Abstract
Medullary motoneurons drive vocalization in many vertebrate lineages including fish, amphibians, birds, and mammals. The developmental history of vocal motoneuron populations in each of these lineages remains largely unknown. The highly conserved transcription factor Paired-like Homeobox 2b (Phox2b) is presumed to be expressed in all vertebrate hindbrain branchial motoneurons, including laryngeal motoneurons essential for vocalization in humans. We used immunohistochemistry and in situ hybridization to examine Phox2b protein and mRNA expression in caudal hindbrain and rostral spinal cord motoneuron populations in seven species across five chordate classes. Phox2b was present in motoneurons dedicated to sound production in mice and frogs (bullfrog, African clawed frog), but not those in bird (zebra finch) or bony fish (midshipman, channel catfish). Overall, the pattern of caudal medullary motoneuron Phox2b expression was conserved across vertebrates and similar to expression in sea lamprey. These observations suggest that motoneurons dedicated to sound production in vertebrates are not derived from a single developmentally or evolutionarily conserved progenitor pool.
Keywords: Vocalization, Respiration, Hypoglossal, Lamprey, Midshipman, Zebra Finch
Introduction
Many vertebrates use sound production to communicate. Comparative analysis of development led to the hypothesis that an evolutionarily conserved neural network in the caudal medulla generates vocalization (Bass, 2014; Bass and Baker, 1997; Bass et al., 2008). Output from this conserved vocal generator projects to hindbrain and cervical spinal cord premotor and motor neurons leading to species-specific vocalization behaviors (Bass, 2014). Whether these premotor and motor neurons are also evolutionarily conserved is unclear.
The variety of motor behaviors encompassing vocalization across vertebrates requires the use of widely different and often species-specific muscles, innervated by different caudal hindbrain and rostral spinal cord motoneuron pools (Bass et al., 2008; Kelley and Bass, 2010; Ladich and Bass, 1998; Onuki and Somiya, 2007; Vasilakos et al., 2005; Wake, 1993; Wild, 1997). Vocalization in mammals employs the larynx, tongue, diaphragm and intercostal muscles for control of air and phonation (Brudzynski, 2009). Vocal production in songbirds similarly uses respiratory airflow through the uniquely avian syrinx (Wild, 1997). The syrinx is innervated by motoneurons of the tracheosyringeal division of the hypoglossal motor nucleus (XIIts) that makes up the posterior two thirds of the XII nuclear group with the remainder innervating the tongue (Manogue and Paton, 1982; Nottebohm et al., 1976).
In contrast, a variety of species have evolved non-respiratory mechanisms for sound production (Bass and Baker, 1997). While terrestrial frogs use air movement to support vocalization, fully aquatic species such as African clawed frogs (Xenopus laevis) communicate while under water using rhythmic contractions of intrinsic laryngeal muscles to produce brief sound pulses when the laryngeal arytenoid disks move (Brahic and Kelley, 2003; Ryan and Guerra, 2014; Tobias and Kelley, 1987; Yager, 1992). In both terrestrial and fully aquatic anurans, the larynx and the glottis are innervated by vagal motoneurons in the caudal medulla (Simpson et al., 1986; Straka et al., 2006).
In some teleost fish, sound is generated by the contraction of muscles that vibrate the swim bladder (Bass et al., 2008). These sonic muscles are innervated by motoneurons that run in either lateral or medial columns beginning at caudal levels of the vagal motor pool and extending into the cervical spinal cord (Bass et al., 2008; Ladich and Bass, 1998). The swim bladder muscles and motoneurons of toadfishes are referred to as vocal, in part, because they innervate muscle dedicated to sound production, like the syringeal muscles of birds (Bass et al., 1994).
Channel and other catfish species can produce sound by moving pectoral spines across grooves in the pectoral girdle, although some catfish species also have a sonic swim bladder mechanism (Fine et al., 1996; Ladich and Bass, 1996). As discussed elsewhere (Bass and Chagnaud, 2012), pectoral-dependent mechanisms for sound production are best designated as sonic, a term inclusive of a broad range of sound producing mechanisms that include the use of the pectoral skeletal-muscular system in fishes and tetrapods (e.g. avian wings) to generate sounds. Hence, sonic mechanisms include vocal ones, but have other functions as well (e.g. locomotion). The pectoral fins are innervated by motoneurons either located or born within the caudal medulla at the same level as dedicated vocal motoneurons (Hale, 2014; Ladich and Bass, 1996; Ma et al., 2010). In sum, sound production across vertebrates uses motoneurons of the caudal medulla and rostral spinal cord.
Based upon relative location and axonal projection patterns, caudal medullary motoneuron populations across different vertebrates have been suggested, using mammals as the example, to be extensions of vagal, hypoglossal, spinal accessory, or spinal motor pools (Benninger and McNeil, 2011; Ma et al., 2010; Tada and Kuratani, 2015; Wake, 1993). It remains unclear, however, the extent to which these different motoneurons might have shared developmental and hence evolutionary origins between species (Bass, 2014; Bass and Chagnaud, 2012; Benninger and McNeil, 2011; Cambronero and Puelles, 2000; Gilland and Baker, 2005; Hale, 2014; Ma et al., 2010).
Evolutionarily conserved patterns of gene expression are being used to identify homologies for structures and neural populations between species, and even between vertebrates and invertebrates. For example, expression of the highly conserved Paired-like Homeobox 2 (Phox2) transcription factor (TF) gene family in branchial motoneurons in vertebrates and ingestion-related motoneurons in invertebrates has been used to argue for homology of these motorneuron populations (Dufour et al., 2006; Nomaksteinsky et al., 2013; Wilson et al., 2009). In mammals, laryngeal motoneurons express Phox2b during development, which persists in some neurons into adulthood. Hypoglossal (XII) motoneurons, however, do not express Phox2b (Bilodeau et al., 2001; Gray, 2013; Kang et al., 2007; Pattyn et al., 1999). The highly conserved nature of the Phox2b protein and advances in genome and transcriptome sequencing led us to wonder whether comparisons of Phox2b expression in sonic/vocal motoneurons across species might provide a clear test to determine whether these diverse neural populations were homologous between species, i.e. derived from an evolutionarily conserved precursor population. We found that while Phox2b was expressed in vagal motoneurons innervating vocal laryngeal muscles in mouse and amphibians, it was not expressed in motoneurons innervating vocal/sonic non-laryngeal muscles in birds (zebra finch) or bony fish (midshipman, channel catfish). This suggests the motoneurons driving sound production in different vertebrate species can be derived from motor pools with divergent developmental and likely divergent evolutionary origins.
Methods
Animals
African clawed frogs (Xenopus laevis): Staged tadpoles were acquired from Xenopus Express (Brooksville, FL). Bullfrog (Lithobates catesbeiana, formerly called Rana catesbeiana): premetamorphic tadpoles were acquired from the Sullivan Company (Nashville, TN). Channel Catfish (Ictalurus punctatus): Paraformaldehyde fixed brains were acquired from Mark Burleson (Department of Biology, University of North Texas). Brain tissue for mRNA isolation was acquired from discarded catfish heads from a local retailer (Seafood City, University City MO). Mouse (Mus musculus): Mice were bred on site with a mixed CD1/C57BL6 background (Jackson Laboratory, Bar Harbor ME). P0 indicates age at birth. Plainfin midshipman (Porichthys notatus): Paraformaldehyde fixed brains and tissue for mRNA isolation were acquired from field-collected specimens (see (Bass et al., 1994)). Sea lamprey (Petromyzon marinus): Paraformaldehyde fixed brains were acquired from field-collected and laboratory raised specimens. Zebra finch (Taeniopygia guttata): Paraformaldehyde fixed brains and tissues for mRNA isolation were acquired from adult male birds ranging in age from 120 to 500 days of age, obtained from Canary Bird Farm, NJ. All the experiments were performed in accordance with guidelines laid down by the NIH in the US and Canadian Council on Animal Care regarding the care and use of animals for experimental procedures, the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals, and in compliance with protocols approved by the Animal Care Committee at the University of Calgary, the Institutional Animal Care and Use Committees at Cornell University, the Université de Montréal, the Université du Québec à Montréal, and the University of Pennsylvania, and the Animal Studies Committee at Washington University School of Medicine.
