Abstract
We examined consumption of different types of infant formula (eg, cow’s milk, soy, gentle/lactose-reduced, and specialty) and regular milk among a nationally representative sample of 1864 infants, 0 to 12 months old, from the National Health and Nutrition Examination Survey, 2003–2010. Among the 81% of infants who were fed formula or regular milk, 69% consumed cow’s milk formula, 12% consumed soy formula, 5% consumed gentle/ lactose-reduced formulas, 6% consumed specialty formulas, and 13% consumed regular milk products. There were differences by household education and income in the percentage of infants consuming cow’s milk formula and regular milk products. The majority of infants in the United States who were fed formula or regular milk consumed cow’s milk formula (69%), with lower percentages receiving soy, specialty, gentle/sensitive, or lactose-free/reduced formulas. Contrary to national recommendations, 13% of infants younger than 1 year consumed regular milk, and the percentage varied by household education and income levels.
Keywords: nutrition, survey, diet, children
Human milk provides the best nutrition for infants.1 The American Academy of Pediatrics (AAP) recommends exclusive breastfeeding for approximately 6 months, whereupon complementary foods should be introduced, and continuation of breastfeeding for approximately 1 year or longer, as desired.1 Of children born in 2011, 79% initiated breastfeeding.2,3 However, only 49% were breastfeeding at all by 6 months and 27% at 12 months.2,3
Generally, regular cow’s milk formula is recommended for infants who are not breastfed.4,5 However, there are multiple types of other infant formulas. Some have clear clinical indications for use, such as special formulas for prematurely born children (eg, Enfacare, Neosure), protein hydrosylate or elemental formulas for children with cow’s milk and soy protein allergies (eg, Nutramigen, Alimentum), or formulas for other specific nutritional needs (eg, Pregestimil for fat malabsorption; Lofenalac for phenylketonuria). Other formulas have rare true clinical indications (soy formula for galactosemia; lactose-free formulas for true lactose intolerance among infants), or no clinical indications (“gentle” or “sensitive” formulas that contain partially hydrolyzed proteins or reduced lactose content). The use of these latter categories is likely the result of parental preference, and these formulas are often marketed for infant fussiness, colic, and perceived gastrointestinal issues.6
Although all formulas on the US market must comply with strict regulations ensuring safety and adequate nutrition,7 there is debate over associations between different formula types and health outcomes. For instance, soy formulas contain isoflavones, which may have estrogenic effects on developing reproductive, neurobehavioral, and immune systems, and thyroid function, although studies have generally found no significant impact.8,9 Soy formulas also contain phytates, which may inhibit absorption of key micronutrients; although the micronutrient status and growth of children fed soy formula is indistinguishable from those fed cow’s milk formula.10,11 Soy formulas also contain higher levels of aluminum than cow’s milk formula, subsequently, the AAP recommends that soy formula not be given to preterm infants.10 A few studies have examined potential protective effects of soy formula on atopic outcomes; however, a Cochrane review reported no effects of soy formula on rates of atopic diseases.12
Lactose is the sugar found in human milk and has a positive effect on gut microbiota and calcium absorption.13 Lactose-free or lactose-reduced formulas contain sugar from other sources (eg, glucose, fructose, and/or sucrose from corn syrup solids or corn maltodextrin), raising questions about the influence of lactose-free or -reduced formulas on the infant microbiome and later health. As a result, formula manufacturers now frequently add prebiotics and probiotics to infant formulas, though the consequences of that are also unknown.14
The AAP recommends that nonformula milk products (eg, cow’s milk, soy milk, flavored milk products) should be avoided for the first year of life.15,16 Nonformula cow’s milk (or other milk products) does not provide adequate iron, linoleic acid, or vitamin E, and provides excess sodium, potassium, and protein compared with formula or breast milk.16 Consumption of regular cow’s milk instead of human milk or formula can lead to iron-deficiency anemia in infants, and exposure to cow’s milk proteins in infancy has been associated with a greater risk of developing type 1 diabetes.16
Despite many studies on the effects of different types of formulas on infant and child health outcomes, there are no existing data on the consumption of different types of formula. The objective of this analysis was to determine the percentage of infants receiving different types of formula, and to examine how formula consumption patterns may differ by family income, given the different cost of various formula types, and by education, as prior studies have suggested that infant feeding practices may be associated with maternal education.17,18
