Abstract
Rationale
Cross-species quantification of physiological behavior enables a better understanding of the biological systems underlying neuropsychiatric diseases such as Bipolar Disorder (BD). Cardinal symptoms of manic BD include increased motor activity and goal-directed behavior, thought to be related to increased catecholamine activity, potentially selective to dopamine homeostatic dysregulation.
Objectives
The objective of this study was to test whether acute administration of amphetamine, a norepinephrine/dopamine transporter inhibitor and dopamine releaser, would replicate the profile of activity and exploration observed in both humans with manic BD and mouse models of mania.
Methods
Healthy volunteers with no psychiatric history were randomized to a one-time dose of placebo (n=25), 10 mg d-amphetamine (n=18), or 20 mg amphetamine (n=23). 80 mice were administered one of 4 doses of d-amphetamine or vehicle. Humans and mice were tested in the Behavioral Pattern Monitor (BPM), which quantifies motor activity, exploratory behavior, and spatial patterns of behavior.
Results
In humans, the 20-mg dose of amphetamine increased motor activity as measured by acceleration without marked effects on exploration or spatial patterns of activity. In mice, amphetamine increased activity, decreased specific exploration, and caused straighter, one-dimensional movements in a dose-dependent manner.
Conclusions
Consistent with mice, amphetamine increased motoric activity in humans without increasing exploration. Given that BD patients exhibit heightened exploration, these data further emphasize the limitation of amphetamine-induced hyperactivity as a suitable model for BD. Further, these studies highlight the utility of cross-species physiological paradigms in validating biological mechanisms of psychiatric diseases.
Keywords: Bipolar Disorder, dopamine, catecholamines, mania, motor activity, exploration, amphetamine
Introduction
Utilizing cross-species models enables the evaluation of parallels between critical phenotypes in humans and non-human animal models of psychiatric disease. In particular, animal models provide a basis of “cross-talk” between focal psychopathology and underlying biological systems. We have applied such a translational approach to the study of Bipolar Disorder (BD), a chronic and disabling disorder that is characterized by states of mania, hypomania, and depression and affects approximately 2% of the population. One of the core symptoms of the mania that defines BD is increased energy (Cheniaux et al. 2014), recently recognized by the DSM-5 as an essential feature that must be present for most of the day to meet criteria for a manic episode (DSM-5 2013). Increased energy can be further operationalized as psychomotor agitation and/or increased goal-directed activity and has been linked to dysregulation of the catecholamine system, notably dopamine (DA). Several lines of evidence suggest that DA is involved the pathology of BD (Cousins et al. 2009); for example BD patients have higher levels of urinary catecholamines (Grossman and Potter 1999; Koslow et al. 1983); DA-transporter (DAT) availability in the striatum is lower in people with BD (Anand et al. 2011); and DA-related genes such as the DAT gene may confer susceptibility to BD (Greenwood et al. 2001; Greenwood et al. 2006). Supporting the role of the catecholamine system in BD (van Enkhuizen et al. 2015) is the observation that drugs that increase catecholamine activity, such as antidepressants, can sometimes induce a manic state (Salvi et al. 2008). Furthermore, marked hyperactivity can be triggered in humans or rodents by psychostimulants such as amphetamine, which act via the norepinephrine (NE) transporter (NET) and the DAT, suggesting that these mechanisms may be involved in these behaviors.
Our previous work has attempted to quantify aspects of increased motor activity in humans with BD and animal models of mania to further delineate the distinctive neurobiology of this disorder. Increased activity can be objectively quantified across species using our human and animal Behavioral Pattern Monitor (BPM). Using this approach, we demonstrated that manic BD humans are characterized by increased motor activity (Minassian et al. 2010), increased goal-directed behavior as evidenced by interactions with novel stimuli (Perry et al. 2010), and a spatial pattern of straight, distance-covering movements (Perry et al. 2009). The studies above suggested that this profile was unique to mania and not seen in schizophrenia, nor was it observed in a small sample of people with attention deficit hyperactivity disorder (ADHD) (Paulus et al. 2007). This phenotype is also seen in an attenuated form in non-manic phases of the disorder (Henry et al. 2013). Studying animal models of BD mania in parallel with the human investigations enabled the refinement of models that best matched the behavioral profile unique to mania. In attempting to recreate the profile, the closest models involved reducing the function of catecholamine clearance mechanisms. Specifically, in mice, either pharmacological treatment with the selective DAT inhibitor GBR12909 or genetically induced knockdown of the DAT mimicked the behavioral profile observed in humans with manic BD. Thus, mice with either an acute or lifelong reduction in DAT function exhibited increased motor activity as evidenced by movement counts, increased goal-directed behavior as measured by holepokes, and longer, straighter patterns of spatial movement (Perry et al. 2009; Ralph et al. 2001a; Young et al. 2010b). Treatment with the mixed NET/DAT inhibitor amphetamine did not fully replicate the profile in mice however, only increasing motor activity and inducing straighter patterns of movement without a marked effect on goal-directed behavior (Perry et al. 2009). These data provide quantitative support for earlier human studies suggesting that amphetamine treatment does not sufficiently recreate BD profiles (Silverstone et al. 1998; Young et al. 2011a).
