Abstract
In a hen, large quantities of the egg yolk proteins apolipoprotein (apo) II and vitellogenin (VG), are expressed in the liver and transported to the oviduct during egg production. Estrogenic stimulation of the hepatic expression of apo II and VG is due to both transcriptional increase and mRNA stabilization. The nucleolytic degradation of apo II mRNA is prevented by estrogen-regulated mRNA stabilizing factor (E-RmRNASF). Gene-specific effects of a select panel of SERMs on the hepatic expression of the estrogen-responsive genes encoding apo II, VG and E-RmRNASF in the chicken liver were investigated. In the present study, 6-week-old roosters were treated with the vehicle, estrogen, the SERMs genistein, resveratrol, tamoxifen, pterostilbene, raloxifene, catechin and clomiphene or a combination of estrogen and a 200-fold excess of each of the SERMs. Results from mRNA stabilization studies, conducted to investigate the stimulation of expression of E-RmRNASF in the liver by these agents showed that the expression of E-RmRNASF in the liver, was stimulated by estrogen, and the SERMs genistein, resveratrol, tamoxifen, pterostilbene, and catechin, but not by the vehicle, clomiphene or raloxifene. The expression of apo II and VG from the above treatments was determined by Northern blot analysis, RNase protection assays and Western blot analysis. The transcription and protein expression of both apo II and VG genes were seen in response to treatment with estrogen but not with the SERMs or combinations of estrogen and each of the SERMs. The SERMs that stimulated the expression of E-RmRNASF, antagonized the stimulation of the expression of both apo II and VG by estrogen, demonstrating a gene-specific, selective regulation of the above genes in the chicken liver by the SERMs. The above panel of SERMs may likely have adverse effects on egg production.
Keywords: estrogen, apolipoprotein II, vitellogenin, E-RmRNASF, SERMs, phytoestrogens, xenoestrogens, gene-specificity
INTRODUCTION
An oviparous animal such as a hen (Gallus gallus domesticus), lays large macrolecithal terrestrial eggs containing large stores of egg yolk. During periods of egg laying, the hen has to produce enormous quantities of the constituent egg white proteins such as ovalbumin and egg yolk proteins that include apolipoprotein (apo) II and vitellogenin (VG) [1-3]. Apo II and VG, are expressed in the liver and transported to the oviduct during egg production. Apo II is packaged in the very low density lipoprotein (VLDL) particle for extrahepatic transport to the oviduct, while VG, a glycophospholipoprotein, is secreted into the blood and taken up in the oviduct by receptor-mediated uptake. The control of the expression of apo II and VG, has been shown to be clearly governed by estrogen with maintenance of increased steady state levels of the respective mRNAs in response to estrogen by means of increased rates of transcription as well as post-transcriptional stabilization of their mRNAs [4-12]. The stability of the apo II mRNA has been shown to be primarily due to a factor, namely the estrogen-regulated mRNA stabilizing factor (E-RmRNASF), expressed in response to estrogen. E-RmRNASF binds to a specific motif in the 3’untranslated region of apo II mRNA to prevent nucleolytic degradation of the mRNA [5].
In the present study, we have undertaken to investigate the gene-specific effects of a selective panel of SERMs on the hepatic expression of estrogen-responsive genes encoding the apo II, VG and E-RmRNASF in chicken. The hepatic expression of E-RmRNASF occurs in response to estrogen as well as several estrogen-mimicking non-steroidal xenobiotics such as Araclor, Kepone and hexachlorophene [8]; certain SERMs such as tamoxifen [8] and phytoestrogens such as resveratrol and genistein [6, 7], but is inhibited by SERMs such as clomiphene and environmental xenotoxicants such as toxaphene and bisphenol-A [8]. Estrogen-mediated stimulation of apo II and VG genes appear to be predominantly through ER-α and not through ER-β or the G-protein coupled receptor signaling pathways [2].
Several Selective Estrogen Receptor Modulators (SERMs) such as tamoxifen, raloxifene, clomiphene, toremifene, ospemifene etc. have been developed for a variety of therapeutic purposes including prevention and treatment of ER-positive breast cancer, treatment of osteoporosis, induction of ovulation in anovulatory women and dyspareunia. Many of them have been shown to have tissue-specific agonistic or antagonistic effects [13-20].
Though, there are numerous studies that have reported the tissue-selectivity of SERMs [13-20], there is very little information available, at present, regarding the gene-specific regulation by SERMs within the same cell/tissue. Farnell and Ing [21] have shown gene-specific and tissue-specific agonistic and antagonistic effects of three SERMs, namely tamoxifen and two experimental agents GW5638 and EM800 on the expression of estrogen–responsive genes, ER, progesterone receptor, glyceraldehyde 3-phosphate dehydrogenase and cyclophylin in sheep uterus. Tamoxifen was shown to stimulate the expression of the gene encoding prolactin but unlike estrogen was unable induce cell proliferation or expression of progesterone receptor or ornithine aminotransferase in GH4C1 pituitary tumor cells [22].
Estrogen binds nuclear receptors, namely, estrogen receptor (ER)-α and ER-β. Both ER-α and ER-β, when activated by estrogen or estrogen mimicking ligands bind as a dimer, directly to the consensus estrogen response element (ERE) located in the promoters of estrogen-responsive genes [23-30] or indirectly to specific transcription factors, bound to their respective transcription elements [23-30]. ER is also activated in a ligand-independent manner by other cell signaling pathways [28, 29]. Within the ER, both the N-terminal transactivation domain [AF-1] and the transactivation domain AF-2 that contains a leucine-rich motif that constitutes a ligand-activated binding site for cofactors, are involved in the interaction with coregulators [13, 14, 27-29]. AF-1 can activate transcription in a ligand-independent manner, while AF-2 is mostly involved in ligand (estrogen or estrogen-mimicking agents)-dependent activation of transcription [27-29]. The ligand-induced conformational changes in the ER, likely determines its interaction with specific coregulators (coactivators, corepressors) resulting in the stimulation or inhibition of transcription at a given promoter site of an estrogen-responsive gene [27, 30].