Methods
The sample consisted of infants 0 to 12 months old, participating in the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2010. NHANES is a nationally representative survey of the civilian, noninstitutionalized US population, consisting of a household interview and subsequent examination component consisting of an additional interview, examination, and laboratory tests completed in a mobile examination center.19 The NHANES protocol was approved by the Research Ethics Review Board of the National Center for Health Statistics and all participants provided informed consent. Response rates for the interview component range from 88% to 92% for this age group over the study period.20 Of the 2022 infants included in the initial interview component, 1864 children completed the examination component and had at least 1 day of dietary recall data (92.2%); another 120 were missing data on key covariates of interest (eg, household income and education), leaving an analytic sample of 1744 (86.3%). Dietary recall interviews were conducted by trained examiners using a computer-assisted dietary interview system, including a multiple-pass format; proxies (most commonly a parent) complete the interview for children 5 years and younger, reporting the type and quantity of all food and beverages consumed in the 24-hour period of the day prior to the examination.21 One day of dietary recall does not represent an individual’s usual, long-term intake, as there is day-to-day variability in diet that is not captured by a single day of recall. However, across the population, these errors are generally assumed to cancel out if the data are collected evenly throughout the year and days of the week.22 For brevity, we will refer to intake as occurring on the day prior to the examination.22 Although children older than 12 months may still consume formula, children typically transition to cow’s milk at 1 year of age, and thus this analysis is restricted to infants 0 to 12 months old.
Data on formula intake and type were obtained from the individual food files. These files provide information on the amount (kilocalories) and type of formula consumed by participating children as described in the Food and Nutrient Database for Dietary Studies (FNDDS) food codes produced by the US Department of Agriculture.23 Formula was categorized into 4 non-overlapping types by food code: regular cow’s milk formula, soy formula, specialty use (eg, formulas for preterm, acid reflux, phenylketonuria; cow’s milk or soy protein allergy), and partially hydrolyzed (ie, “gentle” or “sensitive” formulas) or lactose-reduced/free. In practice, “gentle” or “sensitive” formulas typically had reduced lactose, so these types were combined into one category, hereafter referred to as gentle/lactose-reduced. Additionally, regular cow’s milk (not formula) and other types of milk (eg, soy, flavored/chocolate) were also quantified to examine whether some infants younger than 12 months might be transitioning to nonformula milk products earlier than recommended. Throughout this analysis, “regular” is used alternatively to describe the basic nonspecialty cow’s milk formula or other types of milk products such as cow’s milk (nonformula), soy milk (nonformula), or flavored milk.
Analysis
To examine whether there were income-related differences in the percentage of infants consuming different types of formula, family income-to-poverty ratio (IPR) was categorized into 2 groups: 0% to 185% of the federal poverty threshold (FPT), and 186% FPT or higher. The federal cutoff for WIC (Women, Infant and Children’s Nutrition Program) eligibility is 185% FPT, thus IPR groupings were based on this cutoff.24 Approximately half of all infants in the United States receive WIC benefits.25 Although WIC has a large influence on the brand of formula consumed by infants,25 it should not determine the specific type of formula consumed since WIC contracts generally include all formula types (eg, cow’s milk, soy, gentle/sensitive, specialty). In support of this, we examined consumption of various formula types by WIC status and found no differences in type of formula consumed, thus those results are not reported here.
Estimates were considered reliable if they had a relative standard error of <30%. To examine the relationship between formula consumption and household education, educational attainment of the household reference person was categorized as less than high school, high school degree or GED, or some college or more. The household reference person is defined by NHANES as someone who is 18 years or older and owns or rents the residence where the infant resides.
