Abstract
Exposures to polychlorinated biphenyls (PCBs) during early development have long-lasting, sexually dimorphic consequences on adult brain and behavior. However, few studies have investigated their effects during juvenile development, a time when increases in pubertal hormones influence brain maturation. Here, male and female Sprague Dawley rats were exposed to PCBs (Aroclor 1221, 1 mg/kg/day) or vehicle prenatally, during juvenile development, or both, and their effects on serum hormone concentrations, gene expression, and DNA methylation were assessed in adulthood. Gene expression in male but not female brains was affected by 2-hits of PCBs, a result that paralleled behavioral effects of PCBs. Furthermore, the second hit often changed the effects of a first hit in complex ways. Thus, PCB exposures during critical fetal and juvenile developmental periods result in unique neuromolecular phenotypes, with males most vulnerable to the treatments.
Keywords: endocrine-disrupting chemical, preoptic area, dopamine receptor, mu opioid receptor, androgen receptor, estrogen receptor
1. Introduction
Polychlorinated biphenyls (PCBs) are some of the most widespread environmental endocrine-disrupting chemicals (EDCs), as they persist in the food chain and are detectable in tissues of virtually all humans (Agency for Toxic Substances and Disease Registry, 2000). While banned in the 1970s, recent epidemiological data show that PCB body burdens continue to be associated with impaired reproductive and neurobiological health in humans (Boucher et al., 2009; Buck Louis et al., 2013; Engel and Wolff, 2013). In addition, rodent studies demonstrate that PCBs exert subtle but chronic effects on a range of social and anxiety related behaviors (Elnar et al., 2012; Jolous-Jamshidi et al., 2010; Reilly et al., 2015; Tian et al., 2011). Many of these behaviors are sexually dimorphic and organized by neonatal exposure to steroid hormones (Adler et al., 1999; Auger and Olesen, 2009; Bitran, 1993; Henley et al., 2011; Mora et al., 1996). As some PCB congeners, including those in the current study (Aroclor 1221, A1221) are weakly estrogenic (Jansen et al., 1993), the majority of behavioral studies focus on gestational or neonatal exposure, a life stage when brain sexual differentiation occurs, and when hormonal perturbations were predicted to have the most profound effects (McCarthy et al., 2009).
Juvenile development is also a time of continued sensitivity to organizational effects of gonadal hormones, as well as activation of neural pathways that were organized earlier in life (Schulz et al., 2009; Sisk and Foster, 2004). We recently demonstrated that exposing rats to PCBs during juvenile development, with or without prior prenatal exposure, affected several types of behavior in a sex- and age-specific manner (Bell et al., 2015). Two hits of PCBs, the first in late gestation and the second in juvenile development, resulted in abnormal levels of play and anxiety-like behavior in adolescent females, and caused disruptions of opposite-sex partner preference in adult males. In some cases, juvenile exposure modified or unmasked the effects of a previous prenatal exposure, especially in the male rats.
The goals of this study were to determine how two hits of PCB exposure, given during prenatal or juvenile development, or both, interact to change expression of genes in the adult brain as potential molecular substrates related to the observed behavioral changes. Neural regions were selected based on their roles in sexually dimorphic sociosexual and anxiety-like behaviors and in mediating the rewarding qualities of these social interactions (Burgdorf et al., 2007; Davis et al., 2010; Gordon et al., 2002; Harding and McGinnis, 2005; Newman et al., 1997; Pfaff and Sakuma, 1979). Genes that were studied within these regions included those involved in dopaminergic and endogenous opioid signaling, the vasopressin and oxytocin systems, and steroid hormone receptors that regulate social and anxiety-like behaviors (Bale et al., 2001; Bielsky et al., 2004; Buck et al., 2014; Bychowski et al., 2013; Egashira et al., 2007; Ferguson et al., 2000; Harding and McGinnis, 2004; Lim and Young, 2006; Matochik et al., 1992; Trezza et al., 2010; Veenema et al., 2013). We hypothesized that changes in gene expression would be correlated with changes in behavior, that a second PCB hit would change the developmental trajectory of gene expression in the brain in a manner not predicted by either hit alone, and that the sexes would differ in their sensitivity to PCB effects.
2. Methods
2.1 Animals and Husbandry
All animal protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by The University of Texas at Austin’s Institutional Animal Care and Use Committee. Sprague-Dawley rats were purchased from Harlan Laboratories (Houston, Texas) and were housed in a humidity- and temperature-controlled room with a 12:12 reversed light cycle (lights out at noon) at 21–23°C. 2–3 animals were group-housed in polycarbonate cages (43 × 21 × 25 cm) with aspen bedding (PJ Murphy Forest Products, Sani-Chip), a PVC tube for enrichment, and weekly cage changes. Rats were fed low phytoestrogen Harlan-Teklad 2019 Global Diet (Harlan-Teklad, Indianapolis, Indiana) ad libitum for the duration of the experiment. Upon arrival, rats were handled daily to acclimate them to their new housing conditions, and mating began at least two weeks later.
Females (3–4 months old, virgin) were mated with sexually experienced untreated male rats (~6 month old); for balance, each stud male sired two litters, one that was subsequently treated with the vehicle and the other with PCBs. The morning after successful mating (sperm-positive vaginal smear), termed embryonic day (E) 1, dams were singly housed. Dams were provided with nesting materials several days prior to expected parturition on E23. On the day after birth [postnatal day (P) 1], litters were culled to equal sex ratios, with final litter size ranging from 6 to 8 pups. Weaned pups were housed with same sex littermates (2–3 per cage), and were weighed and handled for at least 5 minutes weekly. Animals were tested for social and anxiety behaviors in adolescence and adulthood, with results published in a sister paper (Bell et al., 2015). Because of the large number of animals necessary for both studies, the animals were raised in 3 cohorts over 1.5 years, with treatments equally distributed across each cohort.
2.2 Treatments
Aroclor 1221 (A1221, AccuStandard, New Haven, CT, Cat No: C-221N-50MG, Lot: 23683) is a mix of ~45 lightly chlorinated PCB congeners with known estrogenic (Layton et al., 2002; Shekhar et al., 1997), anti-aromatase (Woodhouse and Cooke, 2004), and anti-androgen (Schrader and Cooke, 2003) actions, but without effect on aryl dhydrocarbon receptor (Poland and Glover, 1977). It was dissolved in a 4% dimethylsulfoxide vehicle (Veh, Cat No D4540; Sigma, St Louis, Missouri in sesame oil) for intraperitoneal injection at 1 mg/kg dam body weight. Dams were randomly assigned to either Veh (n = 6) or A1221 (n = 6), and each litter contributed no more than two animals per group. On E16, E18, and E20, during the period of sexual differentiation of the rat brain (Breedlove, 1992; Ramaley, 1979; Rhees et al., 1990; Tobet and Fox, 1989; Wagner et al., 1998), dams were weighed and injected with 0.1 ml of Veh or A1221 solution using a 1 ml syringe with a 25 gauge needle, 3 hours prior to lights out. This mixture and dosage is not toxic to dams, does not cause fetal loss, and was selected so that outcomes of the current study could be compared with findings from several other previous studies using a very similar exposure regime (Dickerson et al., 2011a; Reilly et al., 2015; Steinberg et al., 2008; Walker et al., 2014). Although we did not measure body burden, we estimated that each pup is exposed to approximately 2 μg/kg A1221 based on (Takagi et al., 1976). This is within the range of human exposure according to levels found in maternal serum, cord blood and milk fat (Agency for Toxic Substances and Disease Registry, 2000; Karmaus et al., 2002; Lackmann, 2002; Law et al., 2005; Longnecker et al., 2005; Matthews and Anderson, 1975; Patterson et al., 2009; Schantz, 1996).
Rats were given an additional set of juvenile injections, either Veh or A1221 (1 mg/kg), again at 0.1 mL volume, ip, on P24, 26, and 28, when puberty is beginning, estrogen-positive feedback is being established (Andrews et al., 1981) and the brain is highly sensitive to organizational and activational effects of gonadal steroids (Döhler and Wuttke, 1975; Saksena and Lau, 1979; Schulz et al., 2009; Smyth and Wilkinson, 1994; Vetter-O’Hagen and Spear, 2011). Littermates within a cage were given the same treatment to prevent cross-contamination. With both gestational and juvenile exposures, there were four experimental groups in a 2×2 balanced design (first hit prenatal, second hit juvenile): Veh-Veh, A1221-Veh, Veh-A1221, and A1221-A1221. Final Ns per group were between 9 and 12 for all measures, from 6 litters per treatment. The experimenters were blind to treatment throughout the duration of the experiment.
2.3 Tissue collection
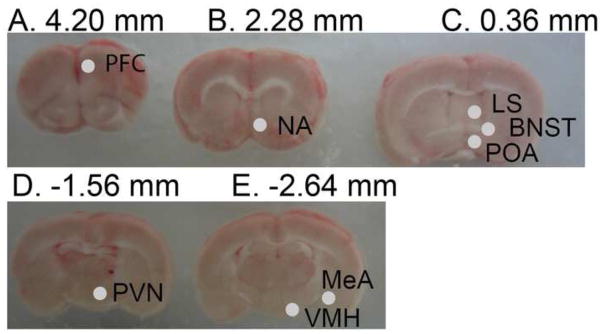
Rats were euthanized in adulthood (between P93-P108) by rapid decapitation 1–3 hours before lights out, on proestrus in females and 3–9 days after the last behavioral test (reported in the companion study; Bell et al., 2015) in both sexes. Brains were immediately removed and placed in ice for 5 minutes prior to placing in an ice-cold stainless steel brain matrix. After cutting the optic chiasm, a razor blade was inserted through the center of this landmark, and three 2-mm (rostral) and two 1-mm (caudal) coronal sections were taken. Sections were placed on an ice-cold microscope slide, and snap frozen on dry ice. One to 11 months later, frozen sections were placed on a freezing stage, allowed to equilibrate to −18°C, and micropunches (0.98 mm diameter) were taken from each region of interest according to Paxinos and Watson, 2009 (Paxinos and Watson, 2009). Photographs were captured of sections before and after punching to ensure consistency across the cohorts (Figure 1). Samples were placed in a cold Eppendorf tube and stored at −80°C for 2–9 months until nucleic acid isolations. Trunk blood samples were collected and allowed to clot for 30 minutes before centrifugation (1500 × g for 5 minutes). Sera were collected and stored at −80°C until use, 1–2 years later.