Our results indicate selective agonistic/antagonistic activity of certain SERMs in a gene-specific manner in the same tissue. The SERMs that stimulated the hepatic expression of E-RmRNASF, not only failed to stimulate the hepatic expression of the estrogen-responsive genes encoding apo II or VG, but inhibited their estrogenic stimulation; hence were antagonistic.
MATERIALS AND METHODS
Synthesis of Uniformly Radiolabeled or Unlabeled, Full-Length ApoIImRNA
Uniformly radiolabeled, authentic capped, and polyadenylated apoII mRNA was synthesized by run-off transcription using the expression plasmid, pT7NAPOII [4, 9]. This expression plasmid contains the full-length cDNA with a unique Hind III restriction site at the end of a poly (A) run of about 50 nucleotides. The plasmid was linearized by digestion with Hind III and transcribed with T7 RNA polymerase (0.8 u/μl; New England Biolabs) at 37°C for 1 hour in a reaction containing 400μM each of ATP, GTP, and CTP, 100μM UTP, 2mM 7MeGpppG, 2μCi/μl [α-32P] UTP, 40mM Tris-HCl, pH 8.0, 8mM MgCl2, 25mM NaCl, 2mM spermidine (HCl), and 2mM DTT. The RNA was purified by oligo-dT affinity chromatography. Non-radiolabeled apo II mRNA was synthesized using the same reaction condition but with 400μM UTP and in the absence of radiolabeled nucleotide.
Estrogen Treatment of Roosters and Preparation of Liver Cytosolic Extracts
Liver cytosolic extracts (S100) were prepared as previously described [9] from livers of 6-week-old white leghorn roosters (SPAFAS, Norwich, CT) who received two intramuscular injections of the vehicle (DMSO), 5μmol/kg of 17β-estradiol or 1000 μmol/kg of the respective SERMS (Sigma Chemical Co, Mo) or a combination of 5 μmol/kg of β-estradiol and 1000 μmol/kg of each of the respective SERMs, at 3-day intervals. A 50% homogenate of liver was prepared with a hand-held, loose-fitting Dounce homogenizer in 10mM HEPES, pH 7.9, 1.5mM MgCl2, 10mM KCl, 0.5mM phenylmethylsulfonyl fluoride (PMSF), 5μg/ml leupeptin, and 5μg/ml aprotinin. The homogenate was centrifuged for 10 min at 800g, and the supernatant was removed and mixed with 0.11 volume of 300 mM HEPES, pH 7.9, 1.4M KCl, 30mM MgCl2, 0.5mM PMSF, 5μg/ml leupeptin, and 5μg/ml aprotinin. Following centrifugation at 100,000 × g for 60 min in a Beckman SW 41 rotor, the supernatant was dialyzed for 5-8 h against 20 volumes of 20mM HEPES, pH 7.9, 20% glycerol, 100mM KCl, 30mM MgCl2, 0.2mM EDTA, 0.5mM dithiothreitol, 0.5mM PMSF, 5μg/ml leupeptin, and 5μg/ml aprotinin, flash frozen in liquid N2 and stored at −80 °C. All procedures were carried out at 4°C. Protein concentrations were determined using Pierce BCA protein assay kit.
In Vitro Assay for Determination of ApoII mRNA Stability
Either unlabeled or radiolabeled apoII mRNA (2.5 × 105 cpm) was incubated for an hour in the presence of 10mM HEPES, pH 7.9, 40mM KCl, 6mM MgCl2, 1mM dithiothreitol, 5mg/ml heparin, 40μg/ml yeast tRNA, and 20μg protein of vehicle-(control), estrogen- or the SERMs-treated liver cytosolic extracts at 26 °C. Reaction was stopped by the addition of 200 μl of stop solution containing 0.1% SDS and 1mM EDTA. In the case of radiolabeled apo II mRNA, the RNA was electrophoresed on an 8M urea-6% polyacrylamide gel, and visualized by autoradiography. The unlabeled apo II mRNA was detected by means of reverse transcriptase aided PCR (RT-PCR), resolving the amplified product on 2.5% TBE-agarose gel containing ethidium bromide and visualizing on UV-transilluminator. Results from experiments with extracts pooled from four similarly treated roosters were identical with that of each of the individual extracts. There appeared to be no individual variation among the different extracts.
Western Blot Analyses of Estrogen Responsive Apolipoprotein II and Vitellogenin
Western blot analyses was performed using cytosolic liver extracts from roosters treated with estrogen, SERMs, a combination of estrogen and each of the SERMs or the vehicle. The protein concentrations were determined using the Pierce BCA protein assay kit. 20μg protein from each of the liver cytosolic extracts was used to determine the expression of apo II and VG by Western blot analysis. The proteins were separated on 7.5% (for VG) or 15% (for apo II) SDS-polyacrylamide gels with prestained standard protein markers. The proteins were transferred to a nitrocellulose membrane. Transfer was checked using 10% Ponceau S solution in 1X TBST. The membranes were washed with 1X TBST and then blocked with 5% non-fat dry milk for one hour. They were then probed with the relevant primary antibodies (polyclonal α-apo II, α-VG and α-β-actin antibodies at a 1:1000 dilution), for about 14-16 hours at 37°C with constant agitation. After incubation with respective primary antibodies, the membranes were washed with TBST and then probed with horseradish-peroxidase conjugated α-rabbit IgG secondary antibody at a 1:5000 dilution for one hour at room temperature with constant agitation. The membranes were then treated with ECL chemiluminisence reagent and the image was captured using the Gel Logic 2200 PRO System. β-actin was used for normalization. The densitometric analyses were performed using the Carestream Molecular Imaging Software.
