Abstract
Background and Objective
Targeted speech therapy can lead to substantial naming improvement in some subjects with anomia following dominant-hemisphere stroke. We investigated whether treatment-induced improvement in naming is associated with post-stroke preservation of structural neural network architecture.
Methods
Twenty-four patients with post-stroke chronic aphasia underwent 30 hours of speech therapy over a two-week period and were assessed at baseline and after therapy. Whole brain maps of neural architecture were constructed from pre-treatment MRI T1-weighted and diffusion tensor MRI to derive measures of global brain network architecture (network small-worldness) and regional network influence (nodal betweenness centrality). Their relationship with naming recovery was evaluated with multiple linear regressions.
Results
Treatment-induced improvement in correct naming was associated with post-stroke preservation of global network small worldness, and of betweenness centrality in temporal lobe cortical regions. Together with baseline aphasia severity, these measures explained 78% of the variability in treatment response.
Conclusions
Preservation of global and left temporal structural connectivity broadly explains the variability in treatment-related naming improvement in aphasia. These findings corroborate and expand on previous classical lesion-symptom mapping studies by elucidating some of the mechanisms by which brain damage may relate to treated aphasia recovery. Favorable naming outcomes may result from the intact connections between spared cortical areas that are functionally responsive to treatment.
Keywords: Aphasia, naming, recovery, structural connectome, magnetic resonance imaging, diffusion tensor imaging
1. Introduction
Stroke is one of the leading causes of disability worldwide and in the United States [1]. Aphasia is an impairment of language processing that often results from strokes affecting the dominant brain hemisphere [2]. A large number of subjects who suffered a left hemisphere stroke remain with some degree of long-term aphasia [2, 3] and this persistent communication impairment is commonly recognized as one of the most debilitating sequelae from stroke [4]. One of the hallmark deficits of aphasia is the inability to name objects or people (anomia) [5]; the severity of anomia is closely related to a poor quality of life [6, 7].
While many patients with aphasia exhibit some recovery of naming in the first days to weeks after the stroke, 30–40% persist with long lasting naming impairments [2]. Speech therapy can be effective to improve language processing for some subjects with chronic aphasia [8–11]. However, as is well recognized among clinicians, speech therapy can be ineffective for many others, and there are no well-defined means to predict which subjects may benefit from treatment. For example, in spite of decades of research, a relationship between linguistic factors and aphasia treatment success has not been revealed, suggesting that baseline behavioral testing may not provide clinicians the necessary prognostic indicators [e.g. see 12]. Accordingly, therapy management could be improved by a better understanding of the neurobiological mechanisms associated with aphasia and naming recovery.
Our group has demonstrated that patients with lesions encompassing areas commonly associated with lexical retrieval and phonological processing, such as Brodmann's areas (BA) 37 and 39, are less likely to show treatment-related improvement in correct naming [13]. Furthermore, the functional modulation (i.e. brain activation) of the peri-lesional left frontal and left temporal cortices are also associated with an increase in the number of items named correctly [14], suggesting that the apparent preservation of cortical regions after the stroke is not, by itself, sufficient to enable recovery. Even though post-treatment brain activation among patients with aphasia differs from baseline [15], some patients with apparently intact temporal or frontal cortical structures fail to achieve a good recovery [16].
Contemporary advances in structural brain mapping, particularly those employing structural Magnetic Resonance Imaging (MRI), have greatly expanded our knowledge regarding the relationship between brain connectivity, neural network integrity, and language. Specifically, whole brain MRI diffusion tensor imaging (DTI) can be quantified to provide a map of neural architecture for each person, i.e., the individual brain connectome [17]. Adapting connectome methods to the study of neural architecture in persons who suffered a stroke, our group has recently demonstrated that white matter destruction can lead to disconnection of remote and apparently intact cortical structures [18]. The destruction of white matter and subsequent disruption of brain connectivity are strong predictors of post-stroke naming impairment [19]. Indeed, a compromised neural network can be as important as direct cortical necrosis in the ensuing severity of naming problems [19].
Thus, it is likely that changes occurring within white matter tracts, leading to cortical disconnection, may account for some of the variability in language recovery previously demonstrated by lesion symptom mapping studies. Specifically, preserved cortical connectivity may permit the functional recruitment of areas modulated by therapy and partially explain the structural framework for recovery.
This hypothesis is consistent with recent findings relating the importance of white matter tracts for language impairment and recovery [20]. Previous studies have shown that lesion load on language-relevant white matter tracts (namely, the arcuate and uncinate fasciculi) contribute to recovery, with changes between pre- and post-treatment of anomia [21–24]. Abnormal patterns and post-treatment changes have also been shown to occur at the level of the functional connectome [25, 26], which describes the synchronous activity of regions in an individual brain. All of the aforesaid results have encouraged the development of interventions incorporating non-invasive brain stimulation (e.g. repetitive transcranial magnetic stimulation), which have preliminarily showed positive immediate-, short-, and long-term effects on naming performance [27–29].
We hypothesized that the post-stroke integrity of neural network architecture is a crucial determinant for naming recovery motivated by speech therapy. Here, we describe a cohort of patients with chronic aphasia and naming impairment after a stroke, who underwent a controlled period of intense speech therapy aimed at improving naming performance. Part of this cohort was previously examined by our group in a classical lesion-symptom mapping study [30]. Here, we aim to complement that study by investigating whether direct cortical disconnection and neural architecture are the mechanisms associated with post-stroke lesions that lead to impairments and permit recovery. All subjects were carefully assessed regarding their language abilities before and after the treatment. Instead of attempting to match treatment type to each individual’s cognitive-linguistic profile, all participants underwent anomia treatment using a protocol that has been amply described in the literature – semantic and phonological cueing hierarchies [31]. The rationale behind this approach is that the relationship between behavioral impairment, optimal treatment type, and treatment success is, at best, very poorly understood at this time. Much like traditional studies that employed lesion-symptom mapping to reveal the relationship between behavioral impairment and structural brain damage, the approach taken here assumes that residual neuroanatomy (i.e. structural connectivity) relates to patients’ ability to improve with the aid of anomia treatment.
We conducted a detailed assessment of global and regional neural network architecture prior to treatment, with the intention to evaluate the relationship between the degree of post-stroke network disruption and the ability to achieve improvement after speech therapy.
2. Methods
2.1. Subjects
We studied 24 individuals recruited from the local community with chronic aphasia as a result of a left hemisphere ischemic stroke that occurred at least six months prior to enrolling in this study. All subjects were right handed prior to the stroke and had no history of other neurological diseases. They also did not have a history or imaging evidence of other strokes. Patients with a history of medication-refractory seizures were not included in this study. Twenty of these patients were included in previous studies by our group.
The behavioral data from twenty patients reported here were also included, in part, in two previous studies from our group [13, 14]. Those studies examined a subject population that overlapped with the subjects reported here. The question addressed here, i.e., the relationship between naming recovery and the integrity of structural brain networks, was not addressed in those studies.
All subjects signed an informed consent to participate in this study. They were recruited and tested at The University of South Carolina (USC). The USC Institutional Review Board approved this study.
2.2. Baseline language testing
A certified speech pathologist administered and scored language performance at baseline based on the Western Aphasia Battery [32] (WAB) and the Philadelphia Naming Test [33] (PNT). Together, these tests provide a measure of: 1) aphasia type and severity (WAB), and 2) naming performance (PNT). Specifically, aphasia severity was defined in accordance with the WAB aphasia quotient (AQ), which is a measure of aphasia severity ranging from 0 to 100. The PNT is a computer-based assessment of naming [33], which includes 175 pictures representing mid- and high-frequency nouns from a word frequency list compiled by Francis and Kucera [34].
Prior to therapy, all subjects underwent two independent complete testing sessions, no further than 2–3 days apart, in order to account for daily variability in performance. The baseline performance scores on the PNT were defined as the average of the two pre-treatment testing sessions.
