Abstract
Oogenesis and spermatogenesis are tightly regulated complex processes that are critical for fertility function. Germ cells undergo meiosis to generate haploid cells necessary for reproduction. Errors in meiosis, including the generation of chromosomal abnormalities, can result in reproductive defects and infertility. Meiotic proteins are regulated by post-translational modifications including SUMOylation, the covalent attachment of small ubiquitin-like modifier (SUMO) proteins. Here, we review the role of SUMO proteins in controlling germ cell development and maturation based on recent findings from mouse models. Several studies have characterized the localization of SUMO proteins in male and female germ cells. However, a deeper understanding of how SUMOylation regulates proteins with essential roles in oogenesis and spermatogenesis will provide useful insight into the underlying mechanisms of germ cell development and fertility.
Keywords: SUMOylation, meiosis, oocyte, spermatocyte, germ cell
Introduction
Germ cells are the specialized reproductive cells that develop into spermatozoa or oocytes. They are unique as they are the only cells that undergo both mitosis and meiosis, producing haploid daughter cells through cellular division and exchanging genetic material through homologous recombination. In mammals, primordial germ cells (PGCs) originate from pluripotent epiblast cells before and during gastrulation (Rossant, et al., 1978). Following rapid proliferation, PGCs differentiate into either oogonia or quiescent prospermatogonia within the ovaries and testes, respectively (Tam and Snow, 1981). Male sex differentiation is induced by the presence of the Sry (sex determining region Y) gene, which initiates supporting cell precursors to develop into Sertoli cells, rather than granulosa cells (Albrecht and Eicher, 2001, Koopman, et al., 1991). The Sertoli and granulosa cells support and maintain testicular and ovarian germ cells. This maintenance of the germ cells is critical for reproduction and fertility.
Oogenesis is the process by which ovarian germ cells undergo meiosis and develop into a mature ovum (Fig. 1). After migrating to the genital ridge, PGCs divide into germ cell cysts through incomplete cytokinesis during mitosis (Pepling and Spradling, 1998). Prior to birth, germ cell cysts enter meiosis and proceed through the leptotene, zygotene and pachytene stages of prophase I, arresting in diplotene until they are released by the luteinizing hormone surge during adult reproductive cycling (Borum, 1967). Germ cell cysts break down to form primordial follicles, which consist of individual primary oocytes surrounded by somatic cells that will eventually differentiate into the granulosa cells of the follicle. Germ cell cyst breakdown occurs just before and after birth in mice (Pepling and Spradling, 1998). During the process of folliculogenesis, oocytes within recruited primordial follicles differentiate into full-grown oocytes within mature follicles (McLaren, 2000, Neal and Baker, 1975). During this growth phase, oocytes acquire competence to resume meiosis and to be fertilized (Sorensen and Wassarman, 1976). The preovulatory luteinizing hormone surge triggers oocytes to resume meiotic maturation until a second arrest at metaphase II (Neal and Baker, 1975, Whitaker, 1996) Following ovulation, fertilization induces the completion of meiosis (Pepling and Spradling, 1998, Schultz, et al., 1983).
Fig. 1.
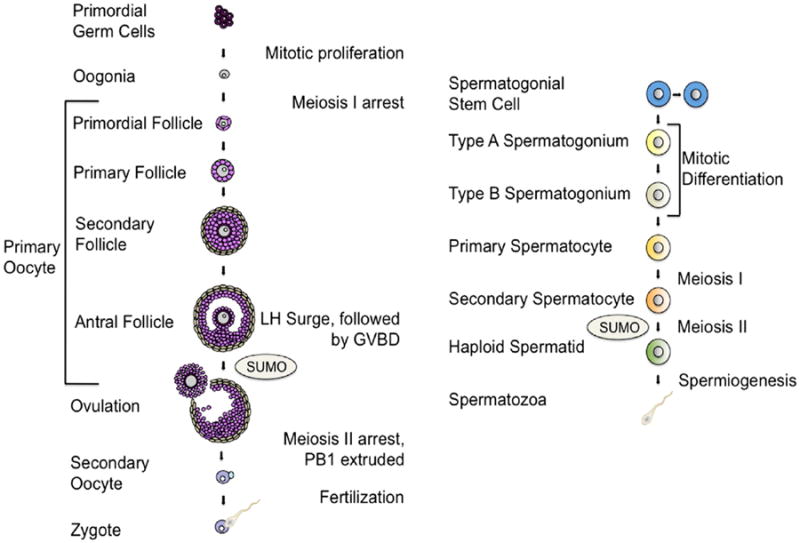
Oogenesis and Spermatogenesis. Within the ovary, primordial germ cells undergo mitosis to form primary oocytes. Primary oocytes, surrounded by somatic cells to form primordial follicles, begin meiosis and arrest in prophase I until the luteinizing hormone (LH) induces resumption of oocyte maturation. Oocytes then undergo germinal vesicle breakdown (GVBD), extrude the first polar body (PB) and become arrested at metaphase II of meiosis. Following ovulation, the secondary oocyte will resume meiosis only if fertilization occurs. Spermatogonial stem cells may either divide to regenerate themselves or to form type A spermatogonium during spermatogenesis. Type A spermatogonium divide mitotically to generate type B spermatogonium, which divide once to produce primary spermatocytes. Primary spermatocytes begin meiosis to form secondary spermatocytes which complete meiosis II to produce haploid spermatids. During spermiogenesis, sperm cells mature and differentiate to form spermatozoa with an acrosomal cap and flagellum. SUMOylation regulates meiotic processes in both female and male germ cells.