Figure 1.
Images of 5 brain sections from rostral to caudal (A–E) showing locations of 0.98 mm diameter punches. Punches are shown only on one hemisphere for ease in viewing, but bilateral punches were used for RNA extraction. Sections A–C are 2 mm thick, D–E are 1 mm thick. Abbreviations: Prefrontal cortex (PFC), nucleus accumbens (NA), lateral septum (LS), bed nucleus of the stria terminalis (BNST), preoptic area (POA), paraventricular nucleus (PVN), ventromedial hypothalamus (VMH), and medial amygdala (MeA).
2.4 Serum hormone quantification
Total serum testosterone (T) was determined in male animals via radioimmunoassay (ImmuChem Double Antibody 125I RIA kit, Cat No 07-189105, Lot# RTK1420, MP Biomedicals, Costa Mesa, CA), according to manufacturer directions. All samples were run in a single assay, and duplicate volumes of 50 μl serum were used. The assay limit of detection was 0.03 ng/mL, and the intraassay C.V. was 1.41%. This assay is not sensitive enough to run T in females. Total serum estradiol (E2) was determined in male and female rats via radioimmunoassay (UltraSensitive Estradiol RIA, Cat No DSL4800, Lot# 150622 C, Beckman Coulter, Pasadena, CA), according to manufacturer directions. Samples were run in a single assay and duplicate volumes of 200 μl of serum were used. Assay sensitivity was 2.2 pg/ml and intraassay C.V. was 1.30%. Progesterone (P4), triiodothyronine (T3), and thyroxine (T4) were also determined from a separate serum aliquot from the same male and female rats via a magnetic bead panel (Milliplex Steroid/Thyroid Hormone Magnetic Bead Panel, Cat No STTHMAG-21K, Lot# 2484258, EMD Millipore Corp, Billerica, MA) according to manufacturer directions. Samples were precipitated with acetonitrile before reconstitution with assay buffer, and were run in duplicate volumes of 25 μl. The limits of detection were 0.09, 0.04, and 0.28 ng/ml and the intra-assay CVs were 2.53%, 4.99%, and 4.16% for P4, T3, and T4, respectively.
2.5 Nucleic acid extraction
Frozen tissue punches were lysed and homogenized using 22 gauge needles and syringes. DNA and RNA were extracted using an Allprep DNA/RNA mini kit (Qiagen Cat No 80204) according to manufacturer instructions and the RNA column was treated with DNase (Qiagen Cat No 79254). RNA was eluted with 100 μl of nuclease free water (Applied Biosystems Cat No AM9937) and DNA was eluted with 200 μl of buffer included in kit. Samples were stored at −20°C in 66% ethanol and 0.5M NaCl for 1–4 weeks before being concentrated as follows. Samples were placed at −80°C for 10 minutes before they were centrifuged at 14000 × g for 20 minutes at 4°C to pellet the nucleic acid. The pellet was washed with 70% ethanol and centrifuged again for 10 minutes before supernatant was discarded and samples were dried via inversion at room temperature for 10 minutes and then in a speedvac at 43°C for 5 minutes. Pellets were resuspended in 12 μl water and quantity was determined via Promega QuantiFluor Systems on the Glomax Multi + Detection System (RNA: Cat No E3310, dsDNA: Cat No E2670), according to manufacturer instructions. 150–1500 ng of RNA, and 15–75 ng of DNA, were isolated, depending on the region. RNA quality was assessed by randomly selecting ~10% of the samples to run on a Bioanalyzer 2100 (Agilent RNA 6000 Pico Kit, Cat No 5067-1513, Agilent Technologies, Santa Clara, California); all tested samples had RNA integrity numbers of 9 and above.
2.6 Gene expression quantification
RNA samples (200 ng) were converted to cDNA in 20 μl reactions using a high-capacity cDNA reverse transcription kit with RNase inhibitor (Life Technologies Cat No 4374966) according to the manufacturer directions. Samples were held at 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 minutes on Applied Biosystems 2720 Thermocycler. Product was stored at −20°C for up to 10 months until use. Predesigned gene expression primer and probe assays were purchased from LifeTech to identify genes of interest (FAM/MGB-NFQ, Cat No 4351370) and reference genes (VIC/MGB-NFQ, Cat No 4448490), described in Table 1. Assays were prevalidated for duplexing to run a target and calibrating gene together, and reaction efficiency was confirmed in the lab to be within an acceptable range (90–110%). Taqman Gene expression master mix (Cat No 4370074) was used in a 20 μl reaction with 10 ng of cDNA, and each sample was run in triplicate. qPCR was carried out on an Applied Biosystems ViiA 7 (Software version 1.2.4) in the Gore Lab and with the following run parameters: 50°C for 2 minutes, 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Quantification cycle (Cq) was determined automatically by the software. Rpl13a and Gapdh were chosen as reference genes because of previous experience in our lab demonstrating that they are not significantly affected by similar PCB treatments. In the current experiments, reference Cqs differed less than 2% between groups. Relative expression was determined for each sample using the comparative Cq method: reference Cqs were subtracted from target Cqs to determine delta Cq within each sample well. Triplicate delta Cqs were averaged together, removing any technical outliers, and the median Delta Cq of the same sex Veh-Veh group was subtracted to determine fold change in expression for each individual.
Table 1. PCR Target and assay information.
Drd1 and Oprm1 assays target more than one transcript variant.
| Gene Symbol | Gene name | Brain Regions assayed | Life Technologies Assay ID | Amplicon Length | Probe Location | Accession Number |
|---|---|---|---|---|---|---|
| Gapdh | Glyceraldehyde 3-phosphate dehydrogenase | MeA, NA | Rn01775763_g1 | 174 | Exon 8 | NM_017008.4 |
| Rpl13a | ribosomal protein L13a | BNST, LS, PFC, POA, PVN, VMH | Rn00821946_g1 | 66 | Exons 4–5 | NM_173340.2 |
| Ar | androgen receptor | LS, MeA, POA | Rn00560747_m1 | 75 | Exons 3–4 | NM_012502.1 |
| Crh | corticotropin releasing hormone | BNST, PVN | Rn01462137_m1 | 112 | Exons 1–2 | NM_031019.1 |
| Esr1 | estrogen receptor alpha | BNST, LS, MeA, NA, POA, PVN, VMH | Rn01640372_m1 | 67 | Exons 6–7 | NM_012689.1 |
| Avp | vasopressin | BNST, MeA, PVN | Rn00690189_g1 | 78 | Exons 2–3 | NM_016992.2 |
| Avpr1a | vasopressin receptor 1a | LS | Rn00583910_m1 | 65 | Exons 1–2 | NM_053019.2 |
| Oxt | oxytocin | PVN | Rn00564446_g1 | 78 | Exons 2–3 | NM_012996.3 |
| Oxtr | oxytocin receptor | MeA, PFC, POA | Rn00563503_m1 | 60 | Exons 1–2 | NM_012871.2 |
| Drd1 | dopamine receptor D1 | NA | Rn03062203_s1 | 83 | Exon 2 | XM_006253599.2, XM_006253600.2 |
| Drd2 | dopamine receptor D2 | NA | Rn00561126_m1 | 64 | Exons 2–3 | NM_012547.1 |
| Oprm1 | mu opioid receptor | MeA, NA, PFC, POA, VMH | Rn01430371_m1 | 64 | Exons 2–3 | NM_001038597.2, NM_001038599.2, NM_001038600.2, NM_001038601.2, NM_013071.2 |
2.7 DNA methylation quantification
Because expression of the mu opioid receptor (Oprm1) was affected by PCBs in the prefrontal cortex (PFC) and preoptic area (POA), and expression of androgen receptor (Ar) was affected in the POA, these targets were selected for follow-up of DNA methylation of cytosine-guanine dinucleotides (CpG) sites within 250 bases of the transcription start sites (TSS) of each gene. 200 ng of DNA in 45 μl water from the PFC and POA of each rat was shipped to EpigenDx (Worcester, MA) for bisulfite conversion and pyrosequencing of Oprm1 (3 CpG sites, −241 to −209 bp from the TSS), and Ar (8 CpG sites, −70 to +39 bp from the TSS) regulatory regions. CpG sites were analyzed by pyrosequencing for percentage of methylation.