Tissue Acquisition
Bullfrog, catfish, lamprey, and Xenopus laevis were anesthetized in deionized water containing tricaine methanesulfonate (50 mg/l). Midshipman fish were anesthetized in 0.025% benzocaine (ethyl p-amino benzoate; Sigma, St. Louis, Mo.). Neonate mice were anesthetized by hypothermia. Zebra finches were anesthetized with 0.04 ml euthasol (Virbac AH, Inc, Fort Worth, Texas). All species were either transcardially perfused with 4% PFA in 0.1M phosphate buffered saline (PBS) for histology or their brains removed to sterile PBS for mRNA isolation. Fixed tissues were postfixed in PFA overnight at 4°C, cryoprotected in 25–30% sucrose in PBS, blocked, frozen in Optimal Cutting Temperature compound (Sakura Finetek, Torrance CA), and stored at −75°C. Brains were sectioned in sets of 4–6 on a Hacker (Winnsboro, SC) cryostat at 20 μm and sections thaw mounted onto Superfrost Plus (Fisher Scientific, Hampton, New Hampshire) slides and stored at −20° C until use.
In situ hybridization (ISH)
As previously described (Gray, 2013), slides were immersed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffered saline (PBS), permeabilized with radioimmunoprecipitation assay buffer, washed in 0.1 M triethanolamine-HCl with 0.25% acetic anhydride, blocked in hybridization buffer at 65°C, then placed into slide mailers containing hybridization buffer with digoxigenin labeled antisense Phox2 or Phox2b cRNA at 1 μg/ml overnight at 65°C. Slides were washed in sodium citrate buffers at 62°C, then washed and incubated in alkaline phosphatase conjugated anti-DIG antibody in 10% normal horse serum and incubated in nitro blue tetrazolium chloride and 5-Bromo-4-chloro-3-indolyl phosphate (NBT-BCIP, Roche, Indianapolis, IN) until cellular labeling is clear. For combined IHC and ISH, slides were stained for mRNA expression prior to immunohistochemical labeling (IHC). All compounds were acquired from Sigma-Aldrich (St. Louis MO).
Immunohistochemistry (IHC)
After ISH, tissue sections were washed in PBS with 0.2% triton X-100, blocked in 10% heat inactivated normal horse sera, incubated in antibody overnight at 4°C, incubated in secondary antibody and coverslipped in Vectashield (Vector Laboratories, Burlingame, CA).
Antibodies
We used goat anti-choline acetyltransferase (ChAT, 1:100, Invitrogen, Carlsbad CA) or goat anti-Phox2b (1:200, Santa Cruz Biotechnology, Santa Cruz CA) antibodies for IHC analysis in mice.
Cloning of ISH probes
Bullfrog, catfish, and midshipman poly-A mRNA was isolated using a Trizol plus RNA purification kit according to manufacturer’s directions (Life Technologies, Grand Island, NY). cDNA fragments for ISH probes were isolated by PCR amplification from whole brain cDNA as previously described (Gray et al., 2004). Channel catfish: a 646 base pair fragment of the Phox2b gene was cloned from catfish brain cDNA using primers AAGGGACGCGAGATGTCAAACC, GGACCCAACCCGAACCCTGC identified by alignment from existing channel catfish EST sequences (85% homology over 295 bp with Danio rerio Phox2b (Wang et al., 2010). Midshipman: a 657 bp fragment of the Phox2b gene was cloned using primers TGAGCGGCATCGGCTTCTGC, CCAGCTGGCCTCCAATGGGC identified by alignment from midshipman transcriptome sequencing analysis (89% homology over 409 bp against 4 cichlids species)(Feng et al., 2015). Bullfrog: a 490 bp fragment of the Phox2b gene was cloned using primers CTTACCTCAATTCCTCCGCC, GCGGTTCTGGAACCAGACC identified by multispecies alignment (89.1% homology over 430 bp against X. laevis Phox2b).
Probe gene synthesis
A 564 bp fragment corresponding to the predicted zebra finch Phox2b gene (XM_002188672) and an 800 bp fragment from the published lamprey Phox2 sequence (Haming et al., 2011) were commercially synthesized and cloned into puc57 vectors to generate ISH probes (Genscript, Piscataway, NJ). A 694 bp fragment of X. laevis Phox2b was amplified from a previous cloned plasmid (BC084305, ThermoFisher, Pittsburg, PA)(Klein et al., 2002).
IHC and ISH Image Acquisition
Fluorescent and brightfield images were acquired using a Nikon 90i microscope (Nikon Instruments, Melville, NY), Roper H2 cooled CCD camera (Photometrics, Tucson, AZ), and Optigrid Structured Illumination Confocal with a Prior (Rockland, MA) motorized translation stage. Pseudo-colored images were acquired in Velocity (Perkin Elmer, Waltham, MA), and modified in Photoshop (Adobe, San Jose, CA) or ImageJ (NIH, Bethesda, MD) (Schneider et al., 2012) and exported as 8 bit JPEG images. Images were filtered and levels were modified for clarity.
Results
Phox2b in Mouse
We analyzed Phox2b and choline acetyltransferase (ChAT) protein expression in perinatal mouse medulla (IHC, n=7). Phox2b was expressed in visceral sensory neurons of the nucleus tractus solitarius (NTS), catecholaminergic neurons of the A1/2, C1/2 groups, and in parasympathetic motoneurons of the dorsal motor nucleus of the vagus (DMV, Figure 1A–D). It was also expressed in ChAT expressing pharyngolaryneal and esophageal motoneurons of both semi-compact and compact formations of the nucleus ambiguus (NA) but not in XII motoneurons innervating the tongue (Figures 1A, C, D). These results were consistent with previously reported patterns of Phox2b protein expression in adult rat and Phox2b mRNA expression in mice (Bieger and Hopkins, 1987; Cambronero and Puelles, 2000; Kang et al., 2007; Qian et al., 2001).
Figure 1.
