Abstract
Purpose
To prospectively follow children treated with craniospinal irradiation to determine critical combinations of radiation dose and volume that would predict for cognitive effects.
Methods and Materials
Between 1996 and 2003, 58 patients (median age 8.14 years, range 3.99–20.11 years) with medulloblastoma received risk-adapted CSI followed by dose-intense chemotherapy and were followed longitudinally with multiple cognitive evaluations (through 5 years post-treatment) that included IQ (estimated-EIQ, full-scale, verbal and performance) and academic achievement (math, reading, spelling) tests. CSI consisted of 23.4Gy for average-risk patients (non-metastatic) and 36–39.6Gy for high-risk patients (metastatic or residual disease > 1.5cm2). The primary site was treated using conformal or intensity-modulated radiation therapy using a 2cm clinical target volume margin. The effect of clinical variables and radiation dose to different brain volumes were modeled to estimate cognitive scores after treatment.
Results
A decline with time for all test scores was observed for the entire cohort. Sex, race and CSF shunt status had a significant impact on baseline scores. Age and mean radiation dose to specific brain volumes, including the temporal lobes and hippocampi, had a significant impact on longitudinal scores. Dichotomized dose distributions at 25Gy, 35Gy, 45Gy and 55Gy were modeled to show the impact of the high-dose volume on longitudinal test scores. The 50% risk of a below-normal cognitive test score was calculated according to mean dose and dose intervals between 25Gy and 55Gy at 10Gy increments according to brain volume and age.
Conclusions
The ability to predict cognitive outcomes in children with medulloblastoma using dose-effects models for different brain sub-volumes will improve treatment planning, guide intervention, and help estimate the value of newer methods of irradiation.
Introduction
The cognitive effects of craniospinal irradiation (CSI) have been a primary concern for investigators and caregivers involved in the treatment of children with medulloblastoma (MB) [1–5] the most common malignant brain tumor in children. Until 25 years ago, the standard of care for all patients included 36Gy CSI followed by irradiation of the posterior fossa to a cumulative dose ≥ 54Gy. To reduce treatment complications, CSI dose levels are now limited to 23.4Gy for patients with minimal residual disease and no evidence of neuraxis metastases while 36Gy remains the standard for other patients including those with residual disease ≥ 1.5cm2 or documented metastases; those treated with 23.4Gy CSI require adjuvant chemotherapy to achieve the same level of disease control observed with higher doses [6]. CSI includes supplemental “boost” irradiation of the primary site. Until recently, the anatomic posterior fossa has been the target volume for patients with MB [7]. Further reducing craniospinal dose and testing the feasibility of focal irradiation of the primary site, in lieu of posterior fossa irradiation, has been the objective of recent and ongoing institutional and cooperative group studies [8;9]]. Despite these changes, the gains have been small leading investigators to question whether further reductions in dose and volume are warranted or whether they are likely to result in an improvement over past results [10;11].
There are limited data correlating regional or volumetric effects of irradiation in children with MB. Investigators from the Childhood Cancer Survivor Study attempted to associate region-specific radiation dose and neurocognitive and quality of life outcomes in adult survivors of central nervous system malignancies including those with MB [12]. High dose irradiation of the temporal region was associated with memory impairment compared to non-irradiated patients; however, no association between dose and outcome was observed for other regions. We were the first to report a volumetric association between radiation dose and cognitive effects in children with MB [13]. We observed, in a series of children who were prospectively followed after risk-adapted post-operative craniospinal irradiation and adjuvant chemotherapy, that radiation dose to the entire brain was associated with longitudinal IQ scores. Although the volume receiving the highest dose had the greatest impact, there was a similar decline in IQ for each Gy exposure. These results supported further reductions in radiation dose and volume with an emphasis on reducing the volume that receives the highest dose, especially for young patients who are at greatest risk for cognitive effects.
In this report we explore the association between 3-dimensional brain dose and cognitive effects in children with MB. We evaluate toxicity thresholds based on dose, volume, and age. We extend our prior results in a larger cohort of children and add academic achievement as a response variable in the models. We have included the dose information about the hippocampus. This has been viewed as a critical functional volume related to neurogenesis and subsequent cognitive effects [14]. The goal of this research was to estimate critical combinations of radiation dose and volume resulting in cognitive impairment. Understanding dose and volume effects will improve radiation therapy planning and our understanding of partial organ tolerances to the effects of irradiation beyond those already published [15].
