Abstract
At physiological conditions, a majority of biomolecules (e.g., amino acids, peptides and proteins) exist predominantly in the zwitterionic form that usually decides the biological functions. However, zwitterionic amino acids are not geometrically stable in gas phase and this seriously hampers the understanding of their structures, properties and biological functions. To this end, one of the recent research focuses is to demonstrate the stabilization effects of zwitterionic amino acids. Relative stabilities of canonical conformers are dependent on water contents, while zwitterionic stability improves monotonously and pronouncedly with increase of water contents. We find that one water molecule can render zwitterionic proline geometrically stable, and stabilities of different zwitterionic amino acids increase as glycine <proline <arginine. In addition, we have determined the numbers of water molecules required for zwitterionic proline to be energetically preferential and conformationally predominant, respectively as four and five. Five water molecules are enough to fill up the first shell of proline functional sites (carboxylic and amido), which is in line with the results of glycine. At any water content, zwitterionic formation will not be hindered kinetically because of rather low activation barriers, and the distribution of zwitterionic amino acids will be largely dependent on their thermodynamic stabilities.
Electronic supplementary material
The online version of this article (doi:10.1186/s40064-015-1661-8) contains supplementary material, which is available to authorized users.
Keywords: Density functional calculations, Conformational analysis, Zwitterionic stabilization, H-bonds
Background
In aqueous solutions, a wide range of biomolecules (e.g., amino acids, peptides and proteins) exist predominantly in the zwitterionic form, and water molecules are essential to maintain their native conformations and physiological functions (Timasheff 1970; Rand 2004). Interaction of biomolecules and water has attracted general interest (Teeter 1991; Levy and Onuchic 2004; Corradini et al. 2013), and amino acids, the structural unit of proteins, are generally the protypes for ab initio and density functional investigation of peptides and proteins (Császár 1992; Hu et al. 1995; Yu et al. 1995; Zhang and Chung-Phillips 1998; Remko and Rode 2001; Hoyau and Ohanessian 1998; Ai et al. 2003; Constantino et al. 2005; Remko and Rode 2006).
On the other hand, amino acids in gas phase consist entirely of canonical conformers (Császár 1992; Hu et al. 1995; Yu et al. 1995), which is totally different from the condition in aqueous solutions. This is obviously an obstacle for us to comprehend the electronic properties and biological functions of zwitterionic structures. Zwitterions can generate strong electric fields around that usually decide the biological functions of biomolecules. Owing to the particular importance, one of the recent research focuses is to demonstrate the stabilization effects of zwitterionic amino acids (Yu et al. 1995; Zhang and Chung-Phillips 1998; Remko and Rode 2001; Hoyau and Ohanessian 1998; Ai et al. 2003; Constantino et al. 2005; Remko and Rode 2006; Corral et al. 2006; Yang et al. 2009; Jensen and Gordon 1995; Snoek et al. 2002; Yamabe et al. 2003; Balta and Aviyente 2004; Im et al. 2008; Gutowski et al. 2000; Rimola et al. 2008; Wu and McMahon 2007; Rimola et al. 2013; Kass 2005; Yang et al. 2008; Tian et al. 2009; Hwang et al. 2011; Li et al. 2011; Kim et al. 2014; Yang and Zhou 2014), and a variety of attempts have been made in this aspect, such as protonation and deprotonation (Yu et al. 1995; Zhang and Chung-Phillips 1998), metalation (Hoyau and Ohanessian 1998; Ai et al. 2003; Constantino et al. 2005; Remko and Rode 2006; Corral et al. 2006; Yang et al. 2009), hydration (Jensen and Gordon 1995; Snoek et al. 2002; Yamabe et al. 2003; Balta and Aviyente 2004; Im et al. 2008; Hwang et al. 2011; Li et al. 2011; Kim et al. 2014) and anion attachment (Kass 2005; Yang et al. 2008; Tian et al. 2009). A minimum of two water molecules is required to stabilize glycine in the zwitterionic form (Jensen and Gordon 1995), while one is enough to cause zwitterionic arginine as the lowest-energy conformer (Im et al. 2008). Interaction of proline and water has been investigated by different groups (Li et al. 2011; Kim et al. 2014), and two water molecules were considered necessary for rendering zwitterionic proline to be geometrically stable.
