Abstract
Liver injuries induced by carbon tetrachloride (CCL4) or thioacetamide (TAA) are dependent on cytochrome P450 2E1 (CYP2E1). CYP2A5 can be induced by TAA but not by CCL4. In this study, liver injury including fibrosis induced by CCL4 or TAA were investigated in wild type (WT) mice and CYP2A5 knockout (cyp2a5−/−) mice as well as in CYP2E1 knockout (cyp2e1−/−) mice as a comparison. Acute and sub-chronic liver injuries including fibrosis were induced by CCL4 and TAA in WT mice but not in cyp2e1−/− mice, confirming the indispensable role of CYP2E1 in CCL4 and TAA hepatotoxicity. WT mice and cyp2a5−/− mice developed comparable acute liver injury induced by a single injection of CCL4 as well as sub-chronic liver injury including fibrosis induced by one month of repeated administration of CCL4, suggesting that CYP2A5 does not affect CCL4-induced liver injury and fibrosis. However, while 200 mg/kg TAA-induced acute liver injury was comparable in WT mice and cyp2a5−/− mice, 75 and 100 mg/kg TAA-induced liver injury were more severe in cyp2a5−/− mice than those found in WT mice. After multiple injections with 200 mg/kg TAA for one month, while sub-chronic liver injury as indicated by serum aminotransferases was comparable in WT mice and cyp2a5−/− mice, liver fibrosis was more severe in cyp2a5−/− mice than that found in WT mice. These results suggest that while both CCL4- and TAA-induced liver injuries and fibrosis are CYP2E1 dependent, under some conditions, CYP2A5 may protect against TAA-induced liver injury and fibrosis, but it doesn’t affect CCL4 hepatotoxicity.
Keywords: Cytochrome P450, liver injury, fibrosis, metabolism, oxidative stress, hepatic stellate cell
INTRODUCTION
The cytochrome P450 (CYP) enzymes are a super-family of hemeprotein that serve as terminal oxidases in the mixed-function oxidase system for metabolizing various endogenous substrates, such as steroids and fatty acids, and xenobiotics, including drugs, toxins, and carcinogens. The enzymes are named CYP for cytochrome P450, followed by an Arabic number denoting the family (more than 40% identity on the amino acid sequence level), a letter designating the subfamily (more than 55% identity), and finally an Arabic numeral representing the individual gene in the subfamily (Lu and Cederbaum, 2008). Many chemicals including carbon tetrachloride (CCL4) and thioacetamide (TAA) cause liver injury via metabolism by CYPs. CCL4-induced lipid peroxidation (LPO) is believed to be dependent on CYP2E1 (Johansson and Ingelman-Sundberg, 1985), because anti-CYP2E1 IgG can block CCL4 induction of LPO (Ekström et al., 1989) and CCL4-induced liver injury was not observed in CYP2E1 knockout (cyp2e1−/−) mice (Wong et al., 1998; Avasarala et al., 2006). Similarly, TAA-induced hepatic necrosis also involves CYPs (Hunter et al., 1977; Porter et al., 1979) and TAA-induced liver injury was lower in cyp2e1−/− mice compared with wild type (WT) mice (Kang et al., 2008). These results suggest thatCYP2E1 is indispensible for TAA- and CCL4-induced liver injury.
CYP2A subfamily includes but not limits to CYP2A6 in humans, CYP2A5 in mice, and 2A3 in rats (Su and Ding, 2004). In rats, CYP2A3, which is orthologous to mouse CYP2A5 and human CYP2A6, is expressed at high levels in the olfactory mucosa but is not detectable in liver. Coumarin, a plant alkaloid, is hydroxylated specifically by coumarin 7-hydroxylase (COH) encoded by the human cyp2a6 gene and mouse cyp2a5 gene, a mouse ortholog to human CYP2A6, and COH activity is considered as a specific marker for catalytic activities of CYP2A5 and CYP2A6 (Su and Ding, 2004; Kirby et al., 2011; Abu-Bakar et al., 2013). In mouse liver, COH activity can be highly induced by pyrazole, but in rat liver COH is not detectable, even after treatments with pyrazole (Raunio et al., 1988). Therefore, mouse rather than rat is applied for studies in the field of human CYP2A6. Animal studies showed that TAA can induce cyp2a5 mRNA and COH catalytic activity in both DBA/2 mice and C57BL/6 mice (Salonpää et al., 1995), but CCL4 can’t induce COH catalytic activity in C57BL/6 mice although it can induce COH in DBA/2 mice with elevated basal COH activity (Pellinen et al., 1993). CYP2A5 knockout (cyp2a5−/−) mice on C57BL/6 background are useful for investigating the role of CYP2A5 in CYP2E1-mediated liver injury induced by TAA and CCL4. Because of the C57BL/6 background, CYP2A5 can be induced by TAA rather than CCL4 in WT mice but not in cyp2a5−/− mice, but CCL4 can’t induce CYP2A5 either in WT mice or in cyp2a5−/− mice. We hypothesize that CYP2A5 may affect liver injury induced by TAA but not by CCL4. Thus, the difference in liver injury should be observed in TAA model but not in CCL4 model.
MATERIALS AND METHODS
Chemicals
Carbon tetrachloride (reagent grade 99.9%) and thioacetamide (ACS reagent >99.0%) were purchased from Sigma-Aldrich, St. Louis, MO, USA. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) assay kits were purchased from Pointe Scientific, Canton, MI, USA.
Animals
SV/129-background CYP2E1 knockout (cyp2e1−/−) mice (Lee et al., 1996) were kindly provided by Dr. Frank J. Gonzalez (Laboratory of Metabolism, National Cancer Institute, Bethesda, MD, USA), and breeding colonies were established at Mount Sinai (Lu et al., 2008; 2010). The C57BL/J6 background CYP2A5 knockout (cyp2a5−/−) mouse colony was established at Mount Sinai by re-derivation from male cyp2a5−/− mice (Zhou et al., 2010) (kindly provided by Dr. Xinxin Ding, Wadsworth Center, New York State Department of Health, Albany, NY, USA) and female C57BL/J6 WT mice (purchased from Charles River Laboratory, MA, USA). All mice were used in this study were male and 2 months old. The mice were housed in temperature-controlled animal facilities with 12-hour light/12-hour dark cycles and were permitted consumption of tap water and Purina standard chow ad libitum. The mice received humane care, and experiments were carried out according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals and with approval of the Mount Sinai Animal Care and Use Committee.
Experimental design and Treatments
The cyp2a5−/− mice are C57BL/J6 background and the cyp2e1−/− are SV129 background, recently we found that there was no difference in acute and chronic liver injury induced by CCL4 and TAA between the C57BL/J6 WT mice and SV129 WT mice (Wang et al, 2014).
For acute liver injury induced by a higher dose of CCL4, the mice were divided into 6 groups of 7 mice each: 1. WT Control; 2. WT CCL4; 3. cyp2a5−/− Control; 4. cyp2a5−/− CCL4; 5. cyp2e1−/− Control; 6. cyp2e1−/− CCL4. In CCL4 groups, the mice were injected with CCL4 (CCL4 in mineral oil) intraperitoneally (i.p.) at 0.5 ml/kg. In Control groups, the mice were injected with mineral oil i.p. at the same volume. The mice were sacrificed 16 h after the injection.
For acute liver injury induced by a lower dose of CCL4, the mice were divided into 4 groups of 5 mice each: 1. WT Control; 2. WT CCL4; 3. cyp2a5−/− Control; 4. cyp2a5−/− CCL4. In CCL4 groups, the mice were injected with CCL4 i.p at 15 µl/kg. In Control groups, the mice were injected with mineral oil i.p. at the same volume. The mice were sacrificed 16 h after the injection.
For acute liver injury induced by different doses of TAA, the mice were divided into 12 groups of 5 mice each: 1. WT Control; 2. WT TAA 10 mg/kg; 3. WT TAA 50 mg/kg; 4. WT TAA 75 mg/kg; 5. WT TAA 100 mg/kg; 6. WT TAA 200 mg/kg; 7. cyp2a5−/− Control; 8. cyp2a5−/− TAA 10 mg/kg; 9. cyp2a5−/− TAA 50 mg/kg; 10. cyp2a5−/− TAA 75 mg/kg; 11. cyp2a5−/− TAA 100 mg/kg; 12. cyp2a5−/− TAA 200 mg/kg. In TAA groups, the mice were injected with TAA (TAA in saline) i.p. at different doses. In Control groups, the mice were injected with saline i.p. at the same volume. The mice were sacrificed 24 h after the injection.