Methods and Materials
The study cohort included 58 patients (median age at diagnosis 8.14 years, range 3.99–20.11 years) treated between 1996 and 2003 diagnosed with MB and longitudinally followed after surgery, radiation therapy, and post-irradiation chemotherapy with multiple (> 2) cognitive evaluations. The group was further characterized by sex (male=40, female=18), race/ethnicity (white=47, black=9, Hispanic=2), extent of resection (GTR=47, <GTR=11), risk-classification (average=34, high=24), CSF shunt (present=8, absent=50), and 10 patients had more than one surgery. At the time of diagnosis, 50/58 patients were right-handed, 6/58 were left-handed, and 2/58 were ambidextrous. After surgery, one right-handed patient became left-handed and one ambidextrous patient became right-handed.
The treatment protocol included resection followed by risk-adapted, post-operative, craniospinal irradiation (CSI), and post-irradiation chemotherapy as described elsewhere [16]. Average risk patients received 23.4Gy CSI, 36Gy conformal posterior fossa irradiation and 55.8Gy primary site irradiation using a 2cm clinical target volume (CTV) margin. High-risk patients received 36–39.6Gy CSI followed by 55.8Gy primary site irradiation using a 2cm CTV margin. When the posterior fossa was irradiated to 36Gy after 23.4Gy CSI, the CTV for that volume was the anatomic posterior fossa. Composite radiation dose data was assembled for all patients and normal tissue volumes were systematically contoured on MR imaging data registered to the treatment planning CT. Dose-volume data for each of the normal tissue structures was extracted in differential form for integration. The median and mean doses were determined for each brain region. [Table 1]
Table 1.
Radiation dose to different brain volumes in 58 patients with medulloblastoma.
| Normal Tissue Volume of Interest | Dose (cGy) | ||||
|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | |
| Brain Total | 4034 | 528.7 | 3797 | 3336 | 5006 |
| Left Hippocampus | 5219 | 421.9 | 5379 | 3749 | 5892 |
| Right Hippocampus | 5189 | 420.6 | 5286 | 4110 | 5885 |
| Infratentorial | 5688 | 159.6 | 5678 | 5349 | 6167 |
| Supratentorial | 3814 | 596.4 | 3596 | 3006 | 4865 |
| Left Temporal | 4558 | 450.7 | 4462 | 3600 | 5507 |
| Right Temporal | 4529 | 422.0 | 4436 | 3749 | 5462 |
Patients underwent serial cognitive testing at baseline (following surgical resection) and annually after the start of CSI. The cognitive tests for this study included IQ and academic achievement. IQ was estimated based on the Information, Similarities, and Block Design subtests from the age-appropriate Wechsler scale (Wechsler Preschool and Primary Scales of Intelligence, Revised [WPPSI-R] [17], Wechsler Intelligence Scale for Children, Third Edition [WISC-III] [18], and Wechsler Adult Intelligence Scale, Revised [WAIS-Revised]) [19]using a formula presented in Sattler [20]. This method for estimating IQ correlates highly with IQs derived from full administration (r = 0.93). Age-based scaled scores, with a mean of 100 and standard deviation of 15, were derived using each standardization sample. Academic testing consisted of three subtests from the Wechsler Individual Achievement Test (WIAT; Word Reading, Spelling, and Math Reasoning) [21]. These subtests are content representative, reliable, and have good convergent/discriminant validity. Performance on each subtest was converted to an age-standardized score with a mean of 100 and standard deviation of 15.
A linear mixed model with random coefficients was used to estimate the impact of the specific clinical variables and non-overlapping dose-volume intervals on the longitudinal trend of the cognitive scores after the start of CSI. A variety of clinical variable were included in the modeling process. Dose variables included mean dose to the contoured normal tissue volumes and dichotomized the dose distributions. We generated pairs of dose volume variables: V0_25Gy and V25Gy+, V0_35Gy and V35Gy+, V0_45Gy and V45Gy+ and V0_55Gy and V55Gy+. We then fit a random coefficient model to investigate the effect of dose volumes on the longitudinal trend of cognitive scores over time. Because of the small volume for the hippocampus, it was not treated with volumetric dose data. We modeled the combined effect of radiation dose and volume and age at the time of irradiation. We then calculated the TD 50/5. The TD 50/5 is the tolerance dose for a given normal tissue that within 5 years will cause a maximal (unacceptable) 50% complication rate. To estimate the TD 50/5 for the normal tissue volumes included in this study we fixed the level of our response variables (cognitive scores) to 85 and dose in 5Gy increments and determined the threshold volume corresponding to a particular dose that would result in a score below 85. For each model, the estimating equation developed by the mixed-model procedure was examined for direction of slope (positive or negative), magnitude of the specific dose-volume coefficients, and the P value of each coefficient. For each fitted model, only the factors significant at P <0.10 were included in the final estimating equation. P values were not adjusted for multiple testing. All analyses were performed by using SAS.
Results
Longitudinal Trends in Cognitive Scores
The longitudinal trends in cognitive scores were modeled during the first 5 years after radiation therapy (RT). The linear models showed that baseline evaluations for IQ and academic achievement were within the range of normal. Longitudinally, there was a statistically significant decline (points per year) in all scores. [Table 2]
Table 2.