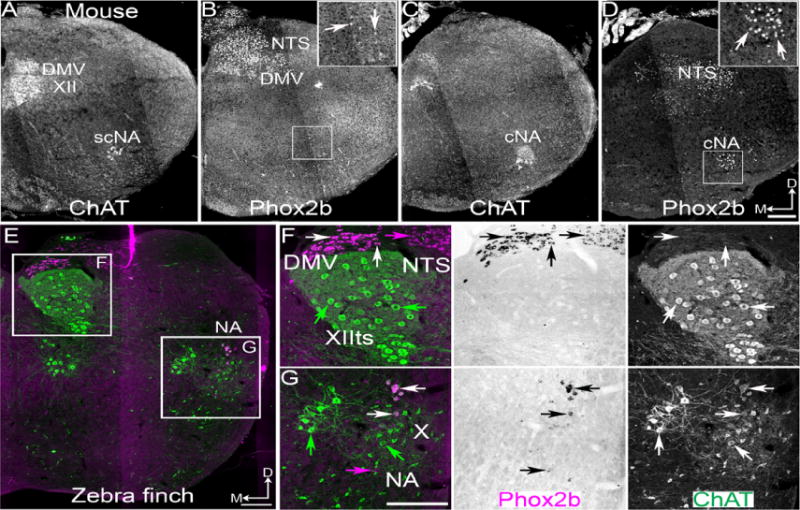
Somatic origin of mouse and zebra finch vocal motoneurons. (A–D) Confocal mosaic images of ChAT (A,C) and Phox2b (B,D) immunohistochemistry (IHC) in neonate mouse mid to rostral medulla. Phox2b is co-expressed with ChAT in DMV and cNA branchial motoneurons. Phox2b is expressed in non-cholinergic NTS and ventrolateral medulla neurons (B,D). E–G. Confocal mosaic images of ChAT IHC (green) and pseudocolor ISH for Phox2b (magenta) in zebra finch caudal medulla. F–G Expansion from E showing combined (left) and single color images (middle – brightfield Phox2b, right – ChAT). F Images showing the absence of Phox2b co-expression in tracheosyringeal hypoglossal (XIIts) ChAT expressing motoneurons. Phox2b is expressed in the NTS. G Images showing Phox2b is co-expressed within a subset of NA motoneurons and in a small number of non-cholinergic interneurons. White arrows indicate Phox2b and ChAT co-expression. Green arrow indicates ChAT single expression. Magenta arrow indicates Phox2b single expression. Scale bars = 200 μm, D – dorsal, M - medial. ChAT – choline acetyltransferase, cNA – compact formation of the nucleus ambiguus, DMV – dorsal motonucleus of the vagus, NTS - nucleus tractus solitarius, scNA – semi-compact formation of the nucleus ambiguus, XII – hypoglossal motonucleus, XIIts – tracheosyringeal hypoglossal motonucleus.
Phox2b in Zebra Finch
Using a cDNA fragment corresponding to the coding region from the predicted zebra finch Phox2b gene sequence, we performed non-radioactive ISH combined with ChAT IHC in the medulla of adult zebra finch (n=4). Consistent with expression in mammals and chicken, Phox2b mRNA was present in NTS and cranial motor pools including the NA (Figure 1E, G) and in the DMV just dorsal to the XII (Figure 1E, F). In the NA, only a subset of motoneurons showed Phox2b expression, consistent with the down regulation of protein expression in the majority of semi-compact formation motoneurons seen in rodents (Figure 1A, B, G) (Kang et al., 2007). Phox2b expression in non-cholinergic neurons within this region likely represents catecholaminergic neurons of the A1/C1 groups (Figure 1G) (Kang et al., 2007; Qian et al., 2001). We found no Phox2b mRNA expression within zebra finch XIIts motoneurons (Figure 1F), or within the spinal cord (not shown). These results are consistent the absence of Phox2b in mammalian XII and with zebra finch XIIts and mammalian XII motoneurons being derived from a shared progenitor domain and representing homologous structures (Wild, 2004).
Phox2b in Teleost Fish
The best-characterized teleost vocalization network is the swim bladder drumming network of the plainfin midshipman (Porichthys notatus) that belongs to a group of highly vocal teleosts commonly referred to as toadfishes (Rice and Bass, 2009). The swim bladder muscles are innervated by motoneurons in the vocal motor nucleus (VMN, Figure 2A), which extends from the caudal medulla into the rostral spinal cord (Bass, 1985; Bass et al., 2008). From midshipman transcriptome data (Feng et al., 2015), we identified the putative midshipman Phox2b homolog and cloned a 657 bp cDNA fragment from midshipman brain tissue. We performed ISH for Phox2b in the medulla of adult male midshipman (n=6). We found no expression of Phox2b mRNA within the ChAT expressing VMN or within the rostral spinal cord (Figure 2A, C, D, not shown) but did find mRNA within the NTS and vagal motor pools (Figure 2B, C). In fish, unlike in rodents or zebra finch, all vagal motoneurons, including both branchial and parasympathetic neurons, are located directly adjacent to the ventricular surface (Herrick, 1905). To verify that all VMN motoneurons lacked Phox2b expression, we applied neurobiotin to the cut end of the vocal nerve innervating a single swim bladder muscle to transneuronally fill the paired midline VMN (see (Bass et al., 1994; Bass et al., 2008) for methods) and then performed Phox2b ISH combined with fluorescent biotin labeling (n=4 males). None of the neurobiotin labeled VMN motoneurons expressed Phox2b although they directly abutted the Phox2b positive vagal motoneurons (Figure 2E, F). These data suggest that VMN motoneurons share a progenitor domain with XII motoneurons in mammals and birds, including the XIIts, thus representing a homologous motoneuron population.
Figure 2.
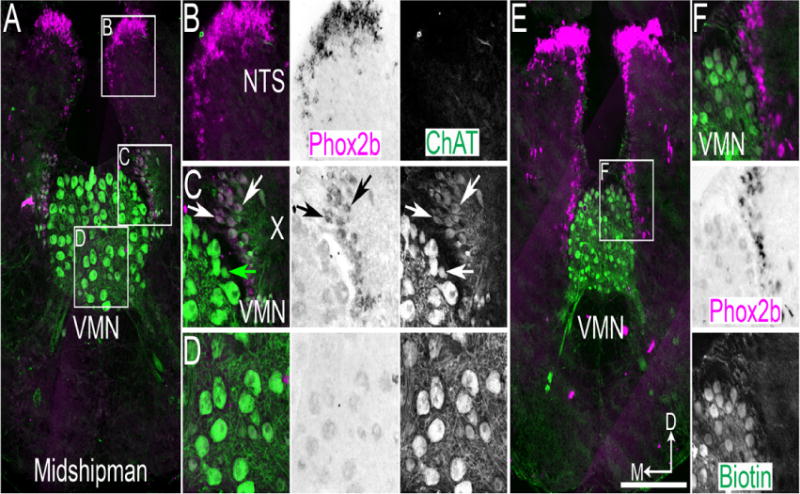
Midshipman vocal motor nucleus (VMN) motoneurons do not express Phox2b. A. Confocal mosaic image of ChAT IHC (green) and pseudocolor inverted bright field ISH for Phox2b (magenta) in adult Midshipman caudal medulla. B–D. Expansions from A showing Phox2b and ChAT co-staining. Left – dual color image, middle – brightfield Phox2b staining, right – ChAT single color staining. Note the absence of ChAT staining in NTS region (B), co-localization of ChAT and Phox2b in vagal motoneurons (C), and absence of Phox2b expression above background in VMN (D). White arrows indicate co-expression, green arrow indicates ChAT only staining. E. Confocal mosaic image of fluorescent biotin labeling (green) and pseudocolor inverted brightfield ISH for Phox2b (magenta) in adult Midshipman caudal medulla. F. Expansion from E showing lack of biotin labeling in Phox2b expressing neurons. Top – color image, middle – brightfield Phox2b staining, bottom – fluorescent biotin labeling single color. Scale bar = 200 μm. D – dorsal, M - medial.