This cross-species approach supported a novel direction for modeling BD in mice by using amphetamine to block both DA and NE uptake. While pharmacological manipulation has been examined extensively, albeit separately, for many years in rodents and humans, no one has verified that broad inhibition of catecholamine reuptake is sufficient to increase activity of both healthy humans and mice with fidelity across species. For example, the majority of human studies using amphetamine to model BD have focused on subjective and/or cardiovascular effects, with no direct comparison to an equivalent animal model or paradigm. The aim of the current study was to assess a cardinal feature of BD that can be quantified in parallel human and animal paradigms and test whether pharmacological manipulations in healthy humans and in mice that non-selectively target the catecholaminergic system could recreate the increased exploratory behavior profile observed in BD. This “reverse-translational” approach has two purposes: 1) to determine whether amphetamine recreates a mania-like profile in humans; and 2) to further validate or invalidate the utility of our cross-species behavioral paradigm in capturing this cardinal symptom. We conducted a double-blind placebo-controlled study where one of two doses of d-amphetamine was administered to healthy volunteers prior to testing in the human version of the BPM (hBPM). We also conducted experiments in mice to replicate our previous findings of the effect of amphetamine in the mouse BPM, elaborating on these studies using a narrower dose range and investigating whether there were sex effects by including both male and female mice. We hypothesized that, in both humans and mice, amphetamine would increase motor activity irrespective of sex, but have no significant effect on goal-directed behavior as measured by novel object interactions in humans and holepokes in mice.
Methods- Humans
Participants
The University of California San Diego (UCSD) School of Medicine’s institutional review board approved the study. Participants were recruited from flyers and on-line advertisements posted in the community and included 66 male and female volunteers with the following inclusion criteria: 1. Ages 18–35. 2. In good general health. 3. No lifetime history of an Axis I or Axis II disorder. 4. No first-degree relative with a history of psychotic or mood disorder. 5. No specific contraindications or previous adverse reactions to amphetamine. Exclusion criteria included: 1. Clinically significant electrocardiogram or physical exam findings as determined by the study physician. 2. Women with a positive serum HCG pregnancy test or who are lactating. 3. History of alcohol or substance (e.g., sedative-hypnotics, cannabis, stimulants, opioids, cocaine, hallucinogens) abuse or dependence within the last 30 days, or a positive result on a urine toxicology screen for illegal substances completed on study entrance. Nicotine abuse or dependence was not an exclusion criterion. 4. Current severe, systemic medical illness that may compromise cognitive functioning or serious cardiac disease. 5. Current or history of neurological disorder such as seizures or stroke, Parkinson’s Disease, dementia, or a history of head injury with loss of consciousness for at least 15 min.
Participants who met all inclusion/exclusion criteria were randomized, in a double-blind fashion, to 1 of 3 groups: placebo, 10 mg d-amphetamine, or 20 mg d-amphetamine. The doses of amphetamine were based upon those used in previous studies (Ballard et al. 2014; de Wit et al. 2002; Hart et al. 2012; Weafer and de Wit 2013); the 10- and 20-mg doses have elicited behavioral effects and have been generally well-tolerated in those reports. As these previous studies illustrate, it is not standard practice to adjust drug doses to weight of the subject in human pharmacological challenge studies.
At study’s end and final unblinding, 25 subjects had been randomized to placebo, 18 subjects to amphetamine 10 mg, and 23 subjects to amphetamine 20 mg. The sample size for the 10-mg dose is lower because an interim unblinding and data analysis indicated no trends for effects of the 10 mg dose; thus in an effort to complete data collection in a timely manner the 10-mg dose was dropped from the study. Demographic data can be found in Table 1.
Table 1.
Demographic information and comparisons for subjects given placebo (n=25), amphetamine 10 mg (n=18), and amphetamine 20 mg (n=23). Values are means (standard deviations) unless otherwise specified.
| Placebo | Amphetamine 10 mg |
Amphetamine 20 mg |
Group difference test |
|
|---|---|---|---|---|
| Age (years) | 22.9 (4.2) | 22.7 (4.8) | 22.4 (3.9) | F(2,63)<1, NS |
| Years of education |
15.0 (1.8) | 15.9 (2.5) | 15.1 (1.4) | F(2,63)=1.4, NS |
| Gender | 12 Male, 13 Female | 7 Male, 11 Female | 11 Male, 12 Female | Fisher’s Exact<1, NS |
| Body Mass Index (Kg/m2) |
23.2 (2.7) | 23.5 (4.8) | 24.3 (4.6) | F(2,63)<1, NS |
| Baseline systolic blood pressure (mm Hg) |
116.2 (10.2) | 118.4 (10.8) | 122.1 (12.7) | F(2,63)=1.67, NS |
| Baseline diastolic blood pressure (mm Hg) |
73.7 (7.3) | 72.1 (8.7) | 74.7 (7.4) | F(2,63)<1, NS |
| Baseline heart rate (beats/min) |
72.0 (11.0) | 69.8 (11.9) | 73.9 (14.0) | F(2,63)<1 NS |
| Post-test systolic blood pressure (mm Hg) |
125.1 (15.2) | 122.8 (7.3) | 136.5 (11.4) | F(2,63)=6.1, p=0.005*@ |
| Post-test diastolic blood pressure (mm Hg) |
73.8 (13.0) | 74.9 (8.1) | 83.7 (8.4) | F(2,63)=5.0, p=0.01*@ |
| Post-test heart rate (beats/min) |
62.8 (8.1) | 69.1 (8.4) | 75.6 (18.6) | F(2,63)=4.1, p=0.02* |
| Change systolic | 7.3 (14.7) | 2.3 (9.9) | 13.5 (12.0) | F(2,63)=3.0, p=0.06@ |
| Change diastolic | −0.45 (11.2) | 0.42 (9.5) | 9.2 (9.8) | F(2,63)=4.8, p=0.01*@ |
p< .05, placebo vs. amphetamine 20 mg.
p < .05, amphetamine 10 mg vs. amphetamine 20 mg.