Longitudinal models of cognitive scores through 5 years after craniospinal irradiation in patients with medulloblastoma.
| Psychology Test | Number of Patients | Baseline | 5 Year Score | Δ Points/Years |
|---|---|---|---|---|
| Estimated IQ | 58 | 93.44 | 89.35 | −0.82 |
| WIAT Math | 52 | 94.50 | 84.11 | −2.08 |
| WIAT Reading | 52 | 94.99 | 83.48 | −2.30 |
| WIAT Spelling | 52 | 93.28 | 82.84 | −2.09 |
Cognitive test score = baseline value + Δ points/year × time in years
Impact of Clinical Variables on Longitudinal Trends in Cognitive Scores
We then investigated the impact of clinical variables on the longitudinal trend of cognitive scores by adding one clinical variable at a time. For significant changes in longitudinal scores we note P-values and absolute differences in the annual rate of change comparing high and low-impact variables. Risk classification: EIQ (P=0.0347, 1.93pts/yr) and math scores (P=0.0050, 2.87pts/yr) declined at a higher rate in HR patients. Sex: Spelling scores declined at a higher rate in female patients (P=0.0207, 2.06pts/yr). Race: EIQ was lower in black patients at baseline (P=0.0151, 14.93pts). CSF shunt: EIQ was higher at baseline (12.58 points) in patients who did not have a CSF shunt (P=0.0478) and those without CSF shunts had a lower rate of decline in math (P=0.0025, 4.79pts/yr) and reading scores (P=0.0319, 2.32pts/yr). Extent of resection: Baseline math scores were higher in patients who underwent <GTR (P=0.0091, 9.97pts). GTR was associated with a slower rate of decline in reading scores (P=0.0269, 2.25pts/yr) than those who underwent <GTR. Age at RT: With the exception of math and reading scores, age (time of diagnosis or irradiation) had a highly significant impact on the rate of decline in all test scores (EIQ, P=0.0141; Math, P=0.1832; Reading, P=0.0688; Spelling, P=0.0424).
Impact of Mean Radiation Dose on Longitudinal Trends in Cognitive Scores
The longitudinal trends in cognitive scores were modeled by time since irradiation and mean dose. Increasing mean dose to all volumes had a statistically significant negative impact on EIQ. Increasing mean dose to all normal tissue volumes except for the infratentorial brain and hippocampi had a statistically significant negative impact on math scores. Increasing mean dose to the right temporal lobe had a statistically significant negative impact on reading scores. The impact of increasing mean dose to the right hippocampus was borderline significant. When age was included it had a significant impact on longitudinal scores in all models. [Table 3]
Table 3.
Effect of increasing mean dose on cognitive test scores by brain volume. P-values are grouped in columns according to the inclusion (+) or exclusion (−) of age in the model.
| Normal Tissue Volume | Estimated IQ | WIAT Math | WIAT Reading | WIAT Spelling | ||||
|---|---|---|---|---|---|---|---|---|
| − | + | − | + | − | + | − | + | |
| Entire Brain | 0.0121 | <0.0001 | 0.0096 | 0.0007 | n.s. | <0.0001 | n.s. | 0.0002 |
| Supratentorial Brain | 0.0161 | <0.0001 | 0.0251 | 0.0009 | n.s. | <0.0001 | n.s. | 0.0002 |
| Temporal Lobe (Left) | 0.0032 | <0.0001 | 0.0184 | 0.0013 | n.s. | <0.0001 | n.s. | 0.0002 |
| Temporal Lobe (Right) | 0.0005 | <0.0001 | 0.0053 | 0.0009 | 0.0184 | <0.0001 | n.s. | <0.0001 |
| Hippocampus (Left) | 0.0751 | <0.0001 | n.s. | 0.0025 | n.s. | <0.0001 | n.s. | 0.0001 |
| Hippocampus (Right) | 0.0130 | <0.0001 | n.s. | 0.0016 | n.s. | <0.0001 | n.s. | <0.0001 |
| Infratentorial Brain | 0.0002 | <0.0001 | n.s. | 0.0034 | n.s. | <0.0001 | n.s. | 0.0001 |
TD 50/5 for below average IQ and academic achievement based on mean normal tissue dose
We calculated the mean dose required for a child to have a 50% risk of a below average IQ or academic achievement test score 5 years after irradiation. The calculation was performed using age-adjusted mean dose models. The estimated mean doses are presented as iso-effect curves in figure 1.
Fig. 1.