In this work, density functional calculations were employed to comprehend the gas-phase interaction of different proline conformers and water with a wide range of contents (n = 0–5). Five water molecules were found enough to fill up the first shell of proline functional sites (carboxylic and amido), as in the case of glycine (Kokpol et al. 1988). We determined that one water molecule can stabilize proline in zwitterionic form; meanwhile, stabilities of zwitterionic glycine, proline and arginine were compared with each other. Then, the numbers of water molecules necessary to render zwitterionic proline energetically preferential and conformationally predominant were respectively determined, and the relationship of zwitterionic stability vs. water content was demonstrated. Finally, transition states for the transformation from canonical to zwitterionic proline at all water contents were located, testifying that zwitterionic formation in gas phase is impeded mainly by the thermodynamic rather than kinetic stability.
Computational methods
B3LYP density functional, in combination with 6-31+G (d,p) basis set, was used for structural optimizations and frequency calculations (Frisch et al. 2013). Energy minima were confirmed to have all positive frequencies while transition states displayed a single imaginary frequency corresponding to the eigenvector along the reaction path, and the assignment of each transition sate was verified by perturbing the structure along both directions of products and reactants with subsequent structural optimizations. Single-point energy calculations at the B3LYP/6-311++G (2df, 2pd) and MP2/6-311++G (2df, 2pd) levels of theory were then carried out on these optimized structures. Afterwards, the 6-31+G(d,p) and 6-311++G (2df, 2pd) basis sets were respectively designated as bs1 and bs2, and unless otherwise noted, all energies were reported at the B3LYP/bs2//B3LYP/bs1 level, which has been sufficiently validated before (Li et al. 2011; Kim et al. 2014; Yang et al. 2009; Rankin et al. 2002; Yang et al. 2010; Ajitha and Suresh 2011) and in this work.
Results and discussion
As shown in Scheme 1, three proline conformers have been considered in this work: two canonical (PA and PB) while the third zwitterionic (PC), which are in line with previous studies (Rankin et al. 2002; Yang et al. 2010; Ajitha and Suresh 2011; Yang et al. 2015). PA and PB are canonical conformers that predominant in gas phase (Eszter et al. 2003; Alln et al. 2004; Kapitán et al. 2006) and become the choice for catalytic studies (Rankin et al. 2002; Yang et al. 2010; Ajitha and Suresh 2011). PA is slightly less stable than PB with the relative energy of 1.6 kcal/mol (Table 1 and Additional file 1: Table S1). PC (zwitterionic) does not represent a local minimum on the potential energy surface (PES) (Eszter et al. 2003; Alln et al. 2004), and so its relative stability is evaluated by fixing the N-H1 distance at 1.030 Å, with production of a higher relative energy than PB (13.1 kcal/mol). Relative energies of these proline conformers are also calculated at other theoretical levels, which are in line with the default B3LYP/bs2//B3LYP/bs1 method (Additional file 1: Table S1).
Scheme 1.
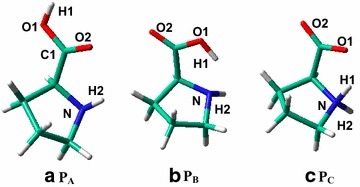
Proline in canonical (a and b) and zwitterionic (c) forms
Table 1.
Relative stabilities for interacted structures of different proline conformers (E RS) and water with a wide range of contents (n = 0–5)
| n = 0a | n = 1 | n = 2 | n = 3 | n = 4 | n = 5 | ||
|---|---|---|---|---|---|---|---|
| Gas phase | P A nW I | 1.6 | −1.2 | −3.3 | −0.1 | 0.4 | −0.5 |
| P C nW I | 13.1 | 8.7 | 2.8 | 1.1 | −2.6 | −6.0 |
Energy units in kcal/mol
For a given water content, P B nW I is used as energy benchmark
aIn zwitterionic proline (P C), the N-H1 distance is fixed at 1.030 Å during structural optimizations
For each proline confirmer, interactions with water can result in the various structures that are discerned by suffixing nWN, wherein n (n = 1, 2, …) represents the number of water molecules and N (=I, II, …) ranks the stability of different structures. For instance, PA2WIII/PC5WI stands for the third/first stable conformer for PA/PC interactions with two/five water molecules.