For acute liver injury induced by a high dose of TAA, the mice were divided into 6 groups of 5 mice each: 1. WT Control; 2. WT TAA; 3. cyp2a5−/− Control; 4. cyp2a5−/− TAA; 5. cyp2e1−/− Control; 6. cyp2e1−/− TAA. In TAA groups, the mice were injected with TAA i.p. at 200mg/kg. In Control groups, the mice were injected with saline at the same volume. The mice were sacrificed 24 h after the injection.
For chronic liver injury and fibrosis induced by CCL4, the mice were divided into 6 groups of 7 mice each: 1. WT Control; 2. WT CCL4; 3. cyp2a5−/− Control; 4. cyp2a5−/− CCL4; 5. cyp2e1−/− Control; 6. cyp2e1−/− CCL4. In CCL4 groups, the mice were injected with CCL4 i.p. at 0.5 ml/kg, twice per week, for one month. In Control groups, the mice were injected with mineral oil i.p. at the same volume. The mice were sacrificed 48 h after the last injection.
For chronic liver injury and fibrosis induced by TAA, the mice were divided into 6 groups of 7 mice each: 1. WT Control; 2. WT TAA; 3. cyp2a5−/− Control; 4. cyp2a5−/− TAA; 5. cyp2e1−/− Control; 6. cyp2e1−/− TAA. In TAA groups, the mice were injected with TAA i.p. at 200mg/kg, twice per week, for one month. In Control groups, the mice were injected with saline at the same volume. The mice were sacrificed 48 h after the last injection.
Tissue preparations
The mice were sacrificed by cervical dislocation after blood was collected via the retro-orbital venous sinus under anesthesia by inhalation of isoflurane. The blood was put in room temperature for 1 h and then serum was isolated following a centrifuge at 3000 cpm for 10 min. Serum activity of ALT and AST were measured using kits (Pointe Scientific, Canton, MI, USA). The livers were rapidly excised into fragments and rinsed with cold saline. One piece of liver tissue from the biggest lobe without gall bladder was put in neutral Formalin solution to fix for paraffin bedding. The other liver tissues were snap-frozen and stored at −80°C.
Hepatic microsomes were prepared by placing liver aliquots in 0.15 M potassium chloride (KCl) and homogenizing in a polytron homogenizer for 10 strokes. The homogenate was centrifuged at 9,000 g for 20 min, and then the resulting supernatant fraction was centrifuged further at 105,000 × g for 60 min. The resulting pellets (microsomes) were re-suspended in 50 mM sodium phosphate buffer (pH 7.4). All procedures were carried out under cold conditions.
Cytochrome P450 2E1 and 2A5 Activity
CYP2E1 activity was measured by the rate of oxidation of 1 mM p-nitrophenol to p-nitrocatechol by 100 µg of microsomal protein for 15 min at 37 °C (Lu and Cederbaum, 2006). CYP2A5 activity was measured by assessing coumarin 7-hydroxylase activity with 100 µM coumarin as substrate plus 100 µg of microsomal protein and incubation for 15 min at 37 °C (Lu and Cederbaum, 2006).
Measurement of reduced glutathione (GSH) levels
Liver homogenate was mixed with trichloroacetic acid (TCA) to a final concentration of 5% TCA and the mixture was incubated at 4° C for 30 min to extract GSH. The TCA extracts (10 µl) were added to 200 µl of methanol containing 1 mg/ml o-phthalaldehyde and then were incubated for 15 min at 37°C in the dark (Hong et al., 2015). Fluorescence was measured at 350/420 nm (excitation/emission). The concentration of GSH was determined from a GSH standard curve.
Determination of Thiobarbituric Acid Reactive Substances (TBARS)
In brief, hepatic homogenates were incubated with 0.2 ml of TCA [15% (wt/vol)]-thiobarbituric acid (TBA) [0.375% (wt/vol)]-hydrogen chloride (HCl) (0.25 N) solution in a boiling water bath for 10 min. After centrifugation at 1,000 rpm for 5 min, the resulting supernatant was used to determine the formation of TBARS by evaluating absorbance at 535 nm (Hong et al., 2015). Malondialdehyde (MDA) was treated as above served as a standard.
Liver Histology and Immunohistochemistry
Liver sections were stained with hematoxylin and eosin (H&E) for necrosis evaluation. Sirius Red staining was performed to evaluate fibrosis. Fast green was used for background staining. Immunohistochemical staining (IHC) for collagen I was performed by using anti-collagen I antibody (Millipore), followed by a Broad Spectrum (AEC) Histostain-Plus kit (Invitrogen). No staining was observed in the absence of the primary antibody. For the computer-assisted quantification assessment, the integrated optical density (IOD) was calculated from 10 random fields per section containing similar size portal tracts and central veins at ×100 and using Image-Pro 7.0 Software (Media Cybernetics, Bethesda, MD, USA). The results were expressed as fold-change over the controls.
Statistics
Results are expressed as means ± SD. Statistical evaluation was carried out by using a two-way analysis of variance with subsequent the Student-Newman-Keuls post hoc test. P<0.05 was considered as statistical significance.
RESULTS
CCL4-induced acute liver injury, sub-chronic liver injury and fibrosis are dependent on CYP2E1 but not on CYP2A5
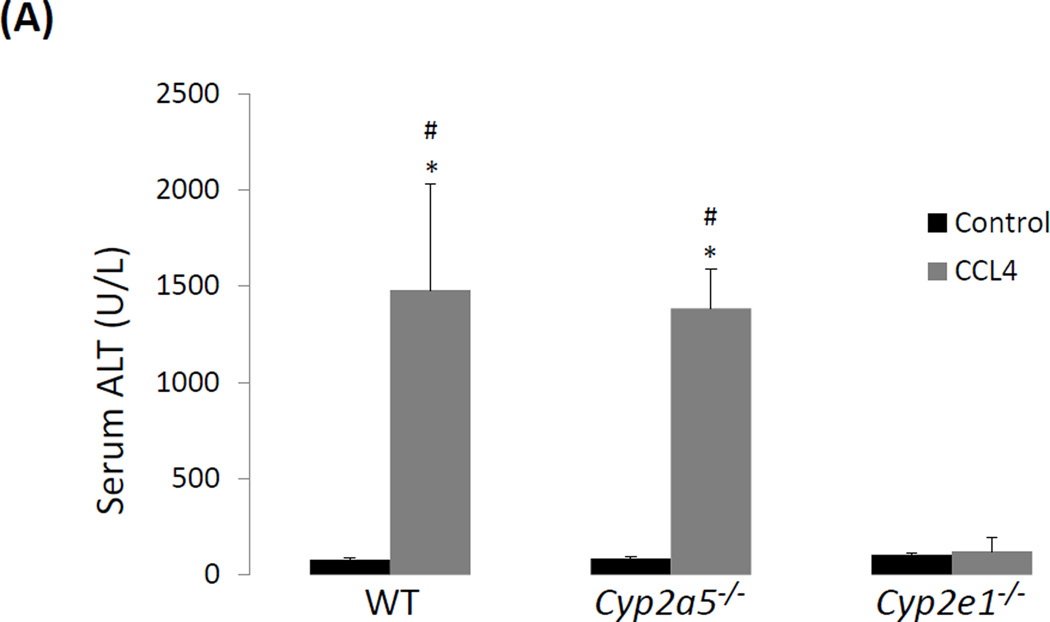
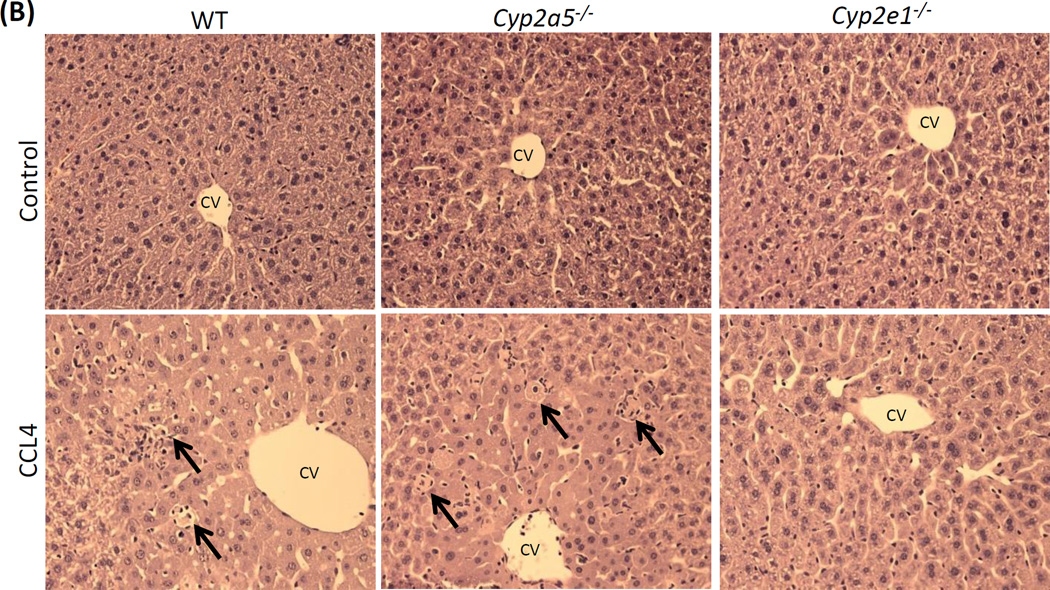
To examine whether CYP2E1-dependent CCL4 liver injury is also related to CYP2A5 activity, WT and cyp2a5−/− mice were injected with CCL4 at 0.5 ml/kg. CCL4-induced liver injury is not observed in cyp2e1−/− mice therefore cyp2e1−/− mice were also treated with same dose of CCL4 as a negative control. As shown in Fig 1A, after 24 h, serum ALT was increased comparably in WT mice and cyp2a5−/− mice, while serum ALT was not increased in cyp2e1−/− mice. Single cell death and necrotic foci were observed comparably in WT mice and cyp2a5−/− mice, while none was observed in cyp2e1−/− mice (Fig 1B). These results suggest that CCL4-induced liver injury was dependent on CYP2E1 rather than CYP2A5.