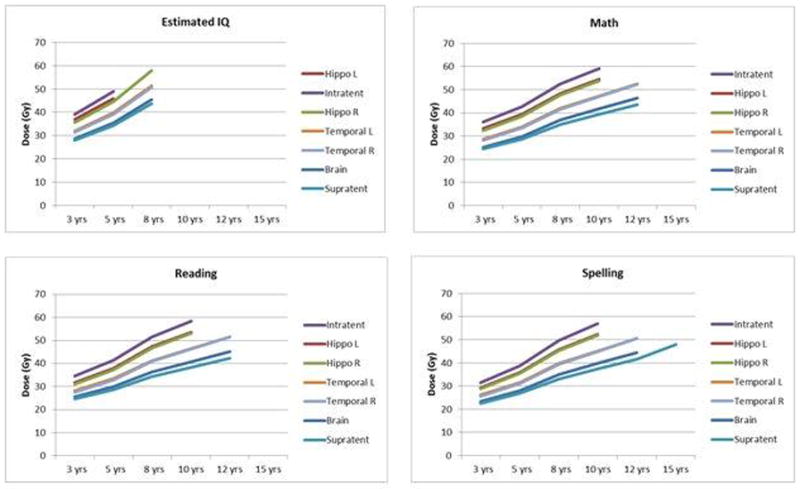
Estimated iso-effect curves of mean dose by brain volume and age at the time of irradiation representing a probability of below average IQ or academic achievement 5 years after treatment. Each graph represents a different cognitive test and each curve represents a different normal tissue volume. Missing estimates indicate that the model calculated a dose that was outside the range of dose used to generate the models.
Hippo L, left hippocampus; Infratent, infratentorial brain; Hippo R, right hippocampus; Temporal L, left temporal lobe; Temporal R, right temporal lobe; Brain, entire brain volume; Supratent, supratentorial brain; EIQ, estimated IQ; Math, WIAT math scores; Reading, WIAT reading scores; Spelling, WIAT spelling scores.
The Impact of Radiation Dose Intervals on Longitudinal Trends in Cognitive Scores
Using the cut-points of 25Gy and 35Gy, the higher dose interval had a consistent and statistically significant impact on the longitudinal trend in cognitive test scores whereas the lower dose interval did not. A similar finding was observed for the infratentorial and temporal lobe volumes at 45Gy. For the other normal tissue volumes evaluated at 45Gy and all normal tissue volumes at 55Gy, the impact of dose was significant only when the dose interval (high or low) included the majority of the volume (data not shown). The only exception was for EIQ. At the high-dose cut points of 45Gy and 55Gy, the higher-dose term was smaller than the lower-dose term and retained statistical significance. [Table 4]
Table 4.
Statistical significance of dose volume intervals on longitudinal cognitive scores after craniospinal irradiation.
| Volume | Test | Cutpoint V25Gy | Cutpoint V35Gy | Cutpoint V45Gy | Cutpoint V55Gy | ||||
|---|---|---|---|---|---|---|---|---|---|
| V<25Gy | V>25Gy | V<35Gy | V>35Gy | V<45Gy | V>45Gy | V<55Gy | V>55Gy | ||
| Brain | EIQ | n.s | 0.0079 | n.s. | 0.0027 | n.s. | 0.0140 | n.s. | 0.0185 |
| Math | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | 0.0010 | n.s. | n.s. | |
| Reading | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | 0.0252 | n.s. | n.s. | |
| Spelling | n.s. | 0.0003 | n.s. | 0.0001 | n.s. | n.s. | n.s. | n.s. | |
| Supratentorial | EIQ | n.s. | 0.0077 | n.s. | 0.0028 | n.s. | 0.0153 | n.s. | n.s. |
| Math | n.s. | 0.0001 | n.s. | <0.0001 | n.s. | 0.0032 | 0.0403 | n.s. | |
| Reading | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | 0.0177 | 0.0051 | n.s. | |
| Spelling | n.s. | 0.0002 | n.s. | <0.0001 | 0.0486 | n.s. | 0.0024 | n.s. | |
| Infratentorial | EIQ | n.s. | n.s. | n.s. | n.s. | 0.0483 | 0.0144 | 0.0018 | 0.0002 |
| Math | n.s. | 0.0002 | n.s. | 0.0003 | n.s. | <0.0001 | n.s. | <0.0001 | |
| Reading | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | |
| Spelling | n.s. | <0.0001 | 0.0309 | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | |
| Temporal Left | EIQ | n.s. | 0.0362 | 0.0152 | 0.0010 | n.s. | 0.0016 | n.s. | 0.0167 |
| Math | n.s. | 0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | 0.0590 | n.s. | |
| Reading | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | 0.0063 | n.s. | |
| Spelling | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | 0.0035 | 0.0033 | n.s. | |
| Temporal Right | EIQ | n.s. | 0.0413 | 0.0094 | 0.0006 | 0.0203 | 0.0003 | n.s. | 0.0019 |
| Math | n.s. | 0.0004 | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | n.s. | |
| Reading | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | 0.0282 | 0.0201 | |
| Spelling | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | 0.0006 | 0.0234 | n.s. | |
EIQ, estimated IQ; Math, WIAT math scores; Reading, WIAT reading scores; Spelling, WIAT spelling scores; n.s., not significant
TD 50/5 for below average IQ and academic achievement based on radiation dose intervals
For the entire brain and left and right temporal lobes we calculated, according to the age of the patient at the time of RT, the threshold volumes receiving dose in excess of 25Gy, 35Gy, 45Gy, and 55Gy would have 50% of cognitive scores falling below 85 for EIQ 5 years after RT. The results show that no additional dose to the entire brain above a specified level would be required for patients with the specified ages or younger to have a 5% probability of EIQ less than 85 at 5 years: age 8 years and 25Gy, age 12 years and 30Gy, age 15 years and 35Gy. For both the left and right temporal lobes these values were age 8 years and 25Gy, age 8 years and 30Gy, age 10 years and 35Gy, age 12 years and 40Gy, age 12 years and 45Gy, and age 15 years and 50Gy. The results show that there is less than a 50% probability of an EIQ less than 85 for the following combination of brain dose and age: < 25Gy and ≥ 8 years, < 30Gy and ≥ 12 years, and < 35Gy and ≥ 15 years. The probability of an EIQ less than 85 at 5 years is less than 50% for the following combinations of left and right temporal lobe dose: <25Gy and 8 years, <30Gy and 8 years, <35Gy and 10 years, and <40Gy and 12 years. [Figures 2a–c]
Fig. 2.
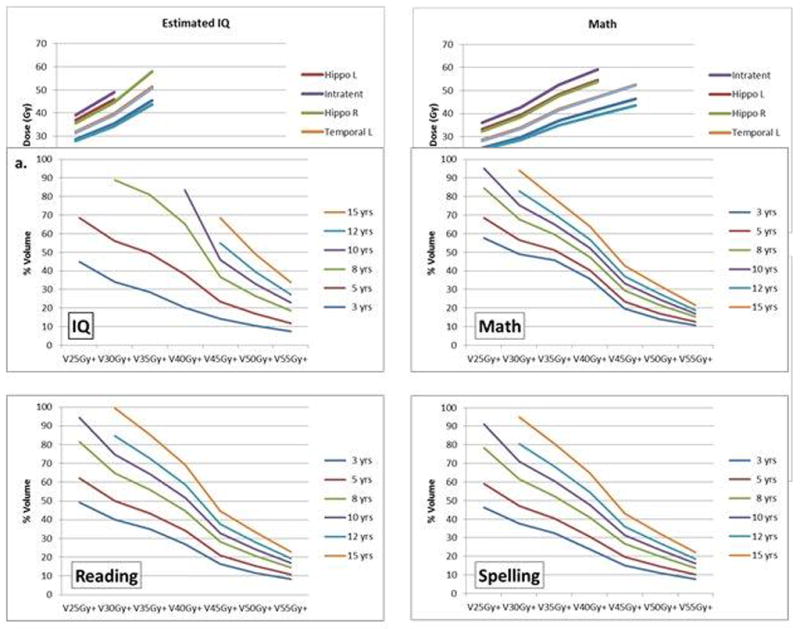
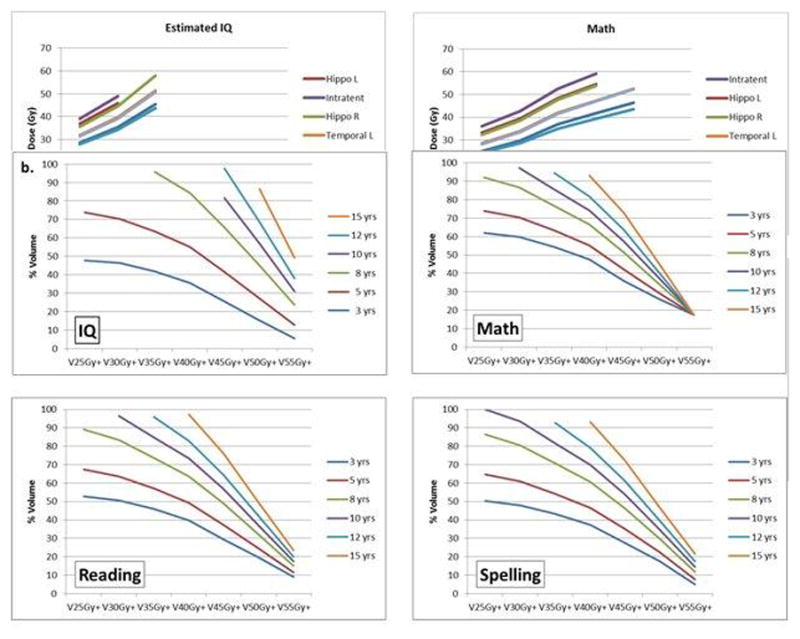
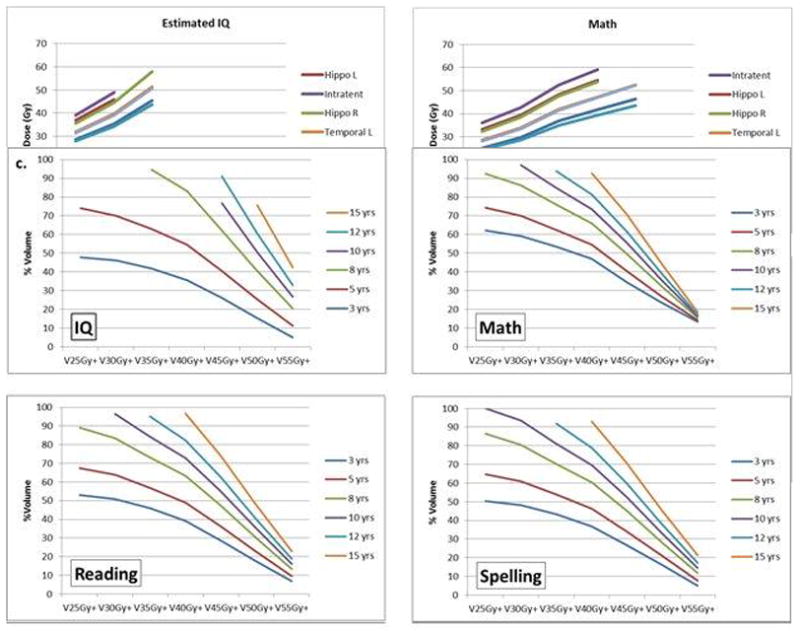
Iso-effect curves of age at the time of irradiation modeled according to the percentage of a specific brain region receiving a mean dose in excess of a specified amount. V25Gy+ represents the percent volume of the brain receiving dose in excess of 25Gy. TD 50/5 for brain (a), left temporal lobe (b) and right temporal lobe (c).
Discussion
Patients with MB treated with post-operative CSI and post-irradiation chemotherapy experience a decline in cognitive test scores during the first 5 years after treatment and a variety of clinical variables contributed to the baseline scores or decline. The presence of CSF shunt, sex, and race had the greatest impacted on baseline IQ scores. High-risk classification, female gender, the presence of CSF shunt, and the extent of resection had a significant impact on decline in scores. Depending on the outcome measure, the decline exceeded 4 points per year in some cases. As anticipated, age had a significant impact on decline in all measures, and the rate of decline was inversely proportional to age at the time of irradiation.