2.3. Baseline MRI scanning
All subjects underwent MRI scanning at USC in a 3T Siemens Trio equipped with a 12-channel head coil in accordance with the following protocol: A) T1-weighted images (3D MPRAGE, TR = 2250ms, TE = 4.15ms, 256×256matrix, 256×256mm FOV, parallel imaging GRAPPA=2, 80 reference lines, TA=377s); B) Diffusion EPI scan (30-directions with b=1000 s/mm2 and b=2000 s/mm2, TR = 6100ms, TE = 101ms, 82×82 matrix, 222×222mm FOV, parallel imaging GRAPPA=2, 80 45 contiguous 2.7mm axial slices, TA=390s).
MRI scanning was performed at baseline before any language therapy was applied.
2.4. Language therapy
After baseline testing was concluded, all subjects underwent a period of speech therapy aimed at improving naming performance. Therapy was performed daily for a period of two weeks. During each 3-hour treatment session, the subject underwent clinician administered anomia treatment using semantic and phonemic cueing hierarchies [31]. As indicated earlier, the treatment approach used here was not selected based on the cognitive-linguistic impairment profile of any single patient or a sub-group of patients. Rather, it was chosen given that studies that have employed similar methods have revealed its potential in improving naming across a large group of patients [35]. All subjects completed the treatment as planned without missing sessions.
The nouns targeted in treatment (n=160) were not included on the PNT. Thus, naming improvement was qualified as an increase in naming of untrained items. During this time, none of the subjects participated in any other form of speech therapy. Likewise, none of the subjects experienced any significant change in health status, or underwent any medication adjustments during the duration of the study.
2.5. Post-therapy language testing
After the conclusion of treatment, all subjects were re-evaluated with the PNT. This post-testing replicated the pre-treatment evaluations, and was also scored by a speech language pathologist. The evaluation occurred no longer than one week after treatment completion. The two post treatment evaluations occurred within 2–3 days of each other, scores again being the average of these post-therapy tests.
2.6. Preprocessing of MRI data and connectome construction
The preprocessing steps involved in the construction of the individual connectome involved: 1) segmentation of the probabilistic gray matter map (from T1 weighted images), further divided into cortical anatomical regions of interest (ROIs) corresponding to Brodmann areas (BA); 2) segmentation of the probabilistic white matter map from T1 weighted images; 3) registration of the individual white matter map and cortical ROIs into the individual DTI space; 4) probabilistic DTI fiber tracking; 5) iterative evaluation of the number of tractography streamlines connecting each possible pair of ROIs; 6) correction of each pair-wise connection strength (i.e., number of streamlines) based on the volume of the connected ROIs and the distance travelled by the streamlines. A summary of these steps is demonstrated in Figures 1 and 2.
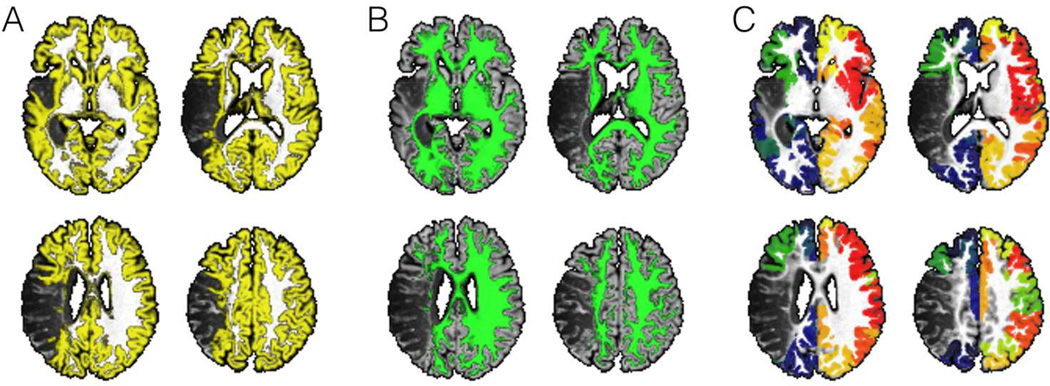
Figure 1.
This figure summarizes the initial preprocessing steps employed to evaluate structural connectivity. Images from one representative subject are shown. The T1 weighted images are segmented into probabilistic maps of gray matter (panel A) and white matter (panel B). The probabilistic map of gray matter is then divided into anatomical regions of interest (ROIs) corresponding to Brodmann Areas (BA) (panel C – each color representing a different ROI);.
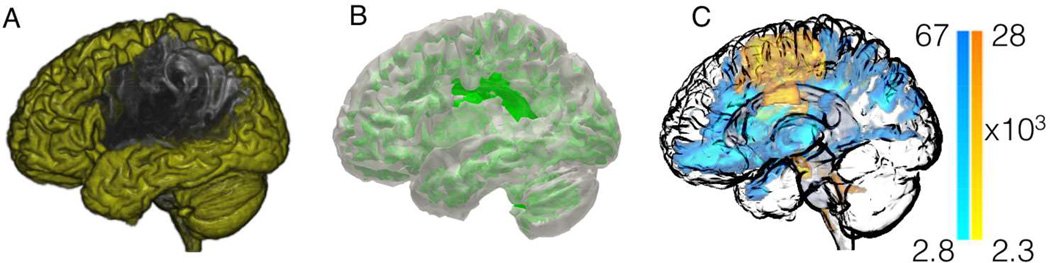
Figure 2.
Following the initial preprocessing steps, special processing is then conducted to exclude the areas involved in the post-stroke necrotic tissue, thus leaving only viable cortex (panel D – areas in yellow) and viable white matter (panel E - areas in green). Using this probabilistic white matter map as a guide, probabilistic white matter DTI streamlines are reconstructed by seeding each ROI at a time, and computing the number of streamlines arriving at the other ROIs. Panel C illustrates the voxel-based counts of probabilistic streamlines generated by seeding BA 6 (pre-motor cortex, with streamlines shown in “warm” color) and 48 (insula, streamlines in “cold” color) in the right hemisphere. The scale bars illustrate the voxel based counts of streamlines.
We employed methods previously described by our group [19] to overcome the distortions caused by the stroke necrotic lesion, aiming to preserve the anatomical authenticity of gray and white matter tissues, while avoiding the computation of fiber tracking in areas corresponding to the necrotic tissue. The area of necrosis was manually drawn on T2 images. The three-dimensional representation of this lesion was then used to calculate the lesion volume (number of voxels × voxel volume) for the purposes of our statistical analyses (see below). These methods are described in detail in the Methods section of the Online Supplementary Material.
In summary, for each subject, the structural connectome was ultimately defined as a connectivity matrix A, where each entry Aij represented the weighted link between cortical ROIs i and j (i.e., between two distinct BA), denoting the number of streamlines connecting these ROIs, adjusted based on the size of the ROIs and the distance travelled by the streamlines.
2.7. Measures of neural network architecture
From the individual connectivity matrix, we derived a measure that reflected the integrity of the global network, namely, normalized small worldness (NSW). This is a widely used measure of structural connectivity, which was calculated in accordance with the methods described by Brown and collaborators [36]. Specifically, for each subject’s structural network, 1,000 random networks (of similar link weight distribution) were generated by means of a computerized algorithm. We then calculated the global clustering coefficient of each network, which is an average of how each node in the network is connected to its neighbors (i.e., a proportion of how many links actually exist between a node and its neighbors and the maximum number of links that could exist). We then computed a ratio of the clustering coefficient from the real network to the average clustering coefficient from the random networks was calculated as the normalized clustering coefficient. We applied a similar procedure to calculate the ratio of characteristic path length (i.e. the number of links counted in the shortest path to transverse all possible pairs of network nodes) between the real network and the 1,000 random networks. NSW resulted then from the ratio between the normalized clustering and the normalized characteristic path length, thus obtaining one unique score that reflects global network integrity.