Figure 1.
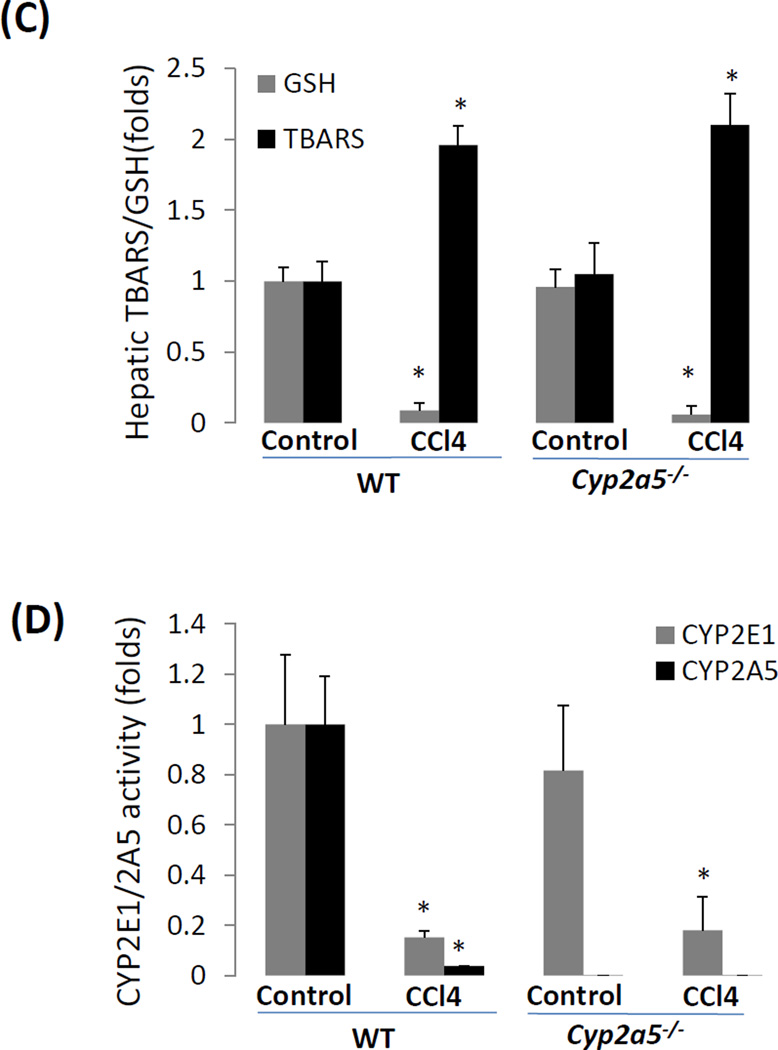
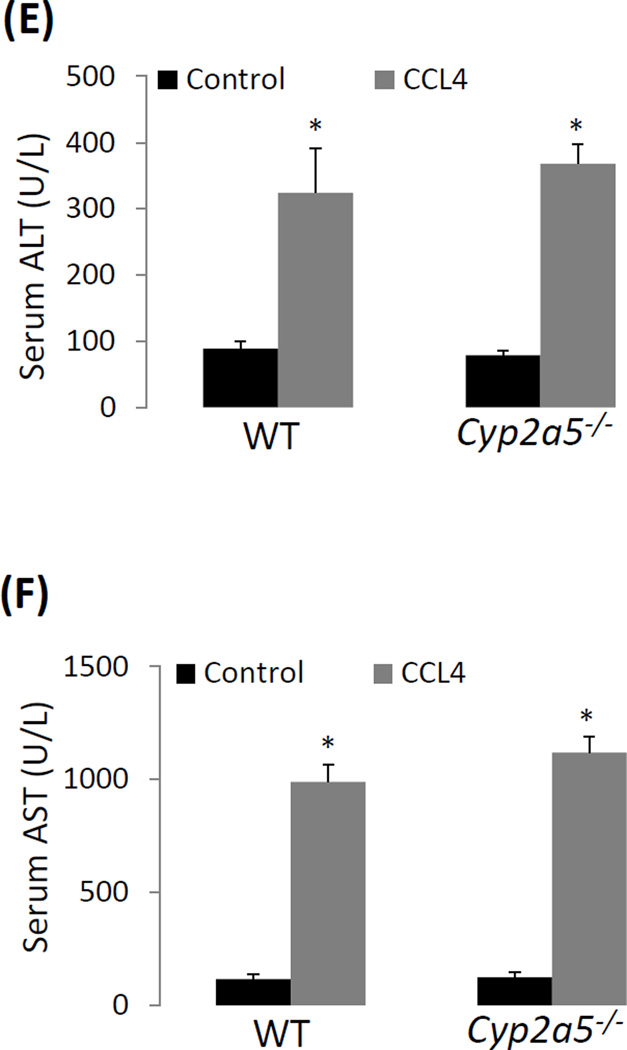
CCL4-induced liver injury and oxidative stress are CYP2E1 dependent but independent of CYP2A5. (A) Serum ALT activity and (B) pathological observation in liver sections with H&E staining (×200) after CCL4 was injected i.p. at 0.5 ml/kg into WT, cyp2a5−/− mice and cyp2e1−/− mice. CV, central vein. Arrows show small single necrotic foci. (C) Liver TBARS and GSH and (D) liver microsomal CYP2E1 and CYP2A5 activity after CCL4 was injected i.p. at 0.5 ml/kg into WT and cyp2a5−/− mice. (E) Serum ALT activity and (F) Serum AST activity after CCL4 was injected i.p. at 15 µl/kg into WT and cyp2a5−/− mice. * P<0.05, compared with Control; # P<0.05, compared with cyp2e1−/− mice group.
CCL4-induced LPO is involved in CCL4-induced liver injury (Johansson and Ingelman-Sundberg, 1985; Ekström et al., 1989). While CCL4-induced liver injury was comparable between WT mice and cyp2a5−/− mice, LPO as indicated by increases in TBARS and decreases in GSH was also comparable between WT mice and cyp2a5−/− mice (Fig 1C). After a single injection of CCL4, CYP activities were inhibited, and the inactivation of CYPs was one of the earliest signs of acute liver injury induced by CCL4 (de Groot H and Haas W, 1980). As shown in Fig. 1 D, both CYP2A5 and CYP2E1 were inhibited by administration of CCL4 in either WT mice or cyp2a5−/− mice.
We also examined liver injury induced by a lower dose of CCL4. As shown in Fig. 1 E and F, when mice were injected CCL4 at 15 µl/kg, serum ALT and AST were elevated comparably in WT mice and cyp2a5−/− mice after 24 h, suggesting that even at a lower dose, CCL4-induced mild liver injury is still independent of CYP2A5.