Infants may consume more than one type of formula (and/or regular milk), thus odds of consumption of each type of formula were examined using separate logistic regressions. Regressions controlled for age (in months) of the infant. Marginal probabilities of consumption from these logistic regressions reflect the percent of infants receiving different types of formula, adjusted for age. Because an infant may consume more than one type of formula (and/or regular milk), the resulting percentages for each type of formula are not mutually exclusive of one another and percentages add to a total of greater than 100%. The denominator in this case was the entire sample of infants consuming any formula or regular milk products regardless of whether they also reported consuming breast milk, though infants who drank only human milk on the given day as their source of milk were not included (n = 277). Differences in the percentage consuming various types of formula by IPR and household education level were assessed using Wald tests.
A second analysis examined the percent of total formula or regular milk consumed in the population accounted for by the various types. For this analysis, we used methods delineated in the NHANES dietary tutorials, whereby the dietary recall weight was multiplied by the total amount of formula or regular milk consumed (in kilocalories) and then proportions by subtype were estimated.26 Infants who were partially breastfed, but also received formula, were included in this analysis, as the amount of formula they consumed contributes to the denominator (ie, total weighted amount of formula in kcal consumed by the study population). All analyses were performed using Stata SE (version 12.1) survey commands and day 1 dietary recall weights to account for the complex, stratified, multistage probability sample design and oversampling and nonresponse.
Results
Descriptive characteristics of the sample can be seen in Table 1. More than 81% of children 0 to 12 months old consumed some formula or milk. Formula or milk fed infants were older (mean age of 6.6 months) compared with infants drinking only human milk (5.4 months; P < .05). A higher percentage of children receiving human milk as their only milk source were non-Hispanic white, while a lower percentage were non-Hispanic black or Mexican American compared with infants receiving formula or regular milk. A higher percentage of infants receiving formula or milk fell into the lower IPR category (≤185% FPT) compared with infants receiving human milk as their only source of milk. Finally, a higher percentage of infants receiving formula or milk had household education levels of less than a high school education, while a lower percentage reported at least some college education compared to infants consuming only human milk as their milk source.
Table 1.
Sociodemographic Characteristics of US infants, 0 to 12 Months Old, Overall and by Type of Milk Consumed: NHANES 2003–2010.
| Type of Milk Consumed |
|||
|---|---|---|---|
| Characteristic | Overall, % (SE) | Formula/Milk, % (SE) | Only Human Milk, % (SE) |
| n | 1864 | 1587 | 277 |
| Weighted % | 81.1 | 18.9 | |
| Male | 49.6 (1.5) | 48.9 (1.5) | 52.7 (4.3) |
| Female | 50.4 (1.5) | 51.1 (1.5) | 47.3 (4.3) |
| Age (mean months)a | 6.4 (0.1) | 6.6 (0.1) | 5.4 (0.3) |
| 0–6a | 54.6 (1.5) | 52.1 (1.6) | 65.0 (3.5) |
| 7–9 | 26.4 (1.5) | 26.8 (1.7) | 24.7 (3.2) |
| 10–12a | 19.0 (1.2) | 21.1 (1.3) | 10.3 (3.0) |
| Race/ethnicityb | |||
| Non-Hispanic whitea | 56.2 (2.8) | 53.1 (3.1) | 69.4 (3.5) |
| Non-Hispanic blacka | 12.9 (1.3) | 14.8 (1.6) | 4.5 (1.2) |
| Mexican Americana | 17.8 (1.6) | 19.1 (1.8) | 12.1 (2.0) |
| IPRc | |||
| 0% to 185% FPTa | 50.6 (2.2) | 54.5 (2.2) | 34.0 (4.2) |
| ≥186% FPTa | 49.4 (2.2) | 45.5 (2.2) | 66.0 (4.2) |
| Household educationc | |||
| <High schoola | 23.1 (1.4) | 26.3 (1.6) | 9.0 (1.8) |
| High school/GED | 23.3 (1.5) | 24.5 (1.6) | 17.9 (3.4) |
| Some college or morea | 50.6 (2.0) | 45.7 (2.0) | 71.9 (4.2) |
Abbreviations: IPR, income-to-poverty ratio; FPT, federal poverty threshold; SE, standard error.
denotes significant difference between infants consuming formula or milk and those whose milk source is human milk, P < .05.