2.8. Involvement of temporal and frontal nodes in the global network
In order to assess the global network participation of temporal, parietal, and frontal nodes, we calculated the average nodal betweenness centrality (BC) of a series of ROIs. BC was calculated employing functions from the Brain Connectivity Toolbox [37]. BC is a measure of regional (i.e., local, as opposed to global) network integration, and it is related to how many of the network’s shortest paths travel through the node, providing a measure of influence of each node into the network [37]. As stated above, a shortest path is the minimal network distance between each possible pair of nodes. Nodes with high BC are commonly traversed by shortest paths linking two other nodes, being thus very influential and central to the network. We selected left-sided ROIs based on recent models of speech processing anatomy [38], and also based on conventional lobar neuroanatomy. Thus, ROIS were grouped as follows: left frontal ROIs (BA= [44, 45, 46]), left parietal ROIs (BA= [7, 39, 40, 41]), and left temporal ROIs (BA=[20, 21, 22, 37, 38]). These ROIs were selected to represent the majority of the dorsolateral aspects of the temporal and parietal lobes, as well as the posterior aspects of the inferior and middle temporal gyri. This approach permits an assessment of large representations of language areas within the left hemisphere, albeit with limited resolution to detect abnormalities within smaller or more localized sub-regions. This process was repeated to assess nodal BC of right hemisphere ROIs corresponding to the homologous BAs of the ROIs described above.
2.9. Statistical analyses
We investigated the relationship between increase in correct naming and measures of neural architecture. We controlled for other confounding variables that could independently influence network architecture or naming improvement, such as age (at the time of the study enrollment), time since the stroke, overall aphasia severity (WAB-AQ), and the volume of the necrotic stroke lesion.
Treatment-related improvement in naming was defined based on the difference between pre and post treatment performance on the PNT, adjusted based on the potential for recovery, as follows: the number of new items correctly named on the PNT was divided by the total number of items in the PNT [175] minus the number of correctly named items at baseline. Thus, an improvement in naming 20 items was weighted differently if the baseline score had been 155 ([20/(175-155)] = 100% improvement), versus 75 ([20/(175-75)] = 20% improvement).
The relationship between naming improvement and neural architecture was investigated through multiple linear regressions with treatment-related improvement in naming set as the dependent variable, and measures of neural architecture along with controlling variables set as independent variables. The explanatory power of the resulting regression model was determined as R2 (proportional reduction in error) with the explanatory factors of interest entered into the regression analysis using a stepwise approach. Control for multiple comparisons was performed through Bonferroni correction: multiple linear regression analyses were performed to evaluate the relationship between the following variables: 1) baseline WAB and left hemisphere BCs, 2) baseline WAB and NSW; 3) PNT improvement and left hemisphere BCs; 4) PNT improvement and NSW; 5) PNT improvement, left BCs and NSW; 6) PNT improvement and right and left BCs. Therefore the level of statistical significance of a given model was set at p < 0.05/6 = 0.008.
3. Results
3.1. Demographics
The mean age of the study sample was 60.2 ±11.9 years. Twelve subjects were male and the mean age at the time of the stroke was 55.5 ±12.1 years. Twenty-one subjects were Caucasian and 3 were African-American (Demographic details are further described in Supplementary Table 1).
3.2. Pre-treatment language performance
As the purpose of this study was to determine the association between treatment related improvement in correct naming and structural connectivity in aphasic patients, it was essential that the patient sample reflect a wide range of aphasia severities and types as well as variability in lesion location and extent of injury. The mean AQ for the group of subjects was 57.94 (SD = 28.27). Based on the WAB sub-scores, the aphasia types were: anomic: 41% (10/24); Broca’s: 33% (8/24); Wernicke’s: 8% (2/24); conduction: 8% (2/24); and global 8% (2/24). At baseline, combining the two pre-treatment testing sessions, among all subjects, the average number of correctly named items on the PNT was 59 ±61 items, ranging from 0 to 154 items. The average number of attempts (i.e., correct and error responses) was 118 ±61 items, and the average number of no responses was 57 ±61 items. Semantic paraphasias accounted for 9.6±7.3 items, while phonemic paraphasias comprised 11.3±14.7 items.
3.3. Post-treatment language performance
Subjects improved, on average, by correctly naming 6.8 ±7.1 more items after treatment (taking into account the average between the two post-treatment sessions in comparison with the average from the two pre-treatment sessions). The treatment-related improvement in naming, as defined above by ratio between number of correctly named items and the number of pre-treatment incorrect responses, was 12.3 ±15.6%, ranging from −4.5% (i.e., worsening after treatment) to 20.5%. There was also an increase in the number of response attempts (12.7 ±18.6 items) after treatment. Interestingly, on average, phonemic paraphasias increased by 1.9±7.2 items, while semantic paraphasias remained unchanged (0±5.5 items). No significant correlation was found between changes in correct naming and time (months) post-stroke (r = .08, p = .71).
The pre-treatment and post-treatment language performance data are presented in Supplementary Table 1.
3.4. Stroke lesion variability
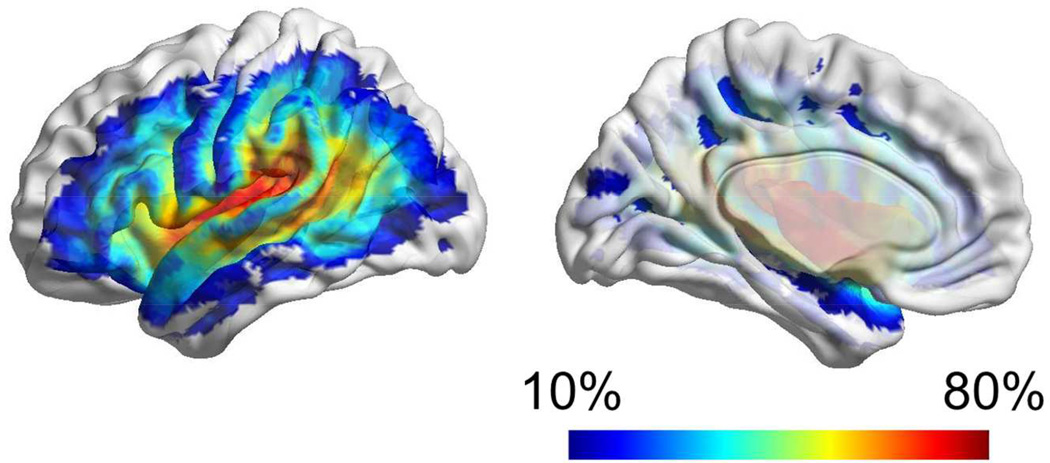
All patients included in this study exhibited a chronic necrotic lesion corresponding to sequelae of liquefactive gliosis due to ischemic injury in the cortical or subcortical vascular territory perfused by the left middle cerebral artery. In all subjects, the lesion was clearly visible on T2 weighted images. As demonstrated by the overlap between lesion sites (Figure 3), the most common location of damage was the left insular cortex, followed by the left temporal and the left frontal opercular regions, and then by other cortical areas in the left dorsolateral frontal, parietal and temporal regions. This overlap pattern is compatible with previously described lesion distributions in patients with aphasia [39].
Figure 3.
The anatomical distribution of the necrotic post-stroke brain lesions is demonstrated through a color-coded map (overlaid onto a cortical surface diagram) where each color represents the frequency, across all subjects, with which the corresponding anatomical region was lesioned.
3.5. Structural connectomes and left hemisphere connectivity abnormalities
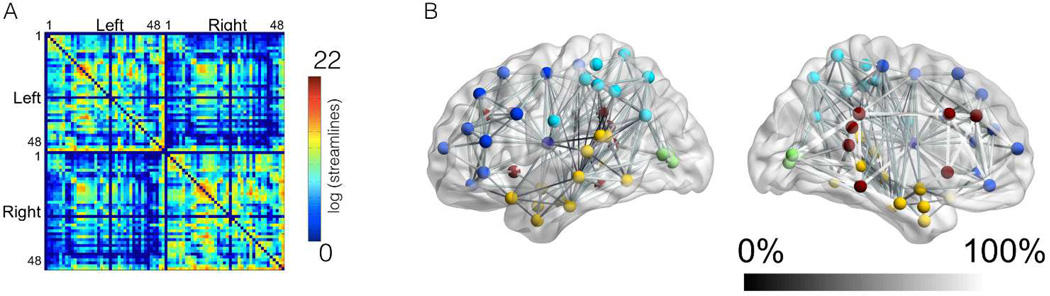
The structural connectome from each subject is demonstrated in Supplementary Figure 1. The average group connectome is demonstrated in Figure 4A. Even though the location of the necrotic lesion is heterogeneous among the study subjects, overall, the connections within the left hemisphere are less numerous or exhibit lower strength compared with connections within the right hemisphere. Figure 4A also demonstrates that the connectome can be abridged into a matrix where each entry corresponds to the connectivity strength between the row and column ROIs.