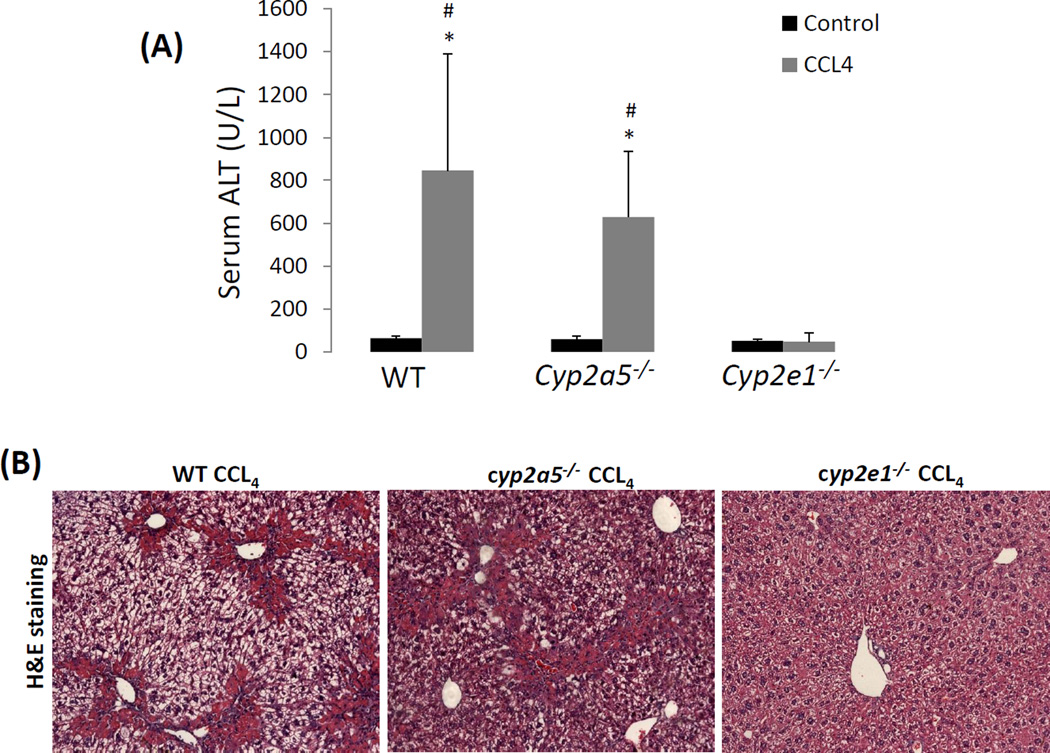
Next, we examined sub-chronic liver injury induced by a long-term (1 month) administration of CCL4 in WT mice, cyp2e1−/− mice and cyp2a5−/− mice. The mice were injected with CCL4 at 0.5 ml/kg, twice a week. After 1 month of injection, the mice didn’t show any evident clinical signs, and the body gain was similar to the control mice. The mice were sacrificed 48 h after the last injection of CCL4. In cyp2e1−/− mice, serum ALT remained normal after a long-term CCL4 administration, however, serum ALT was elevated by a long-term CCL4 in the cyp2a5−/− mice, which was slightly and insignificantly lower than those in WT mice (Fig 2 A). In paraffin liver sections with H&E staining, massive ballooning degeneration was observed in both of WT mice and cyp2a5−/− mice, while normal structure and morphology was observed in cyp2e1−/− mice after the long-term CCL4 treatment (Fig 2 B). These results suggest that sub-chronic liver injury induced by CCL4 is also independent of CYP2A5.
Figure 2.
CCL4-induced chronic liver injury and fibrosis are dependent on CYP2E1 but independent of CYP2A5. CCL4 was injected i.p. at 0.5 ml/kg into WT, cyp2a5−/− mice and cyp2e1−/− mice, twice a week, for one month. The mice were sacrificed 48 h after the last CCL4 injection. (A) Serum ALT activity. (B) H&E staining (×100). (C) Sirius Red/Fast Green staining(×100). Lower panels show the coarse surfaces of liver. Arrows show red stained fibers. (D) Quantification for Sirius Red staining. (E) IHC for collagen I (×100). Arrows show red stained collagen I. (F) Quantification for Collagen I IHC staining. * P<0.05, compared with Control; # P<0.05, compared with WT group; & P<0.05, compared with cyp2a5−/− mice group.
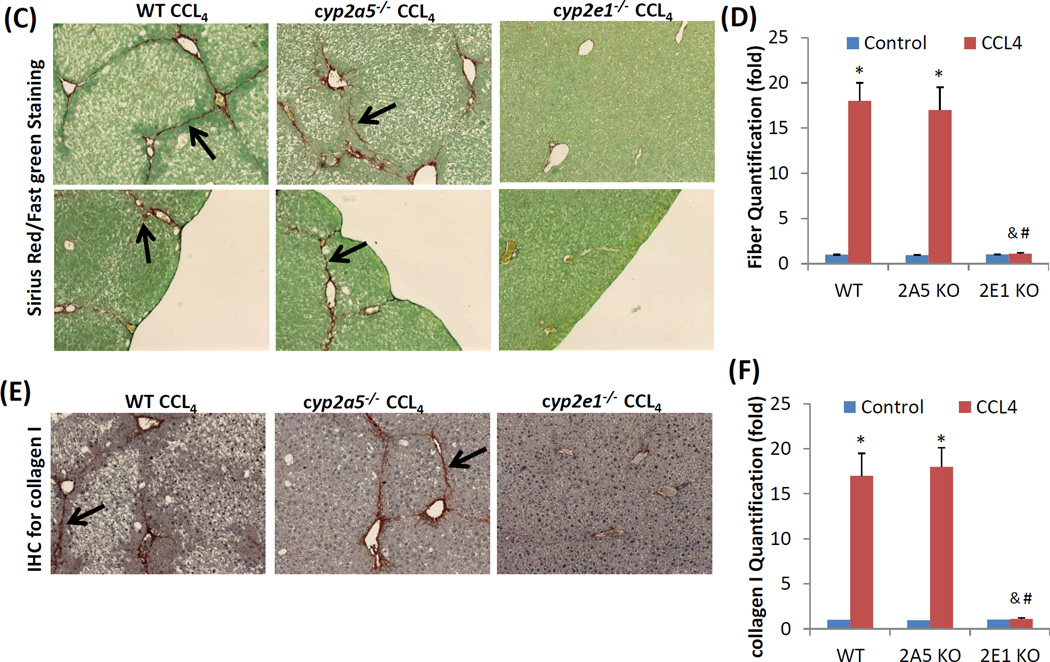
Liver fibrosis is characteristic of extra collagen deposition in extracellular matrix (Mallat and Lotersztajn, 2013). Sirius Red staining in paraffin liver sections was performed for collagen fiber observation. Collagen fibers were observed comparably in WT mice and cyp2a5−/− mice after the long-term CCL4 injection, whereas no fibrosis was observed in cyp2e1−/− mice (Fig 2 C, D). The similar extent of fibrosis in WT mice and cyp2a5−/− mice but not in cyp2e1−/− mice was also confirmed by IHC for collagen I (Fig 2 E, F). In fibrotic liver, the surface is coarse. As shown in Fig 2 C lower panels, after CCL4 treatment, liver surface was coarse in WT mice and cyp2a5−/− mice but not in cyp2e1−/− mice. These results suggest that CCL4-induced fibrosis is dependent on CYP2E1 but independent of CYP2A5.