The most important information from this study was the association between radiation dose and cognitive test scores. Similar to our previous work we were able to show regional differences in radiation dose and effect [13]. In this study we expanded the association between dose, volume, and outcome measures to include additional structures and academic achievement. Increasing mean dose to all volumes except for the left hippocampus impacted IQ. Increasing mean dose to all volumes except the infratentorial brain and either hippocampus had an effect on math scores. Increasing mean dose only to the right temporal lobe had a significant impact on reading scores. There was no association between spelling scores and radiation dose for this cohort. Increasing mean dose to all volumes affected all scores when age was included in the model. This is one of the first large scale studies to demonstrate an effect between hippocampus dose and cognitive outcome in children, although many have supported hypotheses surrounding this association. Age at the time of irradiation, when incorporated into the model, increased the significance of the aforementioned interactions between mean dose and time and contributed additional correlations between radiation dose, all measures of academic achievement, and the normal tissue volumes under evaluation. The latter finding suggests the importance of including clinical variables in the models.
Understanding the association between radiation dose and outcome is important. Most radiation oncologists prefer a simplified approach to treatment optimization relating risk of complications to a specific dose. The calculated TD 50/5 estimates in this report provide this type of data reduction. We estimated that when the brain dose exceeds 25Gy for a patient less than 8 years, 30Gy for a patient less than 12 years and 35Gy for a patient less than 15 years, there is a 50% probability of below average IQ 5 years after treatment.
The infratentorial brain appears to be the most tolerant normal tissue volume amongst those assessed for the outcomes of IQ and academic achievement followed by the temporal lobes and associated hippocampi, and finally the supratentorial brain. The implication of this information is that for the given combinations of dose and volume it may be difficult to reduce side effects. In the setting where CSI is administered, measures taken to reduce dose to normal tissues in the boost phase of treatment might have little impact. This finding supports the need to further reduce or eliminate the use of CSI wherever possible.