Figure 4.
This diagram demonstrates the average connectome in panel A, with axis numbers corresponding to BAs. The scale bar demonstrates the link-wise strength, which corresponds to the log of the number of streamlines connecting the ROIs (corrected based on ROI volume and distance travelled by the streamlines). Panel B demonstrates the link-wise asymmetry in connectivity, illustrating how often links in the left hemisphere were present, in comparison with the homologous right hemisphere links that were present in 100% of subjects. A sphere in its center of mass illustrates the location of each BA ROI; straight lines connecting the nodes demonstrate links; the shade of gray of each link corresponds to its frequency of observation in comparison with the right hemisphere. The colors of the nodes illustrate their anatomical locations (light blue – parietal and inferior frontal; dark blue – frontal; yellow – temporal; dark red – pericingulate regions and insula, green – occipital).
Figure 4B demonstrates a quantification of the relative frequency of disconnection within the left hemisphere, shown as the relative frequency with which links in the left hemisphere were observed, including only links whose right hemisphere homologous was observed in all patients (ranging from 0 – never, to 100% - in all patients). As expected, less connectivity in the left hemisphere was observed involving areas with highest necrotic tissue overlay (insular, opercula and dorsolateral regions).
3.6. Relationship between baseline language performance and structural connectivity
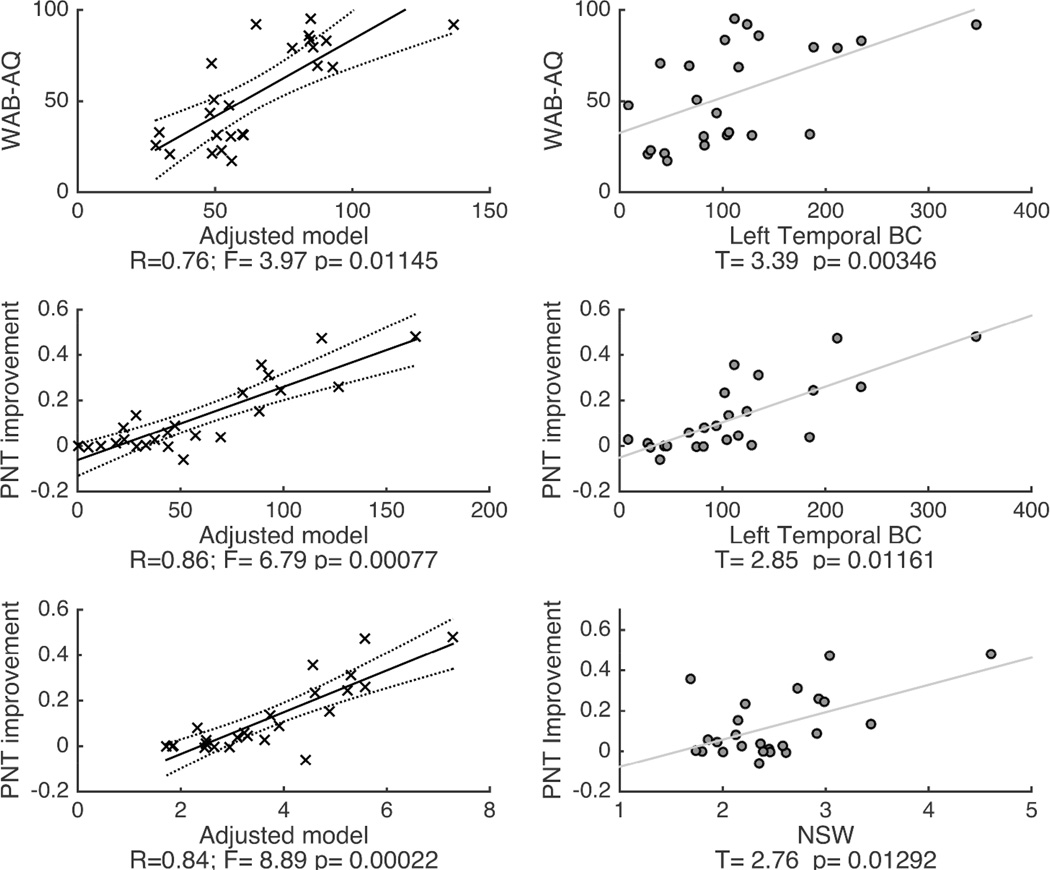
We observed a relationship trend between more severe aphasia (measured as lower WAB-AQ) and lower left temporal BC (F=3.97, p=0.011, model R=0.76, model R2 =0.58), controlling for age, time since the stroke and stroke lesion size. However, this model was not statistically significant due to the control for multiple statistical comparisons. This trend suggests that loss of influence from left temporal nodes onto the general network was associated with more severe forms of aphasia at baseline (Figure 5 and Supplementary Table 2).
Figure 5.
Scatter plots demonstrating the results from the linear regression models in relationship with baseline WAB-AQ (upper panel) and treatment-related improvement in the naming (PNT improvement - middle and lower panels) set as dependent variables. The adjusted models (controlling for age, time since the stroke and lesion size) are shown on the left column, and the association between the independent variables related to global network measures (NSW – normalized small worldness) and regional left temporal network measures (BC – betweenness centrality) are shown on the right column. Supplementary Table 2 provides a comprehensive description of the statistical features of these models.
We did not observe a relationship between baseline aphasia severity and global network NSW (F=2.7, p=0.06; Supplementary Table 2). Moreover, NSW was not correlated with lesion volume (r = −0.04, p = 0.9).
3.7. Relationship between naming improvement and regional and global structural connectivity
We observed a robust relationship between treatment-related improvement in naming and a higher left temporal BC (controlling for age, time since the stroke, stroke lesion size and WAB-AQ) (model F=6.79, p=0.00077, model R=0.86, model R2 =0.74, Figure 5, Supplementary Table 2). In this model, WAB-AQ was also associated with treatment-related improvement in naming (T=2.45, p=0.03); patients with less severe aphasia achieved a higher naming improvement.
We did not observe a significant relationship between treatment-related improvement in naming and regional network measures from the frontal or parietal areas (Supplementary Table 2). Lesion volume was not associated with treatment-induced improvements in naming (r = − 0.28, p = 0.18). Moreover, there was not a direct correlation between lesion burden in the temporal lobes and the network integration of the remaining temporal lobe cortex (BC) (r = − 0.01, p=0.9).
We also observed a robust relationship between PNT improvement and global network NSW. Patients with higher global NSW demonstrated greater treatment-related improvement in naming (controlling for age, time since the stroke, stroke lesion size and WAB-AQ) (model F=8.89, p=0.0002, model R=0.84, model R2 =0.712, Figure 5, Supplementary Table 2). Similarly, WAB-AQ was also associated with PNT improvement.
Finally, if NSW and left temporal BC were both included as independent variables in the same model (again, controlling for age, time since the stroke, stroke lesion size and WAB-AQ), this new model explained 78% of the variance observed in improvement in the PNT (F=10.2, p-value = 0.00007, model R=0.88, model R2 =0.78). We did not evaluate the individual influence of each predictor in this model since left temporal BC and NSW were highly correlated (R=0.62, p=0.001), leading to inaccuracies in individual independent variable contribution due to multicolinearity.