CYP2A5 protects against low doses of TAA-induced mild liver injury
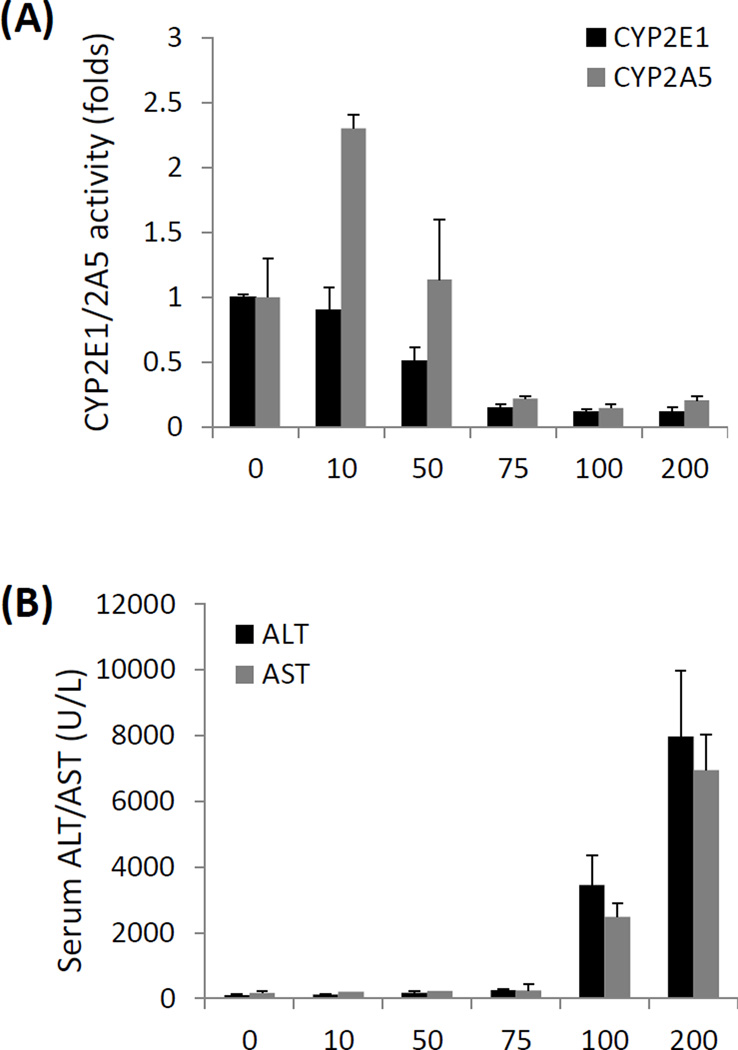
Unlike CCL4, TAA can induce CYP2A5 in C57BL/6 mice (Salonpää et al., 1995). To examine the relationship of TAA-induced liver injury and CYP2A5 activity, various doses of TAA were administrated to WT mice with C57BL/6 background. TAA inhibited CYP2E1 activity in a dose-dependent manner: while 10 mg/kg TAA had no effect on CYP2E1 activity, 50 mg/kg TAA inhibited CYP2E1 activity by 50%, and 75–200 mg/kg further decreased CYP2E1 activity to 90% (Fig. 3 A). However, TAA affected CYP2A5 activity in a different pattern: CYP2A5 activity was also inhibited dramatically by 75–200 mg/kg TAA, but it was induced 2.3-fold by 10 mg/kg TAA and remained unchanged after 50 mg/kg TAA treatment (Fig. 3 A). As for liver injury, 10 – 75 mg/kg TAA didn’t induce significant liver injury as reflected by the levels of serum ALT and AST, but 100 mg/kg TAA increased levels of serum ALT and AST up to around 3000 U/L and 200 mg/kg TAA further increased serum ALT and AST up to 8000 U/L (Fig. 3 B). These results suggest that TAA induces CYP2A5 but not CYP2E1 at a low, nontoxic dose, but TAA inhibits both CYP2A5 and CYP2E1 at a hepatotoxic dose.
Figure 3.
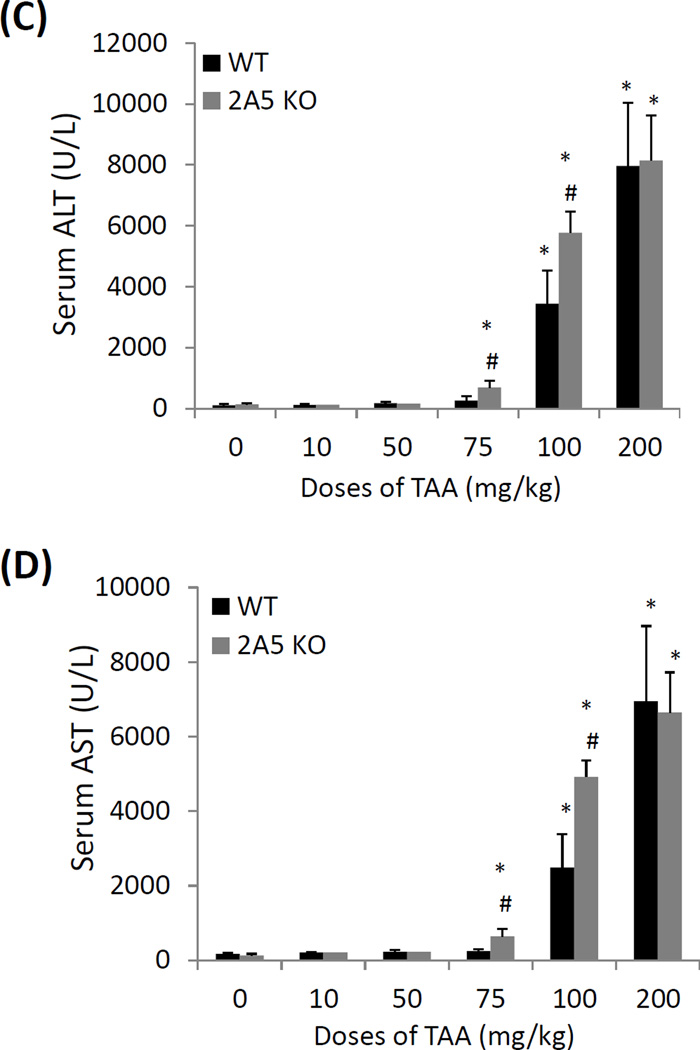
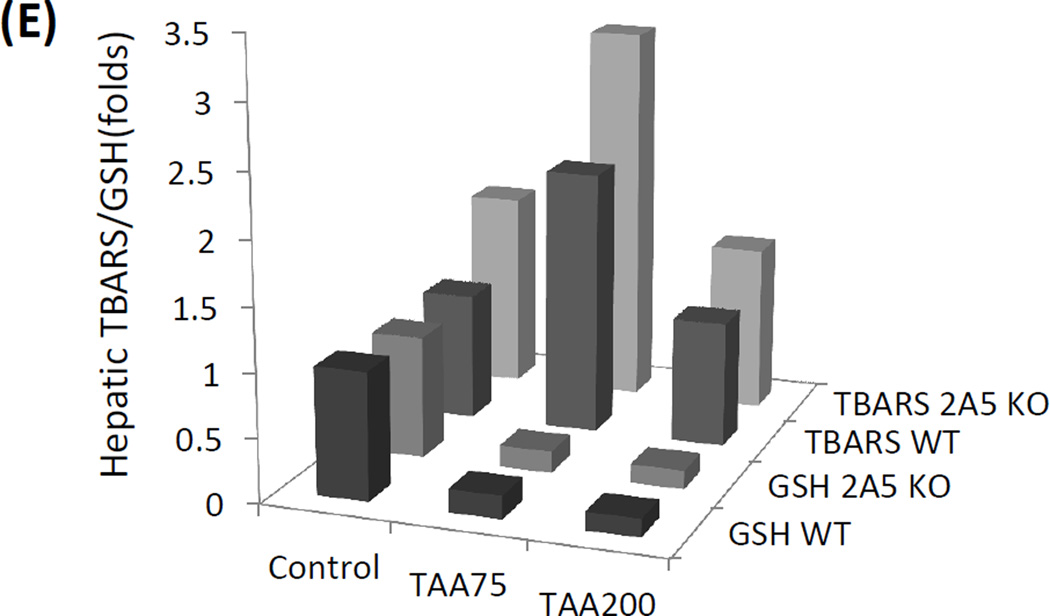
Relationship of CYP2A5 and TAA-induced liver injury and oxidative stress. (A) Liver microsomal activities of CYP2E1 and CYP2A5 and (B) serum levels of ALT and AST after the WT mice were injected with different doses of TAA. (C) Serum levels of ALT, (D) serum AST and (E) hepatic TBARS and GSH after different doses of TAA were injected into WT mice and cyp2a5−/− mice. * P<0.05, compared with Control group (0 mg/kg); # P<0.05, compared with WT group.