Northern Blot Analyses of Apolipoprotein-II and Vitellogenin mRNA
RNA extraction from liver tissue homogenates was performed essentially as previously described [31]. 5μg of total RNA from each sample was resolved by electrophoresis on a 1.2% formaldehyde/agarose gel and transferred to a nylon membrane using a semi-dry electroblotting apparatus (Bio-Rad, CA). The membranes were hybridized overnight at 42°C with biotinylated apo II or VG oligonucleotide probes in the presence of 5× SSC buffer containing Denhard's reagent, washed twice with .5 × SSC buffer, and incubated with avidin peroxidase. The membranes were then washed and treated with ECL chemiluminisence reagent, and the image was captured using the Gel Logic 2200 PRO System. Subsequently, the blots were stripped and re-probed with biotinylated β-actin oligonucleotide probe. β-actin was used for normalization.
Reverse Transcriptase-Aided Polymerase Chain Reaction (RT-PCR)
First strand cDNA Synthesis and PCR amplification were performed using ProtoScript AMV LongAmp Taq RT-PCR kit (New England Biolabs, MA). 1μg whole cell RNA from each of the control and drug-treated rooster livers was mixed with 2 μl of 50μM anchored oligo dT in sterile RNase free microfuge tubes and the volume was adjusted to 8μl with nuclease-free water. The RNA was denatured at 70°C for 5 minutes and flash-cooled promptly on ice. Following the addition of 10μl of the AMVRT reaction mix containing 50mM Tris-HCl (pH 8.3), 40mM KCl, 8.75mM MgCl2, 10mM DTT, 0.1mg/ml acetylated BSA, 0.25mM anchored oligo-dT, 400μM dNTP and 20 units of AMVRT enzyme, the reaction was incubated at 42 °C for an hour followed by heat inactivation of the enzyme by incubating at 80°C for 5 minutes. To 5μl of this reaction mix, 25μl of the LongAmp Taq 2X Master Mix, 1 μl of 10 μM forward primer, 1μl of 10μM reverse primer were added and the reaction volume was brought to 50μl with deionized distilled water and amplified in a Perkin-Elmer thermocycler for 30 cycles. The amplified PCR products were then resolved on a 2.5% TBE-agarose gel containing ethidium bromide and visualized on a UV transilluminator.
Primers used for apo II and β-actin mRNA:
Apo II - Forward: 5’-AACCTCAGCTTCAGCCTGGGAGAG-3’
- Reverse: 5’-CTCTAGTTACATTAATGGGAGCAT-3’
β-actin - Forward: 5’-ACTTTCTACAATGAGCTGAGAGTA-3’
- Reverse: 5’-CTTGATTTTCATTGTGCTAGGTGC-3’
Subcloning truncated cDNA corresponding to 3’UTR upstream domain of apoII mRNA
For purposes of synthesizing biotinylated, antisense riboprobe RNA complimentary to the upstream domain of the 3’-UTR, the cDNA corresponding to this region was sub-cloned into pGEMT vector by nested polymerase chain reaction (PCR) amplification of this region using pT7NAPOII as template containing apo II cDNA. The reverse primer contained Hind III linker sequence at its 5’end in order to create a unique Hind III restriction site in the plasmid at the end of the insert, when transcribed by T7 RNA polymerase. Bam H1 linker sequence was included in the 5’ end of the forward primer for run-off transcription using Sp6 RNA polymerase on Bam H1 linearized plasmid and also for any future sub-cloning of this region of the cDNA. The PCR products were cloned using the T-tailed vector, pGEM-T (Promega, Madison, WI). The ligated plasmids were then used to transform competent JM109 bacterial cells by electroporation and plated on ampicillin/LB plates in soft agar containing X-Gal and IPTG. Mini-plasmid preparations were made from white colonies obtained from the plates, and sequenced by the dideoxy method of DNA sequencing using PCR. The bacterial clones that contained plasmids with the correct inserts in the desired orientation were then grown for maxi-preparation of the respective plasmids using the Qiagen plasmid preparation kit (Qiagen, Valencia, CA). The subcloned plasmids pGEMT-APOII3’UTRUPS contained the cDNA region corresponding to the upstream 3’-UTR domain. Since, pGEM-T plasmid has two promoter sequences, namely T7 and Sp6 in opposite orientations flanking the insert sequence, the sense RNA is transcribed by T7 and the antisense by Sp6 RNA polymerases respectively. These antisense RNA synthesized by run-of transcription using Sp6 RNA polymerase was used for the RNase protection assay.
Run-off Transcription of Antisense RNA of Apo II 3’-upstream UTR
The run-off transcription of apo II 3’ upstream UTR region was performed by incubating a 50μl reaction containing 20μM UTP, 20μM biotinylated UTP, 400μM each of CTP, GTP, and ATP, 3μg BAM H1 cut pGEMTAPOII3’UTRUPS plasmid, and 40 units of SP6 enzyme and SP6 reaction buffer (Promega) at 37 °C for an hour. The RNA was purified by phenol/chloroform extraction and ethanol precipitation, lyophilized and resuspended in TE buffer.
RNase Protection Assay
10μg of whole cell RNA from livers of roosters treated with the vehicle, estrogen or the SERMs were hybridized with 0.5μg biotinylated antisense upstream 3’ UTR RNA and the RNase protection assay was performed as previously described [32, 33] using single strand-specific RNase T1. The protected RNAs were electrophoretically resolved on 8M urea-6% polyacrylamide gel, transferred by electroblotting to nylon membrane, UV cross-linked, probed with avidin-peroxidase and visualized by chemiluminisence. The images were scanned using Gel Logic 2200 PRO System.
RESULTS
Apo II mRNA is Stabilized by the E-RmRNASF
The stimulation of the hepatic expression of the avian apo II and VG is due to increased transcription as well as post-transcriptional stabilization of their mRNAs. The apo II mRNA is stabilized by a stabilizing factor E-RmRNASF that is expressed in response to estrogen, which protects the mRNA from endonucleolytic degradation by binding to the upstream 3’ UTR region of the mRNA [4-8]. This is evidenced by the fact that apo II mRNA, when incubated in the presence of liver cytosolic extracts from roosters injected with the vehicle devoid of estrogen, underwent rapid degradation (4, 5; Fig. 1, lane 2) however, when incubated in the presence of liver cytosolic extracts from estrogen-treated roosters, the mRNA remained highly stable (4, 5; Fig. 1, lane 3) due to the expression of the stabilizing factor E-RmRNASF, in response to estrogen.