F F-statistic, NS not significant
Procedure
After providing informed consent, participants underwent a physical examination by an MD and were administered an ECG. Clinically significant findings on these exams constituted exclusion from the study (n=5). They were then assessed with the SCID to rule out Axis I and Axis II disorders. They were asked for a urine sample for toxicology analysis and pregnancy test if applicable. Randomization and administration of study drug was conducted by the UCSD Investigational Pharmacy.
Participants entered the hBPM 60 min after administration of study drug, based upon the pharmacokinetic profile of amphetamine and evidence that it reaches peak plasma concentrations at 2–2.5 hr post-ingestion (Wong et al. 1998) and broadly consistent with other studies on the behavioral effects of d-amphetamine (60 min-90 min) (Ballard et al. 2014; de Wit et al. 2002; Weafer and de Wit 2013). Assessment of vital signs (heart rate and blood pressure) was conducted prior to ingestion of study drug and immediately prior to the end of the entire test battery. Since physiologic parameters can be impacted by body weight, height and weight were collected for the calculation of Body Mass Index (BMI).
Prior to entering the hBPM, participants were fitted with an ambulatory monitoring device worn on the torso that quantifies motor activity via triaxial accelerometer output (Hidalgo 2010; Vivometrics 2002). Encrypted data were stored on a removable memory card and subsequently extracted and analyzed with the Vivosense™ proprietary PC-based software (version 2.7). Mean acceleration values were generated from a filtered summation of movement on the x, y, and z axes while incorporating force effects due to gravity. After being fit with the monitor, individuals were placed in the hBPM for 15 min without explicit directions except to wait for the experimenter to prepare another task.
The hBPM has been described previously (Henry et al. 2013; Henry et al. 2011; Minassian et al. 2010; Minassian et al. 2011; Perry et al. 2010; Perry et al. 2009). The hBPM consists of a 3.5 m by 4.9 m rectangular room that contains several items of furniture, including two bookcases, several tall filing cabinets, a corkboard mounted on the wall, and a short storage chest, but no chairs. A number of small objects were placed throughout the room to stimulate exploration and invite participant interaction (Henry et al. 2011; Minassian et al. 2011; Perry et al. 2009). These items were selected to meet several criteria, including being safe, colorful, tactile, and manipulable (Pierce and Courchesne 2001); they include a feather mask that could be worn, a kaleidoscope, finger puppets, and a paddle ball game. Participant activity in the room was monitored and recorded by a camera concealed in the ceiling that is equipped with a fish-eye lens capable of viewing the entire room. Digitized videos sampled at 30 frames per second were stored on a computer in an adjacent room for subsequent analysis (Perry et al. 2010).
During the informed consent process, subjects were told that they might be videotaped during a part of their examination, but were not specifically instructed that this recording would occur during the hBPM session. Following the hBPM session, subjects were returned to the laboratory where other testing was completed. To ensure safety after the potential administration of amphetamine, subjects were observed for three additional hours while they remained in the laboratory to complete other testing. Blood pressure and heart rate were measured prior to leaving the laboratory, and a physician confirmed that each subject’s vital signs were normal prior to the subject’s departure.
Data Analysis and Statistics
To assess motor activity, mean acceleration in digital units was derived for each of the three 5-min time periods of the 15-min hBPM session; mean values of acceleration are a common and accepted method with which to determine static and dynamic motor activity (Godfrey et al. 2008).
Object interactions in the hBPM were quantified manually by trained raters blind to group condition who evaluated participant exploration in one-second increments during the video recording. We have previously established interrater reliability for hBPM video ratings; kappa reliability coefficients for rater-coded measures range from 0.91 to 0.96 (Perry et al. 2010). We quantified the total number of object interactions, defined as deliberate physical contact with a novel object with any part of the body, e.g., hand or foot.
To assess spatial patterns of behavior, digitized video images were subjected to frame-by frame analysis with proprietary software (TopScan 1.0; Clever Systems Inc, Washington, DC) that generates x-y coordinates of subjects within a 720 by 480 pixel grid. The x-y data were initially processed with a low-pass Butterworth filter to remove instrumental noise. Spatial d (i.e., dimensionality), measured between values of 1 and 2, indicates the extent to which a subject travels in a straight line (close to 1) or adopts a more convoluted, meandering path, such as very localized, circumscribed movements (close to 2). This measure is calculated by plotting the successive x-y coordinates of the path traveled against varying lengths of measuring resolutions (e.g., measuring the distance traveled with a small versus large ruler). The distance traveled is plotted against the number of movement counts using a double-logarithmic plot, with the slope of this line of fit being used to calculate spatial d (Paulus and Geyer 1991). Values at either end of this range may indicate perseverative behavior, as indicated by the tendency of manic BD individuals and DAT KD mice to engage in abnormally straight and repetitive patterns of activity (Perry et al. 2009; Young et al. 2010b).
The data were inspected for normality and homogeneity of variance. Outliers were defined as values that were 3 or greater standard deviations from the mean (Stevens 1992); these values were truncated to within 3 standard deviations in order to preserve their relative value while retaining power. A total of six outlier values were truncated. After removal of outliers, the distributions were again inspected and hBPM data were found to be normally distributed (skew and kurtosis values < +/−1). Group differences were tested using repeated-measures analyses of variance (ANOVA), with the three 5-min time periods of hBPM exposure as the repeated measure and group (placebo, 10-mg amphetamine, 20-mg amphetamine) as the between-subjects measures. Effect sizes were calculated with partial eta-squared. Statistical analyses were conducted with SPSS version 22.0 (IBM Corporation 2013).