2.8 Analysis and statistics
When tested as a covariate, no effects of litter were detected for any of the significant effects, so individual rats were used as the unit of analysis for statistical purposes. Any outliers were identified via Grubbs test and were removed (maximum of one per group unless notes taken while performing the experiment indicated a technical reason for exclusion, e.g., poor dissection or errors in isolation). Individual hormonal, gene expression, and methylation measures were analyzed using a 2 × 2 analysis of variance (ANOVA) within each sex to determine any main effects of prenatal or juvenile treatment and interactions, with appropriate follow-up t-tests to identify the source of detected interactions. If measures failed to meet normality or homogeneity assumptions (as indicated by Shapiro Wilks and Levene’s tests), a non-parametric Kruskal-Wallis test was used, indicated by KW. In this case, an interaction was identified by testing for effects of one treatment while holding the other constant and vice versa. Prior to tissue collection, the sociosexual behavior of these same animals was assessed (Bell et al 2015). PCB exposure affected the production of ultrasonic vocalizations after being placed in a novel testing apparatus prior to the introduction of a stimulus animal and the time spent with a hormone- or a no-hormone-treated opposite sex stimulus animal in a three chamber test of sociosexual partner preference in adult male animals. Pearson correlations were used to determine relationships between hormone and gene expression outcomes and 1) significant behavioral changes and/or 2) related methylation measures. All endpoints were selected for analysis according to a priori hypotheses and, accordingly, significance levels were not adjusted. Analysis was completed using SPSS (Version 18, IBM), with significance defined as *p < 0.05 and **p < 0.01.
3. Results
3.1 Hormones
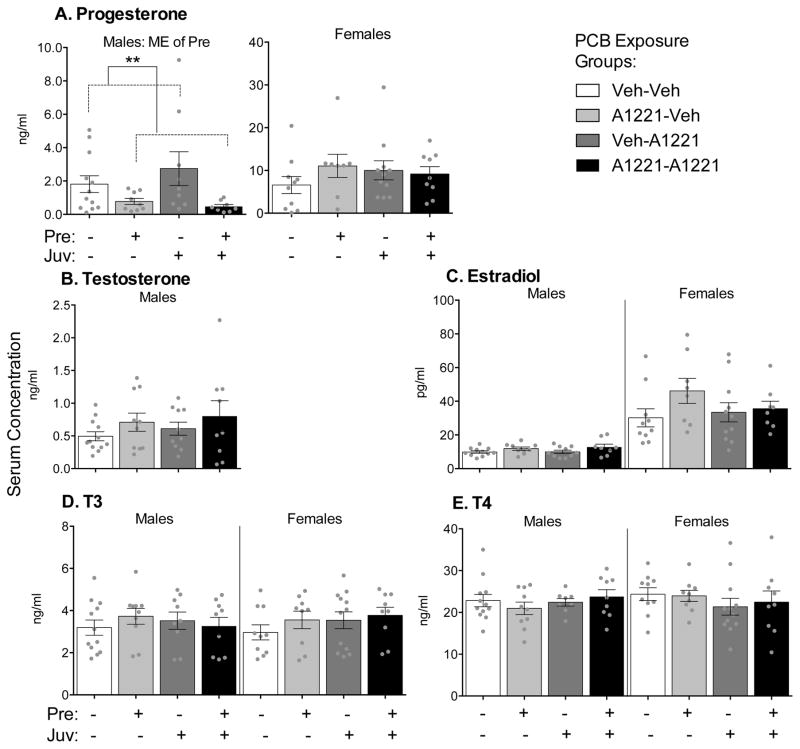
Serum hormone concentrations are shown in Figure 2. P4 and E2 concentrations were much higher in females than males, as expected (Fig 2). The only main effect of prenatal PCB exposure was detected for circulating P4 concentrations, and was limited to males (Fig 2A, KW, p = 0.007). Specifically, males exposed to PCBs during prenatal development had lower serum P4 concentrations than males exposed to vehicle at that time.
Figure 2.
Serum concentrations of progesterone (A), testosterone (B), estradiol (C), T3 (D), and T4 (E) are shown as mean ± SEM, with dots indicating data points from individual rats. Note different y-axis scales between graphs. Within-sex main effects (ME) of prenatal or juvenile exposure, or interactions (Pre x Juv) between the two, are described in each subtitle, with specific group differences indicated by **p < 0.01.
3.2 Gene Expression
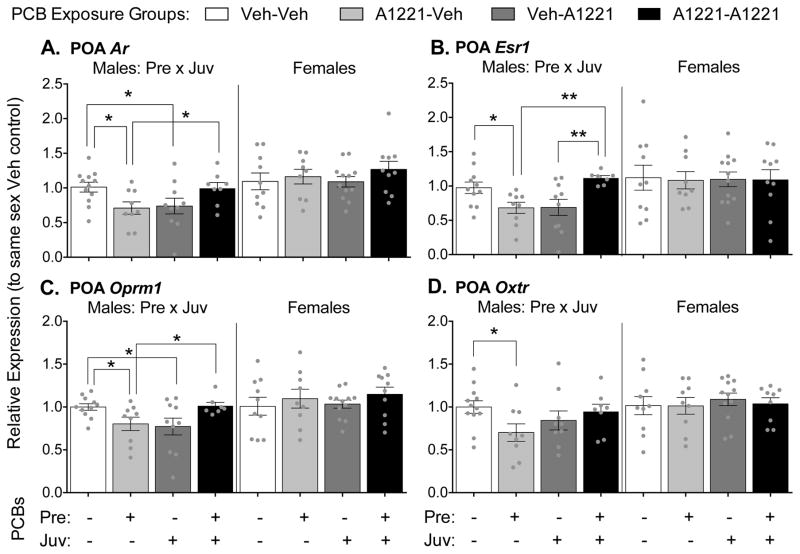
The greatest number of PCB effects on gene expression was found in the preoptic area (POA), and exclusively in males (Figure 3). An interaction between prenatal and juvenile exposure was found in male rats for expression of androgen receptor (Ar, Fig 3A, F(1,35) = 9.48, p = 0.004), estrogen receptor alpha (Esr1, Fig 3B, KW, p < 0.001), mu opioid receptor (Oprm1, Fig 3C, F(1,33) = 9.26, p = 0.005), and oxytocin receptor (Oxtr, Fig 3D, F(1,34) = 4.61, p = 0.039). The interactions can be explained by multiple comparisons where the effect of exposure at one age depended on the exposure status at the other age. Prenatal exposure decreased expression of Ar (F(1,19) = 7.35, p = 0.014), Esr1 (F(1,18) = 6.19, p = 0.023), Oprm1 (F(1,18) = 5.84, p = 0.026), and Oxtr (F(1,19) = 6.00, p = 0.024) only in animals that received the vehicle as juveniles. Juvenile exposure to PCBs also reduced expression of Ar (F(1,20) = 4.54, p = 0.046) and Oprm1 (F(1,19) = 4.98, p = 0.038) only in animals unexposed prenatally. In contrast to effects found in animals exposed at only one developmental period, prenatal exposure increased expression of Esr1 (F(1,15) = 8.74, p = 0.010) in juvenile-exposed animals, and juvenile exposure increased expression of Ar (F(1,15) = 5.45, p = 0.034), Esr1 (F(1,14) = 20.01, p = 0.001), and Oprm1 (F(1,14) = 4.86, p = 0.045) in prenatally exposed animals. Thus, two developmental hits affected gene expression in the opposite direction from one developmental hit.
Figure 3.
Gene expression levels in the preoptic area (POA) are shown. Levels of Ar (A), Esr1 (B), Oprm1 (C) and Oxtr (D) are shown as mean ± SEM, with dots indicating individual data points. Note different y-axis scales between graphs. Within-sex main effects (ME) of prenatal or juvenile exposure, or interactions (Pre x Juv) between the two, are described in each subtitle, with specific group differences indicated by * p < 0.05, ** p < 0.01.
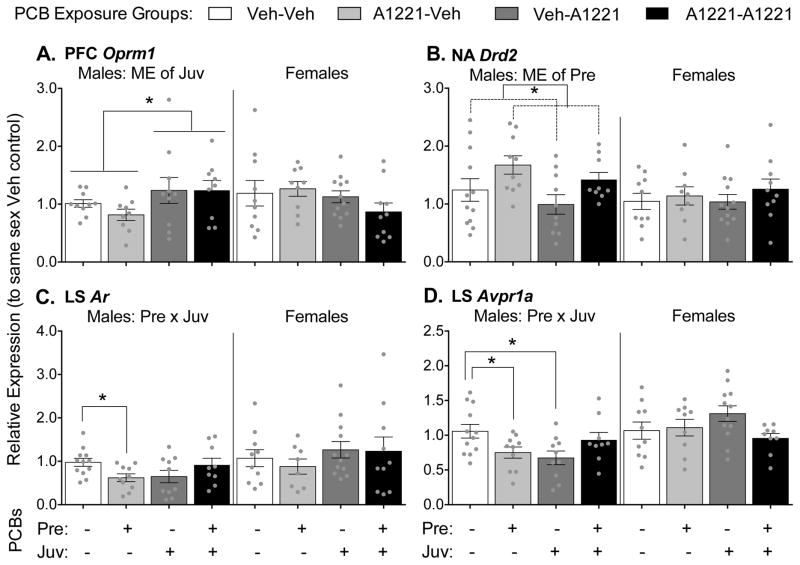
Significant effects of PCBs on gene expression were also found in the prefrontal cortex (PFC, Oprm1), nucleus accumbens (NA, Drd2), and lateral septum (LS, Ar and Avpr1a), again only in male rats (Figure 4). In the PFC (Fig 4A), a main effect of juvenile exposure was found to increase expression of Oprm1 in the PCB-exposed rats (F(1,34) = 4.44, p = 0.042). In the NA (Fig 4B), a main effect of prenatal exposure was found to increase expression of dopamine receptor D2 (Drd2) in the PCB-exposed males (F(1,37) = 6.35, p = 0.016). In the LS (Fig 4C and 4D), a Pre x Juv interaction was found for Ar (F(1,37) = 6.829, p = 0.013) and vasopressin receptor 1a (Avpr1a, F(1,37) = 8.40, p = 0.006). Follow-up tests revealed that the effect of prenatal exposure was present only if animals were unexposed in juvenile development (Ar: F(1,20) = 7.48, p = 0.013; Avpr1a: F(1,20) = 5.47, p = 0.030). An effect of juvenile exposure on Avpr1a expression in the LS was also found, but only if animals were unexposed in prenatal development (F(1,20) = 7.44, p = 0.013). No effects of treatment were found for any genes measured in the bed nucleus of the stria terminalis (BNST), medial amygdala (MeA), paraventricular nucleus (PVN), or ventromedial hypothalamus (VMH) as summarized in Table 2 and presented in Supplemental Table 1.