The iso-effect curves presented have severeal dimensions: patient age at irradiation, radiation dose parameter, brain volume at risk, and psychology outcome measure. The information in the iso-effect plots may be used as a threshold in decline in assessing the potential benefit of delaying irradiation, and to design interventions for populations at risk.
The effects of CSI in long-term survivors of MB are historic [22] and motivation for investigators to test alternatives, including modifications in the sequencing of therapy [23] or general radiation therapy parameters of total dose and fractionation [24]. New information about the biology of MB may identify selected patients for CSI dose reductions or elimination. This information is currently being used to select favorable risk patients for CSI doses as low as 15Gy [25]. As proton therapy promises to further reduce the dose to normal tissue associated with the boost phase of treatment, it is conceivable that with more advanced forms of proton therapy, including intensity-modulated proton therapy [26], selectively reducing dose to critical volumes of the brain during CSI, especially those associated with neurogenesis, might be feasible and safe. Future treatment of children with embryonal tumors may be preferentially administered using proton therapy. Optimally planned intensity-modulated proton therapy might be able to limit the high-dose volume and associated collateral dose to the infratentorial space. This could advantageously limit the dose to the supratentorial structures, including the temporal lobes and hippocampal sub-volumes, to the prescribed CSI dose or below the threshold of effect and lead to improved outcomes [27].
There are limitations to the present study: the number of patients, the number of clinical factors that might affect baseline and longitudinal measures, and the measures themselves which include only global intelligence and academic achievement. The study cohort was treated and followed on a protocol that limited prospective follow-up to 5 years and included patients with high-risk features. There are a number of clinical factors that strongly influence baseline and longitudinal cognition including the effects of age, [28] tumor (hydrocephalus), and surgery [29;30]. Accounting for these factors and the development of comprehensive parametric models, including dose requires more patients.
In summary, there are strong associations between radiation dose, irradiated volume, and cognitive outcomes as measured by standardized tests. When modeling the effect of radiation dose, clinical factors that affect baseline and longitudinal measures should be considered. Future research should be focused on assessing larger datasets, inclusion of patients treated with a wider arrange of CSI dose, and the development of multi-parametric models.
Supplementary Material
Acknowledgments
Research Support: Supported in part by the American Lebanese Syrian Associated Charities (ALSAC)
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
References
- 1.Silverman CL, Palkes H, Talent B, Kovnar E, Clouse JW, Thomas PR. Late effects of radiotherapy on patients with cerebellar medulloblastoma. Cancer. 1984;54(5):825–829. doi: 10.1002/1097-0142(19840901)54:5<825::aid-cncr2820540511>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.LeBaron S, Zeltzer PM, Zeltzer LK, Scott SE, Marlin AE. Assessment of quality of survival in children with medulloblastoma and cerebellar astrocytoma. Cancer. 1988;62(6):1215–1222. doi: 10.1002/1097-0142(19880915)62:6<1215::aid-cncr2820620629>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Hoppe-Hirsch E, Renier D, Lellouch-Tubiana A, Sainte-Rose C, Pierre-Kahn A, Hirsch JF. Medulloblastoma in childhood: progressive intellectual deterioration. Childs Nerv Syst. 1990;6(2):60–65. doi: 10.1007/BF00307922. [DOI] [PubMed] [Google Scholar]
- 4.Kao GD, Goldwein JW, Schultz DJ, Radcliffe J, Sutton L, Lange B. The impact of perioperative factors on subsequent intelligence quotient deficits in children treated for medulloblastoma/posterior fossa primitive neuroectodermal tumors. Cancer. 1994;74(3):965–971. doi: 10.1002/1097-0142(19940801)74:3<965::aid-cncr2820740328>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Radcliffe J, Bunin GR, Sutton LN, Goldwein JW, Phillips PC. Cognitive deficits in long-term survivors of childhood medulloblastoma and other noncortical tumors: age-dependent effects of whole brain radiation. Int J Dev Neurosci. 1994;12(4):327–334. doi: 10.1016/0736-5748(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J Clin Oncol. 1999;17(7):2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 7.Merchant TE, Kun LE, Krasin MJ, Wallace D, Chintagumpala MM, Woo SY, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55. 8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2008;70(3):782–787. doi: 10.1016/j.ijrobp.2007.07.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gajjar A. A Clinical and Molecular Risk-Directed Therapy for Newly Diagnosed Medulloblastoma. ClinicalTrials gov. 2013 NCT01878617 (SJMB03) Available from: http://clinicaltrials.gov/ct2/show/NCT01878617?term=sjmb03&rank=1.