Methods-Mice
Animals
Eighty C57BL/6J mice (40 female, 40 male) were obtained from Jackson Laboratories and tested 9–10 days after their arrival at approximately 3 months of age. Mice were housed four per cage in a reverse-light cycle room (lights off at 8:00 am) at UCSD. Animals had access to food (Harlan, Madison, WI, USA) and water ad libitum except during testing. Mice were allowed to acclimate to a dark testing room for a minimum of 60 min before testing. All testing occurred between 9:00 am and 5:00 pm. This range has been standard practice in our laboratory, since no effect of time of testing was observed and in an analysis of 253 male C57BL/6 mice (Tanaka et al. 2012). Testing took place over two days. All male mice were tested on day one and all females on day two. All testing procedures were approved by the UCSD Institutional Care and Use Committee and all facilities met federal and state requirements for animal care as approved by the American Association for Accreditation of Laboratory Animal Care.
Drugs
d-Amphetamine sulfate (Sigma, St. Louis, MO, USA) was dissolved in saline and administered in a volume of 5 ml/Kg. One of four doses of amphetamine (1.4, 2.5, 4.5, or 7.9 mg/Kg) or vehicle was administered by intraperitoneal (i.p.) injection immediately before placing the mouse into the testing chamber and initiating the test session. For analysis of dose response, drug doses were based on a ¼ logarithmic scale, one dose below and two doses above 2.5 mg/Kg, a dose previously shown to increase locomotor activity in this paradigm (Perry et al. 2009; Young et al. 2010a).
Mouse behavioral pattern monitor
Methods for the mBPM have been described previously (Perry et al. 2009; Risbrough et al. 2006; Young et al. 2010b). Mice were tested in 8 BPM chambers. Each chamber consisted of a 30.5 × 60 × 38-cm arena. A Plexiglas hole-board floor equipped with 3 floor holes, one each in the left, middle, and right of the floor, was enclosed on each side by clear Plexiglas walls with eight holes, one on each short wall and 3 on each long wall. Each hole was equipped with an infrared photobeam to detect holepoking. The chamber was illuminated by a single light (producing 350 lux in the center and 92 lux in each corner) positioned above the center of the arena. The location of the mouse across nine unequal regions (four corners, four walls, and center; (Geyer et al. 1986)) was recorded every 0.1 s by a 12 × 24 grid of infrared photobeams positioned 1 cm above the floor and 2.5 cm apart. Rearing was detected by an array of 16 photobeams placed 2.5 cm above the floor, along the long wall of the arena. Mice were placed in the upper left corner of the chamber at the beginning of a session which was then immediately initiated. The primary dependent measures of interest were total activity counts, holepokes, rearings, and spatial d.
This study was done to determine a dose response effect of amphetamine in male and female C57BL/6J mice administered saline (n=16, 8 male, 8 female) or amphetamine at 1.4 (n=16, 8 male, 8 female), 2.5 (n=16, 8 male, 8 female), 4.4 (n=16, 8 male, 8 female), or 7.9 (n=16, 8 male, 8 female) mg/Kg. Locomotor and behavioral activities of the mice were assessed for 60 min.
Data Analysis and Statistics
Data from the experiment were analyzed using a mixed analysis of variance, with sex and treatment as between-subject variables and time period (three 20-min time bins) as a within-subject variable. Where no main effects of sex were observed, analysis was collapsed across the variable. The data were analyzed using Biomedical Data Programs statistical software (Statistical Solutions Inc., Saugus, MA, USA). The alpha level was set to 0.05.
Results-Humans
The placebo and amphetamine groups did not differ significantly in terms of demographic variables or baseline heart rate and blood pressure (Table 1). Participants given 20-mg amphetamine had significantly higher post-test heart rate and blood pressure than the placebo group, and higher blood pressure than the 10-mg amphetamine group. Change in diastolic blood pressure from baseline to post-test was higher in the 20-mg amphetamine group than in the 10-mg and placebo groups, and change in systolic blood pressure was higher in 20-mg amphetamine subjects compared to 10-mg amphetamine subjects.
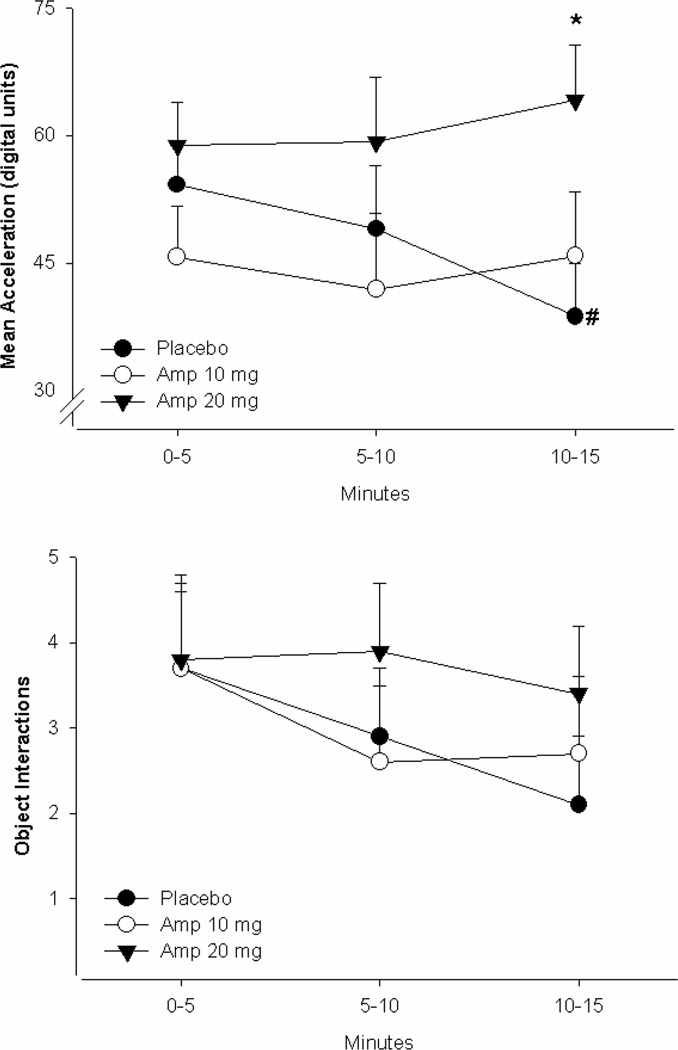
A repeated-measure ANOVA on acceleration indicated a significant time period-by-group interaction, with a medium effect size [F(4,122)=2.6, p=0.04, partial eta-squared =0.07]. In the placebo group there was a linear decrease in acceleration from the first to the final time period, but this decrease was not observed in the 20-mg amphetamine group, who had significantly higher acceleration than either the placebo or 10-mg amphetamine group in the third time period (Figure 1A). There was no significant main effect of time period [F(2,122)<1, NS, partial eta squared=0.01] nor a significant main effect of group [F(2,61)<1, NS, partial eta squared=0.06].