Figure 4.
Gene expression levels in the prefrontal cortex (PFC; A), nucleus accumbens (NA; B), and lateral septum (LS; C and D) are shown. Levels of Oprm1 (A), Drd2 (B), Ar (C) and Avpr1a (D) are shown as mean ± SEM, with dots indicating individual data points. Note different y-axis scales between graphs. Within-sex main effects (ME) of prenatal or juvenile exposure, or interactions (Pre x Juv) between the two, are described in each subtitle, with specific group differences indicated by * p < 0.05.
Table 2. Within-animal Pearson correlations between male genes, hormones and behaviors.
The four behaviors that were significantly affected by PCBs in adulthood (all in males, Bell et al 2015) were correlated with gene expression and hormone concentrations from the same animals. These behaviors were the production of ultrasonic vocalizations (USVs) prior to interacting with a receptive female stimulus animal, and time spent near a hormone- or no-hormone treated female and preference for the hormone-treated female (time with hormone-treated female/time with both females) in sociosexual partner preference test. Effects of PCBs to increase (
 ), decrease (
), decrease (
 ), or interact (Pre x Juv) are shown. Significant correlations between measures that were affected by PCB exposure are highlighted.
), or interact (Pre x Juv) are shown. Significant correlations between measures that were affected by PCB exposure are highlighted.
| USVs prior to female | Time near no-hormone female | Time near both females | Preference for hormone female | ||
|---|---|---|---|---|---|
|
|
|||||
| PCB Effect | Pre

|
Pre
 , Juv , Juv

|
Juv

|
Pre

|
|
| BNST Avp | −0.425 ** | −0.202 | −0.139 | 0.158 | |
| BNST Crh | −0.039 | 0.218 | 0.298 | −0.010 | |
| BNST Esr1 | −0.272 | 0.239 | 0.094 | −0.157 | |
| LS Ar | Pre x Juv | −0.050 | 0.028 | 0.068 | −0.082 |
| LS Esr1 | 0.250 | 0.051 | 0.112 | 0.019 | |
| LS Avpr1a | Pre x Juv | −0.086 | 0.059 | 0.174 | −0.095 |
| MeA Ar | −0.011 | 0.314 | 0.511 ** | −0.021 | |
| MeA Avp | −0.137 | −0.024 | −0.043 | −0.031 | |
| MeA Esr1 | 0.072 | 0.354 * | 0.427 ** | −0.131 | |
| MeA Oprm1 | −0.202 | 0.336 * | 0.287 | −0.238 | |
| MeA Oxtr | 0.000 | 0.102 | 0.275 | 0.029 | |
| NA Drd1 | 0.178 | −0.062 | 0.003 | −0.111 | |
| NA Drd2 | Pre

|
0.338 * | −0.243 | −0.293 | 0.079 |
| NA Esr1 | −0.285 | 0.195 | 0.220 | 0.004 | |
| NA Oprm1 | −0.350 * | −0.113 | −0.016 | 0.180 | |
| PFC Oprm1 | Juv

|
−0.116 | 0.546 ** | 0.346 * | −0.341 * |
| PFC Oxtr | −0.229 | 0.017 | −0.056 | −0.035 | |
| POA Ar | Pre x Juv | −0.284 | −0.057 | −0.062 | −0.022 |
| POA Esr1 | Pre x Juv | −0.188 | −0.101 | 0.003 | 0.055 |
| POA Oprm1 | Pre x Juv | −0.198 | −0.079 | 0.067 | 0.013 |
| POA Oxtr | Pre x Juv | −0.302 | 0.118 | 0.031 | −0.127 |
| PVN Avp | −0.312 | 0.053 | 0.011 | −0.061 | |
| PVN Crh | −0.131 | 0.030 | 0.027 | −0.083 | |
| PVN Esr1 | 0.177 | −0.032 | 0.031 | 0.113 | |
| PVN Oxt | −0.151 | −0.084 | −0.035 | 0.104 | |
| VMH Oprm1 | −0.148 | 0.261 | 0.072 | −0.242 | |
| VMH Esr1 | −0.187 | −0.140 | −0.067 | 0.147 | |
| P | Pre

|
−0.043 | 0.345 * | 0.296 | −0.215 |
| T3 | 0.201 | −0.212 | −0.190 | 0.100 | |
| T4 | −0.008 | 0.107 | 0.134 | −0.077 | |
| T | 0.259 | 0.111 | −0.100 | −0.307 | |
p < 0.01,
p < 0.05
3.4 Gene-Behavior Correlations
In a companion study, we reported the behavioral phenotype of these same rats, and showed that two types of behaviors were significantly affected in the adult male, but not female, rats (Bell et al., 2015). These behaviors included 1) the production of USVs when placed into a novel testing apparatus prior to an interaction with a stimulus animal and 2) the preference for spending time near a hormone- or no-hormone-treated opposite sex stimulus animals. To relate whether the molecular changes described above could be responsible for PCB-induced changes in adult male behavior in the same animals, we conducted Pearson’s correlations between expression of genes and hormone concentrations with significantly affected behaviors in males (Table 2). Results showed that the number of flat USVs was positively correlated with Drd2 expression in the NA (r = 0.338, p = 0.031), both of which were increased by prenatal PCB exposure. Numbers of pre-stimulus USVs were negatively correlated with Avp expression in the BNST (r = −0.425, p = 0.006), and Oprm1 in the NA (r = −0.350, p = 0.027), but these were not affected by PCBs. Time spent near the no-hormone stimulus animal (no-hormone stimulus time) in the sociosexual preference test was positively correlated with Oprm1 in the PFC (r = 0.546, p < 0.001), both of which were increased by juvenile PCB exposure. No-hormone stimulus time was also positively correlated with serum P concentrations (r = 0.345, p = 0.027), both of which were decreased by prenatal PCB exposure, independent of juvenile effects. No-hormone stimulus time was also positively correlated with MeA Esr1 (r = 0.354, p = 0.025) and Oprm1 (r = 0.336, p = 0.048), but these genes were not affected by PCBs. The significant increase in time spent with no-hormone stimulus animal, paired with a non-significant increase in time with the hormone stimulus animal, resulted in an overall increase in time spent with both stimulus animals in animals exposed to PCBs as juveniles. As with no-hormone stimulus time, this measure was positively correlated with Oprm1 expression in the PFC (r = 0.346, p = 0.033), which was also increased by juvenile PCB exposure. The behavior was also positively correlated with MeA Ar (r = 0.511, p = 0.001) and MeA Esr1 (r = 0.427, p = 0.006), but these gene targets were not affected by PCBs. Finally, the preference for the hormone stimulus animal over either stimulus animal was negatively correlated with Oprm1 in the PFC (r = −0.341, p = 0.036).
3.5 DNA Methylation
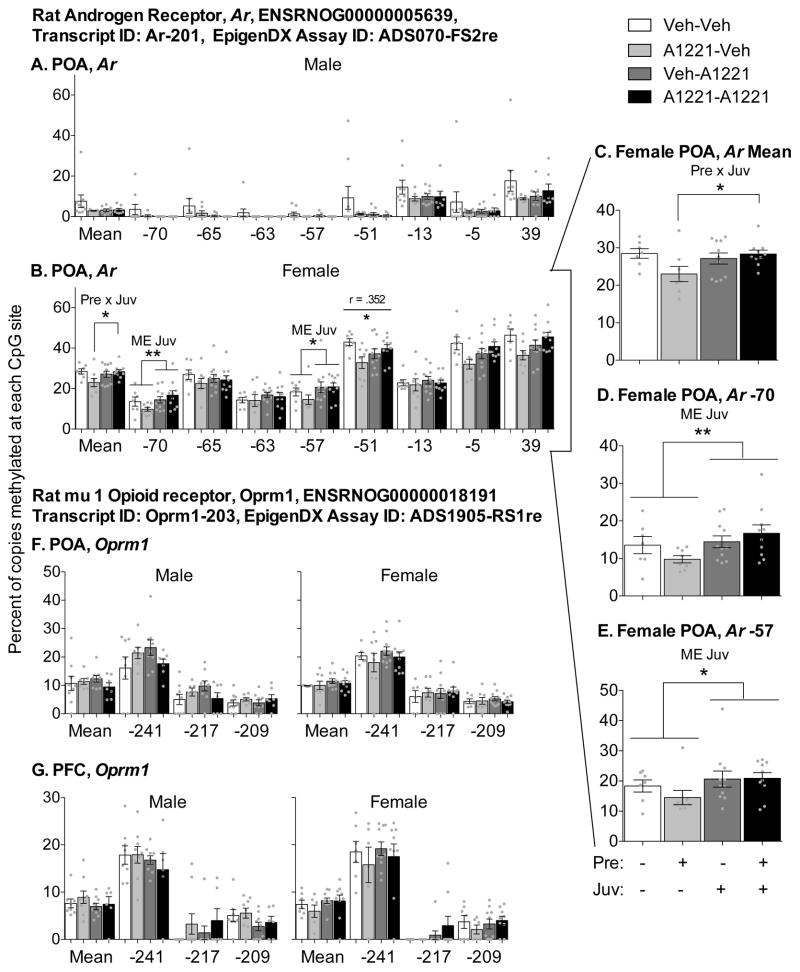
Because PCBs affected expression of Ar and Oprm1 in the POA and Oprm1 in the PFC of male rats, and the expression of these genes are modulated by DNA methylation (Vucetic et al 2011, Nielsen et al 2009, Hao et al 2011, Keil et al 2014), epigenetic analyses were performed on these targets. We decided to conduct work in both sexes as the Ar is on the X-chromosome and should have much higher methylation in females than males (Walker et al., 2014). As expected, % methylation of Ar in the POA was much higher in females than males (Figure 5). Effects of PCBs were found in female (Figure 5B) but not male (Figure 5A) animals. At two of seven sites (Figure 5B, D, E), a main effect of juvenile exposure was found: −70 (F(1,31) = 8.13, p = 0.008) and −57 (F(1,33) = 5.88, p = 0.021). However, this main effect was qualified, as it appears to be driven by low levels of methylation in the A1221-Veh group only. This general pattern was found across several sites, and was detected as a significant Pre x Juv interaction in the average percent methylation combined for the seven sites (Figure 5B, C; F(1,32) = 5.08, p = 0.031). Follow-up tests revealed that juvenile exposure increased methylation levels, but only in prenatally exposed animals, F(1,16) = 5.78, p = 0.029. Methylation at −51 was positively, albeit weakly, correlated with expression of Ar mRNA in female POAs (r = 0.352, p = 0.033), but not males. No significant effects of PCBs were found on Oprm1 CpG sites −241, −217, and −209 in the POA or PFC in either sex (Figure 5F and G), and methylation patterns at these sites were not correlated with gene expression.
