high affective behavior. Physiology & behavior. 2010;99:212–7. doi: 10.1016/j.physbeh.2009.05.014. [DOI] [PubMed] [Google Scholar]