Figure 1. Motor Activity (acceleration) (Panel A) and object interactions (Panel B) in the hBPM in participants given placebo (n=25), amphetamine 10 mg (n=18) or amphetamine 20 mg (n=23).
The effects of d-amphetamine on exploratory behavior in humans were measured using the human behavioral pattern monitor (hBPM). Males and females were treated with either placebo (n=25) or amphetamine at 10 (n=18) or 20 (n=23) mg 1 h prior to assessment in the hBPM. Amphetamine increased activity as measured by acceleration (a), while no effect on object interactions (b) or any other measure was observed. Data presented as mean + SEM, *p?<?0.05 compared to placebo; #, p < 0.05 compared to 20 mg. Amp amphetamine
A repeated-measures ANOVA on object interactions indicated a significant main effect of time period [F(2,126)=3.4, p=0.04, partial eta squared=0.05] such that, in all groups, object interactions decreased across time periods (Figure 1B). There was no main effect of group [F(2,63)<1, NS, partial eta squared=0.01] nor a time period-by-group interaction [F(4,126)<1, NS, partial eta squared=0.02].
A repeated-measures ANOVA on spatial d indicated no significant main effects of time period [F(2,112)=1.05, NS, partial eta squared=0.02] or group [F(2,56) <1, NS, partial eta squared=0.01], nor a time period-by-group interaction [F(4,112)=1.02, NS, partial eta squared=0.04].
Results-Mice
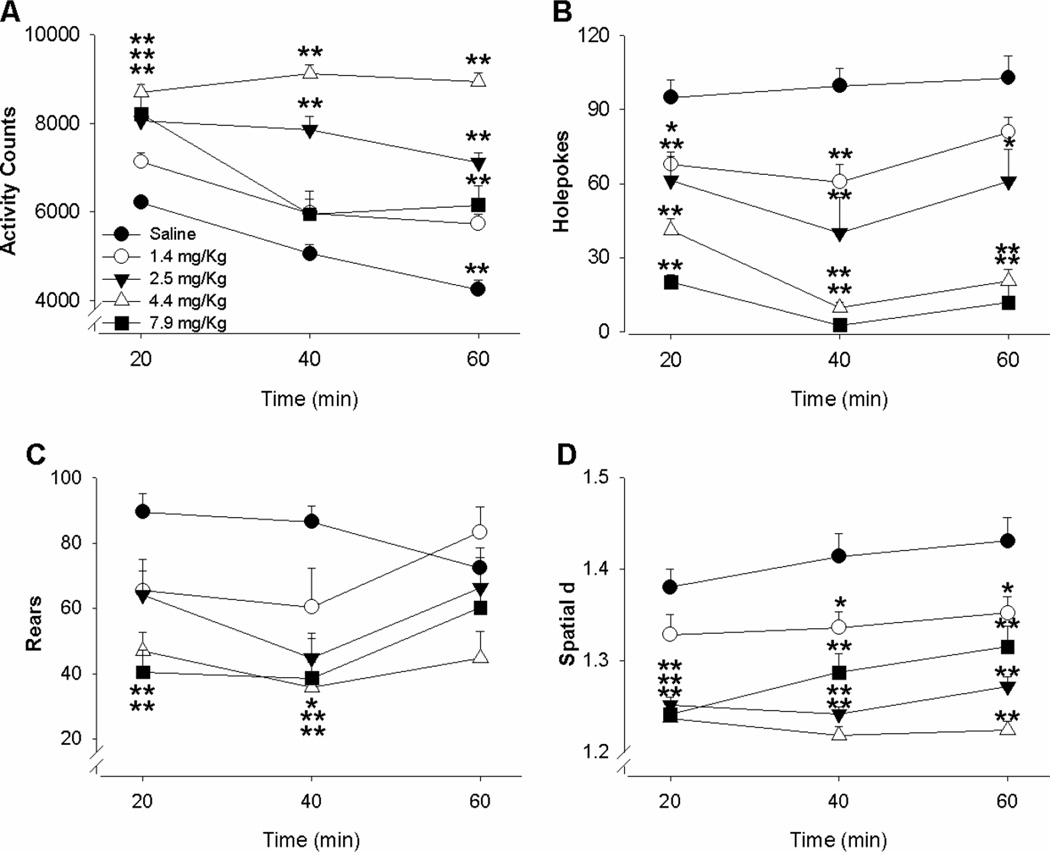
Amphetamine significantly increased overall activity (transitions) in a dose-dependent manner (Figure 2A). A significant main effect of drug (F(4,70)= 30.6, p<0.001) as well significant interaction of drug × time (F(8,140)=11.6, p<0.001) was witnessed. No main effect of sex (F(1,70)=2.16, NS) or interaction of sex × drug (F(4,70)<1, NS) was observed on overall activity. Post-hoc analyses revealed that the 7.9, 4.4, and 2.5 mg/Kg doses significantly elevated overall activity compared to vehicle in the first 20 min, while 4.4 and 2.5 mg/Kg doses elevated activity over the second 20-min bin, and all four doses of amphetamine significantly elevated activity compared to vehicle in the final 20-min bin (p<0.05).