In contrast to female germ cells, male germ cells enter a mitotic arrest and do not begin meiosis in the embryo, but remain quiescent until after birth (Fig. 1). Unlike the oocyte, which may remain arrested in prophase I for years (McLaren, 1984), the male germ cell proceeds through meiosis uninterrupted. Spermatogenesis, the process of mature spermatozoa production from primordial germ cells, occurs inside the epithelium of the seminiferous tubules within the testis (Hess and Renato de Franca, 2008, Oakberg, 1956). This process involves 1) a mitotic proliferation phase of spermatogonia to generate primary spermatocytes, 2) a meiotic phase producing secondary spermatocytes, and 3) haploid round spermatid differentiation into mature spermatozoa during spermiogenesis (Jan, et al., 2012). Primordial germ cells within the walls of the seminiferous tubules divide during mitosis to give rise to type A spermatogonia, which are stem cells necessary for self renewal (de Rooij and Grootegoed, 1998). Type A spermatogonia divide to produce type B spermatogonia, which in turn undergo mitosis to form primary spermatocytes. Primary spermatocytes progress through meiosis I to form secondary spermatocytes, which undergo meiosis II to form round spermatids (Handel, 2004, Nakagawa, et al., 2007, Oakberg, 1971). Both meiotic sex chromosome inactivation (MSCI) and XY chromatin remodeling to form the sex body are key steps necessary to complete meiosis (Handel, 2004, Nakagawa, Nabeshima and Yoshida, 2007, Namekawa, et al., 2006, Oakberg, 1971). During spermiogenesis, or sperm maturation, round spermatids undergo nuclear condensation, formation of the acrosomal cap covering the nucleus, and flagellar formation (de Kretser, et al., 1998). The manchette, a cone-shaped bundle of microtubules surrounding the post-acrosomal spermatid nucleus, develops during the elongation phase of spermatid differentiation and is important for nuclear condensation and shaping as well as tail formation (Kierszenbaum, 2001, Russell, et al., 1991). Mature elongated spermatids are released into the lumen of the seminiferous tubules and subsequently acquire motility in the epididymis (Jan, et al., 2012).
It is well established that germ-cell development is regulated in part by post-translational modifications. Essential meiotic proteins are modified by phosphorylation and ubiquitination in both male and female germ cells (Pomerantz, et al., 2012, Turner, et al., 2006, Visconti, et al., 1995). Recently, SUMOylation has emerged as an important regulator of proteins with critical roles in spermatogenesis and oogenesis (Kuo, et al., 2009, Vigodner, et al., 2013). SUMOylation is a post-translational modification that regulates many cellular processes, and both SUMOylation and deSUMOylation are highly dynamic. The effect on substrates can be to alter protein localization (cytoplasmic or nuclear), activity, protein-protein interactions, or protein stability (Verger, et al., 2003). Four SUMO isoforms have been identified in humans and of these SUMO-1, SUMO-2, and SUMO-3 are evolutionarily conserved and ubiquitously expressed in all developmental stages in eukaryotes. SUMO-4 is mainly found in the kidney and immune tissues (Kerscher, 2007). The conjugatable forms of SUMO-2 and SUMO-3 vary in three N-terminal residues and are functionally indistinguishable as they share 97% identity (Wilson and Heaton, 2008). Conversely, SUMO-2/3 share only 50% sequence homology with SUMO-1. Furthermore, they have distinct functions as SUMO-1 and SUMO-2/3 are conjugated to different target proteins and SUMO-2/3 form polySUMO chains while SUMO-1 does not (Gareau and Lima, 2010).
Similar to ubiquitination, the SUMOylation process involves attachment of a small (11 kDa) polypeptide termed “ubiquitin-like modifier” protein (“SUMO”) (Fig. 2). During SUMOylation, in mammals, one of the SUMO proteins (named SUMO 1-4) is covalently attached to lysine residues within a specific SUMO consensus motif on target proteins. SUMOylation is carried out in three enzymatic reactions that utilize an E1 activating enzyme, an E2 conjugating enzyme, and an E3 protein ligase. SENPs, SUMO specific proteases, regulate the SUMO cycle in multiple ways: 1) by removing SUMO from SUMO modified proteins and 2) processing inactive SUMO to a mature form capable of undergoing conjugation to target proteins (Geiss-Friedlander and Melchior, 2007). The process of SUMOylation is far less complex than ubiquitination, with far fewer redundancies between enzymes. There is no overlap between enzymes in the ubiquitination and SUMOylation conjugation cascades (Geiss-Friedlander and Melchior, 2007).
Fig. 2.