To investigate the role of CYP2A5 in TAA-induced liver injury, WT mice and cyp2a5−/− mice were administrated i.p. with TAA at doses of 10, 50, 75, 100, and 200 mg/kg. After 24 h, TAA at 10 and 50 mg/kg did not cause increases in serum ALT and AST in WT mice or cyp2a5−/− mice. While 75 mg/kg of TAA caused a slight and insignificant increase in serum ALT and AST in WT mice, serum ALT and AST were significantly increased up to 600–700 U/L in cyp2a5−/− mice. At 100 mg/kg of TAA, while serum AST and ALT were increased up to 2500–3500 U/L in WT mice and 5000–6000 U/L in cyp2a5−/− mice (Fig. 3 C and D). However, at dose of 200 mg/kg, serum ALT and AST levels were further increased up to 8000 U/L in WT mice and cyp2a5−/− mice comparably (Fig. 3 C and D). These results suggest that TAA-induced liver injury may be protected by CYP2A5 at lower doses (i.e. 75 and 100 mg/kg) rather than higher dose (200 mg/kg).
TAA also induces oxidative stress (Kang et al., 2008). Administration of 75 mg/kg of TAA caused hepatic GSH depletion comparably in WT mice and cyp2a5−/− mice, but TBARS was produced in cyp2a5−/− mice to a greater extent than in WT mice(Fig. 3 E), suggesting that 75 mg/kg of TAA-induced LPO is stronger in cyp2a5−/− mice, which may be associated with 75 mg/kg TAA-induced liver injury was more severe in cyp2a5−/− mice (Fig. 3 C and D). However, administration of 200 mg/kg of TAA caused hepatic GSH depletion comparably in WT mice and cyp2a5−/− mice, but TBARS production was not increased in either cyp2a5−/− mice or WT mice (Fig. 3 E), which is consistent with comparable and severe liver injury induced by 200 mg/kg of TAA in cyp2a5−/− mice and WT mice.
The cyp2e1−/− mice were also injected with TAA at 200 mg/kg, but serum ALT and AST were almost normal in cyp2e1−/− mice (data not shown), suggesting that CYP2E1 is indispensible for TAA liver injury.
TAA-induced liver fibrosis is more severe in cyp2a5−/− mice than in WT mice
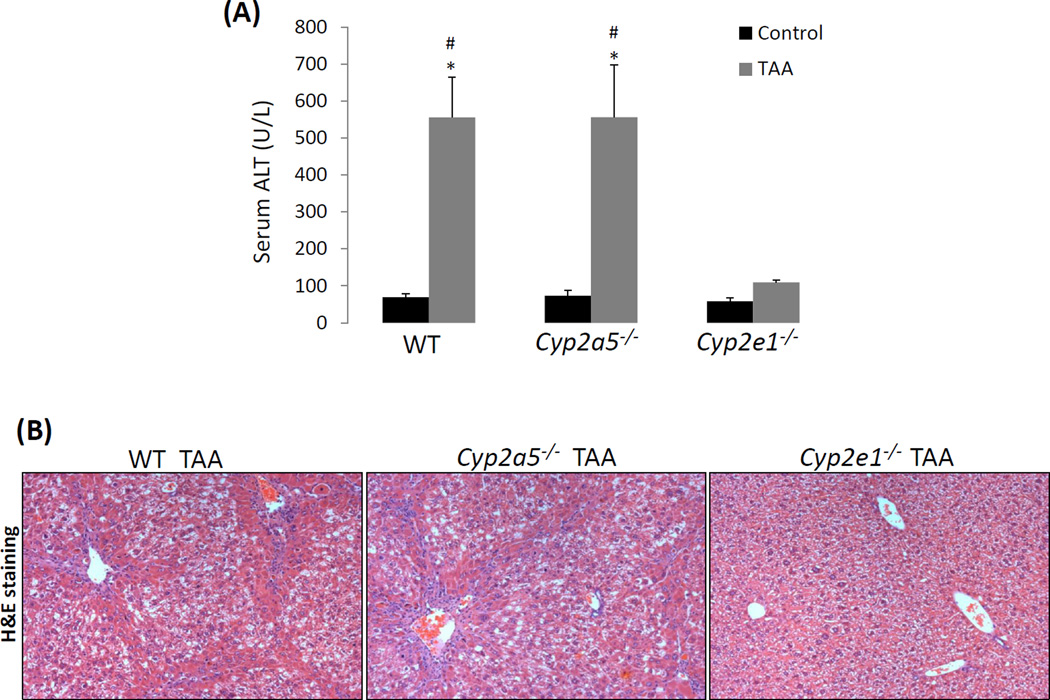
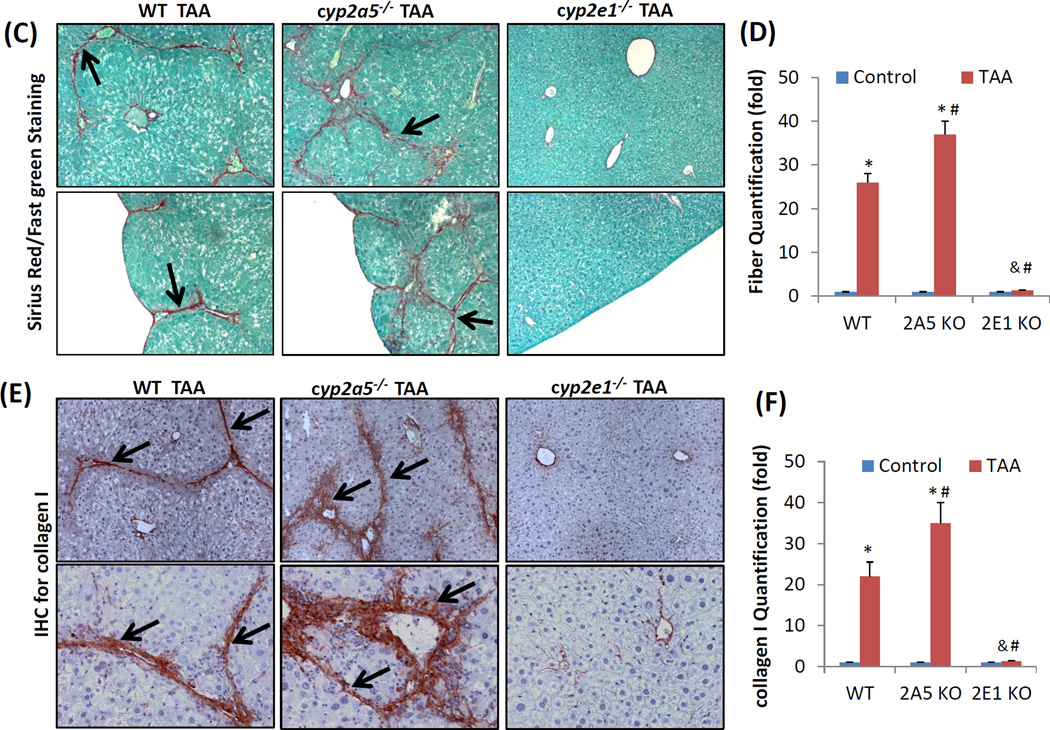
The fact that a low dose of TAA-induced liver injury was more severe in cyp2a5−/− mice led us to examine whether TAA-induced fibrosis is also more pronounced in cyp2a5−/− mice. Fibrosis is a response to chronic liver injury (Mallat and Lotersztajn, 2013). To exclude the possibility that higher grades of fibrosis is due to more severe liver injury, a dose of 200 mg/kg of TAA was selected because there is no difference in liver injury between WT and cyp2a5−/− mice at this high dose. WT and cyp2a5−/− mice were injected with TAA at 200 mg/kg, twice a week. After 1 month of injection with TAA, the mice didn’t show any evident clinical signs, and the body gain was similar to the control mice. The cyp2e1−/− mice were also treated with TAA to confirm the role of CYP2E1in TAA-induced liver fibrosis. The mice were sacrificed at 48 h after the last injection of TAA. Serum ALT was elevated to about 550 U/L in both WT and cyp2a5−/− mice, while it was not significantly increased in cyp2e1−/− mice (Fig 4 A). Consistent with serum ALT, H&E staining showed that massive ballooning degeneration and inflammatory cell infiltration were observed in both WT mice and cyp2a5−/− mice, while normal liver structure and morphology were observed in cyp2e1−/− mice (Fig 4 B), suggesting that high dose of TAA-induced sub-chronic liver injury was also dependent on CYP2E1 but independent of CYP2A5. Sirius Red/Fast Green staining showed that collagen fibers were observed in WT mice but not in cyp2e1−/− mice (Fig 4 C, D), suggesting that TAA-induced fibrosis is also dependent on CYP2E1. Unlike CCL4, TAA-induced fibrosis was stronger in cyp2a5−/− mice than in WT mice (Fig 4 C, D): fiber-containing septa in cyp2a5−/− mice were thicker than in WT mice (Fig 4 C). IHC for collagen I further confirmed the above observation (Fig 4 E, F). These results suggest that CYP2A5 may protect against TAA-induced liver fibrosis.