Children of other Hispanic and other race groups are not described because of the limited sample sizes of these groups.
Sample sizes for these variables are based on the 1744 infants without missing data, of whom 266 reported human milk as their only source of milk.
Of children consuming formula or milk, 68.9% consumed cow’s milk formula on the day prior to the examination, while 11.6% consumed soy formula, 6.3% consumed specialty formula, and 5.4% consumed gentle/lactose-reduced formula (see Table 2). Additionally, 12.6% of children consumed nonformula milk products on the day prior to the examination including cow, soy, or goat milk, as well as flavored milk products (eg, chocolate milk). There were significant associations between family IPR and consumption of cow’s milk formula, soy formula, and regular milk (see Table 2). While 72.4% of infants from lower-income families (IPR ≤185%) consumed regular cow’s milk formula, only 64.9% of infants from higher income families consumed regular cow’s milk formula (P < .05). The percentage of children consuming soy formula was significantly higher among infants from higher income groups compared with the lower income group (P < .05). Consumption of specialty formula or gentle/lactose-reduced formula was not significantly associated with family IPR. Consumption of regular milk or milk products was higher among the lower income group (14.4%) as compared with the higher income group (10.9%; P < .05).
Table 2.
Percentage of Infants 0 to 12 Months Old Consuming Different Types of Formula or Regular Milk, NHANES 2003–2010, by Family Income-to-Poverty Ratio (IPR).a
| Overall (Unweighted n = 1587) | IPR ≤185% (Unweighted n = 1004)b |
IPR >185% (Unweighted n = 474)b |
|
|---|---|---|---|
| Cow’s milk formula | 68.9 (65.1–72.5) | 72.4 (68.5–76.3) | 64.9 (59.5–70.2)c |
| Soy-based | 11.6 (9.6–14.0) | 8.3 (6.1–10.4) | 15.8 (11.9–19.6)c |
| Specialty | 6.3 (4.9–8.1) | 6.5 (4.3–8.9) | 6.0 (3.5–8.5) |
| Gentle/lactose-reduced | 5.4 (3.6–7.8) | 3.8 (2.4–5.1) | 7.3 (3.1–11.4) |
| Regular milk (not formula) | 12.6 (10.3–15.3) | 14.4 (12.4–16.4) | 10.9 (8.1–13.6)c |
Data are presented as percentage (95% confidence interval). Percentages in each column do not sum to 100 because children may consume more than one type of formula. Percentages are adjusted for age (in months) of the child.
Sample sizes for these variables are based on the 1478 infants without missing data on IPR.
Indicates significant difference from reference group, IPR ≤185%, P < .05.
A lower percentage of infants with household education levels of at least some college consumed regular cow’s milk formula (65.3%) and a higher percentage consumed soy formula (13.9%) compared with infants of where household education levels were less than a high school degree (72.8% and 8.2%, respectively, Ps < .05; see Table 3). The percentage of infants consuming gentle/lactose-reduced formula was higher among infants with household education levels of at least some college (8.4%) compared with infants in households reporting less than a high school degree (2.4%; P < .05). The percentage consuming regular milk products was lower among infants with household education levels of at least some college (11.6%) as compared with households reporting less than a high school degree (15.3%; P < .05).
Table 3.
Percentage of Infants 0 to 12 Months Old Consuming Different Types of Formula or Regular Milk, NHANES 2003–2010, by Household Education Level.a
| <High School (Unweighted n = 543)b |
High School/GED (Unweighted n = 412)b |
Some College+ (Unweighted n = 563)b |
|
|---|---|---|---|
| Cow’s milk formula | 72.8 (67.9–77.8) | 68.2 (61.3–75.1) | 65.3 (60.4–70.1)c |
| Soy-based | 8.2 (4.6–11.7) | 11.8 (7.6–15.9) | 13.9 (10.7–17.1)c |
| Specialty | 5.5 (2.9–8.0) | 7.3 (3.6–11.0) | 6.4 (4.1–8.6) |
| Gentle/lactose-reduced | 2.4 (1.2–3.7) | 4.4 (1.9–6.8) | 8.4 (4.4–12.5)c |
| Regular milk (not formula) | 15.3 (12.5–18.1) | 12.2 (8.9–15.6) | 11.6 (9.0–14.1)c |
Data are presented as percentage (95% confidence interval). Percentages in each column do not sum to 100 because children may consume more than one type of formula. Percentages are adjusted for age (in months) of the child.