- 9.Michalski J. A Study Evaluating Limited Target Volume Boost Irradiation and Reduced Dose Craniospinal Radiotherapy (18.00 Gy) and Chemotherapy in Children With Newly Diagnosed Standard Risk Medulloblastoma: A Phase III Double Randomized Trial. ClinicalTrials gov. 2013 NCT00085735 (ACNS0331) Available from: http://clinicaltrials.gov/ct2/show/NCT00085735?term=acns0331&rank=1.
- 10.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19(15):3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 11.Walter AW, Mulhern RK, Gajjar A, Heideman RL, Reardon D, Sanford RA, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17(12):3720–3728. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 12.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong GT, Jain N, Liu W, Merchant TE, Stovall M, Srivastava DK, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12(11):1173–1186. doi: 10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant TE, Kiehna EN, Li C, Shukla H, Sengupta S, Xiong X, et al. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys. 2006;65(1):210–221. doi: 10.1016/j.ijrobp.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62(5):515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence YR, Li XA, el N I, Hahn CA, Marks LB, Merchant TE, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1989. Revised[WPPSI-R] [Google Scholar]
- 19.Wechsler D. Wechsler Intelligence Scale for Children. 3. New York, NY: Psychological Corporation; 1991. WISC-III. [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale. New York, NY: Psychological Corporation; 1981. Revised[WAIS-Revised] [Google Scholar]
- 21.Sattler JH. Assessment of Children. 3. San Diego, CA: Jeome M. Sattler Publisher Inc; 1992. pp. 219–243. [Google Scholar]
- 22.Wechsler D. Wechsler Individual Achievement Test. New York, NY: Psychology Corporation; 1992. [Google Scholar]
- 23.Edelstein K, Spiegler BJ, Fung S, Panzarella T, Mabbott DJ, Jewitt N, D’Agostino NM, Mason WP, Bouffet E, Tabori U, Laperriere N, Hodgson DC. Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro Oncol. 2011 May;13(5):536–45. doi: 10.1093/neuonc/nor015. Epub 2011 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massimino M, Cefalo G, Riva D, Biassoni V, Spreafico F, Pecori E, Poggi G, Collini P, Pollo B, Valentini L, Potepan P, Seregni E, Casanova M, Ferrari A, Luksch R, Polastri D, Terenziani M, Pallotti F, Clerici CA, Schiavello E, Simonetti F, Meazza C, Catania S, Podda M, Gandola L. Long-term results of combined preradiation chemotherapy and age-tailored radiotherapy doses for childhood medulloblastoma. J Neurooncol. 2012 May;108(1):163–71. doi: 10.1007/s11060-012-0822-7. Epub 2012 Feb 16. [DOI] [PubMed] [Google Scholar]
- 25.Gupta T, Jalali R, Goswami S, Nair V, Moiyadi A, Epari S, Sarin R. Early clinical outcomes demonstrate preserved cognitive function in children with average-risk medulloblastoma when treated with hyperfractionated radiation therapy. Int J Radiat Oncol Biol Phys. 2012 Aug 1;83(5):1534–40. doi: 10.1016/j.ijrobp.2011.10.037. Epub 2012 Feb 16. [DOI] [PubMed] [Google Scholar]
- 26.Gajjar A. A Clinical and Molecular Risk-Directed Therapy for Newly Diagnosed Medulloblastoma. NCT01878617. ClinicalTrials gov. 2013 Available from: http://clinicaltrials.gov/ct2/results?term=SJMB12.
- 27.Merchant TE. Clinical controversies: proton therapy for pediatric tumors. Semin Radiat Oncol. 2013 Apr;23(2):97–108. doi: 10.1016/j.semradonc.2012.11.008. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merchant TE. Proton beam therapy in pediatric oncology. Cancer J. 2009 Jul-Aug;15(4):298–305. doi: 10.1097/PPO.0b013e3181b6d4b7. Review. [DOI] [PubMed] [Google Scholar]
- 29.Di Rocco C, Chieffo D, Pettorini BL, Massimi L, Caldarelli M, Tamburrini G. Preoperative and postoperative neurological, neuropsychological and behavioral impairment in children with posterior cranial fossa astrocytomas and medulloblastomas: the role of the tumor and the impact of the surgical treatment. Childs Nerv Syst. 2010 Sep;26(9):1173–88. doi: 10.1007/s00381-010-1166-2. Epub 2010 Jun 16. [DOI] [PubMed] [Google Scholar]
- 30.Palmer SL, Hassall T, Evankovich K, Mabbott DJ, Bonner M, Deluca C, Cohn R, Fisher MJ, Morris EB, Broniscer A, Gajjar A. Neurocognitive outcome 12 months following cerebellar mutism syndrome in pediatric patients with medulloblastoma. Neuro Oncol. 2010 Dec;12(12):1311–7. doi: 10.1093/neuonc/noq094. Epub 2010 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