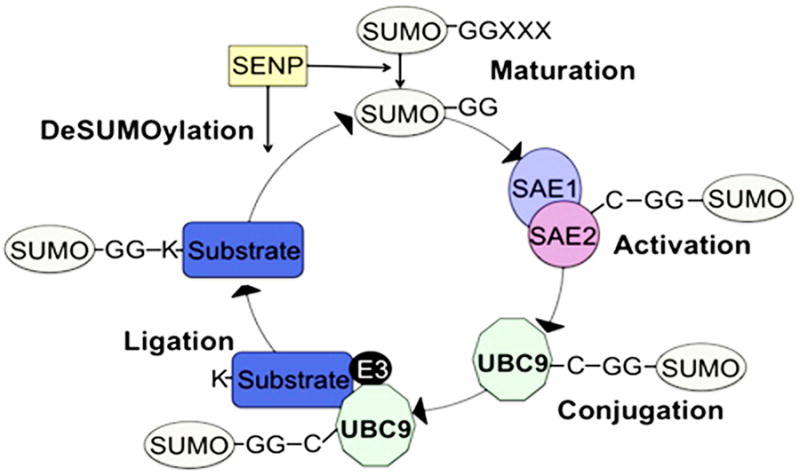
The SUMOylation reaction. The covalent attachment of SUMO proteins to specific lysine residues within substrate proteins is carried out in three enzymatic steps: 1) After SENP-mediated proteoloytic processing of the inactive SUMO precursor, SUMO is activated to the mature SUMO form by the E1 activating heterodimer, 2) SUMO is then conjugated to the E2 conjugating UBC9, and 3) E3 ligases can interact with UBC9 to facilitate SUMO ligation to substrate proteins. SUMO can be reversibly removed by the SENP proteins.
SUMO proteins are localized in the oocyte and may function in regulating gene expression
Multiple studies have characterized the localization of SUMO-1 and SUMO-2/3 in oocytes. The observed distinct localization patterns of SUMO-1 and SUMO-2/3 suggest temporal regulation of specific target proteins by SUMOylation throughout oocyte maturation and growth. While SUMO-1 is predominantly localized on the nuclear membrane, SUMO-2 and SUMO-3 are diffusely localized in nucleoplasm and are not enriched on the nuclear membrane in meiotically competent oocytes (Ihara, et al., 2008). Subsequent studies confirm SUMO-1 localization in the germinal vesicle (GV) of GV-intact oocytes, but did not detect SUMO-1 in the nuclear membrane (Yuan, et al., 2014). Transcriptionally active and inactive oocytes display differences in SUMO localization. Both SUMO-1 and SUMO-2/3 are localized to the nucleoplasm and chromatin in transcriptionally active oocytes. However, in transcriptionally quiescent oocytes, SUMO-1 is only weakly detected with chromatin around the nucleolus and nuclear membrane while SUMO-2/3 is localized throughout the nucleoplasm and on chromatin (Ihara, Stein and Schultz, 2008). During oocyte maturation, SUMO-1 is concentrated at the spindle poles of metaphase I and II, while SUMO-2/3 is localized near centrosomes, an observation also seen in metaphase I spermatocytes in mice (La Salle, et al., 2008, Yuan, et al., 2014). Collectively, the differential localization of SUMO-1 and SUMO-2/3 suggest they are modulating their target substrates in a temporal fashion during the acquisition of meiotic competence in the growing mammalian oocyte. However, more detailed studies are needed to determine the specific identities and roles of these SUMOylated proteins during meiosis.
During their development in the ovarian follicle, oocytes gain the ability to resume meiosis (i.e., are meiotically competent). Meiotically competent oocytes are capable of undergoing germinal vesicle breakdown, and arrest at metaphase I, resume meiosis and arrest at metaphase II (Schultz, Montgomery and Belanoff, 1983, Schultz and Wassarman, 1977, Wickramasinghe, et al., 1991). In contrast, immature oocytes are not able to proceed through meiosis (i.e., are meiotically incompetent). There is a change in transcriptional activity between meiotically competent and incompetent oocytes. Meiotically incompetent oocytes are transcriptionally active during their growth phase. However, fully-grown meiotically competent oocytes are in a transcriptionally quiescent state. The first studies investigating the role of SUMOylation in oocytes demonstrated that SUMOylation may be important for the regulation of gene expression by stimulating transcription and associating with RNA processing machinery in mouse GV-intact oocytes (Ihara, et al., 2008). UBC9, the E2 SUMO conjugating enzyme, is mainly localized in the nucleoplasm in meiotically incompetent and fully grown oocytes (Ihara, et al., 2008). The number and size of UBC9-containing structures increases with GV-intact oocyte growth. Transcriptionally quiescent fully-grown GV-intact oocytes have larger UBC9-containing structures in the nucleus compared to transcriptionally active meiotically incompetent growing oocytes. Inhibition of transcription via α-amanitin treatment in incompetent oocytes causes an increase in the size of UBC9-containing bodies and a decrease in number of UBC9-containing bodies (Ihara, et al., 2008). The use of α-amanitin to selectively inhibit RNAP II and RNAP II and block transcription to cause alterations in UBC9-containing structures suggests a role for SUMOylation in transcriptional regulation. However, more direct methods, such as performing RNA sequencing (RNA-seq) to determine transcript levels after inhibiting SUMO-1 and SUMO-2/3 in meiotically competent oocytes would provide strong supporting evidence. In addition, overexpression of UBC9 stimulates transcription in meiotically incompetent oocytes independent of UBC9 catalytic activity. UBC9 colocalizes with SFRS2, a major component of nuclear speckles, in both incompetent and competent mouse oocytes. These data indicate SUMOylation may be involved in regulating pre-mRNA splicing, mRNA export and gene expression (Ihara, et al., 2008). However, these colocalization observations do not provide direct evidence of a role for UBC9 and SUMOylation in regulating transcription, which awaits further studies.