An alternate sound production system in teleosts relies on movements of the pectoral fins (Ladich and Bass, 1996). From channel catfish (Ictalurus punctatus) EST sequence data, we identified a putative catfish Phox2b homolog and cloned a 684 bp cDNA fragment from catfish brain tissue (Chen et al., 2010; Wang et al., 2010). We examined the localization of catfish Phox2b mRNA and ChAT protein by combined ISH/IHC in adult catfish medulla (n=3). We found Phox2b mRNA expression within the NTS and vagal motor pools (Figure 3A–C). The NTS in catfish was enlarged compared to midshipman, birds, and mammals, likely due to the expanded role of taste in this species (Finger, 2009; Herrick, 1905). Directly ventral and lateral to the Phox2b and ChAT expressing vagal motoneurons we identified a small group of loosely organized, large cholinergic neurons that lacked Phox2b (Figure 3A, C). Whether these neurons are related to XII or VMN motoneurons is unknown. Motoneurons in the region innervating pectoral muscles, however, did not show any Phox2b expression above background (Figure 3A, D) (Hale, 2014; Ladich and Bass, 1998).
Figure 3.
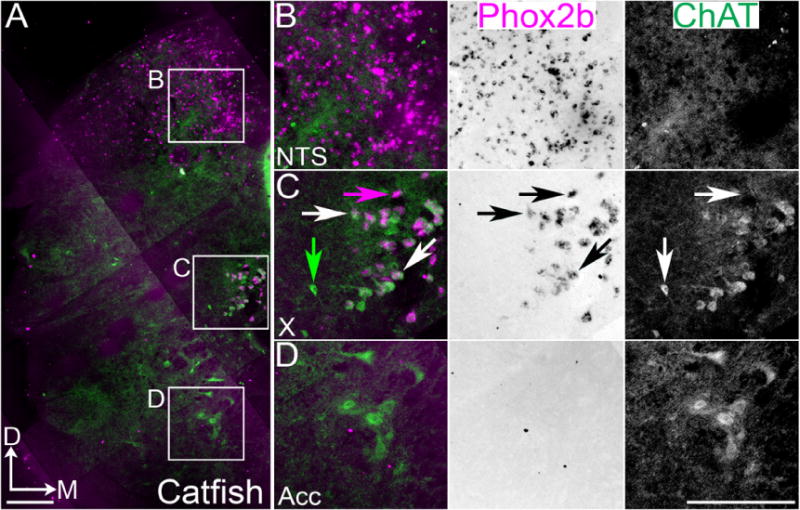
Conserved organization of Phox2b populations in channel catfish caudal medulla. A. Confocal mosaic images of ChAT IHC (green) and inverted pseudocolor ISH for Phox2b (magenta) in adult channel catfish. B–D. Expansions from A showing combined (left) and single color images (middle – brightfield Phox2b, right – ChAT staining). B. Images showing Phox2b expression in non-cholinergic NTS neurons. Note the increased NTS size related to taste sensation. C. Images showing broad co-localization of Phox2b with ChAT in caudal vagal column. D. Images showing the absence of Phox2b mRNA expression in ChAT positive rostral spinal cord populations. White arrows indicate Phox2b and ChAT co-expression. Magenta arrow indicates Phox2b single expression. Green arrow indicates ChAT single expression. Scale bars = 200 μm. D – dorsal, M - medial. Acc – accessory nucleus.
Phox2b in Amphibians
As different anuran amphibians require either the movement of air (terrestrial) or solely mechanical (fully aquatic) mechanisms to produce sound, we asked whether Phox2b expression in motoneurons might differ across species by analyzing Phox2b expression in the terrestrial bullfrog (Lithobates catesbiana) and the fully aquatic African clawed frog (Xenopus laevis)(Martin and Gans, 1972; Ryan and Guerra, 2014; Tobias and Kelley, 1987; Wetzel et al., 1985; Yager, 1992). As there were no bullfrog genome or transcriptome sequences available, we designed degenerate PCR primer pairs based on multi-vertebrate Phox2b protein sequence alignments. We cloned a 439 bp fragment corresponding to the putative bullfrog Phox2b coding sequence from bullfrog brain cDNA. For African clawed frog, we isolated a 694 bp Phox2b fragment from a published sequence (Klein et al., 2002). We performed combined Phox2b ISH and ChAT IHC in premetamorphic bullfrog tadpole (n=5) and stage 50–60 X. laevis (n=6) medulla and spinal cord (Figure 4A–H).
Figure 4.
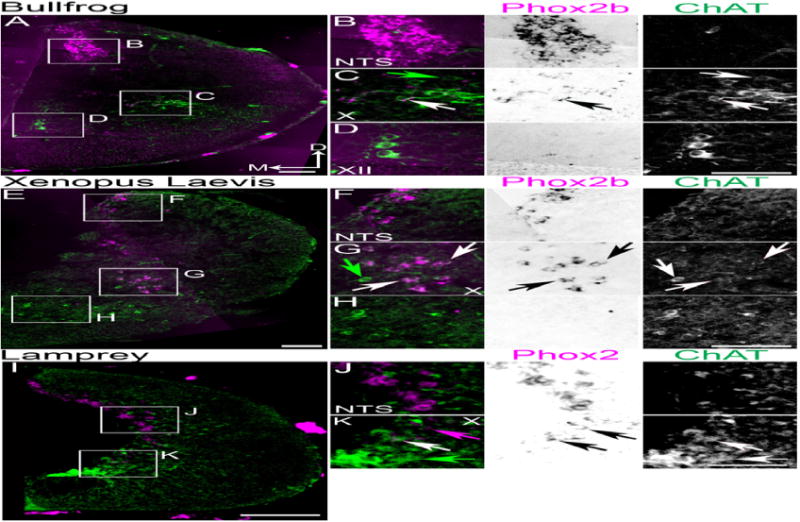
Conserved organization of Phox2b populations in anuran and sea lamprey caudal medulla. Confocal mosaic images of ChAT IHC (green) and pseudocolor ISH for Phox2b (magenta) in premetamorphic bullfrog tadpole (A–D), or stage 55 African clawed frog (X. laevis) (E–H). B–D Expansions from A showing combined (left) and single color images (middle – brightfield Phox2b, right – ChAT) staining. B. Images showing Phox2b expression in non-cholinergic NTS neurons. C. Images showing broad co-localization of Phox2b with ChAT in the caudal vagal column. D. Images showing lack of Phox2b expression in XII motoneurons. F–H Expansions from E showing combined (left) and single color images (middle – Phox2b, left – ChAT) staining. F. Images showing Phox2b expression in non-cholinergic NTS neurons. G. Images showing broad co-localization of Phox2b with ChAT in caudal vagal column. H. Images showing lack of Phox2b expression in ventral motoneurons. I–K Confocal mosaic images of ChAT IHC (green) and pseudocolor ISH for Phox2 (magenta) in transformer sea lamprey. J–K Expansions from I showing combined (left) and single color images (middle brightfield Phox2, right ChAT) staining. J. Images showing Phox2b expression in non-cholinergic NTS neurons. K. Images showing co-localization of Phox2b with ChAT in presumptive vagal motoneurons. Note the presence of ChAT motoneurons lacking Phox2 expression ventral to vagal motoneurons in all species. White arrows indicate Phox2(b) and ChAT co-expression. Green arrows indicate ChAT single expression. Magenta arrow indicates Phox2 single expression. Note: X. laevis does not have a XII motor nucleus. Scale bars = 200 μm. D – dorsal, M - medial. NTS – nucleus, X – vagal motoneurons, XII – hypoglossal motoneurons.