Figure 5.
DNA methylation (%) averaged across all (Mean) or at individual CpG sites in the promoter region of Ar (A–E) and Oprm1 (F–G) is shown in the POA (A–F) and PFC (G). Insert graphs (C–E) show sites within Ar in female POA where significant effects of treatment were found. CpG locations are given relative to the transcriptional state site (TSS), which is −183 and −995 upstream from ATG in Ar and Oprm1, respectively. Bars show means ± SEM, and dots show individual data points. Note different y-axis scales. Within-sex main effects (ME) of prenatal or juvenile exposure, or interactions (Pre x Juv) between the two, are described in each subtitle, with specific group differences indicated by * p < 0.05 and ** p < 0.01. R values and significance (* p < 0.05 and ** p < 0.01) for correlation with gene expression results across all animals are shown by a horizontal line above the CpG site.
4. Discussion
The results of the current study provide novel information about how two periods of PCB exposure interact to affect the neuromolecular phenotype. As with the performance of social and anxiety-like behaviors in a companion study, the effects of prenatal and juvenile PCB exposure on gene expression in the brain were relatively small and sex-dependent. In adulthood, males but not females showed effects of PCB exposure on brain and behavior. As hypothesized, the majority of effects were due to an interaction between the two hits: juvenile exposure masked or revealed effects of prenatal exposure depending upon the endpoint measured. In some cases, similar effects of prenatal and juvenile exposures were observed, suggesting that the juvenile period represents an extension of the developmental processes initiated during prenatal period. Finally, the POA is a key part of the neural network involved in sexual behaviors in male rats (Hull and Dominguez, 2007; Sakuma, 2008) and was particularly responsive to PCBs. These findings are consistent with previous effects of PCBs, known hormone sensitivity, and the fact that these same P90–100 males had sociosexual behavioral changes in adulthood (Gore et al., 2011; Gorski et al., 1980; Bell et al., 2015). The lack of effects of PCBs on POA gene expression (here) or sexual behaviors (Bell et al., 2015) in adult females is consistent with the postulated lesser role of the POA in feminine sexual behavior in rats (Sakuma, 2008; Veening et al., 2014).
4.1 Progesterone, but not other hormones, was affected by A1221
Of the 5 serum hormones measured, only P4 showed an effect of treatment: concentrations of P4 were decreased in males exposed to prenatal A1221 irrespective of the second hit. To our knowledge, this is the first such observation in male animals, as a previous study demonstrated prenatal A1221-induced reductions in P4 at P1 and P60 in female but not male rats (Dickerson et al., 2011a; 2011b). However, a different PCB mix (1mg/kg of PCB #138, 153, and 180, 1:1:1) increased serum P4 in P1 males (Dickerson et al., 2011a). Progesterone treatment for three days prior to testing impaired social recognition in adult male rats, at least in part by reducing vasopressin expression in the BNST, MeA and LS (Bychowski et al., 2013; Bychowski and Auger, 2012). However, the decrease in P4 in the current study was not accompanied by a change in Avp expression, nor was there evidence of better social recognition memory in male animals when tested as juveniles (Bell et al., 2015). Progesterone is also involved in male sociosexual behavior, as PR knockout mice exhibited reduced mount latency (Schneider et al., 2005). Therefore it is possible that reduced circulating P4 levels are related to the moderate and partial increases in sociosexual preferences observed in adult animals in the sister study (Bell et al., 2015). Indeed, P4 and no-hormone stimulus time were positively correlated within animals. The lack of change in serum concentrations of E2 or T is in agreement with some studies (Dickerson et al., 2011b; Steinberg et al., 2008; Walker et al., 2014). Other studies have shown that more highly chlorinated PCB congeners tended to reduce E2 and T concentrations (Hany et al., 1999; Kaya et al., 2002; Muto et al., 2003; Yamamoto et al., 2005). Similarly, A1221 had no effect on T3 or T4 in the current study, but more highly chlorinated congeners are well known to decrease T3 and T4 (Giera et al., 2011; Khan et al., 2002; Sauer et al., 1994; Ulbrich and Stahlmann, 2004).
4.2 Reward-related genes were sensitive to A1221 in male rats
While several genes involved in the regulation of sexually dimorphic social behaviors were assayed, the opioid and dopaminergic genes in reward-related neural circuits were among the most affected in the current study. We demonstrated that Oprm1 expression in the POA was reduced by either prenatal or juvenile PCB exposure. Mu opioid receptor agonists delivered centrally and directly to the POA inhibited sexual behavior and blocked the normal preference to interact with a receptive over a non-receptive female in male rats (Hughes et al., 1990; Parra-Gámez et al., 2009). Therefore, a reduction in Oprm1 expression in the POA could promote sociosexual interests and be a mechanism for the increased preference for hormone-treated stimulus animals observed in adult male rats treated with PCBs prenatally in our companion paper (Bell et al., 2015). However, this possibility is not supported by correlation analysis showing that the two measures were not related within animals. An increase in time spent performing general social investigation in males treated with PCBs during juvenile development was also observed in that study (Bell et al., 2015). However, this cannot be explained by a parallel reduction in POA Oprm1 in juvenile-treated males, as central mu opioid receptor agonists have been shown to increase non-sexual affiliative behavior in male rats (Meyerson, 1981).
In addition to effects in the POA, Oprm1 expression in the PFC was increased by juvenile exposure to PCBs. This effect was not observed in juvenile mice exposed to a mix of six moderately chlorinated and non-dioxin like PCBs during juvenile development (P0-P21) (Elnar et al., 2012), which could be the result of the different congeners used, species tested, or age at analysis. The different directionality of effects between PFC and POA, and the lack of effect on Oprm1 expression in the NA, indicates site-specific effects of PCBs that may depend on specific cell types or coexpression of other PCB-responsive receptors or transcription factors. While no studies, to our knowledge, have specifically demonstrated the role of mu opioid receptor in the PFC in the regulation of social behavior, there is partial evidence suggesting involvement. Opioid receptor stimulation in the PFC is associated with other reward seeking behaviors such as drug and food consumption and craving (Colasanti et al., 2012; Gorelick et al., 2005; Mena et al., 2011; Mitchell et al., 2012) and, in the NA, mu opioid receptors mediated social reward (Trezza et al., 2011). The mPFC is also important in social play (Bell et al 2009), and social isolation affected expression of opioid receptors in the PFC (Vanderschuren et al 1995). Time spent near a no-hormone stimulus animal, total social time (Bell et al 2015), and PFC Oprm1 expression were all increased by juvenile PCB exposure and positively correlated. Therefore, PFC Oprm1 changes could be a mechanism behind the pro-social behavioral effects.
Of the two dopamine receptors assayed, Drd2 but not Drd1 was affected by PCBs. More specifically Drd2 expression was upregulated in the NA by prenatal A1221 in male animals. Noncoplanar PCBs, including PCB4, a component of A1221, cause long-term decreases in dopamine levels in the striatum (Choksi et al., 1997; Seegal et al., 1997; 1994; 1990; Shain et al., 1991). While not measured in the current study, it is possible that DA levels were reduced and resulted in a compensatory upregulation of Drd2 expression. Indeed, 3 mg/kg Fox River PCB mix throughout gestation and weaning increased D2 autoreceptor sensitivity (Fielding et al., 2013). These effects may be specific to the PCB mixture or to the brain region, as A1254 during juvenile development did not affect D1 or D2 receptor binding in the dorsal striatum of female mice (Tian et al., 2011). Dopamine action in the NA is consistently linked to play and USV production in juvenile animals (Vanderschuren et al., 1997); however, no effects of PCBs were observed in these behaviors in male animals in our companion paper (Bell et al., 2015). In addition, D2 receptor agonist action in the NA caused an increase in the production of USVs in adult male rats alone in a novel recording chamber (Brudzynski et al., 2012). Therefore, the increased Drd2 expression in response to prenatal PCBs in the current study could be a mechanism behind the parallel increase in flat USV production in prenatal PCB-exposed adult males in our other study (Bell et al., 2015). Indeed, these measures were positively correlated within animals. Given the importance of corticolimbic opioid and dopaminergic action in substance abuse, these findings may also be a mechanism by which PCBs increased sensitivity to cocaine and amphetamine (Fielding et al., 2013; Poon et al., 2013).