SUMOylation is important in regulating meiosis in the mammalian oocyte
The function of SUMOylation in oocyte development and maturation is largely unknown, although some studies have begun to analyze the role of SUMOylation during meiosis I and II. SENP2, a SUMO-specific protease shown to inhibit SUMOylation and cause prometaphase arrest in HeLa cells, has been overexpressed by Myc-SENP2 microinjection into GV-oocytes cultured until maturation into MII eggs. Overexpression of SENP2 results in both an inhibition and accumulation of SUMOylated proteins in mature eggs (Wang, et al., 2010, Zhang, et al., 2008b). The multiple functions of SENPs, including deSUMOylation and processing SUMO to an active form, may explain the resulting inhibition and accumulation of SUMOylated proteins and indicate SENP manipulation is not likely to be the ideal method for studying the role of SUMOylation in oocyte meiotic maturation. Instead, targeted deletion or inhibition of SUMO-1, SUMO-2/3 or UBC9 in oocytes would provide a more straightforward analysis of meiotic defects due to loss of SUMOylation.
Various studies have demonstrated a role for SUMOylation in regulating spindle function during meiosis II. These studies altered components of the SUMO pathway in the oocyte by RNAi or with neutralizing antibodies. SENP2 overexpression results in MII spindles with defective morphology and MII spindles with branched microtubules still connected to the first polar body, indicating cellular division may be disrupted (Wang, et al., 2010). These results suggest that SUMOylation of meiotic proteins is important for MII spindle organization and potentially cytokinesis in mature eggs. However, as mentioned previously, it is unclear if these defects are due to excessive or decreased SUMOylation due to the dual role of SENPs in processing SUMO to an active form as well as cleaving SUMO from target proteins. Inhibition of SUMO components by microinjecting SUMO-1 or UBC9 specific antibodies or siRNAs also results in an increase in abnormal spindles characterized by the absence of spindles, spindles with disordered poles, or elongated spindles in both immature and mature oocytes. Additionally, subcellular localization of γ-tubulin, a spindle-pole protein known to function in spindle organization was altered in both immature and mature oocytes (Lee, et al., 2000, Yuan, et al., 2014). These studies imply that proteins regulating spindle formation are modified by SUMOylation and a potential candidate for further investigation may be γ-tubulin. Both GVBD and polar body 1 (PB1) extrusion are inhibited by the loss of SUMO-1, although not completely, indicating possible compensation by SUMO-2/3. This could be further investigated by additionally inhibiting SUMO-2/3 to investigate the effects of spindle formation in oocytes. Stage-specific SUMO-1 antibody injections into GV-oocytes and in vitro cultured oocytes determined that SUMO-1 functions prior to anaphase I during PB1 extrusion. Overexpression of SUMO-1 via His-Sumo-1 mRNA microinjection into GV-stage oocytes is not detrimental to meiotic maturation (Yuan, et al., 2014). This may indicate that when SUMO-1 is in excess, the target substrates are saturated and consequently there is no negative effect on meiotic maturation. In summary, while inhibition of SUMO-1 results in multiple meiotic defects, excess SUMO-1 does not significantly alter oocyte maturation during meiosis.
Additional research indicates that SUMO-1 functions in both kinetochore-microtubule attachment and centromere localization of BUBR1, a spindle-assembly checkpoint protein, in GV-oocytes. Depletion of SUMO-1 by Sumo-1-specific siRNA microinjection disrupted kinetochore-microtubule attachment at metaphase I and reduced BUBR1 signal at centromeres, suggesting SUMO-1 could function in regulating the spindle-assembly checkpoint and BUBR1 stability or degradation (Yuan, et al., 2014). Furthermore, SUMOylation is important for the regulation of the cohesion protein REC8, which forms a complex with Securin, an inhibitor of separase, to regulate chromosome segregation during meiotic metaphase-anaphase transition. Loss of SUMO-1 alters subcellular localization of REC8 in chromosomes and Securin at spindles (Yuan, et al., 2014). SUMO-1 antibody microinjection into GV-stage oocytes that were then cultured for 8 hours did not alter REC8 and Securin protein abundance (Yuan, et al., 2014). Because these two proteins are known to be important for chromosome segregation prior to anaphase, SUMO-1 could be critical for the prevention of aneuploidy. However, additional studies are needed to demonstrate that BUBR1 and REC8 are direct targets of SUMO modification. From the published studies, it is clear that SUMO-1 appears to regulate multiple processes during meiotic oocyte maturation; however, a role for SUMO-2/3 has yet to be investigated (Yuan, et al., 2014).
To date, very few SUMO modified target proteins in the mouse oocyte have been identified, and most of these are involved in meiosis. In particular, Septin 2, a GTP binding protein important during mitosis,, is 1) modified by SUMO-1 during meiosis, and 2) functions in chromosome congression and meiotic progression, but not cytokinesis of the first PB in mouse oocytes (Zhu, et al., 2010). Identifying oocyte-specific substrates is critical for understanding additional roles of SUMOylation in regulating oocyte development and ovarian folliculogenesis, which is controlled in part by oocyte-specific genes and morphogens.
SUMO proteins are expressed during meiosis in male germ cells
The initial identification of SUMO protein expression in the XY body of rat pachytene spermatocytes suggested SUMOylation may function in male meiosis (Rogers, et al., 2004). Specifically, SUMO-1 is localized to the sex chromosomes of meiotic spermatocytes, the centrosome and manchette of spermatids, and specific domains of the somatic cells of the mouse and rat testes (Vigodner and Morris, 2005). Further studies observed perinuclear SUMO-1 localization in the cytoplasm of spermatogonia during germ cell development in mouse and rat testes. SUMO-1 is detected on gonosomal chromatin during the zygotene phase of prophase I when synapsis or pairing of homologous chromosomes occurs (Vigodner, et al., 2014). SUMO-1 localization increases in the sex body of early and mid-pachytene spermatocytes when chromosome condensation occurs, but not in diplotene spermatocytes before the first meiotic division (Vigodner and Morris, 2005). SUMO-1 is not detected in secondary spermatocytes undergoing meiosis II (Vigodner and Morris, 2005). These collective observations of SUMO-1 localization in spermatogonia and primary spermatocytes suggest that SUMO modification may regulate proteins involved in meiosis I and spermatid elongation. Combined with the SUMO localization data from meiotic oocytes, these studies provide compelling evidence for a general function of SUMOylation in meiosis.