In both amphibian species tested, we found strong Phox2b mRNA expression within the NTS (Figure 4A, B, E, F) although the NTS was larger in bullfrog. Similarly, Phox2b was present in ChAT expressing laryngeal motoneurons similar to its expression in mammalian laryngeal motoneurons important for vocalization (Figure 4C, G) (Simpson et al., 1986). In amphibians, as in birds and mammals, these neurons were located away from the ventricular space. Bullfrogs, having a well-developed tongue, had a clear XII motor nucleus, which did not express Phox2b (Figure 4D). X. laevis lacks a tongue and the XII was not evident, although a small population of scattered, large ChAT-expressing neurons was present adjacent to the ventral midline (Figure 4H). Phox2b was not expressed in amphibian spinal cord.
Phox2 in the Sea Lamprey
Our comparative data suggested that the caudal medulla contains at least two distinct classes of motoneurons; Phox2b+ vagal motoneurons dorsal to a population of Phox2b− motoneurons in the same position as those used for vocalization in many vertebrates. We wanted to know if this pattern of Phox2b localization was present in jawless vertebrates, in part, because they are not thought to produce vocalization (Bass, 2014). To test this hypothesis, we synthesized a 800 bp cDNA fragment corresponding to the coding region of the sea lamprey (Petromyzon marinus) single Phox2 family gene (Haming et al., 2011). Combined Phox2 ISH with ChAT IHC in larval (n=2) and transformer (n=4) sea lamprey caudal medulla and spinal cord showed no Phox2 mRNA expression in spinal cord (Fritzsch and Northcutt, 1993). Phox2 expression that resembled Phox2b expression in other vertebrates was present in the caudal medulla; a thin population likely corresponding to the NTS was present along the dorsal ventricular rim (Figure 4I, J). We also found two distinct motoneuron populations; a dorsal subset that co-expressed both Phox2 and ChAT consistent with motoneurons innervating the gill arches and a ventral Phox2 negative, ChAT expressing population consistent with the identification of a putative lamprey hypoglossal homolog (Figure 4I, K) (Fritzsch and Northcutt, 1993; Kusakabe and Kuratani, 2007).
Discussion
Communication using sounds is a behavior shared by many vertebrates (Bass, 2014). It is hypothesized that an evolutionarily conserved vocal oscillator is located within the caudal ventral medulla of all the major lineages of vocal vertebrates (Bass et al., 2008). While the genetic identities of most vocal oscillators are unknown, the overall structure of hindbrain motor pools is highly conserved between vertebrates (Feldman et al., 2013; Gray et al., 2010; Tupal et al., 2014a). Cranial and spinal motoneuron organization shows a high concordance between lamprey, fish, amphibians, birds, and mammals (Cambronero and Puelles, 2000; Dufour et al., 2006; Fritzsch and Northcutt, 1993; Gilland and Baker, 2005; Gray, 2013; Koyama et al., 2011; Straka et al., 2006). The correspondence of motoneuron pools between species is less clear within the transition zone between the caudal end of the vagal motor pool and the cervical spinal cord (Benninger and McNeil, 2011; Cambronero and Puelles, 2000; Gray, 2013; Noden and Francis‐West, 2006; Tada and Kuratani, 2015). Motoneuron pools within this region vary in location and organization between species and identifying exact evolutionary homologs at a cellular level has been difficult (Tada and Kuratani, 2015). This is especially true for populations involved in the production of vocal/sonic behaviors and an underlying reason for the experiments described here.
In the caudal medulla, mammals have a tightly clustered XII motor nucleus located just ventral to the Phox2b expressing dorsal motor nucleus of the vagus (DMV) neurons. In addition, they have a Phox2b− spinal accessory nucleus contiguous with the more rostral Phox2b expressing vagal motoneurons of the NA essential for swallowing and vocalization in humans (Bieger and Hopkins, 1987; Kang et al., 2007; Ullah et al., 2007). The organization of motoneurons in avian caudal medulla is nearly identical. The major difference is that birds have a unique motor pool, the XIIts, that makes up the caudal two thirds of the XII nuclear group and innervates the avian vocal apparatus known as the syrinx (Nottebohm et al., 1976; Wild, 1997). Similar to the XII in mammals, XIIts axons project ventrally through the brainstem but separate as a long tract through the neck to the syrinx (Manogue and Paton, 1982; Nottebohm et al., 1976; Wild, 1997). The XIIts nerve contains primary sensory fibers similar to those found in visceral cranial nerves (Bottjer and Arnold, 1982). Thus the exact homology of this nucleus has been somewhat unresolved (Cambronero and Puelles, 2000; Wild, 2004).
In a subset of teleosts including midshipman and other vocal teleost species, this same transitional region between the hindbrain and spinal cord contains VMN motoneurons innervating the sonic muscles (Bass, 1985; Bass et al., 2015; Bass et al., 2008). These motoneurons can lie either near or on the midline (e.g. Figure 2A) or more lateral depending upon the species (Ladich and Bass, 1996; Ladich and Bass, 1998). In addition, this region also contains motoneurons controlling the pectoral appendages that are also sonic in some species of fish (Bass and Chagnaud, 2012; Hale, 2014; Ma et al., 2010). The axons of pectoral and VMN motoneurons exit via the occipital nerve (Ma et al., 2010; Onuki and Somiya, 2007). As with XIIts, the exact homology of these motor pools to those in other vocal vertebrates has remained unclear.
The distinction between branchial and somatic motoneuron identity is proposed to be highly evolutionarily conserved (Butler and Hodos, 2005; Nomaksteinsky et al., 2013). Branchial, still often referred to as “special visceral”, motoneurons share a functional phenotype with motoneurons traditionally referred to as general somatic efferents that innervate muscle derived from somites, namely that both groups innervate skeletal, i.e. somatic, muscle. Nevertheless, branchial motoneurons, which include the motoneurons of cranial nerves V, VII, IX and X, are distinct from other somatic motoneurons in their developmental origin, location within the brainstem, and transcriptional profiles (Diogo and Ziermann, 2014; Gilland and Baker, 2005; Matsuoka et al., 2005; Noden and Francis‐West, 2006; Piekarski and Olsson, 2007; Tada and Kuratani, 2015).
Phox2b is required for the appropriate differentiation of both visceral and branchial motoneurons (Dufour et al., 2006; Kang et al., 2007; Nomaksteinsky et al., 2013) and is expressed by vagal motoneurons that innervate laryngeal muscle (Bieger and Hopkins, 1987; Edgeworth, 1920). Intrinsic laryngeal muscles used for sound production are derived from somitic mesoderm as are other muscles associated with the branchial arches (Noden and Francis‐West, 2006; Sambasivan et al., 2011). The targeted loss of the TF T-box 1 (Tbx1) eliminates laryngeal as well as 1st and 2nd pharyngeal arch muscles suggesting a conserved relationship between laryngeal muscles and branchial muscles derived from head mesoderm (Kelly et al., 2004; Sambasivan et al., 2011). The tongue muscles are also derived from occipital somatic mesoderm, but in contrast with laryngeal motoneurons, XII motoneurons do not express Phox2b (Kusakabe and Kuratani, 2007). Despite the complicated evolution and development of vocal motoneurons, and more generally cranial motoneurons and their peripheral targets, we wondered whether comparisons of the highly conserved transcription factor gene Phox2b might provide a clear way to test the homology of vocal motoneurons across vertebrates. If Phox2b expressing motoneurons in the caudal medulla of mammals innervate somatic muscles used for vocalization, we posited that motoneurons innervating functionally or evolutionarily related muscles in other vocal vertebrate species might also express Phox2b.