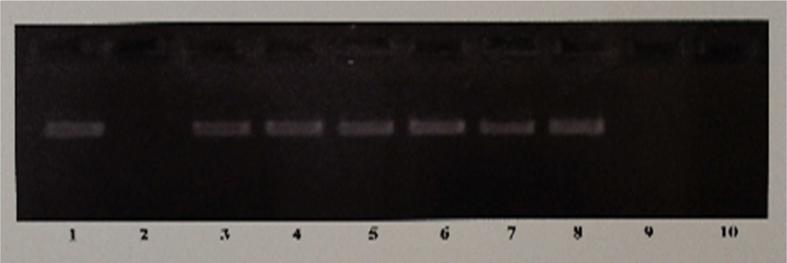
Fig. 1. The Effects of Estrogen or the Test SERMs on the Hepatic Expression of the Estrogen-Regulated mRNA Stabilizing Factor.
Figure shows an ethidium bromide stained 2.5% agarose gel of the RT-PCR products of apo II cDNA. The in vitro transcribed apo II mRNA was used in mRNA stability assays to determine the effects of estrogen or the SERMs on the expression of E-RmRNASF in rooster livers. Lane 1 represents incubation of apo II mRNA in the absence of any added liver cytosolic extract, and lane 2 represent incubation of apo II with control liver cytosolic extract from roosters who received only the vehicle. Lanes 3-10, represent incubation of apo II mRNA with liver cytosolic extracts from roosters who were treated with estrogen (lane 3), resveratrol (lane 4), genistein (lane 5), catechin (lane 6), tamoxifen (lane 7), pterostilbene (lane 8), raloxifene (lane 9) or clomiphene (lane 10)
The Hepatic Expression of the E-RmRNASF that Stabilizes Apo II mRNA in Response to Estrogen, is also Stimulated by SERMs Such as Resveratrol, Genistein, Catechin, Pterostilbene and Tamoxifen But Not by Raloxifene or Clomiphene
RT-PCR analysis of unlabeled apo II mRNA incubated in liver cytosolic extracts from roosters treated with resveratrol (Fig. 1, lane 4), genistein (Fig. 1, lane 5), catechin (Fig.1, lane 6), tamoxifen (Fig. 1, lane 7), pterostilbene (Fig. 1, lane 8), showed that the mRNA was protected from degradation just as in the case of the estrogen-treated extract (Fig. 1, lane 3) indicating the stimulation of expression of E-RmRNASF by these agents, which protected the apo II mRNA from nucleolytic degradation. Raloxifene (Fig. 1, lane 9) and clomiphene (Fig. 1, lane 10) however, failed to stimulate the expression of E-RmRNASF, resulting in the degradation of the apo II mRNA and hence was not detected in the RTPCR.
The Hepatic Expression of Apolipoprotein II and Vitellogenin proteins is Stimulated by Estrogen, but Inhibited by the SERMs
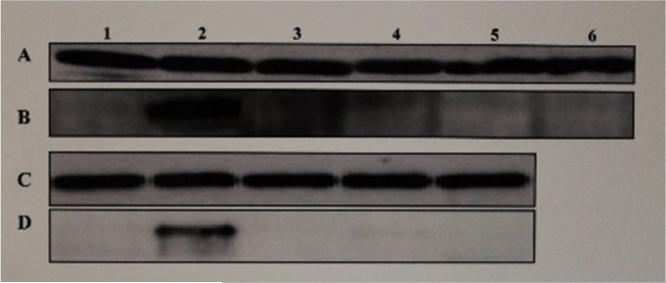
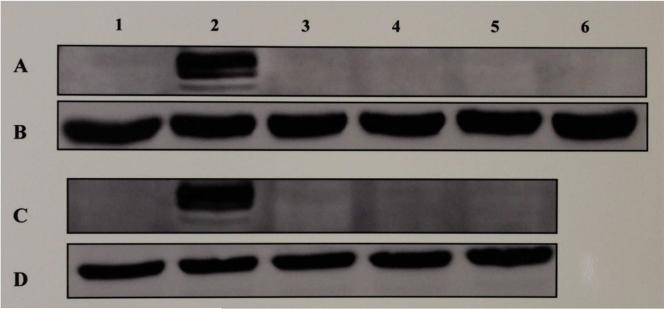
The effect of estrogen, the test SERMs or combinations of estrogen and each of the test SERMs on the hepatic expression of apo II and VG proteins, when examined by means of Western blot analyses of the liver cytosolic extracts from roosters treated with the vehicle, estrogen, the test SERMs or combinations of estrogen and each of the test SERMs, showed both apo II (Fig. 2, lane 2B & 2D) and VG (Fig. 3, lanes 2A & 2C) to be expressed in the liver in response to estrogen as expected. Surprisingly however, none of the SERMs tested (Fig. 2, lanes 3B-6B, 3D- 5D; Fig. 3, lanes 3A-6A, 3C-5C), including resveratrol (Fig. 2, lane 3B; Fig 3. lane 3A), genistein (Fig. 2, lane 4B; Fig 3. lane 4A), catechin (Fig. 2, lane 5B; Fig 3. lane 5A), tamoxifen (Fig. 2, lane 6B; Fig 3. lane 6A), and pterostilbene (Fig. 2, lane 3D; Fig 3. lane 3C) that stimulated the expression of E-RmRNASF, were able to turn on their expression in the liver. Previously, we had shown clomiphene to behave as an estrogenic antagonist in chicken [8]. Clomiphene (Fig. 2, lane 5D; Fig. 3, lane 5C) as well as raloxifene (Fig. 2, lane 4D; Fig. 3, lane 4C) also failed to stimulate the hepatic expression of apo II and VG. When estrogen was co-administered with 200 fold excess of resveratrol, genistein, catechin, tamoxifen, pterostilbene, clomiphene or raloxifene, all of these SERMs inhibited the stimulation of expression of apo II and VG by estrogen (data not shown). Based on the above results, it is evident that all of above SERMs, even the ones which were agonistic with regard to stimulation of expression of E-RmRNASF, were antagonistic/antiestrogenic with regard to the expression of apo II and VG respectively. House-keeping β-actin gene, which is constitutively expressed was used as an internal control for normalization.