SUMOylation regulates several meiotic components in male germ cells
Although the role of SUMOylation during male meiosis is not well characterized, studies have provided initial insight into possible meiotic regulatory functions of SUMOylation. SUMO and phosphorylated γH2A.X, a histone protein important for meiotic sex chromosome inactivation (MSCI) and sex body formation, colocalize in the sex body of mouse pachytene spermatocytes prior to the first meiotic division. The authors suggests SUMO-1 may regulate synapsis and condensation of sex chromosomes, MSCI, and XY body formation (Vigodner and Morris, 2005). The possible role of SUMO regulation of XY body formation is further supported by SUMO-1 and SUMO-2/3 localization to the XY body and chromocenters in pachytene and diplotene spermatocytes (La Salle, et al., 2008). Only SUMO-2/3 are detected near centromeres in metaphase I spermatocytes. This observation suggests SUMO-1 and SUMO-2/3 function at different stages of meiosis in males. Further investigation with functional assays is required to strengthen the validity of the correlative localization results. For example, studying the effects of SUMO-1 inhibition on condensation of sex chromosomes and MSCI formation would address this concern.
Comparisons of human and mouse male germ cells have revealed other potential functions of SUMO-1 in meiotic function. During human meiotic prophase, SUMO-1 localizes to sex chromosomes and centromeric and pericentromeric chromatin, but not centromeres, in male germ cells (Vigodner, et al., 2006). As human spermatocytes complete prophase in meiosis I, SUMO-1 is not detected in the sex body or pericentromeric heterochromatin, but is localized solely within centromeres. Haploid round spermatid nuclei display consistent SUMO-1 association with multiple centromeric clusters (Vigodner, et al., 2006). During spermatid elongation, SUMO-1 is detected at the manchette initiation site. In mouse spermatids, SUMO-1 is observed at a single chromacenter, or aggregated heterochromatin, region of certain round spermatids and perinuclear ring of the manchette, as well as in the centrosomes of elongating mouse spermatids, two initiation sites for microtubule assembly. These findings suggest SUMO-1 may function in centromeric chromatin condensation and microtubule nucleation and nuclear reshaping (Vigodner and Morris, 2005). However, the target proteins important for manchette formation, spermatid elongation, and microtubule assembly that are potentially modified by SUMOylation have not been identified.
During the pachytene substage in men, SUMO-1 modification of the synaptonemal complex (SC) and SC proteins (SCP)-1 and SCP2, but not SCP3, is observed (Brown, et al., 2008). While SUMO-1 is not present prior to pachytene in normal tissue, SUMO-2/3 is observed as early as leptotema and zygonema and in some, but not all, pachytene stage cells in men. Therefore, SUMO-1 may be more important for the maintenance of the autosomal SC scaffold than SUMO-2/3 (Brown, et al., 2008). More recent studies demonstrate that SUMO-1 and SUMO-2/3 are prominently localized in the neck area of human sperm that is associated with the redundant nuclear envelope (RNE), as well as in the flagella and head regions, but not centrioles or mitochondria (Vigodner, et al., 2013. These observations are similar to SUMO localization in mice, suggesting conserved function between the two species. Interestingly, excessive SUMOylation is present in defective spermatozoa. Nonmotile, deformed microcephalic (small head), and aciphalic (no head) spermatozoa with two-tails or curled tails display significantly higher levels of SUMOylation in their neck and tail regions compared to normal sperm (Vigodner, et al., 2013). These results implicate SUMOylation levels could be a potential indicator of impaired sperm function and unregulated, excessive SUMOylation could likely cause the sperm defects leading to male infertility. Currently, 55 SUMO targets in human sperm have been identified by mass spectrometry analysis. These target proteins are involved in several functional groups including: 1) stress response and protein folding, 2) cytoskeletal and flagella proteins include major flagella proteins, 3) sperm motility and function, 4) transcription and protein synthesis, and 5) glycolysis and mitochondrial function (Vigodner, et al., 2014). Other groups have also confirmed that proteins such as topoisomerase 2α, are SUMOylated in response to stress during spermatogenesis (Shrivastava, et al., 2010). However, these proteins have yet to be individually validated as SUMO targets. Following validation, the functional significance of these SUMOylated candidates in spermatogenesis can be determined.
Conclusions
Current studies have demonstrated that SUMOylation may regulate multiple processes in the oocyte including gene expression and transcription, MII spindle organization, germinal vesicle breakdown, and polar body extrusion, as well as kinetochore-microtubule attachment (Table 1). While SUMO-1 may function in facilitating cytokinesis and in maintaining the spindle-assembly checkpoint in meiosis II, the specific role of SUMO-2/3 during meiotic maturation is unknown. Current studies have mainly focused on understanding SUMOylation in postovulatory oocytes. The function of SUMOylation in developing oocytes remains uninvestigated. Additionally, the identities of SUMOylated target proteins expressed in the oocyte are unclear. Possible candidate proteins thus far include γ-tubulin, BUBR1, REC8, Securin and Septin2. Discovering the substrate proteins that are SUMOylated in the oocyte will likely provide more depth and insight into the mechanistic roles of SUMOylation in regulating oocyte development and function.