The linear regression analysis combining nodal BC from left and right hemisphere BAs as independent variables demonstrated an association with improvement in the PNT (F=5.62, p-value = 0.003, model R=0.82, model R2 =0.68); nonetheless, none of the right hemisphere regions (i.e., right frontal, right temporal and right parietal ROIs) were independently associated with improvement.
4. Discussion
In this study, we examined the relationship between regional and global brain neural network architecture in the chronic state after an ischemic stroke, and the ability to improve in naming performance after treatment in subjects with aphasia. Our findings can be summarized as described below.
First, preserved global network architecture (NSW) is associated with a more pronounced treatment-related improvement in naming. Second, if temporal lobe regions remain integrated with the remaining network (i.e., temporal lobe BC), a higher treatment-related improvement is also more likely to occur. These two measures (NSW and left temporal BC) are highly associated with each other, but independently, each one of these measures could predict more than 70% of the variance in naming improvement. Third, subjects with more severe aphasia prior to treatment (lower WAB-AQ) are also less likely to improve with treatment. Combined with NSW and left temporal BC, a model composed by these three measures could predict 78% of the treatment-related improvement. Fourth, we did not observe a relationship between structural integrity of other brain regions (frontal or parietal) and treatment-related improvement.
4.1. Treatment-related improvement is still possible in the chronic stages of aphasia
Even though the prognosis for recovery for some patients with aphasia can be unfavorable, our study corroborates previous observations reporting that a subset of subjects may still experience recovery in the chronic stages of aphasia [8, 40–42]. As it has been suggested in treatment studies of aphasia [13, 14], the identification of patients with the potential to recover could lead to a positive impact in the clinical management of aphasia, enabling therapy to a targeted population, thus providing access to speech therapy that is not routinely offered in the chronic post-stroke stage, and the possibility of further recovery.
So far, the crucial limitation in this approach has been the identification of those patients who can actually improve. Besides aphasia severity, there are probably few behavioral variables that can discriminate patients with a good prognosis as accurately. For example, recovery has not been shown be related to age during therapy, age at the time of the stroke, necrotic lesion size or even aphasia characteristics [e.g. 43, 44]. Nonetheless, the patients who benefit from anomia therapy seem to have in common some basic neurobiological apparatus that enables improved naming as a result of treatment. Our findings suggest that the integrity of structural brain networks (e.g. as reflected by NSW and BC measures) is a biomarker for the mechanisms that support treated aphasia recovery. As such, it can be quantified and potentially used in clinical settings to better understand and guide aphasia treatment in a more tailored fashion for each patient. Future studies should further assess cognitive variables such as motivation, attention, learning and memory, as well as comprehension in order to better predict treatment-induced recovery. Moreover, potential research in this area should attempt to replicate the present framework by using alternative interventions. This includes not only other rehabilitation strategies, but also different time frames. For example, language therapy used in this study was rather intense (3 hour training sessions, 5 days a week), albeit short in duration (2 weeks). It is possible that some patients require similarly intense training during longer periods of time in order for improvement to emerge on post-treatment testing. Importantly, we found no correlation between time post-stroke and changes in naming before and after treatment, suggesting that the significant gains in naming that are reported here are probably not driven by spontaneous recovery. The systematic studying of treatment logistics (i.e., treatment session length, duration, number/duration of breaks in between sessions, etc.) is certainly warranted.
4.2. Structural network integrity as a framework for functional recovery
Our group has recently demonstrated that cell loss and disruption of network connectivity is a pervasive phenomenon in patients with regional gliosis after an ischemic stroke [18]. White matter damage is well known to occur in experimental models of stroke [45] but it has been underestimated in humans thus far due to methodological limitations in measuring structural connectivity. However, with improvement in spatial registration of brains with lesions [46] and in DTI fiber tracking techniques, it is now possible to measure radiographic correlates of Wallerian degeneration [47]. Furthermore, measurements of white matter pathways also allow for the comprehensive assessment of brain connectivity and construction of the brain connectome [48, 49], which permits unprecedented analysis of whole-brain neural network architecture. The structural brain connectome is the scaffolding supporting brain function, and significant insight into this framework can be gained by investigating the relationship between behavioral measures and regional connectome variability, as well as graph theory network analyses of connectome conformation [17, 50]. Using brain connectome methodology, optimized to take into account anatomical deformations associated with post-stroke gliosis [46], our group recently demonstrated a direct association between disrupted connectivity and chronic stage aphasia severity [19]. To our knowledge, that was the first study assessing the relationship between connectome integrity and aphasia severity in a group study. Therefore, it is possible that structural connectivity also supports brain plasticity necessary for naming improvement. In fact, the extent of structural damage to gray matter components of the language network has been seen as the main limiting factor for treatment-induced naming improvement [13, 30]. Consequently, there is vast potential for correlating behavioral performance with the “strength” (i.e., density of streamlines in the case of structural connectomes) between different brain areas. This connectivity approach complements conventional voxel-based lesion-symptom mapping (VLSM) [39] by elucidating some of the neurobiological mechanisms underlying the relationship between brain damage and loss of function. Specifically, this connectivity approach provides information about how white matter loss contributes to impairment and recovery. The connectivity approach should not be seen as antagonist to VLSM, but rather as its complement, because it permits the quantification of one of the elements of the lesion, i.e., cortical disconnection. Since cortical disconnection can exist in regions that are located outside the lesion, the disconnection approach may hypothetically provide more information compared with VLSM in specific cases where disconnection may be more prominent than the necrotic lesion.
Moreover, the connectivity approach demonstrated here may also provide information about the underlying neural network responsible for orchestrating recovery. For instance, if a cluster of voxels in two different brain areas were shown to correlate with naming performance on VLSM, a correlation between behavior and the link between these two regions favors their concerted action in contributing to performance. On the contrary, if the link between areas relevant to behavior revealed by VLSM does not correlate with performance, their contribution is ought to be independent from one another. As a third possibility, if connectivity analyses demonstrate a significant contribution of areas not lesioned by the stroke, this would be suggestive of connectional diaschisis, the process by which structural and functional connectivity change between brain regions as a result of a distant lesion [51]. Our approach thus calls for follow-up studies directly comparing the ability of VLSM- and connectivity-derived measures to predict prognosis. Specifically, it prompts for the assessment as to whether there are subgroups of patients where one approach may be particularly suited at explaining recovery.
In this study, we demonstrated that preservation of global neural network architecture and, in particular, preservation of the left temporal neocortex influence onto the brain networks (i.e., left temporal BC) are both important determinants of recovery. This was achieved by conducting analyses both at the whole-brain (NSW) and at the nodal level (BC). For the latter, we selected specific ROIs that would be relevant for naming. This requires a priori criteria, which we based on extensive research showing the involvement of left frontal, temporal, and parietal cortices as the core structures involved in naming. This is of course an arbitrary decision, as naming is a very complex function, relying on an extensive network that may extend beyond the structures selected for our analyses. In fact, recent evidence has proposed a potential role of dominant-homologous right hemisphere regions in aphasia recovery [20]. The 12 left hemisphere Brodmann areas we included, however, cover the vast majority of the language network and are in agreement with current neurobiological models of speech processing and naming [38, 52, 53]. It is thus likely that predicting accuracy of recovery can be further improved by pooling ROIs beyond those studied here, extending beyond the dominant hemisphere. Taken together, therefore, our results show that preserved cortical connectivity is key for enabling anomia recovery, likely because it supports functional modulation during the reestablishment of language networks motivated by therapy
4.3. Left temporal cortex influence on the global network
We observed that the average BC from specific left temporal cortical ROIs was associated with naming recovery. Interestingly, nodal BC is a measure of participation, or influence [50], of a given node on the global network, measuring how important that node is to the structural integrity of the network; BC denotes the ratio of the shortest paths within the network that involve a specific node [37]. That is, it quantifies how many routes between two other nodes require passing through a specific node. Therefore, BC is not necessarily high in highly connected nodes. For example, in a network with many random links, a node with a high number of connections may still remain isolated from most of the remaining connections. Conversely, if a node is central to the network and participates in many connections, it will have a high BC. In biological networks, a balance between efficiency and economy of connections likely determines the overall organization of the network. Areas with great influence (or high BC) are strategically placed to facilitate information transfer and reduce the randomness of connections.