Figure 2. Effects of d-amphetamine in mice in the BPM.
The effects of d-amphetamine on exploratory behavior in mice measured using the mouse behavioral pattern monitor (BPM). Male and female C57BL/6J mice (n=40 per sex) were treated with vehicle or d-amphetamine (1.4, 2.5, 4.5, or 7.9 mg/kg; n=8 per dose) immediately prior to their introduction to the mouse BPM. Amphetamine treatment significantly increased activity (a), while reducing holepoking (b), rearing (c), and lowering spatial d (d), in mice irrespective of sex. These effects were seen in each domain even at the lowest dose, except on activity levels. Data presented as mean + SEM, *p < 0.05 compared to saline, **p < 0.01 compared to saline
Specific exploration (holepoking) was significantly decreased by amphetamine in a dose-dependent manner (Figure 2B). A significant main effect of drug (F(4,70)=27.3, p<0.001) as well as a significant interaction of drug × time (F(8,140)=4.3, p<0.001) was observed. A significant main effect of sex (F(1,70)=5.5, p<0.05) was observed, however, sex did not significantly interact with drug (F(4,70)<1, NS) or time (F(2,140)=2.6, NS) as measured by holepoking. Post-hoc analysis revealed that all doses of amphetamine significantly decreased holepoking compared to vehicle in the first two 20-min time periods (p<0.05). In the final 20-min time period, 1.4 mg/Kg was the only dose to not differ significantly from vehicle.
Amphetamine dose dependently decreased another aspect of specific exploration, rearing (Figure 2C). A significant main effect of drug (F(4,70)= 3.8, p<0.005) as well significant interaction of drug × time (F(8,140)= 3.2, p<0.005) was observed on rearing. Post-hoc analyses revealed that rearing in the first 20-min time period was decreased by 7.9 and 4.4 mg/Kg amphetamine compared to vehicle (p<0.05). Over the second 20 min, 2.5 mg/Kg as well as 7.9 and 4.4 mg/Kg amphetamine significantly decreased rearing compared to vehicle (p<0.05). There were no significant differences in rearing over the final 20-min time period.
Finally, spatial d was significantly decreased by amphetamine in a dose-dependent manner (Figure 2D). A significant main effect of drug (F(4,70)= 24.7, p<0.001) as well as a significant interaction of drug × time (F(8,140)=3.4, p<0.001) was witnessed. No main effect of sex (F(1,70)=3.2, NS) or interaction of sex × drug (F(4,70)=1.4, NS) was witnessed. Post-hoc analysis revealed that spatial d was significantly decreased compared to vehicle by the 7.9. 4.4, and 2.5 mg/Kg dose of amphetamine over the first 20-min time period (p<0.05). All doses decreased spatial d compared to vehicle over the next two 20-min time periods.
Discussion
The goal of this study was to capitalize on our cross-species work to delineate biological mechanisms that contribute to the increased activity/energy profile characteristic of BD. In healthy humans, the 20-mg dose of amphetamine increased motor activity without increasing specific exploration and without marked effects on spatial patterns of activity. Previous work in the mouse version of the BPM demonstrated that a one-time dose of d-amphetamine increased motor activity while decreasing specific exploration (operationalized as holepokes) and increasing straight-line movements (operationalized as lower spatial d) (Perry et al. 2009; Ralph et al. 2001b). The current study replicates our previous findings in mice. In contrast, this amphetamine profile was inconsistent with the profiles observed in either the manic (Minassian et al. 2010; Minassian et al. 2011; Perry et al. 2009) or non-manic phases of BD (Henry et al. 2013), which are characterized by both increased motor activity and increased specific exploration. Thus, acute amphetamine as a model for mania has modest face validity (hyperactivity alone, no effect on object exploration or spatial d) and limited predictive validity [lithium treatment did not reverse amphetamine effects in humans; (Silverstone et al. 1998)].
Acute amphetamine treatment has often been used to model mania in mice and rats (Einat 2006; Machado-Vieira et al. 2004; Post and Weiss 1989; Shaldivin et al. 2001). The suitability of this model has been called into question (Silverstone et al. 1998; Young et al. 2011a). The current findings underscore the limited validity of the model, given that treatment with the non-selective NET/DAT inhibitor amphetamine did not recreate the behavioral profile of BD mania in either mice or humans (Perry et al. 2009). Although subchronic amphetamine dosing in rodents has also been used as a model of mania (Frey et al. 2006) and schizophrenia (Hijzen et al. 1991), these studies are fewer and have not been conducted in humans. Interestingly, former chronic methamphetamine use in humans did not increase activity but increased object interactions in the hBPM (Henry et al. 2011). In contrast to combined DAT/NET inhibition, both selective acute pharmacologic (e.g., GBR12909) and genetic (e.g., KD) reductions in DAT function in mice better mimicked the human BD mania profile, including increased exploration (Perry et al. 2009; Young et al. 2010a; b). The relative non-specificity of amphetamine to the DAT, e.g., its higher affinity for NET in humans and mice (Han and Gu 2006), and the fact that NET inhibition alone does not increase activity or selective exploratory behavior (Viggiano et al. 2004), underscores the likely greater importance of the DAT as a biological mechanism underlying abnormal behavior in BD. Importantly, the potential synergistic interactions between DAT and NET in influencing behavior have not been explored; such work could be conducted with genetic and pharmacological manipulations in rodents and potentially cross-validated by studying the potential epistasis of DAT- and NET-related genotypes in humans.