Table 1.
Suggested roles for SUMOylation in female and male germ cells.
| Oogenesis | Spermatogenesis | Reference | |
| Meiotic processes | Germinal vesicle breakdown (GVBD) | Synapsis and condensation of chromosomes | Yuan, et al., 2014, Vigodner and Morris, 2005 |
| Metaphase II spindle formation and organization | Meiotic sex chromosome inactivation (MSCI) | Wang, et al., 2010, Vigodner and Morris, 2005 | |
| Polar body 1 (PB1) extrusion | XY body formation | Yuan, et al., 2014, Rogers, et al., 2004 | |
| Chromosome segregation & prevention of aneuploidy | Synaptonemal complex scaffold maintenance during pachytene stage | Yuan, et al., 2014, Brown, et al., 2008 | |
| Cytokinesis | Microtubule Assembly | Wang et al., 2010, Vigodner and Morris, 2005 | |
| Non-meiotic processes | Pre-mRNA splicing, mRNA export and transcriptional activity | Spermatid elongation & manchette formation | Ihara, et al., 2008, Vigodner and Morris, 2005 |
Similar to the oocyte, SUMOylation also functions in regulating meiosis in males (Table 1). The characterization of SUMO-1 localization implicates SUMO modification as a possible modulator of microtubule nucleation, nuclear reshaping, and maintaining the synaptonemal complex scaffold. The discovery that abnormal spermatozoa display excessive SUMOylation strongly indicates this modification is important in regulating male fertility. The SUMO target proteins identified through mass spectrometry analysis provides a broad understanding of the diverse roles of SUMOylation in sperm cells. However, more investigative mechanistic research is required to confirm these targets are modified by SUMOylation and assess how SUMO modification regulates their functions.
Localization studies in both spermatocytes and oocytes demonstrate that SUMO-1 and SUMO-2/3 display some unique expression patterns. These data indicate that the different SUMO isoforms function in modifying distinct proteins in germ cells. While excessive SUMOylation has been identified as a potential marker of defective human sperm, comparable studies investigating the human oocyte have not been performed. Additionally, high throughput screening, similar to the mass spectrometry analysis of human sperm, to globally determine potential SUMOylation targets in the mammalian oocyte is required. Additionally, more thorough studies are needed to understand how specific SUMO modification regulates target proteins and affects fertility function in male and female. Few studies have also indicated dysregulation of SUMOylation in the progression of testicular and prostate cancer, as well as epithelial ovarian cancer (Dong, et al., 2013, Mukherjee, et al., 2012). The role of SUMOylation in these reproductive diseases is not well understood. Further characterization would assist in uncovering the function of SUMOylation in regulating fertility.
The findings summarized above indicate the SUMO pathway regulates meiosis in male and female germ cells. These studies support work performed in other species, including yeast and Drosophila, demonstrating a role for SUMOylation in controlling synaptonemal complex assembly and spindle formation during meiosis (Apionishev, et al., 2001, Cheng, et al., 2006). The overall significance of SUMOylation is evident by the embryonic lethality resulting in severe mitosis defects in Ubc9 mutant embryos (Nacerddine, et al., 2005). Numerous studies have demonstrated roles for SUMOylation in cell cycle regulation, replication, transcription, translation, DNA repair, and subcellular transport in other cell types. However, the significance of SUMOylation during embryonic development requires further investigation. Manipulation of the SUMO cycle during germ cell development is limited due to difficulties transfecting and culturing oocytes and spermatocytes. Furthermore, the regulation of SUMOylation levels by UBC9, E3 ligases, and SENPs during development is not understood. Understanding how SUMO proteins are regulated as well as how SUMOylation regulates germ cell-expressed proteins will expand our understanding of germ cell development and fertility maintenance.
Table 2.
Functions of SUMO pathway components in embryonic development and fertility.
| Mouse Knockout | Viability/Fertility | Reference |
| PIASx (E3 ligase) | Both sexes fertile, males display reduced testis weight, increased apopotic testicular cells | Santti, et al., 2005 |
| PIASy (E3 ligase) | Both sexes fertile | Wong, et al., 2004 |
| Senp1 (SUMO- specific protease) | Embryonic lethal at E13.5 due to placental abnormalities | Yamaguchi, et al., 2005 |
| Senp2 (SUMO- specific protease) | Embryonic lethal at E10 due to cardiac developmental defects | Kang, et al., 2010 |
| Sumo-1 | Both sexes fertile | Zhang, et al., 2008a |
| Sumo-2 | Embryonic lethal at E10.5 due to severe growth retardation | Wang, et al., 2014 |
| Sumo-3 | Both sexes fertile | Wang. et al., 2014 |
| Ubc9 (E2 enzyme) | Embryonic lethal by E7.5 due to mitotic chromosome defects | Nacerddine, et al., 2005 |
Acknowledgments
Studies in the Pangas Laboratory are funded by NIH R01 CA138628, R01 HD076980, and T32 HD007165.