Necessary for zwitterionic stabilization
Figure 1 shows the interacted structures of proline conformers (PA, PB and PC) and one water molecule. PA and PB each result in four stable interacted structures, and their relative stabilities increase in the order of PA1WIV (5.6) <PA1WIII (3.2) <PB1WIV (2.9) <PB1WIII (2.6) <PB1WII (1.4) <PA1WII (1.3) <PB1WI (0) <PA1WI (−1.2), see Table 1 and Additional file 1: Table S2. Note that relative energies (unit kcal/mol) are given in parentheses, and PB1WI is used as benchmark. It can be seen that the conformational preference is altered by presence of one water molecule, and the lowest-energy structure is from PA instead of PB that represents the most stable conformer in the isolated state (Eszter et al. 2003; Alln et al. 2004). In PA1WI, water forms two H-bonds with the carboxylic site of proline (O1H1•••O3 and O3H3•••O2). Both PA1WII and PA1WIV have only one intermolecular H-bond, while H-bonding interactions of the former is apparently stronger as reflected from their distances (O3H3•••N: 1.868 Å vs. O3H3•••O1: 2.057 Å). In PB1WI, water interacts mainly with one of the two carboxylic-O atoms, while structures involving both carboxylic-O atoms cannot be located as energy minima. The less efficient intermolecular H-bonding interactions in PB1WI cause it to have a higher relative energy than PA1WI.
Fig. 1.
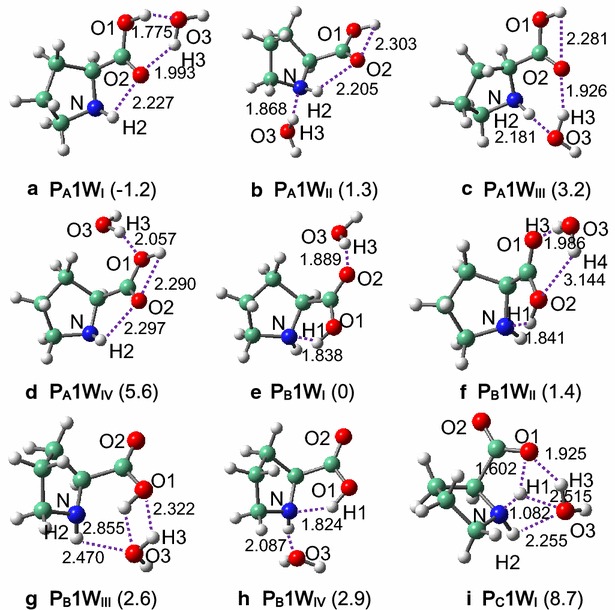
Interacted structures of proline conformers (P A, P B and P C) with one water molecule. Relative energies (kcal/mol) are given in parentheses, using P B 1W I as benchmark. H-bonds (Å) are marked with dashed lines
It is interesting to find that one water molecule can stabilize proline in the zwitterionic form. In PC1WI, two H-bonds are constructed for water with the carboxylic and amido sites of proline (NH2•••O3 and O3H3•••O1) that resemble the condition in PB1WIII; however, both H-bonds have been significantly reinforced, with the distances being optimized respectively at 2.255 and 1.925 Å vs. 2.470 and 2.322 Å in PB1WIII. Despite that, PC1WI still has a much higher energy than PB1WI (8.7 kcal/mol, see Table 1 and Additional file 1: Table S2). For glycine, arginine and proline, relative stabilities of their zwitterionic conformers should differ from each other. Both zwitterionic proline and arigine require one water molecule in order to remain geometrically stable, while zwitterionic glycine necessitates two water molecules (Jensen and Gordon 1995; Im et al. 2008). In addition, one water molecule is already sufficient to cause zwitterionic arginine as the global energy minimum (Im et al. 2008). Accordingly, relative stabilities of these zwitterionic conformers should increase in the order as glycine <proline <arginine.
Introduction of more water molecules
Introduction of a second water molecule to proline results in a more conformational diversity, and PA, PB and PC respectively result in 8, 7 and 7 interacted structures, see Figs. 2, 3, 4 with their relative energies being listed in Table 1 and Additional file 1: Table S3. Structure (i.e., PA2WI) corresponding to PA remains the most stable as in the case of n = 1, wherein two water molecules are both situated at the carboxylic site of proline. Although with two resembling intermolecular H-bonds, interactions in PA2WI are pronouncedly stronger than in PA1WI (1.647 and 1.800 Å vs. 1.775 and 1.993 Å). Structures of two water molecules may be combinatorially obtained from those of one water molecule; e.g., combination of PA1WI and PA1WII leads to PA2WII.
Fig. 2.