Sample sizes for these variables are based on the 1518 infants without missing data.
Indicates significant difference from reference group, <high school, P < .05.
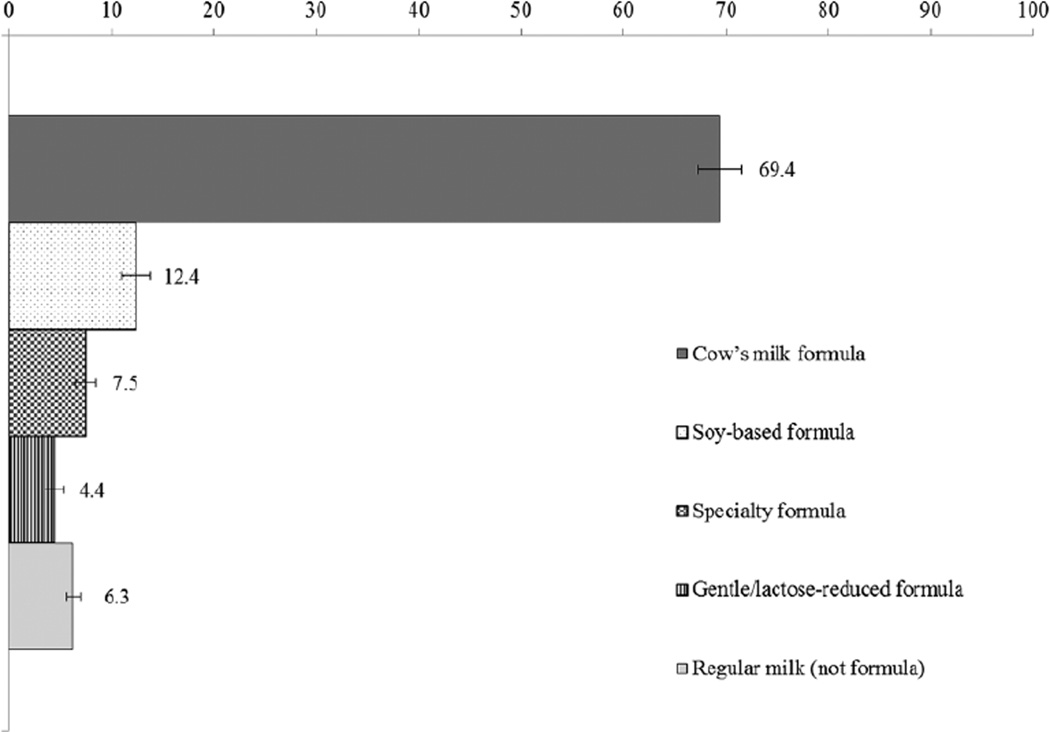
Findings from the second analysis, examining the contribution of specific formula types to overall formula or milk intake among infants, were largely consistent with results presented above (see Figure 1, Tables 4 and 5). The majority of formula or regular milk consumed by US infants consisted of cow’s milk formula (69.4%), followed by soy (12.4%). A lower percentage consisted of specialty (7.5%), and gentle/lactose-reduced formula types (4.4%). More than 6% was not formula but regular milk or milk products (eg, soy milk, chocolate milk). The percentage accounted for by cow’s milk formula was lower among infants from higher income families (62.4%) as compared to infants in the lowest income group (74.3%; P < .05). The percentage accounted for by soy-based formula was higher among infants in the higher income group (18.5%) as compared with the lower income group (8.2%; P < .05). The percentage accounted for by cow’s milk formula was also lower among infants with households reporting a high school degree or GED (69.9%), or some college or more (61.5%) compared with those reporting less than a high school education (78.2%, Ps < .05). The percentages of formula accounted for by soy and gentle/lactose-reduced were higher among infants with household education levels of at least some college (16.5%) as compared with infants of households reporting less than a high school education (8.0%, P < .05). Finally, gentle/lactose-reduced formula accounted for a higher percentage of total formula or milk intake among infants with household education levels of at least some college (7.6%) as compared to infants in households with less than a high school degree (1.1%, P<0.05).