Funding: Supported by NIH R01 CA138628, R01 HD076980, and T32 HD007165.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Apionishev S, Malhotra D, Raghavachari S, Tanda S, Rasooly RS. The Drosophila UBC9 homologue lesswright mediates the disjunction of homologues in meiosis I. Genes Cells. 2001;6:215–224. doi: 10.1046/j.1365-2443.2001.00413.x. [DOI] [PubMed] [Google Scholar]
- Borum K. Oogenesis in the mouse. A study of the origin of the mature ova. Exp Cell Res. 1967;45:39–47. doi: 10.1016/0014-4827(67)90110-3. [DOI] [PubMed] [Google Scholar]
- Brown PW, Hwang K, Schlegel PN, Morris PL. Small ubiquitin-related modifier (SUMO)-1, SUMO-2/3 and SUMOylation are involved with centromeric heterochromatin of chromosomes 9 and 1 and proteins of the synaptonemal complex during meiosis in men. Hum Reprod. 2008;23:2850–2857. doi: 10.1093/humrep/den300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Lo YH, Liang SS, Ti SC, Lin FM, Yeh CH, Huang HY, Wang TF. SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes Dev. 2006;20:2067–2081. doi: 10.1101/gad.1430406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N. Spermatogenesis. Hum Reprod. 1998;13(Suppl 1):1–8. doi: 10.1093/humrep/13.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- Dong M, Pang X, Xu Y, Wen F, Zhang Y. Ubiquitin-conjugating enzyme 9 promotes epithelial ovarian cancer cell proliferation in vitro. Int J Mol Sci. 2013;14:11061–11071. doi: 10.3390/ijms140611061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol. 2010;11:861–871. doi: 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Handel MA. The XY body: a specialized meiotic chromatin domain. Exp Cell Res. 2004;296:57–63. doi: 10.1016/j.yexcr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- Ihara M, Stein P, Schultz RM. UBE2I (UBC9), a SUMO-conjugating enzyme, localizes to nuclear speckles and stimulates transcription in mouse oocytes. Biol Reprod. 2008;79:906–913. doi: 10.1095/biolreprod.108.070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan SZ, Hamer G, Repping S, de Rooij DG, van Pelt AM, Vormer TL. Molecular control of rodent spermatogenesis. Biochim Biophys Acta. 2012;1822:1838–1850. doi: 10.1016/j.bbadis.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Kang X, Qi Y, Zuo Y, Wang Q, Zou Y, Schwartz RJ, Cheng J, Yeh ET. SUMO-specific protease 2 is essential for suppression of polycomb group protein-mediated gene silencing during embryonic development. Mol Cell. 2010;38:191–201. doi: 10.1016/j.molcel.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O. SUMO junction-what's your function? New insights through SUMO interacting motifs. EMBO Rep. 2007;8:550–555. doi: 10.1038/sj.embor.7400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum AL. Spermatid manchette: plugging proteins to zero into the sperm tail. Mol Reprod Dev. 2001;59:347–349. doi: 10.1002/mrd.1040. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Kuo FT, Bentsi-Barnes IK, Barlow GM, Bae J, Pisarska MD. Sumoylation of forkhead L2 by Ubc9 is required for its activity as a transcriptional repressor of the Steroidogenic Acute Regulatory gene. Cell Signal. 2009;21:1935–1944. doi: 10.1016/j.cellsig.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Salle S, Sun F, Zhang XD, Matunis MJ, Handel MA. Developmental control of sumoylation pathway proteins in mouse male germ cells. Dev Biol. 2008;321:227–237. doi: 10.1016/j.ydbio.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Miyano T, Moor RM. Spindle formation and dynamics of gamma-tubulin and nuclear mitotic apparatus protein distribution during meiosis in pig and mouse oocytes. Biol Reprod. 2000;62:1184–1192. doi: 10.1095/biolreprod62.5.1184. [DOI] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol. 2000;163:3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Cruz-Rodriguez O, Bolton E, Iniguez-Lluhi JA. The in vivo role of androgen receptor SUMOylation as revealed by androgen insensitivity syndrome and prostate cancer mutations targeting the proline/glycine residues of synergy control motifs. J Biol Chem. 2012;287:31195–31206. doi: 10.1074/jbc.M112.395210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, Pandolfi PP, Dejean A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell. 2005;9:769–779. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Namekawa SH, Park PJ, Zhang LF, Shima JE, McCarrey JR, Griswold MD, Lee JT. Postmeiotic sex chromatin in the male germline of mice. Curr Biol. 2006;16:660–667. doi: 10.1016/j.cub.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Neal P, Baker TG. Response of mouse graafian follicles in organ culture to varying doses of follicle-stimulating hormone and luteinizing hormone. J Endocrinol. 1975;65:27–32. doi: 10.1677/joe.0.0650027. [DOI] [PubMed] [Google Scholar]
- Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99:507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat Rec. 1971;169:515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Pomerantz Y, Elbaz J, Ben-Eliezer I, Reizel Y, David Y, Galiani D, Nevo N, Navon A, Dekel N. From ubiquitin-proteasomal degradation to CDK1 inactivation: requirements for the first polar body extrusion in mouse oocytes. FASEB J. 2012;26:4495–4505. doi: 10.1096/fj.12-209866. [DOI] [PubMed] [Google Scholar]
- Rogers RS, Inselman A, Handel MA, Matunis MJ. SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma. 2004;113:233–243. doi: 10.1007/s00412-004-0311-7. [DOI] [PubMed] [Google Scholar]