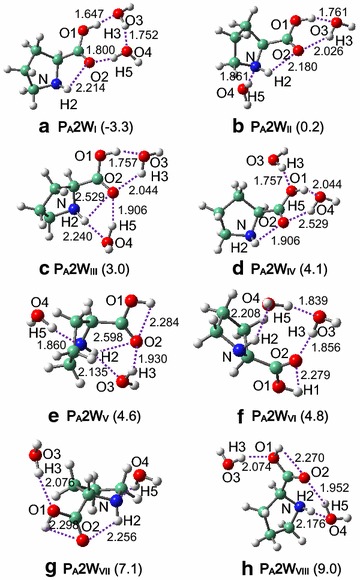
Interacted structures of P A and two water molecules. Relative energies (kcal/mol) are given in parentheses, using P B 2W I as benchmark. H-bonds (Å) are marked with dashed lines
Fig. 3.
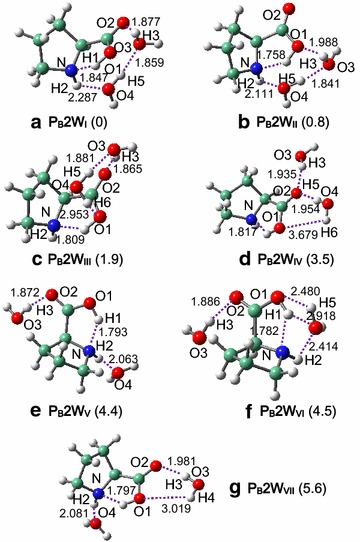
Interacted structures of P B and two water molecules. Relative energies (kcal/mol) are given in parentheses, using P B 2W I as benchmark. H-bonds (Å) are marked with dashed lines
Fig. 4.
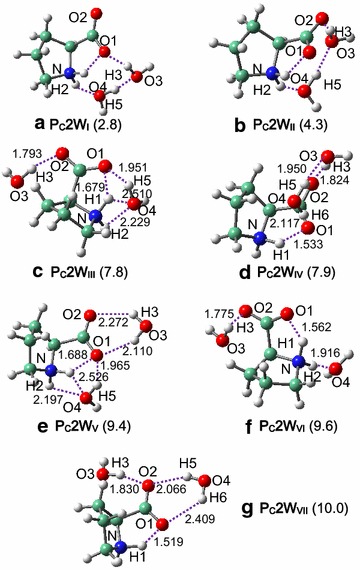
Interacted structures of P C and two water molecules. Relative energies (kcal/mol) are given in parentheses, using P B 2W I as benchmark. H-bonds (Å) are marked with dashed lines
In PB2WI and PB2WII, one water molecule forms H-bond with the carboxylic-O site of proline while the other forms H-bond with the amido site, and two water molecules are connected with each other by one strong H-bond. In PC2WI, the intermolecular H-bonding interactions between proline and water resemble those in PB2WII and also in PC1WI while are greatly consolidated as compared to PC1WI, and the zwitterionic stability improves accordingly (Table 1). Unlike the case of one water, zwitterions (PC2WIV and PC2WVII) that have H-bonds only at the carboxylic site of proline remain geometrically stable, as a result of enhanced intermolecular interactions.
Scheme 2 and Additional file 1: Figures S1–S8 show that with increase of water contents, the functional sites (carboxylic and amido) of proline are gradually saturated. Some structures of PB and PC interactions with 2–4 water molecules have been reported by Lee et al. (Kim et al. 2014) that are in good agreement with the present results. It indicates that five water molecules are enough to fill up the first shell of proline functional sites (carboxylic and amido), consistent with the results of glycine (Kokpol et al. 1988). Relative energies in Table 1 and Additional file 1: Table S3–S6 demonstrate that the leading position of PA begins to be challenged since the third water molecule. PB3WI has comparable stability with PA3WI, while PB4WI rather than PA4WI is slightly energetically preferential. Instead, stability of zwitterionic proline improves monotonously and pronouncedly with the gradual increase of water contents. Zwitterionic structures ascend to be the most stable configuration at n = 4 (PC4WI) and becomes the predominant configuration since n = 5 (PC5WI), where rather complex H-bonding networks are constructed between water and proline.
Scheme 2.