We found that zebra finch XIIts, midshipman VMN, and channel catfish pectoral motoneurons did not express Phox2b mRNA, strongly supporting the hypothesis that these motoneuron groups are not homologs of mammalian laryngeal motoneurons. Both XIIts and VMN neurons were located directly ventral to Phox2b expressing cholinergic motoneurons in a location identical to XII motoneurons in mammals and strongly consistent with all of these motoneuron pools being homologous structures. Interestingly, all species tested, including those lacking tongues (i.e. Xenopus and fish), showed the presence of large Phox2b−/ChAT+ neurons directly ventral to midline medullary Phox2(b)+/ChAT+ neurons. We suggest these are likely to be motoneurons as this equivalent rostro-caudal region in mice lacks cholinergic interneurons (Gray, 2013). This further suggests the maintenance of a highly conserved motoneuron population whose size, organization, and function is species-specific (Kusakabe and Kuratani, 2007).
These data are consistent with a transition zone between medulla and spinal cord originating from either a distinct rhombomere 8 or from “pseudorhombomeres”(Bass et al., 2008; Cambronero and Puelles, 2000). One common feature of vagal, hypoglossal and spinal accessory nerve motor neurons is that they innervate muscles attached to bones and connective tissues generated from post-otic neural crest even though the muscles themselves are derived from paraxial mesoderm (Benninger and McNeil, 2011; Matsuoka et al., 2005). In tetrapods, the XII motor nucleus is limited to the hindbrain while both the VMN and pectoral fin motoneuron pools in fish extend into the rostral cervical spinal cord (Bass et al., 2008; Ma et al., 2010). This same region in mammals contains phrenic motoneurons innervating the mammalian specific diaphragm. Interestingly, muscles of both the pectoral fin and diaphragm have been proposed to be derived from shoulder muscle precursors (Hirasawa and Kuratani, 2013; Matsuoka et al., 2005).
Spinal cord motoneurons migrate laterally and are organized as continuous columns (Dasen et al., 2005). Phrenic motoneuron cell bodies, in contrast, migrate longitudinally to form tight but separate clusters with highly constrained dendritic trees (Greer et al., 1999; Philippidou et al., 2012). XII motoneurons migrate dorsally and cluster into a discrete nucleus. Mutation of the evolutionarily conserved transcription factor Motoneuron and Pancreas Homeobox 1 (MNX1, also known as HB9) does not block motoneuron differentiation, but does eliminate the formation of the phrenic nerve as well producing a diffuse organization of the XII nucleus (Arber et al., 1999; Thaler et al., 1999). Similarly, the phrenic nucleus requires an extended period of Hox5 gene expression to maintain cell number and connectivity (Philippidou et al., 2012). These data, in addition to evolutionary modifications of the head, trunk, and neck region in vertebrates, suggest species-specific modifications of genetic programs play an important role in shaping the final migration, axon projection, and organization of vocal motoneurons (Bass et al., 2008; Benninger and McNeil, 2011; Cambronero and Puelles, 2000; Diogo and Ziermann, 2014; Gray, 2013; Ma et al., 2010; Piekarski and Olsson, 2007)
Taken together, our findings suggest the motoneurons generating vocalization in vertebrates are not derived from a single, evolutionarily conserved progenitor pool. Both mammals and amphibians use Phox2b-expressing vagal motoneurons in controlling laryngeal-dependent vocalization. In contrast vocal motoneurons in birds and teleost fish, as well as pectoral-dependent sonic motoneurons in fish and likely all tetrapods, lack Phox2b expression. Mammalian vocalization, it should be pointed out, also clearly involves non-Phox2b expressing neurons from XII and phrenic motor pools. Whether the pattern generating premotoneuron populations involved in vocalization are similarly developmentally flexible is an interesting question for future analysis (Bass and Baker, 1997; Bass et al., 2008; Bouvier et al., 2010; Gray et al., 2010; Sweeney and Kelley, 2014; Tupal et al., 2014b).
Figure 5.
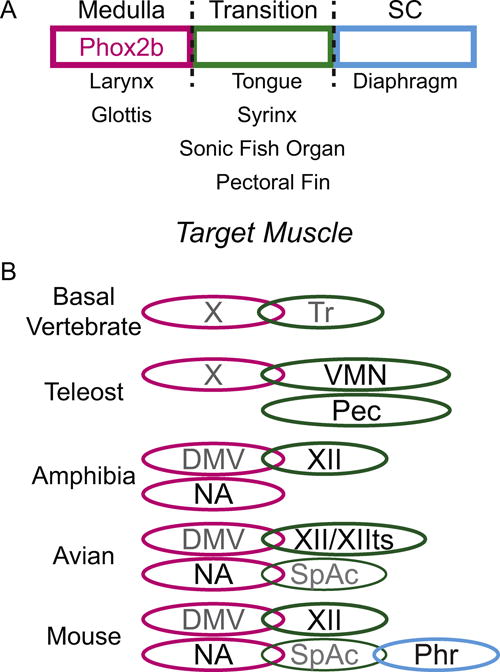
Evolutionary organization of vocal motoneurons. A. Schematic showing simplified anatomical organization of vocal motoneurons in vertebrates. Left column indicates muscles innervated by Phox2b expressing vagal motoneurons (magenta). Middle column indicates target muscles innervated by motoneurons within the medullary/spinal cord transition zone (green). Right column indicates target muscles innervated by motoneurons within the spinal cord. B. Schematic indicating hypothesized evolutionary expansion and localization of medullary (magenta), transition zone (green), and rostral spinal cord (cyan) motoneurons important for vocalization, from basal vertebrate to mammal. Multiple ovals indicate clearly separate nuclei. Location of ovals indicates relative location within brain. Grey text indicates unknown role in vocalization. Note: the phrenic nucleus is not a continuation of the spinal accessory nucleus. Abbreviations: DMV – dorsal motor nucleus of the vagus, NA – nucleus ambiguus, Phr – phrenic nucleus, Pec – pectoral fin motoneurons, SpAc – spinal accessory nucleus, Tr – transition zone motoneurons, VMN – vocal motor nucleus, XII – hypoglossal motor nucleus, XIIts - tracheosyringeal motor nucleus, X – vagal motoneurons.
Highlights.
Motoneurons important for vocalization in vertebrates are not derived from a single developmental progenitor pool.
Vocal motoneurons in birds and some teleost fish likely share origins from a single developmental progenitor pool that includes the hypoglossal (XII) motoneurons of tetrapods.