Fig. 2. Western Blot Analyses for the Expression of Apo II in Liver Cytosolic Extracts From Roosters Treated With Estrogen or Test SERMs.
The figure shows a chemiluminograph of a Western blot of liver cytosolic extracts from roosters who received treatment with the vehicle (lane 1, panels A, B, C & D), estrogen (lane 2, panels A, B, C & D), resveratrol (lane 3, panels A & B), genistein (lane 4, panels A & B), catechin (lane 5, panels A & B), tamoxifen (lane 6, panels A & B), pterostilbene (lane 3, panels C & D), raloxifene (lane 4, panels C & D), clomiphene (lane 5, panels C & D). Panels A and C were probed with α-chicken β-actin rabbit polyclonal antibody and panels B and D were probed with α-chicken apo II rabbit polyclonal antibody.
Fig. 3. Western Blot Analyses for the Expression of Vitellogenin in Liver Cytosolic Extracts from Roosters Treated With Estrogen or Test SERMs.
The figure shows a chemiluminograph of a Western blot of liver cytosolic extracts from roosters who received treatment with the vehicle (lane 1, panels A, B, C & D), estrogen (lane 2, panels A, B, C & D), resveratrol (lane 3, panels A & B), genistein (lane 4, panels A & B), catechin (lane 5, panels A & B), tamoxifen (lane 6, panels A & B), pterostilbene (lane 3, panels C & D), raloxifene (lane 4, panels C & D), clomiphene (lane 5, panels C & D). Panels A and C were probed with α-chicken vitellogenin rabbit polyclonal antibody and panels B and D were probed with α-chicken β-actin rabbit polyclonal antibody.
Northern Blot Analyses for Hepatic Expression of Apo II and Vitellogenin mRNA in Response to Treatment with Estrogen, Test SERMs or Combinations of Estrogen and Test SERMs
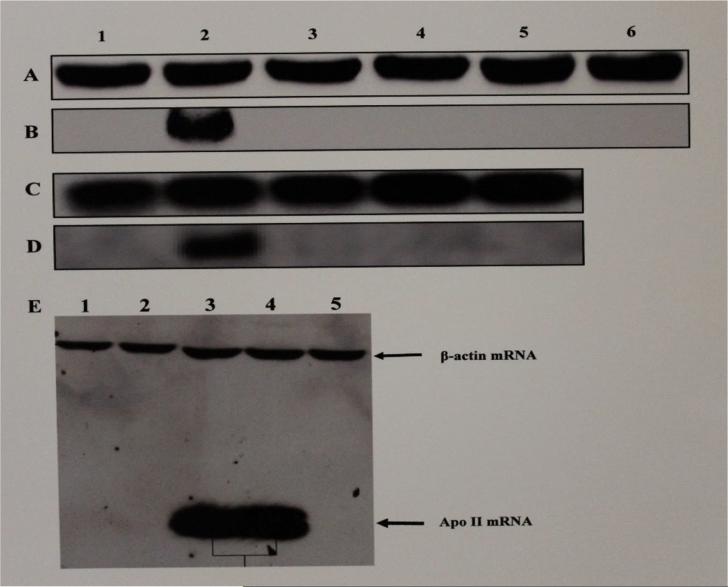
Northern blot analyses of the whole cell RNAs from the livers of roosters treated with the vehicle, estrogen, the test SERMs or combinations of estrogen and the test SERMs, showed expression of mRNAs of apo II and VG in response to estrogen (Fig 4, lanes 2B, 2D, 3E, 4E; Fig. 5, 2A, 2C, 2E, respectively) but not to resveratrol (Fig. 4, lanes 3B, 1E; Fig 5. lane 3A, 3E, respectively), genistein (Fig. 4, lanes 4B, 2E; Fig 5. lanes 4A, 4E, respectively), catechin (Fig. 4, lane 5B; Fig 5. lane 5A, 5E, respectively), tamoxifen (Fig. 4, lane 6B; Fig. 5, lane 6A), pterostilbene (Fig. 4, lane 3D; Fig 5, lane 3C, respectively), raloxifene (Fig. 4, lane 4D; Fig 5, lane 4C, respectively) and clomiphene (Fig. 4, lane 5D; Fig. 5, lane 5C, respectively) indicating the lack of stimulation of transcription of the respective estrogen-responsive genes in response to the SERMs tested. When co-administered with estrogen, resveratrol (Fig. 4, panel E, lane 1; Fig 5, panel E, lane 3), genistein (Fig. 4, panel E, lane 2; Fig 5, panel E, lane 4), catechin (Fig. 4, panel E, lane 5; Fig 5, panel E, lane 5), tamoxifen (data not shown), pterostilbene (data not shown), clomiphene (data not shown) and raloxifene (data not shown) inhibited stimulation of transcription of apo II and VG genes respectively by estrogen, indicating that these SERMs were in fact antagonistic at these two promoters.
Fig. 4. Northern Blot Analysis for Transcription of Apolipoprotein II mRNA in Livers of Roosters Treated With Estrogen, Test SERMs or Combinations of Estrogen and Test SERMs.
The figure shows a chemiluminograph of a Northern blot of liver cytosolic extracts from roosters who received treatment with the vehicle (lane 1, panels A, B, C & D), estrogen (lane 2, panels A, B, C & D), resveratrol (lane 3, panels A & B), genistein (lane 4, panels A & B), catechin (lane 5, panels A & B), tamoxifen (lane 6, panels A & B), pterostilbene (lane 3, panels C & D), raloxifene (lane 4, panels C & D), clomiphene (lane 5, panels C & D). Panels A and C were probed with biotinylated antisense chicken β-actin DNA oligonucleotide probe and panels B and D were probed with biotinylated antisense chicken apo II DNA oligonucleotide probe. Panel E shows the antiestrogenic effect of the SERMs on apo II gene expression and the relative electrophoretic mobility of apo II mRNA in relation to the β-actin mRNA. Lanes 1-5 in panel E correspond to whole cell RNA from livers of roosters treated with estrogen + resveratrol (lane 1), estrogen + genistein (lane 2), estrogen (lanes 3 & 4), and estrogen + catechin (Lane 5), probed with biotinylated apo II and β-actin antisense DNA oligonucleotides.