Figure 1.
The overall percentage of formula/milk (in kilocalories) intake by type among infants 0 to 12 months old in the United States: NHANES, 2003–2010.
Table 4.
Percentage of Total Formula/Milk Intake (in Kilocalories) Among Infants 0 to 12 Months Old Who Reported Consuming Formula or Regular Milk, NHANES 2003–2010, by Family Income-to-Poverty Ratio.a
| ≤185% FPT | >185% FPT | |
|---|---|---|
| Cow’s milk formula | 74.3 (69.4–79.1) | 62.4 (55.8–69.0)b |
| Soy-based | 8.2 (5.6–10.8) | 18.5 (13.2–23.7)b |
| Specialty | 7.8 (4.7–10.9) | 7.2 (3.7–10.7) |
| Gentle or lactose-reduced/-free | 3.1 (1.6–4.6) | 5.7 (2.0–9.4) |
| Regular milk (not formula) | 6.6 (4.5–8.7) | 6.2 (3.9–8.6) |
Data are presented as percentage (95% confidence interval).
Indicates significant difference from reference group, ≤185% federal poverty threshold (FPT), P < .05.
Table 5.
Percentages of Total Formula/Milk Intake (in Kilocalories) Among Infants 0 to 12 Months Old Who Reported Consuming Formula or Regular Milk, NHANES 2003–2010, by Household Education Level.
| < High School | High School/GED | Some College+ | |
|---|---|---|---|
| Cow’s milk formula | 78.2 (73.1–83.3) | 69.9 (62.7–77.0)b | 61.5 (54.9–68.1)b |
| Soy-based | 8.0 (3.7–12.3) | 11.3 (6.8–15.9) | 16.5 (11.8–21.2)b |
| Specialty | 6.0 (2.6–9.4) | 8.4 (3.5–13.3) | 8.5 (5.5–11.5) |
| Gentle or lactose-reduced/free | 1.1 (0.5–1.8) | 3.7 (1.1–6.3) | 7.6 (3.5–11.6)b |
| Regular milk (not formula) | 6.6 (3.7–9.5) | 6.6 (3.7–9.5) | 5.9 (4.0–7.8) |
Data are presented as percentage (95% confidence interval).
Indicates significant difference from reference group, ≤high school, P < .05.
Discussion
Findings of the current analysis using 1 day of dietary recall data suggest that while the majority of infants receiving formula in the United States receive regular cow’s milk formula, approximately 12% consumed soy-based formula, 5% consumed gentle or lactose-reduced formulas, 6% consumed specialty formulas, and 13% report consumption of regular milk prior to 1 year of age. Higher family income was associated with a lower percentage of infants consuming regular cow’s milk formula and regular milk products, and a higher percentage consuming soy-based formula. Similarly, higher levels of household education (at least some college) were associated with lower odds of consuming regular cow’s milk formula and regular milk products, and higher odds of consuming soy formula and gentle/lactose-reduced formulas compared with infants from households where the household education level was less than a high school degree.