- Rossant J, Gardner RL, Alexandre HL. Investigation of the potency of cells from the postimplantation mouse embryo by blastocyst injection: a preliminary report. J Embryol Exp Morphol. 1978;48:239–247. [PubMed] [Google Scholar]
- Russell LD, Russell JA, MacGregor GR, Meistrich ML. Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am J Anat. 1991;192:97–120. doi: 10.1002/aja.1001920202. [DOI] [PubMed] [Google Scholar]
- Santti H, Mikkonen L, Anand A, Hirvonen-Santti S, Toppari J, Panhuysen M, Vauti F, Perera M, Corte G, Wurst W, Janne OA, Palvimo JJ. Disruption of the murine PIASx gene results in reduced testis weight. J Mol Endocrinol. 2005;34:645–654. doi: 10.1677/jme.1.01666. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Wassarman PM. Biochemical studies of mammalian oogenesis: Protein synthesis during oocyte growth and meiotic maturation in the mouse. J Cell Sci. 1977;24:167–194. doi: 10.1242/jcs.24.1.167. [DOI] [PubMed] [Google Scholar]
- Shrivastava V, Pekar M, Grosser E, Im J, Vigodner M. SUMO proteins are involved in the stress response during spermatogenesis and are localized to DNA double-strand breaks in germ cells. Reproduction. 2010;139:999–1010. doi: 10.1530/REP-09-0492. [DOI] [PubMed] [Google Scholar]
- Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976;50:531–536. doi: 10.1016/0012-1606(76)90172-x. [DOI] [PubMed] [Google Scholar]
- Tam PP, Snow MH. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol. 1981;64:133–147. [PubMed] [Google Scholar]
- Turner JM, Mahadevaiah SK, Ellis PJ, Mitchell MJ, Burgoyne PS. Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev Cell. 2006;10:521–529. doi: 10.1016/j.devcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Verger A, Perdomo J, Crossley M. Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 2003;4:137–142. doi: 10.1038/sj.embor.embor738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigodner M, Ishikawa T, Schlegel PN, Morris PL. SUMO-1, human male germ cell development, and the androgen receptor in the testis of men with normal and abnormal spermatogenesis. Am J Physiol Endocrinol Metab. 2006;290:E1022–1033. doi: 10.1152/ajpendo.00527.2005. [DOI] [PubMed] [Google Scholar]
- Vigodner M, Morris PL. Testicular expression of small ubiquitin-related modifier-1 (SUMO-1) supports multiple roles in spermatogenesis: silencing of sex chromosomes in spermatocytes, spermatid microtubule nucleation, and nuclear reshaping. Dev Biol. 2005;282:480–492. doi: 10.1016/j.ydbio.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Vigodner M, Shrivastava V, Gutstein LE, Schneider J, Nieves E, Goldstein M, Feliciano M, Callaway M. Localization and identification of sumoylated proteins in human sperm: excessive sumoylation is a marker of defective spermatozoa. Hum Reprod. 2013;28:210–223. doi: 10.1093/humrep/des317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Wang L, Wansleeben C, Zhao S, Miao P, Paschen W, Yang W. SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep. 2014;15:878–885. doi: 10.15252/embr.201438534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZB, Ou XH, Tong JS, Li S, Wei L, Ouyang YC, Hou Y, Schatten H, Sun QY. The SUMO pathway functions in mouse oocyte maturation. Cell Cycle. 2010;9:2640–2646. doi: 10.4161/cc.9.13.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. Control of meiotic arrest. Rev Reprod. 1996;1:127–135. doi: 10.1530/ror.0.0010127. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol. 1991;143:162–172. doi: 10.1016/0012-1606(91)90063-9. [DOI] [PubMed] [Google Scholar]
- Wilson VG, Heaton PR. Ubiquitin proteolytic system: focus on SUMO. Expert Rev Proteomics. 2008;5:121–135. doi: 10.1586/14789450.5.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KA, Kim R, Christofk H, Gao J, Lawson G, Wu H. Protein inhibitor of activated STAT Y (PIASy) and a splice variant lacking exon 6 enhance sumoylation but are not essential for embryogenesis and adult life. Mol Cell Biol. 2004;24:5577–5586. doi: 10.1128/MCB.24.12.5577-5586.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sharma P, Athanasiou M, Kumar A, Yamada S, Kuehn MR. Mutation of SENP1/SuPr-2 reveals an essential role for desumoylation in mouse development. Mol Cell Biol. 2005;25:5171–5182. doi: 10.1128/MCB.25.12.5171-5182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YF, Zhai R, Liu XM, Khan HA, Zhen YH, Huo LJ. SUMO-1 plays crucial roles for spindle organization, chromosome congression, and chromosome segregation during mouse oocyte meiotic maturation. Mol Reprod Dev. 2014;81:712–724. doi: 10.1002/mrd.22339. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Mikkonen L, Toppari J, Palvimo JJ, Thesleff I, Janne OA. Sumo-1 function is dispensable in normal mouse development. Mol Cell Biol. 2008a;28:5381–5390. doi: 10.1128/MCB.00651-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Goeres J, Zhang H, Yen TJ, Porter AC, Matunis MJ. SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Mol Cell. 2008b;29:729–741. doi: 10.1016/j.molcel.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JL, Lin SL, Li M, Ouyang YC, Hou Y, Schatten H, Sun QY. Septin2 is modified by SUMOylation and required for chromosome congression in mouse oocytes. Cell Cycle. 2010;9:1607–1616. doi: 10.4161/cc.9.8.11463. [DOI] [PubMed] [Google Scholar]