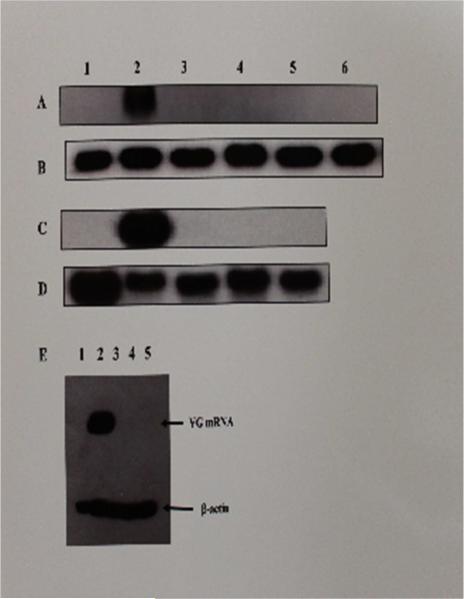
Fig. 5. Northern Blot Analysis for Transcription of Vitellogenin mRNA in Livers of Roosters Treated With Estrogen, Test SERMs or combinations of estrogen and Test SERMs.
The figure shows a chemiluminograph of a Northern blot of liver cytosolic extracts from roosters who received treatment with the vehicle (lane 1, panels A, B, C, & D), estrogen (lane 2, panels A, B, C, & D), resveratrol (lane 3, panels A, & B), genistein (lane 4, panels A, & B), catechin (lane 5, panels A, & B), tamoxifen (lane 6, panels A & B), pterostilbene (lane 3, panels C & D), raloxifene (lane 4, panels C & D), clomiphene (lane 5, panels C & D). Panels A and C were probed with biotinylated antisense chicken VG DNA oligonucleotide probe and panels B and D were probed with biotinylated antisense chicken β-actin DNA oligonucleotide probe. Panel E shows the antiestrogenic effect of the SERMs on VG gene expression and the relative electrophoretic gel mobility of the VG mRNA in relation to β-actin mRNA. Lanes 1-5 in panel E correspond to whole cell RNA from livers of roosters treated with the vehicle (lane 1), estrogen (lane 2), estrogen + resveratrol (lane 3), estrogen + genistein (lane 4), and estrogen + catechin (lane 5).
An RNA protection assay using RNA corresponding to the antisense sequence of the upstream 3’UTR of apo II mRNA showed protection from RNase T1 only with the whole cell RNA from livers of roosters treated with estrogen (Fig. 6, lane 2) but not the SERMs (Fig. 6, lanes 3-7) or the vehicle (fig. 6, lane 8) indicating stimulation of transcription of the apo II gene by estrogen and not the test SERMs.
Fig. 6. RNase T1 protection assay.
Figure shows a chemiluminograph of 8M urea-6% polyacrylamide gel of RNase protection assay performed to detect apo II mRNA. Lane 1 shows the in vitro transcribed biotinylated antisense probe of upstream 3’-UTR of apo II mRNA that has irrelevant 5’ and 3’ overhangs, in the absence of RNase T1 digestion. Lanes 2-8, show RNase protection assays of whole cell RNA from livers of roosters treated with estrogen, resveratrol, genistein, catechin, tamoxifen, clomiphene and the vehicle, respectively. The band in lane 2 corresponds to the nuclease-protected biotinylated antisense probe of upstream 3’UTR of apo II mRNA.
When the antagonistic effect of the test SERMs on the expression of the genes encoding apo II and VG were determined by Western blot (data not shown) and Northern blot analyses (data for tamoxifen, clomiphene, raloxifene and pterostilbene not shown) of livers from roosters treated with a combination of estrogen and each of the SERMs, they all inhibited their stimulation by estrogen, indicating that all the SERMs tested including those that stimulated the expression of E-RmRNASF behaved as antagonists at these two gene promoters.
Discussion
Apo II and VG are egg yolk proteins expressed in abundance in the liver and transported to the oviduct in an egg-laying hen [1-3]. Their elevated expression in the chicken liver in response to estrogen is attributable to estrogen-mediated increased rate of transcription and post-transcriptional mRNA stabilization [4-12]. The mRNA stabilization is due to a stabilizing factor namely, the E-RmRNASF that is expressed in the chicken liver in response to estrogen [4-9].
SERMs have been shown to exert either an agonistic or antagonistic effect on the expression of estrogen responsive genes [4-8, 13-30]. Many of the SERMs have been shown to exert a tissue-specific estrogenic or antiestrogenic effect [6-8, 13-30]. Tamoxifen for instance, works in a tissue-specific manner; while it functions as an estrogenic antagonist in the human breast tissue, it is agonistic in uterine and bone tissues [13-20, 30]. SERMS such as raloxifene, ospemifene, etc., also show such tissue-specific estrogenic or antiestrogenic effects [13-22]. Such tissue specificity is largely attributed to whether or not the ligand-bound ER, recruits coactivators or corepressors [23-30]. When SERMs bind to the ER, the conformational changes of the ER that they induce, determines their interactions with co-regulator proteins as shown by x-ray crystallographic studies [30]. For instance, the binding of tamoxifen to the ER, precludes its interaction with the coactivator protein p300 [27-29]. In fact, microarray analyses have shown that the ER bound to tamoxifen regulates a set of genes substantially different from those regulated by estrogen-liganded ER [27].
In the present study, we have investigated the effects of estrogen and a select panel of SERMs on the hepatic expression of estrogen-responsive genes E-RmRNASF, apo II and VG in the chicken. Apo II and the VG genes share a lot of commonality in the promoter regions with respect to the estrogen response elements (ERE). Their promoters contain two closely spaced ERE sequences, one of which is a perfect consensus sequence and the other that is slightly imperfect [35, 36]. Both regions act in a concerted manner and are required for efficient transcription of the respective genes [35, 36]. Promoter analyses have revealed the presence of several positive and negative sequence motifs in addition to the ERE sequences that influence estrogenic response on these promoters [36].