Figure 4.
TAA-induced liver fibrosis but not chronic liver injury is enhanced in the cyp2a5−/− mice compared to WT mice. TAA was injected at 200 mg/kg into WT mice, cyp2a5−/− mice and cyp2e1−/− mice, twice a week, for one month. The mice were sacrificed 48 h after the last TAA injection. (A) Serum ALT activity. (B) H&E staining (×100). (C) Sirius Red/Fast Green staining(×100). Lower panels show the coarse surfaces of liver. Arrows show red stained fibers. (D) Quantification for Sirius Red staining. (E) IHC for collagen I (×100 upper panels; ×200 lower panels). Arrows show red stained collagen I. (F) Quantification for Collagen I IHC staining. * P<0.05, compared with Control; # P<0.05, compared with WT group; & P<0.05, compared with cyp2a5−/− mice group.
DISCUSSION
It has long been known that CYP2E1 contributes to liver injuries induced by CCl4 and TAA. CYP2A subfamily is in association with CYP2E1in some aspect. For example, pyrazole can induce CYP2A5 as well as CYP2E1, and enhancement by pyrazole of lipopolysaccharides-induced liver injury in mice may involve both CYP2E1 and CYP2A5 in mice (Lu and Cederbaum, 2006). A major cause of boar taint in pigs is accumulation of 3-methylindole, which can be metabolized by both CYP2E1 and CYP2A (Terner et al., 2006). In this study we examined whether CYP2A5 affects liver injuries induced by CCl4 and TAA. We found that CYP2A5 does not affect CCL4-induced liver injury, but CYP2A5 may protect against moderate liver injury induced by TAA. While CYP2A5 has no effect on CCL4-induced liver fibrosis, it can protect against TAA-induced fibrosis.
CYP2E1 contributes to liver injury induced by TAA and CCL4 because CYP2E1 metabolizes TAA and CCL4 to hepatotoxic active metabolites (Johansson and Ingelman-Sundberg, 1985; Ekström et al., 1989; Hunter et al., 1977; Porter et al., 1979). CYP3A and other CYP isoforms including CYP2B may contribute to CCL4 metabolism (Zangar et al., 2000; Frank et al., 1982). However, unlike CYP2E1, other CYPs may detoxify CCL4 because CCL4-induced liver injury was almost negligible (Wong et al., 1998; Avasarala et al., 2006). It is well known that CYP2E1 is ethanol inducible (Lu and Cederbaum, 2008). Ethanol administration is known to increase CCL4- and TAA-induced liver injury, and the underlying mechanism involves the induction of CYP2E1 that accelerates the bio-activation of CCL4 and TAA (Maling et al., 1975; Strubelt et al., 1978). Recently we found that besides CYP2E1, mouse CYP2A5 can also be induced by ethanol, and very interestingly, ethanol induction of CYP2A5 is CYP2E1-dependent (Lu et al., 2011). It has never been examined whether CYP2A5 metabolizes TAA and CCL4. Due to the comparable liver injury between CCL4-treated WT mice and cyp2a5−/− mice, it seems that CYP2A5 has little, if any, effect on CCL4 metabolism. In contrast, TAA-induced liver injury and fibrosis were less severe than those observed in WT mice. Therefore, it will be interesting to address whether CYP2A5 metabolizes TAA.
Liver injury induced by TAA and CCL4 involves oxidative stress (Brattin et al., 1985; Kang et al., 2008). The redox sensitive transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) usually protects against oxidative injury via regulating a panel of antioxidant genes (Cederbaum, 2009). CYP2A6, the human ortholog of CYP2A5, is regulated by Nrf2 (Yokota et al., 2011; Jin et al., 2012). CYP2A5 levels in liver are lower in Nrf2−/− mice compared to WT mice (Lu et al., 2008; Lämsä et al., 2010). CYP2A5 might be among the panel of Nrf2-regulated antioxidants to inhibit oxidative liver injury. Indeed, ethanol-induced CYP2A5, which is regulated by Nrf2, protected against alcoholic liver injury (Lu et al., 2012; Hong et al., 2015). Likewise, TAA-induced liver injury and LPO were enhanced in cyp2a5−/− mice (Fig. 3). CCL4 does not induce CYP2A5, which seems to be a reason why CYP2A5 didn’t protect CCL4-induced liver injury and oxidative stress (Fig. 1). However, heavy metal cadmium can also induce CYP2A5, and the cadmium induction of CYP2A5 is also regulated by Nrf2 (Abu-Bakar et al., 2004), but CYP2A5 appeared to promote cadmium-induced liver injury (unpublished observation). It needs to further study on the relationship between CYP2A5 and oxidative liver injury.
The liver fibrogenic response is characterized by progressive accumulation of extracellular matrix components enriched in collagens (Mallat and Lotersztajn, 2013). In the present study, 200 mg/kg of TAA-induced fibrosis was more severe in cyp2a5−/− mice than in WT mice, suggesting that CYP2A5 has a protective effect against TAA-induced liver fibrosis. This protective effect of CYP2A5 is not due to its protective effect on liver injury, because 200 mg/kg of TAA induced similar acute and chronic liver injury in cyp2a5−/− mice and WT mice. Hepatic stellate cells (HSC) are a major cell type responsible for liver fibrogenesis. Upon chronic liver injury and inflammatory cell infiltration in parenchyma, normally quiescent HSCs become activated and acquire a myofibroblast-like phenotype. The activated HSCs, which become proliferative, contractile, and migratory, synthesize and secrete extra fibrillar collagen-I, which contributes to liver fibrosis (Mallat and Lotersztajn, 2013). Very interestingly, while we failed to detect CYP2E1 expression by western blotting analysis, we observed an expression of CYP2A5 in primary isolated mouse HSCs (Hong et al., 2015). Liver CYP2A5 in C57BL/6 mice can be induced by TAA but not by CCL4 (Salonpää et al., 1995; Pellinen et al., 1993). It is possible that CYP2A5 in HSCs can also be induced by TAA but not by CCL4. Whether CYP2A5 induction in HSC prevents HSC activation and the consequent fibrogenesis needs further study.
CONCLUSION
In conclusion, CCL4-induced oxidative liver injuries including fibrosis are independent of CYP2A5. Oxidative liver injuries induced by lower doses of TAA are protected by CYP2A5. CYP2A5 cannot protect against liver injury induced by a high dose of TAA, but it protects against liver fibrosis induced by the same dose of TAA.
ACKNOWLEDGEMENT
We thank Dr. Xinxin Ding for cyp2a5−/− mice and Dr. Gonzalez for cyp2e1−/− mice.
Grants: These studies were supported by the National Institute on Alcohol Abuse and Alcoholism (AA020877) and the National Natural Science Foundation of China (81170395).
ABBREVIATION
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CCL4
carbon tetrachloride
- COH
coumarin 7-hydroxylase
- CYPs
cytochrome P450s
- HSC
hepatic stellate cell
- IHC
Immunohistochemical staining
- LPO
lipid peroxidation
- TAA
thioacetamide
- Nrf2
nuclear factor-erythroid 2-related factor 2
Footnotes
Contribution:
Experiment design: C.S., A.I.C., H.X., Y.L.
Experiment performance: F.H., P.G., Y.L.
Data analyses and interpretation: F.H., C.S., P.G., A.I.C., H.X., Y.L.
Manuscript: A.I.C., Y.L.
The authors declare that there are no conflicts of interest.