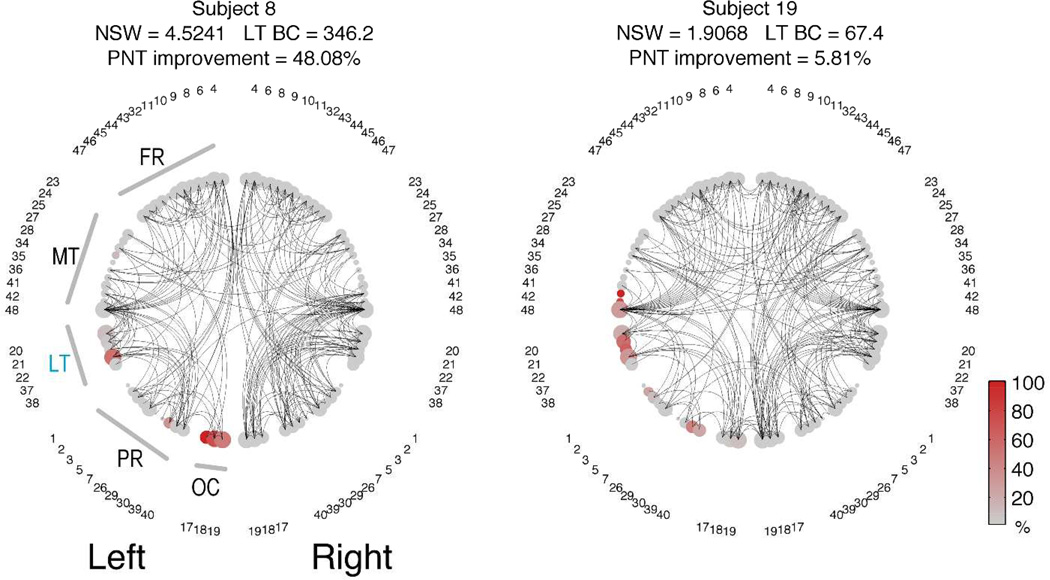
To facilitate the visualization of network organization, Figure 6 provides examples of network configurations from two patients with relatively similar necrotic lesion burden, but with different network metrics and different levels of improvement in the PNT. The complete overview of network configurations from all patients is demonstrated in Supplementary Figure 2.
Figure 6.
The network configurations of two representative patients are demonstrated as 2-dimensional circular diagrams, where each anatomical ROI is symbolized by a circle corresponding to the network node (with numbers indicating the BA), and the links between nodes are represented as curved lines. The nodes are organized in accordance with their anatomical distribution, i.e, left ROIs are placed in the left side of the figure and ROIs are grouped based on lobes. The node size is proportional to the size of the anatomical ROI (log2[size of the ROI]). To improve visualization, the network was made sparser by preserving only the links above the 95% link-weight percentile. The color of the node represents the percentage of the ROI that was damaged by the stroke (in accordance with the colorbar). Note the subject 8 has higher LT BC because there are more connections traversing the LT area (i.e., to travel between two other ROIs, the path “bounces of” LT). Legend: FR=frontal regions; MT = medial and inferior temporal regions; LT = lateral temporal regions; PR = parietal regions; OC = occipital regions.
4.4. The role of the left temporal cortex in language recovery
Naming impairment may occur as a result of lesions affecting several aspects of the cortical language network. Hillis and colleagues demonstrated that several regions are essential for distinct processes underlying naming [54]. Specifically, anomia may arise from combined dysfunction involving the left anterior, inferior and posterior middle/superior temporal cortex, posterior inferior frontal and inferior parietal cortices [54, 55]. Dronkers and colleagues demonstrated that damage to the left mid-posterior middle temporal gyrus (MTG) prevents the retrieval of names associated with objects (i.e. lexical-semantic retrieval) [56]. These findings complement observations from Schwartz et al. demonstrating that semantic errors during naming are associated with damage to the left anterior and mid MTG [57, 58], while phonological errors are associated with lack of integrity of the left supramarginal gyrus and inferior frontal cortex [59]. Furthermore, Hillis et al. have demonstrated in several studies that BA 37 (i.e., located in the posterior portion of the mediobasal and lateral surfaces of the temporal lobe, including the fusiform gyrus and inferior temporal gyrus) is crucial for amodal lexical retrieval [60–62]. Our results are in accordance with these observations. They are also in accordance with a recent model of speech proposed by Hickok and Poeppel [52].
This model suggests that speech is associated with two distinct major processing pathways, a ventral and a dorsal stream. The white matter pathways connecting the posterior and anterior aspects of the temporal lobes likely correspond to the ventral stream, which processes sound-to-meaning relationships and supports lexical retrieval and semantic associations. Conversely, the dorsal stream, which links perisylvian parietotemporal, inferior frontal gyrus, anterior insula, and premotor cortex, supports sound-to-articulation processing [52].
Our results demonstrate that temporal lobe regions directly involved in the ventral stream pathways (i.e., within the middle inferior and superior temporal gyri) are directly associated with naming recovery. Moreover, recovery is not solely associated with the preservation of connectivity of these regions, but also with how much these regions influence the brain network. This is a novel observation that directly supports and complements the dual stream model, particularly as it relates to the role of temporal cortex in speech processing.
Interestingly, our findings regarding global network preservation suggest that connectivity integrity extending beyond the left temporal lobe is also important for aphasia recovery. This observation is in accordance with a recent study by Forkel et al. [20], which demonstrated that integrity of the left and right arcuate fasciculi play a role in spontaneous aphasia recovery. Together, their findings and ours support the notion that regional left hemisphere connectivity, homologous right hemisphere and global network configurations, are determinants of aphasia recovery. These results prompt for future studies exploring the ability of structural connectivity measures in predicting treatment-induced recovery in an independent sample of patients, for example, undergoing different intervention regimes. It will also be important to assess structural connectivity in predicting score improvement among healthy controls. Usually, however, naming tasks such as the one used in this study tend to show a ceiling effect among control participants.
4.5. Global network integrity and language recovery
The relationship between global network integrity (NSW) and recovery can shed additional light on the importance of the preservation of non-random structural organization of neural networks for recovery. Interestingly, the mechanisms underlying preservation of global network architecture may be related to complex interaction between pre-stroke “brain health” and the disruptive effect of large stroke lesions on global neural network. Pre- and post-stroke “brain-health” may be an important predictor of recovery and this may be a promising line for research in neurological rehabilitation. Clearly, further research is needed to substantiate this notion.
4.6. Limitations and future studies
The conclusions derived from the present study favor the use of graph theory measures derived from the structural connectivity in assessing prognosis of aphasia due to chronic stroke. Our findings corroborate the exciting and evolving field of brain connectomics, and we acknowledge a number of limitations that should be taken into account for future studies of this nature. First, we recruited a heterogeneous population of chronic stroke patients, pooling together different aphasic syndromes. Second, we focused on just one aspect of aphasia, namely, anomia. While these can be caveats from certain perspectives, they also constitute important advantages for the purposes of the present study. This is because by focusing on one particular behavioral measure across a diverse population of patients, we were able to obtain a broad range in language performance (i.e., naming ability). The variability in such measure was used in network-based lesion-symptom mapping to identify which links were relevant in the recovery of anomia. Importantly, this is not an all-or-none, binomial categorization. Anomia and its recovery are graded phenomena, and so is the connectivity strength between two brain structures. Thus, by recruiting a heterogeneous sample, we demonstrate that brain connectivity can be useful in predicting changes in performance, despite the clinical label used to categorize each patient. This is especially important because performance variability is also observed across patients within the same clinical group. Nonetheless, future studies with larger sample sizes should attempt to determine whether connectivity measures are better suited for predicting language recovery in different sub-groups of patients, as well as using various measures of language performance.