4.3 Steroid hormone receptor expression was affected by A1221 in male but not female rats
In the current study, Ar gene expression was decreased in the POA and LS by prenatal exposure, and in the POA by juvenile exposure. In the MeA, Ar expression was unaffected. The sensitivity of Ar expression to A1221 agrees with previous findings using a similar exposure regime; however there are some age, sex, and region-specific effects. While A1221 increased AR expression in the anteroventral periventricular (AVPV) region of the hypothalamus in P90 female rats (Walker et al., 2014), it reduced Ar mRNA in the POA of P60 females (Dickerson et al., 2011b) and the arcuate nucleus (ARC) of aged female (Walker et al., 2013) and P90 male rats (Walker et al., 2014). Protein levels of AR were also reduced in embryonic female rat hypothalamus in response to gestational treatment with another PCB mixture, A1254 (Colciago et al., 2006). It is not clear why effects in females were not detected in the current study.
Esr1 was affected in the POA but not in the other six brain regions in which it was assayed. In the POA, either prenatal or juvenile A1221 exposure reduced expression in males, but not females. The literature shows mixed effects of PCBs on Esr1 expression: prenatal A1221 reduced ERα cell number in the adult female AVPV while having no effect on Esr1 expression in the adult female POA (Dickerson et al., 2011b), or adult male or female AVPV or ARC (Walker et al., 2014). Mixed effects were also found in aged female animals, with A1221 increasing expression in the ARC and decreasing expression in the median eminence (Walker et al., 2013). While, human studies show both estrogenic and anti-estrogenic effects of PCBs (Bonefeld-Jørgensen et al., 2001; Tavolari et al., 2006) no relationship was detected between several classifications of PCBs and Esr1 expression itself (Warner et al., 2012). Therefore, the current finding is the first evidence for effects of A1221 on Esr1 expression in male animals, and requires replication.
4.4 The effects of A1221 on social nonapeptides, vasopressin and oxytocin, were limited
In the current study, Avp mRNA was not affected in the BNST, PVN or MeA. This is the first study to assess PCB effects on gene expression of vasopressin; only one other study tested for effects of PCBs on vasopressin action, where dehydration-induced vasopressin release from the supraoptic nucleus is blunted in rats treated with high doses of A1254 for 15 days in adulthood (Coburn et al., 2007). In contrast to Avp, expression of Avpr1a was reduced by either prenatal or juvenile exposure to A1221 in the LS. In the LS, the vasopressin receptor 1a is important for social play in juvenile rats (Veenema et al., 2013) and social recognition memory (Ferguson et al., 2002); however, female, but not male, juvenile animals showed changes in play behavior and no changes in social memory in our sister paper, and therefore the functional implications of these Avpr1a effects in males is unknown.
Expression of the oxytocin receptor, Oxtr, was reduced by prenatal exposure to A1221 in the male POA. This is in agreement with findings from a similar exposure paradigm in adult male ARC (Walker et al., 2014), and was region-specific; no changes in Oxtr expression were seen in the PFC or MeA. Oxt was also not affected by A1221 in the PVN. In a similar studies, prenatal A1221 increased Oxt expression in P90 male and female AVPV (Walker et al., 2014) and aged female ARC (Walker et al., 2013), suggesting regional specificity of A1221 effects within the brain. Oxytocin facilitates sexual behavior, with actions in the PVN, VMH and POA (Argiolas and Melis, 2013). However, PCBs tended to increase sociosexual preferences and general social interest in adult male animals, so, like the Avpr1a effects, the behavioral relevance of the Oxtr change is undetermined.
4.5 DNA methylation was not a major mechanism of PCB-induced change in gene expression
Hormones and early life influences affect long-term brain development by modifying DNA methylation (Champagne et al., 2006; Hao et al., 2011; Vucetic et al., 2010). Early life PCB exposure is also known to reduce global DNA methylation and expression of a DNA methyltransferase (Desaulniers et al., 2009) and histone demethylase (Casati et al., 2012) in liver. Therefore, in order to determine whether the mRNA changes in Ar and Oprm1 were due to epigenetic programming caused by the A1221 exposure, we measured DNA methylation in regulatory regions of these genes.
Our prediction was not substantiated, as juvenile PCB exposure significantly increased methylation in only two of seven CpG sites on the Ar in female POA and there were no effects in males. When all sites were averaged together, an interaction was detected, such that juvenile PCB exposure increased methylation only when females were also exposed prenatally. These effects were distinct from those observed in a similar study, in which prenatal PCBs increased methylation at one different site in males and not females (Walker et al., 2014). DNA methylation of CpG sites is typically thought to reduce accessibility to transcriptional factors and ultimately decrease mRNA expressed (Razin, 1998), and this classic inverse relationship appears to true for Ar in vivo and in reproductive tissues (Jarrard et al., 1998; Keil et al., 2014; J. Tian et al., 2012). However, this might not be the case in heterogeneous neural cells. Indeed, methylation and gene expression were positively correlated at only one of seven sites in POA Ar. The same positive relationship at CpG site −51 was found in a similar study (Walker et al., 2014), suggesting that other epigenetic and non-epigenetic regulatory factors may be more important in regulating gene expression than methylation at these specific sites.
No effects of PCBs were seen on methylation patterns in Oprm1 in the PFC or POA. Oprm1 is epigenetically regulated via DNA methylation in a variety of in vivo models (Andria and Simon, 1999; Hwang et al., 2007), and its expression is inversely correlated with methylation status in other studies (Chorbov et al., 2011; Doehring et al., 2013; Nielsen et al., 2009; Vucetic et al., 2010). Moreover, dietary methyl donor supplementation reverses the effects of high fat diet on PFC Oprm1 expression (Carlin et al., 2013). Therefore, the lack of correlation between methylation levels and gene expression in any of the 3 CpG sites in Oprm1 was surprising. This may be explained by the CpG sites assayed, which were chosen based on the quality of the assay and are different from those in the other published studies.
5. Conclusion
This study demonstrates that neural gene expression was affected by discrete exposures to PCBs during prenatal or juvenile development. Moreover, the two exposure periods interacted to either mask or reveal effects of exposure at one developmental period. These effects were modest and occurred in male but not female animals, in parallel with the behavioral changes observed in adulthood in the same male animals (Bell et al., 2015). Specifically, changes in NA Drd2, PFC Oprm1, and serum P4 might moderate those behavioral effects. We demonstrated that the brain is differentially sensitive to PCBs during prenatal and juvenile periods of development depending on the age and previous exposure history. While our treatment windows occurred during periods of prenatal and postnatal life critical for gonadal hormone action, there may be other periods of development sensitive to long-lasting PCB effects that were not explored herein. Indeed, it is possible that gene expression in the brain remains sensitive to effects of PCBs throughout the lifespan, especially if PCBs are acting via non-hormonal mechanisms, a testable hypothesis. With that said, the effects of PCBs would likely differ depending on the regulatory processes that are occurring at that time, and is an interesting question for future study. Overall, these results demonstrate the need for toxicological testing to take sex, developmental stage, and lifetime exposure history into account when considering adverse outcomes of EDCs.
Supplementary Material
Highlights.
Prenatal and juvenile PCB exposures affect gene expression in adult rat brains.
The effect of a single exposure is often reversed by a second hit.
Males are more sensitive to long-term effects of PCBs than females.
Gene expression in the medial preoptic area was the most affected by PCBs.
Acknowledgments
The authors wish to thank Spurthi Tarugu, Ariel Dryden, Michael Reilly, Dr. Weiling Yin, Alex Garcia and Lindsay Thompson for their assistance with tissue collection and molecular work.