Nearly 13% of infants younger than 12 months consumed some form of regular milk product (eg, cow’s milk, soy milk, flavored milk) on the day prior to the examination, contrary to recommendations issued by the AAP to avoid cow’s milk for the first year of life.15,16 Prior studies, including the Feeding Infants and Toddlers Study (FITS) from 2002, have reported that 7% to 12% of infants 7 to 11 months old consumed cow’s milk on a given day.27 This latter estimate is similar to results presented here, despite the different years and age ranges examined. In our study, a higher percentage of infants from lower income and lower education households reported consuming regular milk products on a given day compared to infants from higher education or income households. These differences were consistent with findings from the Infant Feeding Practices Study II, a mail-based consumer opinion panel study conducted in 2005–2007.17,18
To our knowledge, this is the first population-based study describing the types of formula consumed by US infants, and how consumption differs by family income and education. Existing data and statistics quoted in AAP guidelines come from market share studies, which do not account for the variation in the amount of formula consumed by different children.5 Moreover, there may be discrepancies between what types of formula are purchased and how much is actually consumed. These differences may account for market share studies concluding that soy formulas account for a quarter of the formula market,5 while we found that soy formula represents less than 13% of caloric intake attributable to formula or regular milk.
There are limitations to the current analysis. The number of infants receiving specialty formulas is small in some cases; thus we were not able to examine patterns for some of the less commonly used formulas. Given some of the small subgroup sample sizes, there may have been limited power to detect differences between some subgroups. We combined several years of data to increase sample size for subgroup comparisons, but this may have masked changes in consumption patterns over time. No assertions can be made as to why certain formula types are used or whether they are necessary (eg, the presence of true lactose intolerance, cow’s milk protein or soy protein allergy). Other formula types and additives of interest (eg, prebiotics, probiotics, docosahexaenoic acid, and arachidonic acid) cannot be assessed using the dietary data in NHANES. Finally, infants who sporadically receive formula or regular milk, but received only human milk on the day before the exam, may result in misclassification.
There do not appear to be clear advantages or disadvantages to different commonly used formulas in terms of growth and nutritional status for most healthy infants.10–12 However, ongoing research into the possible differential effects of various formula types, as well as studies that link the microbiome to health sequelae, highlights the importance of examining population-level estimates of formula consumption. This analysis provides a description of formula consumption patterns among US infants who consume formula or regular milk. More than two-thirds of these infants consumed cow’s milk formula, while approximately 12% consumed soy, 6% consumed specialty, and 5% consume gentle/lactose-reduced formula on the day before the exam. Based on 1 day of dietary recall data, nearly 13% of infants younger than 12 months consumed regular cow’s milk or milk products, in contrast to AAP recommendations to avoid these milk products for the first year of life.
Acknowledgments
This work was performed under employment of the US Federal Government and the authors did not receive any outside funding. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Center for Health Statistics, Centers for Disease Control and Prevention.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Author Contributions
LMR, KAH, and AES designed the research, LMR analyzed data, and LMR, KAH, and AES wrote the article and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.American Academy of Pediatrics, Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Accessed June 4, 2015];Breastfeeding among U.S. children born 2000–2010, CDC National Immunization Survey. http://www.cdc.gov/BREASTFEEDING/data/NIS_data/index.htm.
- 3.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Nutrition, Physical Activity, and Obesity. [Accessed June 4, 2015];Breastfeeding report card, United States. 2014 http://www.cdc.gov/breastfeeding/pdf/2014breastfeedingreportcard.pdf.
- 4.O’Connor NR. Infant formula. Am Fam Physician. 2009;79:565–570. [PubMed] [Google Scholar]
- 5.Bhatia J, Greer F American Academy of Pediatrics Committee on Nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121:1062–1068. doi: 10.1542/peds.2008-0564. [DOI] [PubMed] [Google Scholar]
- 6.Stang J, Hoss K, Story M. Health statements made in infant formula advertisements in pregnancy and early parenting magazines: a content analysis. Infant Child Adolesc Nutr. 2010;2:16–25. [Google Scholar]
- 7.US Food and Drug Administration. [Accessed June 4, 2015];Guidance for industry: demonstration of the quality factor requirements under 21 CFR 106.96(i) for “eligible” infant formulas. 2014 http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm400036.htm.