In addition, all patients underwent identical treatment, despite their individual baseline performance or type of aphasia. This constitutes a limitation inherent to rehabilitation studies seeking to provide a consistent and homogeneous intervention that reduces confounders. In everyday clinical practice, naturally, we should make every possible attempt to tailor rehabilitation to the needs of each patient. There is converging evidence demonstrating, however, that the same patients reliably show improvements when undergoing different treatment modalities [e.g. 63, 64]. Accordingly, future research could, for instance, employ more than one intervention and, by using a counterbalanced design, determine whether connectivity measures are able to superiorly predict recovery when patients undergo an intervention that better suits their individual needs. In the same vein, we acknowledge that there are several ways to determine what exactly constitutes an improvement in naming. Here, we used a similar approach to Lasar and et al. [65], but alternative definitions can be considered.
Despite very recent advances offering an alternative approach [66], structural connectivity relies on the segmentation of neural tissue into pre-defined regions of interest. This tethers analyses to a priori anatomical assumptions, depending on factors such as number of ROIs and volume. Such segmentation based on previously defined atlases can be convenient, however, as a large number of studies use these predetermined regions to describe structural and functional changes in the brain. In our study, we decided to employ Brodmann areas because of their widespread use both in research and clinical settings among patient populations with language deficits. We acknowledge, nonetheless, that the ROIs of this atlas are large, usually encompassing different brain regions that serve distinct functions (e.g. anterior and posterior aspects of the temporal gyri). For this reason, future studies should attempt to replicate this approach using other atlases that, by segmenting the brain into a larger number of ROIs, ensure that each one of these areas is confined to a more restricted volume of neural tissue.
Another important issue to take into consideration is the time of assessment post-therapy. Here, we evaluated patients within a week after a two-week intense intervention. Longitudinal follow-up of these patients is crucial, and more studies are needed to determine whether medium- and long-term outcomes can also be predicted using brain connectivity measures.
4.7. Conclusions
Preserved global neural network architecture, with maintenance of the temporal lobe influence in the configuration of the brain neural networks, is crucial to support naming recovery in individuals with chronic aphasia. These are neurobiological markers that explain more than three quarters of the variability in treatment-related naming improvement and suggest that preserved network integrity relates to potential brain plasticity the drives treatment induced improvements in naming in aphasia.
These results have the potential to encourage the translation of brain connectivity studies into real-life clinical contexts, by providing health practitioners with a tool to assess the potential for recovery. In doing so, physicians, speech pathologists, and caregivers can plan for relevant non-pharmacological interventions to subjects who are more likely to benefit from treatment, even in the chronic stages of aphasia. Better strategies can also be adopted for subjects with aphasia and their relatives to readjust their expectations about recovery and outcome, which has the potential to positively contribute to mental health and quality of life.
Supplementary Material
Acknowledgements
This study was supported by The National Institute on Deafness and Other Communication Disorders (NIDCD) (grants number DC008355 and DC014021).
Footnotes
Disclosure: None
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008 May 10;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. Journal of neurology, neurosurgery, and psychiatry. 1986 Jan;49(1):11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croquelois A, Bogousslavsky J. Stroke aphasia: 1,500 consecutive cases. Cerebrovascular diseases. 2011;31(4):392–399. doi: 10.1159/000323217. [DOI] [PubMed] [Google Scholar]
- 4.Hilari K, Byng S. Health-related quality of life in people with severe aphasia. International journal of language & communication disorders / Royal College of Speech & Language Therapists. 2009 Mar-Apr;44(2):193–205. doi: 10.1080/13682820802008820. [DOI] [PubMed] [Google Scholar]
- 5.Kohn SE, Goodglass H. Picture-naming in aphasia. Brain and language. 1985 Mar;24(2):266–283. doi: 10.1016/0093-934x(85)90135-x. [DOI] [PubMed] [Google Scholar]
- 6.Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? A systematic review. Archives of physical medicine and rehabilitation. 2012 Jan;93(1 Suppl):S86–S95. doi: 10.1016/j.apmr.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Code C, Hemsley G, Herrmann M. The emotional impact of aphasia. Seminars in speech and language. 1999;20(1):19–31. doi: 10.1055/s-2008-1064006. [DOI] [PubMed] [Google Scholar]
- 8.Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke; a journal of cerebral circulation. 2003 Apr;34(4):987–993. doi: 10.1161/01.STR.0000062343.64383.D0. [DOI] [PubMed] [Google Scholar]
- 9.Katz RC, Hallowell B, Code C, Armstrong E, Roberts P, Pound C, et al. A multinational comparison of aphasia management practices. International journal of language & communication disorders / Royal College of Speech & Language Therapists. 2000 Apr-Jun;35(2):303–314. doi: 10.1080/136828200247205. [DOI] [PubMed] [Google Scholar]
- 10.Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. Journal of speech, language, and hearing research : JSLHR. 1998 Feb;41(1):172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- 11.Holland AL, Fromm DS, DeRuyter F, Stein M. Treatment efficacy: aphasia. Journal of speech and hearing research. 1996 Oct;39(5):S27–S36. doi: 10.1044/jshr.3905.s27. [DOI] [PubMed] [Google Scholar]
- 12.Hillis AE. Treatment of naming disorders: new issues regarding old therapies. Journal of the International Neuropsychological Society : JINS. 1998 Nov;4(6):648–660. doi: 10.1017/s135561779846613x. [DOI] [PubMed] [Google Scholar]
- 13.Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Sep 1;30(35):11558–11564. doi: 10.1523/JNEUROSCI.2227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. NeuroImage. 2012 Apr 2;60(2):854–863. doi: 10.1016/j.neuroimage.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochon E, Leonard C, Burianova H, Laird L, Soros P, Graham S, et al. Neural changes after phonological treatment for anomia: An fMRI study. Brain and language. 2010 Sep;114(3):164–179. doi: 10.1016/j.bandl.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridriksson J, Bonilha L, Rorden C. Severe Broca's aphasia without Broca's area damage. Behavioural neurology. 2007;18(4):237–238. doi: 10.1155/2007/785280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sporns O. The human connectome: origins and challenges. NeuroImage. 2013 Oct 15;80:53–61. doi: 10.1016/j.neuroimage.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Bonilha L, Nesland T, Rorden C, Fillmore P, Ratnayake RP, Fridriksson J. Mapping remote subcortical ramifications of injury after ischemic strokes. Behavioural neurology. 2014;2014:215380. doi: 10.1155/2014/215380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonilha L, Rorden C, Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke; a journal of cerebral circulation. 2014 Apr;45(4):988–993. doi: 10.1161/STROKEAHA.113.004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forkel SJ, Thiebaut de Schotten M, Dell'Acqua F, Kalra L, Murphy DG, Williams SC, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain : a journal of neurology. 2014 Jul;137(Pt 7):2027–2039. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]
- 21.van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabilitation and neural repair. 2014 May;28(4):325–334. doi: 10.1177/1545968313508654. [DOI] [PubMed] [Google Scholar]