The results presented here indicate a gene-specific effect of the test SERMs on estrogen-responsive genes associated with egg-production, in the chicken liver. Interestingly, while estrogen stimulated the expression of E-RmRNASF as well as apo II and VG, the SERMS that stimulated the expression of E-RmRNASF were unable to stimulate the expression of either apo II or VG. Instead, they inhibited the stimulation of these genes by estrogen. The plausible reason for gene-specificity is most likely determined by the presence or the lack thereof promoter elements in the apo II and VG genes that enable the ER complex to recruit coactivators when bound to estrogen but not to the SERMs in question. It is likely that when bound to the SERMs, the ER most likely recruited co-repressors instead of co-stimulators and hence were antagonistic. The positive and negative regulator elements present in their promoter regions have been shown to impact transcription at these promoters [34-36]. One possible explanation for the above results is that the ER complex, when interacting with E-RmRNASF gene promoter elements, in all likelihood, was able recruit coactivators when bound to estrogen or the agonistic SERMs.
Based on our results it is reasonable to speculate that certain genes are stimulated by both estrogen as well as a full spectrum of agonistic SERMs, while others are restricted to stimulation by estrogen or a limited array of SERMs.
Conclusion
Based on the above results, we can conclude that the genes encoding apo II, VG and E-RmRNASF, which are associated with egg production in chicken, are stimulated by estrogen, but are differentially regulated by the test SERMs. SERMs such as tamoxifen, genistein, resveratrol, pterostilbene and catechin that were agonistic with respect to the expression of E-RmRNASF behaved as antagonists with regard to the expression of apo II and VG in the liver.
SERMs have been shown to exert tissue-specific estrogenic or antiestrogenic effects on gene expression
Gene-specific effects of SERMs were investigated on 3 estrogen-responsive genes associated with egg production in chicken
All test SERMs except raloxifene and clomiphene stimulated E-RmRNASF expression
All SERMs agonistic on E-RmRNASF, were antagonistic on egg yolk proteins apo II and VG, gene expression
Acknowledgements
This work was supported by NIH Grants 1 R15 DK52584-01 and 5 S06 GM54650-3 and support from Long Island University, NY, U.S.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deeley RG, Mulinix DP, Wetekam W, Kronenberg HM, Meyers M, Eldridge JD, Goldberger RF. Vitellogenin Synthesis in the Avian Liver, Vitellogenin is the Precursor of the Egg Yolk Phosphoproteins. J Biol Chem. 1976;250:9060–66. [PubMed] [Google Scholar]
- 2.Li J, Leghari IH, He B, Zeng W, Mi Y, Zhang C. Estrogen Stimulates Expression of Chicken Hepatic Vitellogenin II and Very Low Density Apolipoprotein II Through ER-α. Theriogenology. 2014;82:517–24. doi: 10.1016/j.theriogenology.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Cochrane AW, Deeley RG. Estrogen-Dependent activation of the Avian Apolipoprotein II and Vitellogenin Genes: Transient Alterations in mRNA Polyadenylation and Stability Early During Induction. J Mol Biol. 1988;203:555–67. doi: 10.1016/0022-2836(88)90192-1. [DOI] [PubMed] [Google Scholar]
- 4.Ratnasabapathy R. In Vitro Characterization of an Estrogen-Regulated mRNA Stabilizing Activity in the Avian Liver. Cell Mol Biol Res. 1995;41:583–94. [PubMed] [Google Scholar]
- 5.Ratna WN, Oyeamalu C. The Upstream Stem-Loop Domain of the 3’Untranslated Region of Apolipoprotein II mRNA Binds the Estrogen-Regulated mRNA Stabilizing Factor. J. Steroid Biochem Molec Biol. 2002;80:383–98. doi: 10.1016/s0960-0760(02)00035-3. [DOI] [PubMed] [Google Scholar]
- 6.Ratna WN,J, Simonelli J. The Action of Dietary Phytochemicals, Quercetin, Catechin, Resveratrol and Naringenin on Estrogen-Mediated Gene Expression. Life Sciences. 2002;70:1577–89. doi: 10.1016/s0024-3205(01)01531-4. [DOI] [PubMed] [Google Scholar]
- 7.Ratna WN. Inhibition of Estrogenic Stimulation of Gene Expression by Genistein. Life Sciences. 2002;71:865–77. doi: 10.1016/s0024-3205(02)01770-8. [DOI] [PubMed] [Google Scholar]
- 8.Ratnasabapathy R, Tom M, Post C. Modulation of the hepatic expression of the estrogen-regulated mRNA stabilizing factor by estrogenic and antiestrogenic xenobiotics. Biochem Pharmacology. 1997;53:1425–34. doi: 10.1016/s0006-2952(97)00084-1. [DOI] [PubMed] [Google Scholar]
- 9.Ratnasabapathy R, Hwang S-P, Williams DL. The 3′ untranslated region of apoII mRNA contains two Independent domains that bind distinct cytosolic factors. J Biol Chem. 1990;265:14050–55. [PubMed] [Google Scholar]
- 10.Binder R, Hwang S-P, Ratnasabapathy R, Williams DL. Degradation of apoII mRNA occurs via Endonucleolytic cleavage at 5′-AAU-3′, 5'-UAA-3′ elements in single strand loop domains of the 3' noncoding region. J Biol Chem. 1989;264:16910–18. [PubMed] [Google Scholar]
- 11.Binder R, Hwang SP, Ratnasabapathy R, Eisenberg M, Williams DL. In: Molecular Biology of atherosclerosis. Attie AD, editor. Elsevier Science Publishing Co. Inc.; 1990. pp. 97–107. [Google Scholar]
- 12.Nielson DA, Shapiro DJ. Insights into hormonal control of messenger RNA stability. Mol Endocrinol. 1990;4:953–57. doi: 10.1210/mend-4-7-953. [DOI] [PubMed] [Google Scholar]