- 8.Gilchrist JM, Moore MB, Andres A, Estroff JA, Badger TM. Ultrasonographic patterns of reproductive organs in infants fed soy formula: comparisons to infants fed breast milk and milk formula. J Pediatr. 2010;156:215–220. doi: 10.1016/j.jpeds.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 9.Boland M, Critch J, Kim JH, Marchand V, Prince T, Robertson MA. Concerns for the use of soy-based formulas in infant nutrition. Paediatr Child Health. 2009;14:109–113. [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics, Committee on Nutrition. Soy protein–based formulas: recommendations for use in infant feeding. Pediatrics. 1998;101(1 pt 1):148–153. [PubMed] [Google Scholar]
- 11.Andres A, Cleves MA, Bellando JB, Pivik RT, Casey PH, Badger TM. Developmental status of 1-year-old infants fed breast milk, cow’s milk formula, or soy formula. Pediatrics. 2012;129:1134–1140. doi: 10.1542/peds.2011-3121. [DOI] [PubMed] [Google Scholar]
- 12.Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2006;18:CD003741. doi: 10.1002/14651858.CD003741.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francavilla R, Calasso M, Calace L, et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr Allergy Immunol. 2012;23:420–427. doi: 10.1111/j.1399-3038.2012.01286.x. [DOI] [PubMed] [Google Scholar]
- 14.Joeckel RJ, Phillips SK. Overview of infant and pediatric formulas. Nutr Clin Pract. 2009;24:356–362. doi: 10.1177/0884533609335309. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics, Committee on Nutrition. The use of whole cow’s milk in infancy. Pediatrics. 1992;89:1105–1109. [PubMed] [Google Scholar]
- 16.Leung AK, Sauve RS. Whole cow’s milk in infancy. Paediatr Child Health. 2003;8:419–421. doi: 10.1093/pch/8.7.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clayton HB, Li R, Perrine CG, Scanlon KS. Prevalence and reasons for introducing infants early to solid foods: variations by milk feeding type. Pediatrics. 2013;131:e1108–e1114. doi: 10.1542/peds.2012-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fein SB, Labiner-Wolfe J, Scanlon KS, Grummer-Strawn LM. Selected complementary feeding practices and their association with maternal education. Pediatrics. 2008;122(suppl 2):S91–S97. doi: 10.1542/peds.2008-1315l. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: analytic guidelines 1999–2010. Vital Health Stat 2. 2013;161:1–24. [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. [Accessed June 4, 2015];National Health and Nutrition Examination Survey: NHANES response rates and CPS totals. http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm.
- 21.National Health and Nutrition Examination Survey. [Accessed June 4, 2015];MEC in-person dietary interviewers procedures manual. http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/DietaryInterviewers_Inperson.pdf.
- 22.National Center for Health Statistics. [Accessed June 4, 2015];Important statistical considerations regarding dietary data analyses. http://www.cdc.gov/nchs/tutorials/dietary/Basic/StatisticalConsiderations/intro.htm.
- 23.US Department of Agriculture, Agricultural Research Service, Food Surveys Group. USDA Food and Nutrient Database for Dietary Studies. 4.1 ed. Beltsville, MD: US Department of Agriculture, Agricultural Research Service; 2010. [Google Scholar]
- 24.US Department of Agriculture, Food and Nutrition Service. [Accessed June 4, 2015];Women, Infants and Children (WIC) eligibility requirements. http://www.fns.usda.gov/wic/wic-eligibility-requirements.
- 25.Oliveira V, Frazão E, Smallwood D. The Infant Formula Market: Consequences of a Change in the WIC Contract Brand (Economic Research Report 124) Beltsville, MD: US Department of Agriculture, Economic Research Service; 2011. [Accessed June 4, 2015]. http://www.ers.usda.gov/publications/err-economic-research-report/err124.aspx. [Google Scholar]
- 26.National Center for Health Statistics. [Accessed June 4, 2015];Dietary tutorial: estimate ratios and identify important food group sources of nutrients. http://www.cdc.gov/nchs/tutorials/dietary/Basic/Ratios/intro.htm.
- 27.Ponza M, Devaney B, Ziegler P, Reidy K, Squatrito C. Nutrient intakes and food choices of infants and toddlers participating in WIC. J Am Diet Assoc. 2004;104(1 suppl 1):s71–s79. doi: 10.1016/j.jada.2003.10.018. [DOI] [PubMed] [Google Scholar]