- 22.Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke; a journal of cerebral circulation. 2011 Aug;42(8):2251–2256. doi: 10.1161/STROKEAHA.110.606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinoshita M, Nakada M, Okita H, Hamada J, Hayashi Y. Predictive value of fractional anisotropy of the arcuate fasciculus for the functional recovery of language after brain tumor resection: a preliminary study. Clinical neurology and neurosurgery. 2014 Feb;117:45–50. doi: 10.1016/j.clineuro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Marchina S, Norton AC, Wan CY, Schlaug G. Predicting speech fluency and naming abilities in aphasic patients. Frontiers in human neuroscience. 2013;7:831. doi: 10.3389/fnhum.2013.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA. A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post-stroke aphasia. Human brain mapping. 2014 Aug;35(8):3919–3931. doi: 10.1002/hbm.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair VA, Young BM, La C, Reiter P, Nadkarni TN, Song J, et al. Functional connectivity changes in the language network during stroke recovery. Annals of clinical and translational neurology. 2015 Feb;2(2):185–195. doi: 10.1002/acn3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotelli M, Fertonani A, Miozzo A, Rosini S, Manenti R, Padovani A, et al. Anomia training and brain stimulation in chronic aphasia. Neuropsychological rehabilitation. 2011 Oct;21(5):717–741. doi: 10.1080/09602011.2011.621275. [DOI] [PubMed] [Google Scholar]
- 28.Ren CL, Zhang GF, Xia N, Jin CH, Zhang XH, Hao JF, et al. Effect of low-frequency rTMS on aphasia in stroke patients: a meta-analysis of randomized controlled trials. PloS one. 2014;9(7):e102557. doi: 10.1371/journal.pone.0102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiel A, Hartmann A, Rubi-Fessen I, Anglade C, Kracht L, Weiduschat N, et al. Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke; a journal of cerebral circulation. 2013 Aug;44(8):2240–2246. doi: 10.1161/STROKEAHA.111.000574. [DOI] [PubMed] [Google Scholar]
- 30.Fridriksson J, Bonilha L, Baker JM, Moser D, Rorden C. Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cerebral cortex. 2010 May;20(5):1013–1019. doi: 10.1093/cercor/bhp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linebaugh CW. Cueing hierarchies and word retrieval. In: Brookshire RH, editor. Clinical Aphasiology. Minneapolis, MN: BRK Publishers; 1977. pp. 19–31. [Google Scholar]
- 32.Kertesz A. The Western Aphasia Battery - Revised. New York: Grune & Stratton; 2007. [Google Scholar]
- 33.Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia Naming Test: Scoring and rationale. Clinical Aphasiology. 1996;24:121–133. [Google Scholar]
- 34.Francis WN, Kučera H, Mackie AW. Frequency analysis of English usage : lexicon and grammar. Boston: Houghton Mifflin; 1982. [Google Scholar]
- 35.Maher LM, Raymer AM. Management of anomia. Topics in stroke rehabilitation. 2004 Winter;11(1):10–21. doi: 10.1310/318R-RMD5-055J-PQ40. [DOI] [PubMed] [Google Scholar]
- 36.Brown JA, Terashima KH, Burggren AC, Ercoli LM, Miller KJ, Small GW, et al. Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proceedings of the National Academy of Sciences of the United States of America. 2011 Dec 20;108(51):20760–20765. doi: 10.1073/pnas.1109038108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010 Sep;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Hickok G. Computational neuroanatomy of speech production. Nature reviews Neuroscience. 2012 Feb;13(2):135–145. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nature neuroscience. 2003 May;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 40.Fridriksson J, Hubbard HI, Hudspeth SG, Holland AL, Bonilha L, Fromm D, et al. Speech entrainment enables patients with Broca's aphasia to produce fluent speech. Brain : a journal of neurology. 2012 Dec;135(Pt 12):3815–3829. doi: 10.1093/brain/aws301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridriksson J, Baker JM, Whiteside J, Eoute D, Jr, Moser D, Vesselinov R, et al. Treating visual speech perception to improve speech production in nonfluent aphasia. Stroke; a journal of cerebral circulation. 2009 Mar;40(3):853–858. doi: 10.1161/STROKEAHA.108.532499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brady MC, Kelly H, Godwin J, Enderby P. Speech and language therapy for aphasia following stroke. The Cochrane database of systematic reviews. 2012;5 doi: 10.1002/14651858.CD000425.pub3. CD000425. [DOI] [PubMed] [Google Scholar]
- 43.Lazar RM, Speizer AE, Festa JR, Krakauer JW, Marshall RS. Variability in language recovery after first-time stroke. Journal of neurology, neurosurgery, and psychiatry. 2008 May;79(5):530–534. doi: 10.1136/jnnp.2007.122457. [DOI] [PubMed] [Google Scholar]
- 44.Plowman E, Hentz B, Ellis C., Jr Post-stroke aphasia prognosis: a review of patient-related and stroke-related factors. Journal of evaluation in clinical practice. 2012 Jun;18(3):689–694. doi: 10.1111/j.1365-2753.2011.01650.x. [DOI] [PubMed] [Google Scholar]
- 45.Iizuka H, Sakatani K, Young W. Corticofugal axonal degeneration in rats after middle cerebral artery occlusion. Stroke; a journal of cerebral circulation. 1989 Oct;20(10):1396–1402. doi: 10.1161/01.str.20.10.1396. [DOI] [PubMed] [Google Scholar]
- 46.Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. NeuroImage. 2012 Jul 16;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones KC, Hawkins C, Armstrong D, Deveber G, Macgregor D, Moharir M, et al. Association between radiographic Wallerian degeneration and neuropathological changes post childhood stroke. Developmental medicine and child neurology. 2013 Feb;55(2):173–177. doi: 10.1111/dmcn.12010. [DOI] [PubMed] [Google Scholar]
- 48.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS biology. 2008 Jul 1;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sporns O. The human connectome: a complex network. Annals of the New York Academy of Sciences. 2011 Apr;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 50.Sporns O. From simple graphs to the connectome: networks in neuroimaging. NeuroImage. 2012 Aug 15;62(2):881–886. doi: 10.1016/j.neuroimage.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 51.Carrera E, Tononi G. Diaschisis: past, present, future. Brain : a journal of neurology. 2014 Sep;137(Pt 9):2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- 52.Hickok G, Poeppel D. The cortical organization of speech processing. Nature reviews Neuroscience. 2007 May;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 53.Gleichgerrcht E, Fridriksson J, Bonilha L. Neuroanatomical foundations of naming impairments across different neurological conditions. Neurology. 2015 doi: 10.1212/WNL.0000000000001765. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain : a journal of neurology. 2007 May;130(Pt 5):1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 55.Newhart M, Ken L, Kleinman JT, Heidler-Gary J, Hillis AE. Neural networks essential for naming and word comprehension. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2007 Mar;20(1):25–30. doi: 10.1097/WNN.0b013e31802dc4a7. [DOI] [PubMed] [Google Scholar]
- 56.Baldo JV, Arevalo A, Patterson JP, Dronkers NF. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex; a journal devoted to the study of the nervous system and behavior. 2013 Mar;49(3):658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker GM, Schwartz MF, Kimberg DY, Faseyitan O, Brecher A, Dell GS, et al. Support for anterior temporal involvement in semantic error production in aphasia: new evidence from VLSM. Brain and language. 2011 Jun;117(3):110–122. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain : a journal of neurology. 2009 Dec;132(Pt 12):3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain : a journal of neurology. 2012 Dec;135(Pt 12):3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain : a journal of neurology. 2011 Oct;134(Pt 10):3094–3105. doi: 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen R, Hillis AE, Pawlak M, Herskovits EH. Voxelwise Bayesian lesion-deficit analysis. NeuroImage. 2008 May 1;40(4):1633–1642. doi: 10.1016/j.neuroimage.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cloutman L, Gottesman R, Chaudhry P, Davis C, Kleinman JT, Pawlak M, et al. Where (in the brain) do semantic errors come from? Cortex; a journal devoted to the study of the nervous system and behavior. 2009 May;45(5):641–649. doi: 10.1016/j.cortex.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fridriksson J, Holland AL, Beeson P, Morrow L. Spaced retrieval treatment of anomia. Aphasiology. 2005 Feb;19(2):99–109. doi: 10.1080/02687030444000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fridriksson J, Moser D, Bonilha L, Morrow-Odom KL, Shaw H, Fridriksson A, et al. Neural correlates of phonological and semantic-based anomia treatment in aphasia. Neuropsychologia. 2007 Apr 9;45(8):1812–1822. doi: 10.1016/j.neuropsychologia.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lazar RM, Minzer B, Antoniello D, Festa JR, Krakauer JW, Marshall RS. Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke; a journal of cerebral circulation. 2010 Jul;41(7):1485–1488. doi: 10.1161/STROKEAHA.109.577338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Gray matter axonal connectivity maps. Frontiers in Psychiatry. 2015;6:e35. doi: 10.3389/fpsyt.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.