Acknowledgments
This work was supported by a National Heart, Lung, and Blood Institute Grant to J.A., A.B., E.R.S., G.M. and P.A.G. (R01HL089742), NSF IOS1120925 to A.H.B., a Discovery Grant from National Science and Engineering Council of Canada and the Alberta Innovates Health Solutions to R.J.A.W., a National Institute on Deafness and Other Communication Disorders to M.F.S. (R01DC6102), a National Institutes of Health R01 to D.B.K. (NS23684), a Fulbright International Science and Technology Graduate Fellowship to I.H.B., a Discovery Grant from National Science and Engineering Council of Canada (Grant number: 217435 to R.D.), a grant from the Canadian Institutes of Health Research Grants to R.D, and a Vanier studentship to K.M.. We would like to thank Mark Burleson for providing catfish brain tissue, Margaret Marchaterre for midshipman fish tissue, Marianne Bronner-Fraser for providing the lamprey Phox2 sequence, and J. Martin Wild for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [see comments] [DOI] [PubMed] [Google Scholar]
- Bass A. Sonic motor pathways in teleost fishes: a comparative HRP study. Brain, behavior and evolution. 1985;27:115–131. doi: 10.1159/000118725. [DOI] [PubMed] [Google Scholar]
- Bass A, Marchaterre M, Baker R. Vocal-acoustic pathways in a teleost fish. The Journal of neuroscience. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH. Central pattern generator for vocalization: is there a vertebrate morphotype? Current Opinion in Neurobiology. 2014;28:94–100. doi: 10.1016/j.conb.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Baker R. Phenotypic specification of hindbrain rhombomeres and the origins of rhythmic circuits in vertebrates. Brain Behav Evol. 1997;50(Suppl 1):3–16. doi: 10.1159/000113351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Chagnaud BP. Shared developmental and evolutionary origins for neural basis of vocal–acoustic and pectoral–gestural signaling. Proceedings of the National Academy of Sciences. 2012;109:10677–10684. doi: 10.1073/pnas.1201886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Chagnaud BP, Feng NY. Comparative Neurobiology of Sound Production in Fishes, Sound Communication in Fishes. Springer. 2015:35–75. [Google Scholar]
- Bass AH, Gilland EH, Baker R. Evolutionary Origins for Social Vocalization in a Vertebrate Hindbrain-Spinal Compartment. Science. 2008;321:417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger B, McNeil J. Transitional nerve: A new and original classification of a peripheral nerve supported by the nature of the accessory nerve (CN XI) Neurology research international. 2011;2010 doi: 10.1155/2010/476018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. Journal of Comparative Neurology. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Bilodeau M, Boulineau T, Greulich JM, Hullinger R, Andrisani O. Differential expression of sympathoadrenal lineage-determining genes and phenotypic markers in cultured primary neural crest cells. In Vitro Cell Dev Biol -Animal. 2001;37:185–192. doi: 10.1290/1071-2690(2001)037<0185:DEOSLD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Arnold AP. Afferent neurons in the hypoglossal nerve of the zebra finch (Poephila guttata): localization with horseradish peroxidase. Journal of Comparative Neurology. 1982;210:190–197. doi: 10.1002/cne.902100209. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nature Neuroscience. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- Brahic CJ, Kelley DB. Vocal circuitry in Xenopus laevis: telencephalon to laryngeal motor neurons. Journal of Comparative Neurology. 2003;464:115–130. doi: 10.1002/cne.10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM. Handbook of mammalian vocalization: An integrative neuroscience approach. Academic Press; 2009. [Google Scholar]
- Butler AB, Hodos W. Comparative vertebrate neuroanatomy: evolution and adaptation. John Wiley & Sons; 2005. [Google Scholar]
- Cambronero F, Puelles L. Rostrocaudal nuclear relationships in the avian medulla oblongata: a fate map with quail chick chimeras. The Journal of Comparative Neurology. 2000;427:522–545. doi: 10.1002/1096-9861(20001127)427:4<522::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Chen F, Lee Y, Jiang Y, Wang S, Peatman E, Abernathy J, Liu H, Liu S, Kucuktas H, Ke C. Identification and characterization of full-length cDNAs in channel catfish (Ictalurus punctatus) and blue catfish (Ictalurus furcatus) PLoS One. 2010;5:e11546. doi: 10.1371/journal.pone.0011546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Diogo R, Ziermann JM. Development, Metamorphosis, Morphology and Diversity: The Evolution of Chordate muscles and the Origin of Vertebrates. Developmental Dynamics. 2014 doi: 10.1002/dvdy.24245. [DOI] [PubMed] [Google Scholar]
- Dufour HD, Chettouh Z, Deyts C, de Rosa R, Goridis C, Joly JS, Brunet JF. Precraniate origin of cranial motoneurons. Proceedings of the National Academy of Sciences. 2006;103:8727–8732. doi: 10.1073/pnas.0600805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgeworth F. On the development of the hypobranchial and laryngeal muscles in Amphibia. J Anat. 1920;54:125. [PMC free article] [PubMed] [Google Scholar]
- Feldman J, Del Negro L, Gray PA C. Understanding the Rhythm of Breathing: So Near, Yet So Far. In: Julius D, Clapham DE, editors. Annual Review of Physiology. Vol. 75. Palo Alto: 2013. pp. 423–452. (Annual Reviews). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Fergus D, Bass AH. Neural transcriptome reveals molecular mechanisms for temporal control of vocalization across multiple timescales. BMC Genomics. 2015;16:408. doi: 10.1186/s12864-015-1577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine ML, McElroy D, Rafi J, King CB, Loesser KE, Newton S. Lateralization of pectoral stridulation sound production in the channel catfish. Physiology & behavior. 1996;60:753–757. doi: 10.1016/0031-9384(96)00092-3. [DOI] [PubMed] [Google Scholar]
- Finger TE. Evolution of gustatory reflex systems in the brainstems of fishes. Integrative zoology. 2009;4:53–63. doi: 10.1111/j.1749-4877.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Northcutt RG. Cranial and spinal nerve organization in amphioxus and lampreys: evidence for an ancestral craniate pattern. Cells Tissues Organs. 1993;148:96–109. doi: 10.1159/000147529. [DOI] [PubMed] [Google Scholar]
- Gilland E, Baker R. Evolutionary patterns of cranial nerve efferent nuclei in vertebrates. Brain, behavior and evolution. 2005;66:234–254. doi: 10.1159/000088128. [DOI] [PubMed] [Google Scholar]
- Gray PA. Transcription Factors Define the Neuroanatomical Organization of the Medullary Reticular Formation. Frontiers in Neuroanatomy. 2013;7 doi: 10.3389/fnana.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse Brain Organization Revealed Through Direct Genome-Scale TF Expression Analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MCD, Ross SE, Hirata T, Corbin JG, Eugenín J. Developmental origin of preBötzinger Complex respiratory neurons. The Journal of Neuroscience. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Allan DW, Martin-Caraballo M, Lemke RP. An overview of phrenic nerve and diaphragm muscle development in the perinatal rat. Journal of Applied Physiology. 1999;86:779–786. doi: 10.1152/jappl.1999.86.3.779. [DOI] [PubMed] [Google Scholar]
- Hale ME. Developmental Change in the Function of Movement Systems: Transition of the Pectoral Fins between Respiratory and Locomotor Roles in Zebrafish. Integr Comp Biol. 2014;54:238–249. doi: 10.1093/icb/icu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haming D, Simoes-Costa M, Uy B, Valencia J, Sauka-Spengler T, Bronner-Fraser M. Expression of sympathetic nervous system genes in Lamprey suggests their recruitment for specification of a new vertebrate feature. PLoS One. 2011;6:e26543. doi: 10.1371/journal.pone.0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick CJ. The central gustatory paths in the brains of bony fishes. Studies from the Neurological Laboratory of Denison University. No. XVIII. Journal of Comparative Neurology and Psychology. 1905;15:375–456. [Google Scholar]