REFERENCES
- Abu-Bakar A, Satarug S, Marks GC, Lang MA, Moore MR. Acute cadmium chloride administration induces hepatic and renal CYP2A5 mRNA, protein and activity in the mouse: involvement of transcription factor NRF2. Toxicol Lett. 2004;148:199–210. doi: 10.1016/j.toxlet.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Abu-Bakar A, Hakkola J, Juvonen R, Rahnasto-Rilla M, Raunio H, Lang MA. Function and regulation of the Cyp2a5/CYP2A6 genes in response to toxic insults in the liver. Curr. Drug Metab. 2013;14:137–150. [PubMed] [Google Scholar]
- Avasarala S, Yang L, Sun Y, Leung AW, Chan WY, Cheung WT, Lee SS. A temporal study on the histopathological, biochemical and molecular responses of CCl(4)-induced hepatotoxicity in Cyp2e1-null mice. Toxicology. 2006;228:310–322. doi: 10.1016/j.tox.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Brattin WJ, Glende EA, Jr, Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. Nrf2 and antioxidant defense against CYP2E1 toxicity. Expert Opin. Drug Metab. Toxicol. 2009;5:1223–1244. doi: 10.1517/17425250903143769. [DOI] [PubMed] [Google Scholar]
- de Groot H, Haas W, et al. O2-independent damage of cytochrome P450 by CCl4-metabolites in hepatic microsomes. FEBS Lett. 1980;115:253–256. doi: 10.1016/0014-5793(80)81180-x. [DOI] [PubMed] [Google Scholar]
- Ekström G, von Bahr C, Ingelman-Sundberg M. Human liver microsomal cytochrome P-450IIE1. Immunological evaluation of its contribution to microsomal ethanol oxidation, carbon tetrachloride reduction and NADPH oxidase activity. Biochem Pharmacol. 1989;38:689–693. doi: 10.1016/0006-2952(89)90217-7. [DOI] [PubMed] [Google Scholar]
- Frank H, Haussmann HJ, Remmer H. Metabolic activation of carbon tetrachloride: induction of cytochrome P450 with phenobarbital or 3-methylcholoanthrene and its effect on covalent binding. Chem. Biol. Interact. 1982;40:193–208. doi: 10.1016/0009-2797(82)90101-6. [DOI] [PubMed] [Google Scholar]
- Hong F, Liu X, Ward SS, Xiong H, Cederbaum AI, Lu Y. Absence of CYP2A5 enhances alcohol-induced liver injury in mice. Dig Liver Dis. 2015;47:470–477. doi: 10.1016/j.dld.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AL, Holscher MA, Neal RA. Thioacetamide-induced hepatic necrosis. I. Involvement of the mixed-function oxidase enzyme system. J Pharmacol Exp Ther. 1977;200:439–448. [PubMed] [Google Scholar]
- Jin M, Kumar A, Kumar S. Ethanol-mediated regulation of cytochrome P450 2A6 expression in monocytes: role of oxidative stress-mediated PKC/MEK/Nrf2 pathway. PLoS One. 2012;7:e35505. doi: 10.1371/journal.pone.0035505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Ingelman-Sundberg M. Carbon tetrachloride-induced lipid peroxidation dependent on an ethanol-inducible form of rabbit liver microsomal cytochrome P-450. FEBS Lett. 1985;183:265–269. doi: 10.1016/0014-5793(85)80790-0. [DOI] [PubMed] [Google Scholar]
- Kang JS, Wanibuchi H, Morimura K, Wongpoomchai R, Chusiri Y, Gonzalez FJ, Fukushima S. Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicol Appl Pharmacol. 2008;228:295–300. doi: 10.1016/j.taap.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Kirby GM, Nichols KD, Antenos M. CYP2A5 induction and hepatocellular stress: an adaptive response to perturbations of heme homeostasis. Curr. Drug Metab. 2011;12:186–197. doi: 10.2174/138920011795016845. [DOI] [PubMed] [Google Scholar]
- Lamlé J, Marhenke S, Borlak J, von Wasielewski R, Eriksson CJ, Geffers R, Manns MP, Yamamoto M, Vogel A. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159–1168. doi: 10.1053/j.gastro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Lämsä V, Levonen AL, Leinonen H, Yla-Herttuala S, Yamamoto M, Hakkola J. Cytochrome P4502A5 constitutive expression and induction by heavy metals is dependent on redox-sensitive transcription factor Nrf2 in liver. Chem. Res. Toxicol. 2010;23:977–985. doi: 10.1021/tx100084c. [DOI] [PubMed] [Google Scholar]
- Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: role of cytochrome P450 2E1 and 2A5. Hepatology. 2006;44:263–274. doi: 10.1002/hep.21241. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Gong P, Cederbaum AI. Pyrazole induced oxidative liver injury independent of CYP2E1/2A5 induction due to Nrf2 deficiency. Toxicology. 2008;252:9–16. doi: 10.1016/j.tox.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic. Biol. Med. 2010;49:1406–1416. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang XH, Cederbaum A. Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway. Toxicol Sci. 2012;128:427–438. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wu D, Cederbaum AI. Ethanol induction of CYP2A5: permissive role for CYP2E1. Drug Metab. Dispos. 2011;39:330–336. doi: 10.1124/dmd.110.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maling HM, Stripp B, Sipes IG, Highman B, Saul W, Williams MA. Enhanced hepatotoxicity of carbon tetrachloride, thioacetamide, and dimethylnitrosamine by pretreatment of rats with ethanol and some comparisons with potentiation by isopropanol. Toxicol. Appl. Pharmacol. 1975;33:291–308. doi: 10.1016/0041-008x(75)90096-4. [DOI] [PubMed] [Google Scholar]
- Mallat A, Lotersztajn S. Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am J Physiol Cell Physiol. 2013;305:C789–C799. doi: 10.1152/ajpcell.00230.2013. [DOI] [PubMed] [Google Scholar]
- Porter WR, Gudzinowicz MJ, Neal RA. Thioacetamide-induced hepatic necrosis: II. Pharmacokinetics of thioacetamide and thioacetamide-S-oxide in the rat. J. Pharmacol. Exp. Ther. 1979;208:386–391. [PubMed] [Google Scholar]
- Raunio H, Syngelma T, Pasanen M, Juvonen R, Honkakoski P, Kairaluoma MA, Sotaniemi E, Lang MA, Pelkonen O. Immunochemical and catalytical studies on hepatic coumarin 7-hydroxylase in man, rat, and mouse. Biochem. Pharmacol. 1988;37:3889–3895. doi: 10.1016/0006-2952(88)90070-6. [DOI] [PubMed] [Google Scholar]
- Pellinen P, Stenbäck F, Rautio A, Pelkonen O, Lang M, Pasanen M. Response of mouse liver coumarin 7-hydroxylase activity to hepatotoxins: dependence on strain and agent and comparison to other monooxygenases. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:435–443. doi: 10.1007/BF00171345. [DOI] [PubMed] [Google Scholar]
- Salonpää P, Krause K, Pelkonen O, Raunio H. Up-regulation of CYP2A5 expression by porphyrinogenic agents in mouse liver. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:446–452. doi: 10.1007/BF00169087. [DOI] [PubMed] [Google Scholar]
- Strubelt O, Obermeier F, Siegers CP. The influence of ethanol pretreatment on the effects of nine hepatotoxic agents. Acta Pharmacol. Toxicol. (Copenh) 1978;43:211–218. doi: 10.1111/j.1600-0773.1978.tb02257.x. [DOI] [PubMed] [Google Scholar]
- Su T, Ding X. Regulation of the cytochrome P4502A genes. Toxicol. Appl. Pharmacol. 2004;199:285–294. doi: 10.1016/j.taap.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Terner MA, Gilmore WJ, Lou Y, Squires EJ. The role of CYP2A and CYP2E1 in the metabolism of 3-methylindole in primary cultured porcine hepatocytes. Drug Metab Dispos. 2006;34:848–854. doi: 10.1124/dmd.105.008128. [DOI] [PubMed] [Google Scholar]
- Wang X, Lopategi A, Ge X, Lu Y, Kitamura N, Urtasun R, Leung TM, Fiel MI, Nieto N. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut. 2014;63:1805–1818. doi: 10.1136/gutjnl-2013-306373. [DOI] [PubMed] [Google Scholar]
- Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153:109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- Yokota S, Higashi E, Fukami T, Yokoi T, Nakajima M. Human CYP2A6 is regulated by nuclear factor-erythroid 2 related factor 2. Biochem. Pharmacol. 2011;81:289–294. doi: 10.1016/j.bcp.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Zangar RC, Benson JM, Burnett VL, Springer DL. Cytochrome P450 2E1 is the primary enzyme responsible for low-dose carbon tetrachloride metabolism in human liver microsomes. Chem Biol Interact. 2000;125:233–243. doi: 10.1016/s0009-2797(00)00149-6. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhuo X, Xie F, Kluetzman K, Shu YZ, Humphreys WG, Ding X. Role of CYP2A5 in the clearance of nicotine and cotinine: insights from studies on a CYP2a5-null mouse model. J. Pharmacol. Exp. Ther. 2010;332:578–587. doi: 10.1124/jpet.109.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]