- 13.Maximov PY, Lee TM, Jordan CV. The Discovery and Development of Selective Estrogen Receptor Modulators (SERMs) for Clinical Practice Curr Clin Pharmacol. 2013;8:135–55. doi: 10.2174/1574884711308020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang Y. Molecular Mechanisms of Estrogen and SERMs in Endometrial Carcinogenesis. Nature Reviews Cancer. 2006;6:360–68. doi: 10.1038/nrc1879. [DOI] [PubMed] [Google Scholar]
- 15.Taylor HS. Designing the Ideal Selective Estrogen Modulator-an Achievable Goal. Menopause. 2009;16:609–15. doi: 10.1097/gme.0b013e3181906fa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riggs BL, Hartmann LC. Selective Estrogen-Receptor Modulators–Mechanisms of Action and Application to Clinical Practice. N Engl J Med. 2003;348:618–29. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 17.Wu O. Postmenopausal Hormone Replacement Therapy and Venous Thromboembolism. Gend Med. 2005;2:18–27. doi: 10.1016/s1550-8579(05)80061-0. [DOI] [PubMed] [Google Scholar]
- 18.Cole MP, Jones CT, Todd ID. A New Anti-Oestrogenic Agent in Late Breast Cancer. An Early Clinical Appraisal of ICI46474. Br. J. Cancer. 1971;25:270–75. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward HW. Anti-Oestrogen Therapy for Breast Cancer: a Trial of Tamoxifen at Two Dose Levels. Br Med J. 1973;1:13–14. doi: 10.1136/bmj.1.5844.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagani O, Gelber S, Price K, Zahrieh D, Gelber R, Simoncini E, Castiglione-Gertsch M. (International Breast Cancer Study Group), Torimifene and Tamoxifen are Equally Effective for Early-Stage Breast Cancer: First Results of International Breast Cancer Study Group Trials 12-93 and 14-93. Ann Oncol1. 2004;5(12):1749–59. doi: 10.1093/annonc/mdh463. [DOI] [PubMed] [Google Scholar]
- 21.Farnell YZ, Ing NH. Endometrial Effects of Selective Estrogen Receptor Modulators (SERMs) on Estradiol-Responsive Gene Expression are Gene and Cell Specific. Steroid Biochem Mol Biol. 2003;84:513–26. doi: 10.1016/s0960-0760(03)00076-1. [DOI] [PubMed] [Google Scholar]
- 22.Shull JD, Beams FE, Baldwin TM, Gilchrist CA, Hrbek MJ. The Estrogenic and Antiestrogenic Properties of Tamoxifen in GH4C1 Pituitary Tumor Cells are Gene-Specific. Mol Endocrinol. 1992;6:529–35. doi: 10.1210/mend.6.4.1584221. [DOI] [PubMed] [Google Scholar]
- 23.Nisson S, Makela S, Treuter E, Tujague M, Thornsen J, Anderson G, Enmark E, Peterson K, Warner M, Gustafsson J-A. Mechanism of Estrogen Action. Physiol Rev. 2001;81:1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 24.McDonnell DP, Norris JD. Connections and Regulation of the Human Estrogen Receptor. Science. 2002;32:1642–44. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 25.Klinge CM. Estrogen Receptor Interaction with Estrogen Response Elements. Nucleic Acids Res. 2001;29:2905–19. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein-Hitpass L, Tsai SY, Greene GL, Clark JH, Tsai M-J, O'Malley BW. Specific Binding of Estrogen Receptor to the Estrogen Response Element. Mol Cell Biol. 1989;9:43–49. doi: 10.1128/mcb.9.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanstein B,R, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M. p300 is a Component of an Estrogen receptor Coactivator Complex. Proc Natl Acad Sci USA. 1996;93:11540–45. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwijsen RML, Buckle RS, Hijmans EM, Loomans CJM, Bernards R. Ligand-Independent Recruitment of Steroid Receptor Coactivators to Estrogen Receptor by Cyclin D1. Genes & Dev. 1998;12:3488–98. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trowbridge JM, Rogatsky I, Garabedian MJ. Regulation of Estrogen Receptor Transcriptional Enhancement by the Cyclin A/Cdk2 Complex. Proc Natl Acd. Sci USA. 1997;94:10132–37. doi: 10.1073/pnas.94.19.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of this Interaction by Tamoxifen. Cell. 1998;95:927–37. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P N, Sacchi N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal Biochem. 1987;162:156–59. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Ratnasabapathy R,M, Sheldon M,L, Johal L,N, Hernandez N. The HIV-1 LTR Contains an Unusual Element that Induces the Synthesis of Short RNAS From Various mRNA and snRNA Promoters. Genes & Dev. 1990;4:2061–74. doi: 10.1101/gad.4.12a.2061. [DOI] [PubMed] [Google Scholar]
- 33.Sheldon M, Ratnasabapathy R,N, Hernandez N. Characterization of Short Transcripts, a Human Immunodeficiency virus Type I Transcriptional Element that Activates the Synthesis of Short RNAS. Mol Cell Biol. 1993;13:1251–63. doi: 10.1128/mcb.13.2.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beekman JM,J, Wijnholds J,IJ, Shippers IJ,W, Pot W,M, Gruber M,GABG. Regulatory Elements and DNA-Binding Proteins Mediating Transcription From the Chicken-Very-Low Density Apolipoprotein II Gene. Nucleic Acids Res. 1991;19:5371–77. doi: 10.1093/nar/19.19.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burch JBE,MI, Evans MI,TM, Friedman TM,PJ, O'Malley PJ. Two Functional Estrogen Response Elements are Located Upstream of the Major Chicken Vitellogenin Gene. Mol Cell Biol. 1988;8:1123–31. doi: 10.1128/mcb.8.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seal SN,DL, Davis DL,JB, Burch JB. Mutational Studies Reveal a Complex Set of Positive and Negative Control Elements Within the Chicken Vitellogenin II Promoter. Mol Cell Biol. 1991;11:2704–17. doi: 10.1128/mcb.11.5.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]