Grant support: NIH RO1 ES020662 (ACG), RO1 ES023254 (ACG), NIH T32 ES07247 (MRB), NIH F32 ES023291 (MRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polychlorinated Biphenyls. 2000. Section 6.5–6.7. [PubMed] [Google Scholar]
- Andrews WW, Mizejewski GJ, Ojeda SR. Development of estradiol-positive feedback on luteinizing hormone release in the female rat: a quantitative study. Endocrinology. 1981;109(5):1404–13. doi: 10.1210/endo-109-5-1404. [DOI] [PubMed] [Google Scholar]
- Andria ML, Simon EJ. Localization of promoter elements in the human mu-opioid receptor gene and regulation by DNA methylation. Brain Res Mol Brain Res. 1999;70:54–65. doi: 10.1016/s0169-328x(99)00126-6. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. Neuropeptides and central control of sexual behaviour from the past to the present: a review. Prog Neurobiol. 2013;108:80–107. doi: 10.1016/j.pneurobio.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Auger AP, Olesen KM. Brain sex differences and the organization of juvenile social play behavior. J Neuroendocrinol. 2009;21:519–525. doi: 10.1111/j.1365-2826.2009.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell HC, McCaffrey DR, Forgie ML, Kolb B, Pellis SM. The role of the medial prefrontal cortex in the play fighting of rats. Behavioral Neuroscience. 2009;123(6):1158–68. doi: 10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- Bell MR, Thompson LM, Rodriguez K, Gore AC. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Hormones and Behavior. 2015 doi: 10.1016/j.yhbeh.2015.11.007. publication pending. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bitran D. Treatment with an Anabolic-Androgenic Steroid Affects Anxiety-Related Behavior and Alters the Sensitivity of Cortical GABAA Receptors in the Rat. Horm Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158:141–153. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Bastien CH. Prenatal Exposure to Polychlorinated Biphenyls: A Neuropsychologic Analysis. Environ Health Perspect. 2009;117:7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM. Sexual dimorphism in the vertebrate nervous system. Journal of Neuroscience. 1992;12:4133–4142. doi: 10.1523/JNEUROSCI.12-11-04133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM, Komadoski M, St Pierre J. Quinpirole-induced 50 kHz ultrasonic vocalization in the rat: role of D2 and D3 dopamine receptors. Behav Brain Res. 2012;226:511–518. doi: 10.1016/j.bbr.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Maisog J, Kim S, Chen Z, Barr DB. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121:231–236. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CL, Vendruscolo LF, Koob GF, George O. Dopamine D1 and μ-opioid receptor antagonism blocks anticipatory 50 kHz ultrasonic vocalizations induced by palatable food cues in Wistar rats. Psychopharmacology (Berl) 2014;231:929–937. doi: 10.1007/s00213-013-3307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Bychowski ME, Auger CJ. Progesterone impairs social recognition in male rats. Horm Behav. 2012;61:598–604. doi: 10.1016/j.yhbeh.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychowski ME, Mena JD, Auger CJ. Vasopressin infusion into the lateral septum of adult male rats rescues progesterone-induced impairment in social recognition. Neuroscience. 2013;246:52–58. doi: 10.1016/j.neuroscience.2013.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin J, Hill-Smith TE, Lucki I, Reyes TM. Reversal of dopamine system dysfunction in response to high-fat diet. Obesity. 2013;21:2513–2521. doi: 10.1002/oby.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati L, Sendra R, Colciago A, Negri-Cesi P, Berdasco M, Esteller M, Celotti F. Polychlorinated biphenyls affect histone modification pattern in early development of rats: a role for androgen receptor-dependent modulation? Epigenomics. 2012;4:101–112. doi: 10.2217/epi.11.110. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Choksi NY, Kodavanti P, Tilson HA, Booth RG. Effects of polychlorinated biphenyls (PCBs) on brain tyrosine hydroxylase activity and dopamine synthesis in rats. Fundam Appl Toxicol. 1997;39:76–80. doi: 10.1006/faat.1997.2351. [DOI] [PubMed] [Google Scholar]
- Chorbov VM, Todorov AA, Lynskey MT, Cicero TJ. Elevated levels of DNA methylation at the OPRM1 promoter in blood and sperm from male opioid addicts. J Opioid Manag. 2011;7:258–264. doi: 10.5055/jom.2011.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn CG, Currás-Collazo MC, Kodavanti PRS. Polybrominated diphenyl ethers and ortho-substituted polychlorinated biphenyls as neuroendocrine disruptors of vasopressin release: Effects during physiological activation in vitro and structure-activity relationships. Toxicol Sci. 2007;98:178–186. doi: 10.1093/toxsci/kfm086. [DOI] [PubMed] [Google Scholar]
- Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D, Erritzoe D, Tziortzi AC, Reed LJ, Lingford-Hughes AR, Waldman AD, Schruers KRJ, Matthews PM, Gunn RN, Nutt DJ, Rabiner EA. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol Psychiatry. 2012;72:371–377. doi: 10.1016/j.biopsych.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Colciago A, Negri-Cesi P, Pravettoni A, Mornati O, Casati L, Celotti F. Prenatal Aroclor 1254 exposure and brain sexual differentiation: effect on the expression of testosterone metabolizing enzymes and androgen receptors in the hypothalamus of male and female rats. Reprod Toxicol. 2006;22:738–745. doi: 10.1016/j.reprotox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Davis JF, Loos M, Di Sebastiano AR, Brown JL, Lehman MN, Coolen LM. Lesions of the medial prefrontal cortex cause maladaptive sexual behavior in male rats. Biol Psychiatry. 2010;67:1199–1204. doi: 10.1016/j.biopsych.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaulniers D, Xiao G-H, Lian H, Feng Y-L, Zhu J, Nakai J, Bowers WJ. Effects of mixtures of polychlorinated biphenyls, methylmercury, and organochlorine pesticides on hepatic DNA methylation in prepubertal female Sprague-Dawley rats. Int J Toxicol. 2009;28:294–307. doi: 10.1177/1091581809337918. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol Appl Pharmacol. 2011a;252:36–46. doi: 10.1016/j.taap.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011b;152:581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehring A, Oertel BG, Sittl R, Lötsch J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain. 2013;154:15–23. doi: 10.1016/j.pain.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Elnar AA, Diesel B, Desor F, Feidt C, Bouayed J, Kiemer AK, Soulimani R. Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (6 NDL-PCBs) in mice. Toxicology. 2012;299:44–54. doi: 10.1016/j.tox.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wolff MS. Causal inference considerations for endocrine disruptor research in children’s health. Annu Rev Public Health. 2013;34:139–158. doi: 10.1146/annurev-publhealth-031811-124556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Fielding JR, Rogers TD, Meyer AE, Miller MM, Nelms JL, Mittleman G, Blaha CD, Sable HJK. Stimulation-Evoked Dopamine Release in the Nucleus Accumbens Following Cocaine Administration in Rats Perinatally Exposed to Polychlorinated Biphenyls. Toxicol Sci. 2013;136:144–153. doi: 10.1093/toxsci/kft171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual Polychlorinated Biphenyl (PCB) Congeners Produce Tissue- and Gene-Specific Effects on Thyroid Hormone Signaling during Development. Endocrinology. 2011;152:2909–2919. doi: 10.1210/en.2010-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res Bull. 2002;57:651–659. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25:2157–2168. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, Endres CJ, Dannals RF, Frost JJ. Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Presented at the Biological psychiatry. 2005:1573–1582. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Härer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G. Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol. 1999;158:231–243. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- Hao Y, Huang W, Nielsen DA, Kosten TA. Litter gender composition and sex affect maternal behavior and DNA methylation levels of the Oprm1 gene in rat offspring. Front Psychiatry. 2011;2:21. doi: 10.3389/fpsyt.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiol Behav. 2004;81:671–680. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Microlesions of the ventromedial nucleus of the hypothalamus: effects on sociosexual behaviors in male rats. Behav Neurosci. 2005;119:1227–1234. doi: 10.1037/0735-7044.119.5.1227. [DOI] [PubMed] [Google Scholar]
- Henley CL, Nunez AA, Clemens LG. Hormones of choice: the neuroendocrinology of partner preference in animals. Front Neuroendocrinol. 2011;32:146–154. doi: 10.1016/j.yfrne.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Hughes AM, Everitt BJ, Herbert J. Comparative effects of preoptic area infusions of opioid peptides, lesions and castration on sexual behaviour in male rats: studies of instrumental behaviour, conditioned place preference and partner preference. Psychopharmacology (Berl) 1990;102:243–256. doi: 10.1007/BF02245929. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CK, Song KY, Kim CS, Choi HS, Guo X-H, Law P-Y, Wei L-N, Loh HH. Evidence of endogenous mu opioid receptor regulation by epigenetic control of the promoters. Mol Cell Biol. 2007;27:4720–4736. doi: 10.1128/MCB.00073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen HT, Cooke PS, Porcelli J, Liu TC, Hansen LG. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod Toxicol. 1993;7:237–248. doi: 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- Jarrard DF, Kinoshita H, Shi Y, Sandefur C, Hoff D, Meisner LF, Chang C, Herman JG, Isaacs WB, Nassif N. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res. 1998;58:5310–5314. [PubMed] [Google Scholar]
- Jolous-Jamshidi B, Cromwell HC, McFarland AM, Meserve LA. Perinatal exposure to polychlorinated biphenyls alters social behaviors in rats. Toxicol Lett. 2010;199:136–143. doi: 10.1016/j.toxlet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmaus W, Huang S, Cameron L. Parental concentration of dichlorodiphenyl dichloroethene and polychlorinated biphenyls in Michigan fish eaters and sex ratio in offspring. J Occup Environ Med. 2002;44:8–13. doi: 10.1097/00043764-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Kaya H, Hany J, Fastabend A, Roth-Härer A, Winneke G, Lilienthal H. Effects of maternal exposure to a reconstituted mixture of polychlorinated biphenyls on sex-dependent behaviors and steroid hormone concentrations in rats: Dose-response relationship. Toxicol Appl Pharmacol. 2002;178:71–81. doi: 10.1006/taap.2001.9318. [DOI] [PubMed] [Google Scholar]
- Keil KP, Abler LL, Laporta J, Altmann HM, Yang B, Jarrard DF, Hernandez LL, Vezina CM. Androgen receptor DNA methylation regulates the timing and androgen sensitivity of mouse prostate ductal development. Dev Biol. 2014;396:237–245. doi: 10.1016/j.ydbio.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Lichtensteiger CA, Faroon O, Mumtaz M, Schaeffer DJ, Hansen LG. The hypothalamo-pituitary-thyroid (HPT) axis: a target of nonpersistent ortho-substituted PCB congeners. Toxicol Sci. 2002;65:52–61. doi: 10.1093/toxsci/65.1.52. [DOI] [PubMed] [Google Scholar]