The human and mouse phenotypes of amphetamine-induced BPM profiles were not identical in that reduced specific exploration and straighter movements were observed in the mice but not in humans. Certainly the characteristics of the human BPM, which does contain furniture and objects (versus the mouse BPM, which apart from the holes is an empty chamber) may have limited the ability for amphetamine to induce straight movement patterns in the humans. It may also be relevant that the higher dose in humans, 20 mg, is equivalent to the mid-range doses administered in mice (2.5 – 4.4 mg/Kg in mice is equivalent to 0.20 – 0.35 mg/Kg in humans) (Reagan-Shaw et al. 2008), because the human participants in this study weighed between 60 to 80 Kg. Alternatively, the timing of dosing prior to testing may have introduced a lack of comparable results with some studies focusing on testing 90 min after drug administration (de Wit et al. 2002; Weafer and de Wit 2013). The briefer interval used here (60 min) is one limitation of this study; more pronounced effects on exploratory behavior may have been seen with a longer (e.g., 90 or 120 minute interval). Biological effects of the 20-mg dose were observed at the 60-minute interval however, with increased activity and increased heart rate in healthy humans irrespective of gender. The 10-mg dose was insufficient to produce either biological or behavioral effects in this study.
The administration of d-amphetamine in healthy humans has previously been associated with increased spontaneous motor activity (Greenwald et al. 1998). Amphetamine does, however, decrease impulsivity on cognitive tests (de Wit et al. 2002), which is consistent with its well-known pro-cognitive effects in humans and in mice (but see (Ballard et al. 2014)) and is in contrast to robust findings of increased impulsive behavior in people with BD, even in the euthymic phase (Najt et al. 2007). More recently, there is evidence that the behavioral effects of amphetamine may vary with the subjective experience of the drug by the subject (Weafer and de Wit 2013), which in turn seems to be genetically mediated (Hart et al. 2012). Given the limitations of amphetamine as a model for mania, the effects of catecholaminergic agents that are relatively more selective to DAT than NET on the phenotypes of activity and exploration (e.g., modafinil) would be important to study in humans. Modafinil was shown to increase the quantity of spontaneous motor activity in people with schizophrenia when compared to placebo (Farrow et al. 2006), but its effects on other dimensions of activity, e.g., specific exploration, have not been quantified in humans. These studies are in progress. In mice however, modafinil recreates the profile of high activity, high specific exploration, and straighter line movements seen in BD mania patients (Young et al. 2011b).
In conclusion, the present studies demonstrate modest predictive validity for the reverse-translated BPM. Consistent with mice, amphetamine treatment in humans increased activity without increasing specific exploration. These effects were observed irrespective of sex or gender. At higher doses in mice however, amphetamine treatment reduced specific exploration and induced straighter patterns of movement. Overall, these data indicate that non-selective inhibition of DAT and NET does not recreate the abnormal high-energy exploratory profile of BD mania patients, unlike that which occurs in mice following more selective DAT inhibition. These data therefore support the hypothesis that a selective hyperdopaminergic tone likely occurs during periods of mania.
Acknowledgements
This study was supported by funding from the National Institute of Mental Health (R01 MH071916), as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. The authors do not have a financial relationship with the funding organizations, and had full control of all primary data. The authors thank Dr. Harriet De Wit for her assistance in designing this study, Dr. Martin Paulus for his consultation on data analysis, and Dustin Kreitner, Elise Winbrock, and Karen Kloezeman for their contributions to data collection and analysis.
References
- Anand A, Barkay G, Dzemidzic M, Albrecht D, Karne H, Zheng QH, Hutchins GD, Normandin MD, Yoder KK. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar disorders. 2011;13:406–413. doi: 10.1111/j.1399-5618.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Ballard ME, Gallo DA, de Wit H. Amphetamine increases errors during episodic memory retrieval. Journal of clinical psychopharmacology. 2014;34:85–92. doi: 10.1097/JCP.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniaux E, Filgueiras A, Silva Rde A, Silveira LA, Nunes AL, Landeira-Fernandez J. Increased energy/activity, not mood changes, is the core feature of mania. Journal of affective disorders. 2014;152–154:256–261. doi: 10.1016/j.jad.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar disorders. 2009;11:787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- DSM-5. Diagnostic and statistical manual of mental health disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- Einat H. Modelling facets of mania--new directions related to the notion of endophenotypes. Journal of psychopharmacology (Oxford, England) 2006;20:714–722. doi: 10.1177/0269881106060241. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Hunter MD, Haque R, Spence SA. Modafinil and unconstrained motor activity in schizophrenia: double-blind crossover placebo-controlled trial. The British journal of psychiatry : the journal of mental science. 2006;189:461–462. doi: 10.1192/bjp.bp.105.017335. [DOI] [PubMed] [Google Scholar]