- Hirasawa T, Kuratani S. A new scenario of the evolutionary derivation of the mammalian diaphragm from shoulder muscles. J Anat. 2013;222:504–517. doi: 10.1111/joa.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. The Journal of Comparative Neurology. 2007;503:627–641. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Bass AH. Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Current Opinion in Neurobiology. 2010;20:748–753. doi: 10.1016/j.conb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RG, Jerome-Majewska LA, Papaioannou VE. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Human Molecular Genetics. 2004;13:2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P. Genetic and genomic tools for Xenopus research: The NIH Xenopus initiative. Developmental Dynamics. 2002;225:384–391. doi: 10.1002/dvdy.10174. [DOI] [PubMed] [Google Scholar]
- Koyama M, Kinkhabwala A, Satou C, Higashijima SI, Fetcho J. Mapping a sensory-motor network onto a structural and functional ground plan in the hindbrain. Proceedings of the National Academy of Sciences. 2011;108:1170–1175. doi: 10.1073/pnas.1012189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe R, Kuratani S. Evolutionary perspectives from development of mesodermal components in the lamprey. Developmental Dynamics. 2007;236:2410–2420. doi: 10.1002/dvdy.21177. [DOI] [PubMed] [Google Scholar]
- Ladich F, Bass AH. Biocytin Study in Mochokid Catfish. The Journal of Comparative Neurology. 1996;374:493–505. doi: 10.1002/(SICI)1096-9861(19961028)374:4<493::AID-CNE2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ladich F, Bass AH. Sonic/vocal motor pathways in catfishes: comparisons with other teleosts. Brain, behavior and evolution. 1998;51:315–330. doi: 10.1159/000006545. [DOI] [PubMed] [Google Scholar]
- Ma LH, Gilland E, Bass AH, Baker R. Ancestry of motor innervation to pectoral fin and forelimb. Nat Commun. 2010;1:49. doi: 10.1038/ncomms1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manogue KR, Paton JA. Respiratory gating of activity in the avian vocal control system. Brain Research. 1982;247:383–387. doi: 10.1016/0006-8993(82)91265-3. [DOI] [PubMed] [Google Scholar]
- Martin WF, Gans C. Muscular control of the vocal tract during release signaling in the toad Bufo valliceps. J Morphol. 1972;137:1–27. doi: 10.1002/jmor.1051370102. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM, Francis‐West P. The differentiation and morphogenesis of craniofacial muscles. Developmental dynamics. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Nomaksteinsky M, Kassabov S, Chettouh Z, Stoeklé HC, Bonnaud L, Fortin G, Kandel ER, Brunet JF. Ancient origin of somatic and visceral neurons. BMC biology. 2013;11:53. doi: 10.1186/1741-7007-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. Journal of Comparative Neurology. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Onuki A, Somiya H. Innervation of sonic muscles in teleosts: occipital vs. spinal nerves. Brain, behavior and evolution. 2007;69:132–141. doi: 10.1159/000095202. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Philippidou P, Walsh CM, Aubin J, Jeannotte L, Dasen JS. Sustained Hox5 gene activity is required for respiratory motor neuron development. Nature neuroscience. 2012;15:1636–1644. doi: 10.1038/nn.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski N, Olsson L. Muscular derivatives of the cranialmost somites revealed by long-term fate mapping in the Mexican axolotl (Ambystoma mexicanum) Evolution & Development. 2007;9:566–578. doi: 10.1111/j.1525-142X.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes & Development. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AN, Bass AH. Novel vocal repertoire and paired swimbladders of the three-spined toadfish, Batrachomoeus trispinosus: insights into the diversity of the Batrachoididae. J Exp Biol. 2009;212:1377–1391. doi: 10.1242/jeb.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ, Guerra MA. The mechanism of sound production in túngara frogs and its role in sexual selection and speciation. Current Opinion in Neurobiology. 2014;28:54–59. doi: 10.1016/j.conb.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development. 2011;138:2401–2415. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW, Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S. NIH image to imageJ: 25 years of image analysis. Nat Methods. 2012;9 doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Tobias ML, Kelley DB. Origin and identification of fibers in the cranial nerve IX–X complex of Xenopus laevis: Lucifer Yellow backfills in vitro. Journal of Comparative Neurology. 1986;244:430–444. doi: 10.1002/cne.902440403. [DOI] [PubMed] [Google Scholar]
- Straka H, Baker R, Gilland E. Preservation of segmental hindbrain organization in adult frogs. The Journal of Comparative Neurology. 2006;494:228–245. doi: 10.1002/cne.20801. [DOI] [PubMed] [Google Scholar]
- Sweeney LB, Kelley DB. Harnessing vocal patterns for social communication. Current Opinion in Neurobiology. 2014;28:34–41. doi: 10.1016/j.conb.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada MN, Kuratani S. Evolutionary and developmental understanding of the spinal accessory nerve. Zoological Letters. 2015;1:4. doi: 10.1186/s40851-014-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Tobias ML, Kelley DB. Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. Journal of Neuroscience. 1987;7:3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S, Huang WH, Picardo M, Ling GY, Del Negro CA, Zoghbi HY, Gray PA. Atoh1-dependent rhombic lip neurons are required for temporal delay between independent respiratory oscillators in embryonic mice. Elife. 2014a:e02265–e02265. doi: 10.7554/eLife.02265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S, Rieger MA, Ling GY, Park TJ, Dougherty JD, Goodchild AK, Gray PA. Testing the role of preBötzinger Complex somatostatin neurons in respiratory and vocal behaviors. European Journal of Neuroscience. 2014b;40:3067–3077. doi: 10.1111/ejn.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah M, Mansor O, Ismail ZIM, Kapitonova MY, Sirajudeen K. Localization of the spinal nucleus of accessory nerve in rat: a horseradish peroxidase study. J Anat. 2007;210:428–438. doi: 10.1111/j.1469-7580.2007.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakos K, Wilson RJ, Kimura N, Remmers JE. Ancient gill and lung oscillators may generate the respiratory rhythm of frogs and rats. Journal of Neurobiology. 2005;62:369–385. doi: 10.1002/neu.20102. [DOI] [PubMed] [Google Scholar]
- Wake M. Evolutionary diversification of cranial and spinal nerves and their targets in the gymnophione amphibians. Cells Tissues Organs. 1993;148:160–168. doi: 10.1159/000147535. [DOI] [PubMed] [Google Scholar]
- Wang S, Peatman E, Abernathy J, Waldbieser G, Lindquist E, Richardson P, Lucas S, Wang M, Li P, Thimmapuram J. Assembly of 500,000 inter-specific catfish expressed sequence tags and large scale gene-associated marker development for whole genome association studies. Genome biology. 2010;11:R8. doi: 10.1186/gb-2010-11-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel DM, Haerter UL, Kelley DB. A proposed neural pathway for vocalization in South African clawed frogs, Xenopus laevis. Journal of Comparative Physiology A. 1985;157:749–761. doi: 10.1007/BF01350072. [DOI] [PubMed] [Google Scholar]
- Wild J. Functional neuroanatomy of the sensorimotor control of singing. Annals of the New York Academy of Sciences. 2004;1016:438–462. doi: 10.1196/annals.1298.016. [DOI] [PubMed] [Google Scholar]
- Wild JM. Neural pathways for the control of birdsong production. Journal of Neurobiology. 1997;33:653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Wilson R, Taylor B, Harris M. Evolution of vertebrate respiratory neural control. In: A G, BH S, editors. Encyclopedia of Neuroscience. 4th. Springer; 2009. pp. 67–75. [Google Scholar]
- Yager DD. Underwater acoustic communication in the African pipid frog Xenopus borealis. Bioacoustics. 1992;4:1–24. [Google Scholar]