- Lackmann GM. Polychlorinated biphenyls and hexachlorobenzene in full-term neonates. Reference values updated. Biol Neonate. 2002;81:82–85. doi: 10.1159/000047188. [DOI] [PubMed] [Google Scholar]
- Law DCG, Klebanoff MA, Brock JW, Dunson DB, Longnecker MP. Maternal serum levels of polychlorinated biphenyls and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and time to pregnancy. Am J Epidemiol. 2005;162:523–532. doi: 10.1093/aje/kwi240. [DOI] [PubMed] [Google Scholar]
- Layton AC, Sanseverino J, Gregory BW, Easter JP, Sayler GS, Schultz TW. In vitro estrogen receptor binding of PCBs: measured activity and detection of hydroxylated metabolites in a recombinant yeast assay. Toxicol Appl Pharmacol. 2002;180:157–163. doi: 10.1006/taap.2002.9395. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Brock JW, Guo X. Maternal levels of polychlorinated biphenyls in relation to preterm and small-for-gestational-age birth. Epidemiology. 2005;16:641–647. doi: 10.1097/01.ede.0000172137.45662.85. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Barfield RJ, Nyby J. Regulation of sociosexual communication in female Long-Evans rats by ovarian hormones. Horm Behav. 1992;26:545–555. doi: 10.1016/0018-506x(92)90021-m. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Anderson MW. Effect of chlorination on the distribution and excretion of polychlorinated biphenyls. Drug Metabolism and Disposition. 1975;3:371–380. [PubMed] [Google Scholar]
- McCarthy MM, Wright CL, Schwarz JM. New tricks by an old dogma: mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 2011;31:3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson BJ. Comparison of the effects of beta-endorphin and morphine on exploratory and socio-sexual behaviour in the male rat. Eur J Pharmacol. 1981;69:453–463. doi: 10.1016/0014-2999(81)90449-0. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4:116ra6. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Díaz-Véliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Muto T, Imano N, Nakaaki K, Takahashi H, Hano H, Wakui S, Furusato M. Estrous cyclicity and ovarian follicles in female rats after prenatal exposure to 3,3′,4,4′,5-pentachlorobiphenyl. Toxicol Lett. 2003;143:271–277. doi: 10.1016/s0378-4274(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Newman SW, Parfitt DB, Kollack-Walker S. Mating-induced c-fos expression patterns complement and supplement observations after lesions in the male Syrian hamster brain. Ann N Y Acad Sci. 1997;807:239–259. doi: 10.1111/j.1749-6632.1997.tb51924.x. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Yuferov V, Hamon S, Jackson C, Ho A, Ott J, Kreek MJ. Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology. 2009;34:867–873. doi: 10.1038/npp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Gámez L, García-Hidalgo AA, Salazar-Juárez A, Antón B, Paredes RG. Endomorphin-1, effects on male sexual behavior. Physiol Behav. 2009;97:98–101. doi: 10.1016/j.physbeh.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Wong L-Y, Turner WE, Caudill SP, Dipietro ES, McClure PC, Cash TP, Osterloh JD, Pirkle JL, Sampson EJ, Needham LL. Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ Sci Technol. 2009;43:1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. 6. Elsevier Inc; London, UK: 2009. [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol (Lond) 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Poland A, Glover E. Chlorinated biphenyl induction of aryl hydrocarbon hydroxylase activity: a study of the structure-activity relationship. Mol Pharmacol. 1977;13:924–938. [PubMed] [Google Scholar]
- Poon E, Monaikul S, Kostyniak PJ, Chi LH, Schantz SL, Sable HJK. Developmental exposure to polychlorinated biphenyls reduces amphetamine behavioral sensitization in Long-Evans rats. Neurotoxicol Teratol. 2013;38:6–12. doi: 10.1016/j.ntt.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaley JA. Development of Gonadotropin regulation in the prepubertal mammal. Biol Reprod. 1979;20:1–31. doi: 10.1093/biolreprod/20.1.1. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, Gore AC. The effects of prenatal PCBs on adult social behavior in rats. Horm Behav. 2015;73:47–55. doi: 10.1016/j.yhbeh.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21:781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- Saksena SK, Lau IF. Variations in serum androgens, estrogens, progestins, gonadotropins and prolactin levels in male rats from prepubertal to advanced age. Exp Aging Res. 1979;5:179–194. doi: 10.1080/03610737908257197. [DOI] [PubMed] [Google Scholar]
- Sakuma Y. Neural substrates for sexual preference and motivation in the female and male rat. Ann N Y Acad Sci. 2008;1129:55–60. doi: 10.1196/annals.1417.009. [DOI] [PubMed] [Google Scholar]
- Sauer PJ, Huisman M, Koopman-Esseboom C, Morse DC, Smits-van Prooije AE, van de Berg KJ, Tuinstra LG, Van Der Paauw CG, Boersma ER, Weisglas-Kuperus N. Effects of polychlorinated biphenyls (PCBs) and dioxins on growth and development. Hum Exp Toxicol. 1994;13:900–906. doi: 10.1177/096032719401301213. [DOI] [PubMed] [Google Scholar]
- Schantz SL. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol. 1996;18:217–227. doi: 10.1016/s0892-0362(96)90001-x. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O’Malley B, Levine JE. Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology. 2005;146:4340–4348. doi: 10.1210/en.2005-0490. [DOI] [PubMed] [Google Scholar]
- Schrader TJ, Cooke GM. Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reprod Toxicol. 2003;17:15–23. doi: 10.1016/s0890-6238(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Okoniewski RJ. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl on dopamine function. Toxicol Appl Pharmacol. 1997;146:95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Brosch KO. Decreases in dopamine concentrations in adult, non-human primate brain persist following removal from polychlorinated biphenyls. Toxicology. 1994;86:71–87. doi: 10.1016/0300-483x(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Shain W. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol Appl Pharmacol. 1990;106:136–144. doi: 10.1016/0041-008x(90)90113-9. [DOI] [PubMed] [Google Scholar]
- Shain W, Bush B, Seegal R. Neurotoxicity of polychlorinated biphenyls: structure-activity relationship of individual congeners. Toxicol Appl Pharmacol. 1991;111:33–42. doi: 10.1016/0041-008x(91)90131-w. [DOI] [PubMed] [Google Scholar]
- Shekhar PV, Werdell J, Basrur VS. Environmental estrogen stimulation of growth and estrogen receptor function in preneoplastic and cancerous human breast cell lines. J Natl Cancer Inst. 1997;89:1774–1782. doi: 10.1093/jnci/89.23.1774. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Smyth C, Wilkinson M. A Critical Period for Glutamate Receptor-Mediated Induction of Precocious Puberty in Female Rats. J Neuroendocrinol. 1994;6:275–284. doi: 10.1111/j.1365-2826.1994.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78:1091–1101. doi: 10.1095/biolreprod.107.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Otake T, Kataoka M, Murata Y, Aburada S. Studies of the transfer and distribution of [14C]polychlorinated biphenyls from maternal to fetal and suckling rats. Toxicol Appl Pharmacol. 1976;38:549–558. doi: 10.1016/0041-008x(76)90186-1. [DOI] [PubMed] [Google Scholar]
- Tavolari S, Bucci L, Tomasi V, Guarnieri T. Selected polychlorobiphenyls congeners bind to estrogen receptor alpha in human umbilical vascular endothelial (HUVE) cells modulating angiogenesis. Toxicology. 2006;218:67–74. doi: 10.1016/j.tox.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Tian J, Lee SO, Liang L, Luo J, Huang C-K, Li L, Niu Y, Chang C. Targeting the unique methylation pattern of androgen receptor (AR) promoter in prostate stem/progenitor cells with 5-aza-2′-deoxycytidine (5-AZA) leads to suppressed prostate tumorigenesis. J Biol Chem. 2012;287:39954–39966. doi: 10.1074/jbc.M112.395574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YH, Hwan Kim S, Lee SY, Jang CG. Lactational and postnatal exposure to polychlorinated biphenyls induces sex-specific anxiolytic behavior and cognitive deficit in mice offspring. Synapse. 2011;65:1032–1041. doi: 10.1002/syn.20934. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Fox TO. Sex- and hormone-dependent antigen immunoreactivity in developing rat hypothalamus. Proc Natl Acad Sci USA. 1989;86:382–386. doi: 10.1073/pnas.86.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJM, Vanderschuren LJMJ. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci. 2011;31:6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich B, Stahlmann R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol. 2004;78:252–268. doi: 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Stein EA, Wiegant VM, Van Ree JM. Social isolation and social interaction alter regional brain opioid receptor binding in rats. European Neuropsychopharm. 1995;5:119–127. doi: 10.1016/0924-977X(95)00010-M. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38:2554–2561. doi: 10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, Gerrits PO. Neural mechanisms of female sexual behavior in the rat; comparison with male ejaculatory control. Pharmacol Biochem Behav. 2014;121:16–30. doi: 10.1016/j.pbb.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The Effects of Gonadectomy on Age-and Sex-Typical Patterns of Ethanol Consumption in Sprague Dawley Rats. Alcohol Clin Exp Res. 2011;35:2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology. 1998;139:3658–3661. doi: 10.1210/endo.139.8.6223. [DOI] [PubMed] [Google Scholar]
- Walker DM, Goetz BM, Gore AC. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol Endocrinol. 2014;28:99–115. doi: 10.1210/me.2013-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of Reproductive Aging in Female and Male Rats by Gestational Exposure to Estrogenic Endocrine Disruptors. Endocrinology. 2013;154:2129–2143. doi: 10.1210/en.2012-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J, Osuch JR, Karmaus W, Landgraf JR, Taffe B, O’Keefe M, Mikucki D, Haan P. Common classification schemes for PCB congeners and the gene expression of CYP17, CYP19, ESR1 and ESR2. Sci Total Environ. 2012;414:81–89. doi: 10.1016/j.scitotenv.2011.10.044. [DOI] [PubMed] [Google Scholar]
- Woodhouse AJ, Cooke GM. Suppression of aromatase activity in vitro by PCBs 28 and 105 and Aroclor 1221. Toxicol Lett. 2004;152:91–100. doi: 10.1016/j.toxlet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Narita A, Kagohata M, Shirai M, Akahori F, Arishima K. Effects of maternal exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) or 3,3′,4,4′,5,5′-hexachlorobiphenyl (PCB169) on testicular steroidogenesis and spermatogenesis in male offspring rats. J Androl. 2005;26:205–214. doi: 10.1002/j.1939-4640.2005.tb01087.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.