- Frey BN, Valvassori SS, Reus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J. Changes in antioxidant defense enzymes after d-amphetamine exposure: implications as an animal model of mania. Neurochemical research. 2006;31:699–703. doi: 10.1007/s11064-006-9070-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacology, biochemistry, and behavior. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Godfrey A, Conway R, Meagher D, G OL. Direct measurement of human movement by accelerometry. Med Eng Phys. 2008;30:1364–1386. doi: 10.1016/j.medengphy.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Schuster CR, Johanson CE, Jewell J. Automated measurement of motor activity in human subjects: effects of repeated testing and d-amphetamine. Pharmacology, biochemistry, and behavior. 1998;59:59–65. doi: 10.1016/s0091-3057(97)00387-0. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, Kelsoe JR. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. American journal of medical genetics. 2001;105:145–151. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1161>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Molecular psychiatry. 2006;11:125–133. 115. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Grossman F, Potter WZ. Catecholamines in depression: a cumulative study of urinary norepinephrine and its major metabolites in unipolar and bipolar depressed patients versus healthy volunteers at the NIMH. Psychiatry research. 1999;87:21–27. doi: 10.1016/s0165-1781(99)00055-4. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC pharmacology. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Engelhardt BE, Wardle MC, Sokoloff G, Stephens M, de Wit H, Palmer AA. Genome-wide association study of d-amphetamine response in healthy volunteers identifies putative associations, including cadherin 13 (CDH13) PloS one. 2012;7:42646. doi: 10.1371/journal.pone.0042646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Patt VM, Hua J, Young JW, Geyer MA, Perry W. Inhibitory deficits in euthymic bipolar disorder patients assessed in the human behavioral pattern monitor. Journal of affective disorders. 2013;150:948–954. doi: 10.1016/j.jad.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, van Rhenen M, Young JW, Geyer MA, Perry W. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacology. 2011;215:697–707. doi: 10.1007/s00213-011-2170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo. Equivital lifemonitor. Cambridgeshire, UK: 2010. [Google Scholar]
- Hijzen TH, Broersen LM, Slangen JL. Effects of subchronic d-amphetamine on prepulse and gap inhibition of the acoustic startle reflex in rats. Biol Psychiatry. 1991;29:1119–1128. doi: 10.1016/0006-3223(91)90254-j. [DOI] [PubMed] [Google Scholar]
- IBM Corporation. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp.; 2013. [Google Scholar]
- Koslow SH, Maas JW, Bowden CL, Davis JM, Hanin I, Javaid J. CSF and urinary biogenic amines and metabolites in depression and mania. A controlled, univariate analysis. Archives of general psychiatry. 1983;40:999–1010. doi: 10.1001/archpsyc.1983.01790080081011. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Kapczinski F, Soares JC. Perspectives for the development of animal models of bipolar disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2004;28:209–224. doi: 10.1016/j.pnpbp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. Journal of affective disorders. 2010;120:200–206. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PloS one. 2011;6:24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt P, Perez J, Sanches M, Peluso MA, Glahn D, Soares JC. Impulsivity and bipolar disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17:313–320. doi: 10.1016/j.euroneuro.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Progress in neuro-psychopharmacology & biological psychiatry. 1991;15:903–919. doi: 10.1016/0278-5846(91)90018-v. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Minassian A, Masten V, Feifel D, Geyer MA, Perry W. Human Behavioral Pattern Monitor differentiates activity patterns of bipolar manic and attention deficit hyperactivity subjects. Biological Psychiatry. 2007;61:227. [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA. Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry research. 2010;178:84–91. doi: 10.1016/j.psychres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Archives of general psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biological Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SR. Sensitization, kindling, and anticonvulsants in mania. The Journal of clinical psychiatry. 1989;50(Suppl):23–30. discussion 45–7. [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001a;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Geyer MA. Strain-specific effects of amphetamine on prepulse inhibition and patterns of locomotor behavior in mice. The Journal of pharmacology and experimental therapeutics. 2001b;298:148–155. [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Salvi V, Fagiolini A, Swartz HA, Maina G, Frank E. The use of antidepressants in bipolar disorder. The Journal of clinical psychiatry. 2008;69:1307–1318. doi: 10.4088/jcp.v69n0816. [DOI] [PubMed] [Google Scholar]
- Shaldivin A, Kaptsan A, Belmaker RH, Einat H, Grisaru N. Transcranial magnetic stimulation in an amphetamine hyperactivity model of mania. Bipolar disorders. 2001;3:30–34. doi: 10.1034/j.1399-5618.2001.030104.x. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Pukhovsky A, Rotzinger S. Lithium does not attenuate the effects of D-amphetamine in healthy volunteers. Psychiatry research. 1998;79:219–226. doi: 10.1016/s0165-1781(98)00037-7. [DOI] [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. New Jersey: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behav Brain Res. 2012;233:55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Janowsky DS, Olivier B, Minassian A, Perry W, Young JW, Geyer MA. The catecholaminergic-cholinergic balance hypothesis of bipolar disorder revisited. European journal of pharmacology. 2015;753:114–126. doi: 10.1016/j.ejphar.2014.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Arcieri S, Sadile AG. Involvement of norepinephrine in the control of activity and attentive processes in animal models of attention deficit hyperactivity disorder. Neural Plast. 2004;11:133–149. doi: 10.1155/NP.2004.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivometrics. The LifeShirt System™. Ventura, CA: 2002. [Google Scholar]
- Weafer J, de Wit H. Inattention, impulsive action, and subjective response to D-amphetamine. Drug and alcohol dependence. 2013;133:127–133. doi: 10.1016/j.drugalcdep.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YN, Wang L, Hartman L, Simcoe D, Chen Y, Laughton W, Eldon R, Markland C, Grebow P. Comparison of the single-dose pharmacokinetics and tolerability of modafinil and dextroamphetamine administered alone or in combination in healthy male volunteers. Journal of clinical pharmacology. 1998;38:971–978. doi: 10.1002/j.1552-4604.1998.tb04395.x. [DOI] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology. 2010a;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacology, biochemistry, and behavior. 2010b;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. British journal of pharmacology. 2011a;164:1263–1284. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kooistra K, Geyer MA. Dopamine receptor mediation of the exploratory/hyperactivity effects of modafinil. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011b;36:1385–1396. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]