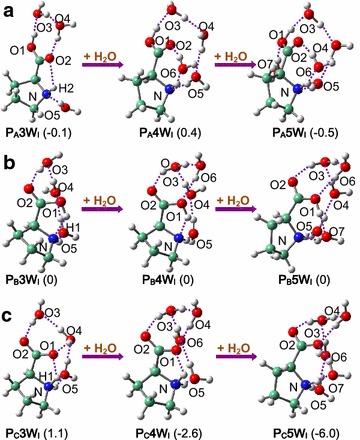
Gradual increase of water to interact with different proline conformers: a P A 3W I → P A 4W I → P A 5W I, b P B 3W I → P B 4W I → P B 5W I and c P C 3W I → P C 4W I → P C 5W I. Relative energies (kcal/mol) are given in parentheses, using P B nW I as benchmark (n = 3, 4, 5). H-bonds are marked with dashed lines
Transformation to zwitterionic proline
Transition state structures for the transformation from canonical to zwitterionic proline with presence of different water contents (n = 1–5) are shown in Additional file 1: Figures S9–S13, and their activation barriers (EA) and reaction heats (ER) are listed in Table 2. In transition state structures, O-H1 bonds have already ruptured while N-H1 bonds are being constructed. At a given water content, there may exist several zwitterionic structures, and the activation barriers (EA) that are dependent on reaction paths may differ significantly; e.g., at n = 3, the EA values vary in the range of 2.6–8.6 kcal/mol, and the maximum (8.6 kcal/mol) is even larger than those of n = 1 and 2. Generally, with increase of water contents, transformation to zwitterionic structures becomes more thermodynamically favourable, as indicated by the calculated reaction heats (ER), see Table 2.
Table 2.
Activation barriers (E A) and reaction heats (E R) for transformation from canonical (P B) to zwitterionic (P C) proline with presence of water molecules
| Reaction paths | E A | E R |
|---|---|---|
| One water molecule | ||
| P B 1W III → [TS1W III–I]≠ → P C 1W I | 6.7 | 6.1 |
| Two water molecules | ||
| P B 2W II → [TS2W II–I]≠ → P C 2W I | 4.2 | 2.0 |
| P B 2W I → [TS2W I–II]≠ → P C 2W II | 6.1 | 4.3 |
| P B 2W VI → [TS2W VI–III]≠ → P C 2W III | 4.9 | 3.2 |
| P B 2W III → [TS2W III–IV]≠ → P C 2W IV | 6.4 | 6.0 |
| P B 2W VII → [TS2W VII–V]≠ → P C 2W V | 6.2 | 3.8 |
| P B 2W V → [TS2W V–VI] → P C 2W VI | 5.8 | 5.2 |
| P B 2W IV → [TS2W IV–VII]≠ → P C 2W VII | 6.7 | 6.5 |
| Three water molecules | ||
| P B 3W II → [TS3W II–I]≠ → P C 3W I | 3.8 | 0.7 |
| P B 3W I → [TS3W I–II ] ≠ → P C 3W II | 4.3 | 1.9 |
| P B 3W III → [TS3W III–III ] ≠ → P C 3W III | 6.3 | −1.1 |
| P B 3W III → [TS3W III–IV ] ≠ → P C 3W IV | 8.6 | 0.7 |
| P B 3W IV → [TS3W IV–V ] ≠ → P C 3W V | 2.6 | −1.1 |
| Four water molecules | ||
| P B 4W I → [TS4W I–I ] ≠ → P C 4W I | 1.9 | −2.6 |
| P B 4W II → [TS4W II–I ] ≠ → P C 4W I | 5.8 | −3.4 |
| P B 4W II → [TS4W II–III ] ≠ → P C 4W III | 5.3 | −2.4 |
| P B 4W III → [TS4W III–IV ] ≠ → P C 4W IV | 2.7 | −2.1 |
| Five water molecules | ||
| P B 5W I → [TS5W I–I ] ≠ → P C 5W I | 4.2 | −6.0 |
| P B 5W II → [TS5W II–II ] ≠ → P C 5W II | 4.8 | −6.4 |
Energy units in kcal/mol
Figure 5 gives the activation barriers (EA) for transformation to the most stable zwitterionic structures (PCnWI) (n = 1–5), which are equal to 6.7, 4.2, 3.8, 1.9 and 4.2 kcal/mol for n = 1, 2, 3, 4 and 5, respectively (Table 2). The larger EA value for PC5WI than for PC4WI is due to the alteration of direct transformation path from O1 to N and the involvement of one water molecule (H11O7H12) (Yamabe et al. 2003). The changing trends of EA at MP2/bs2//B3LYP/bs1 level are identical to that of B3LYP/bs2//B3LYP/bs1; in addition, the exact EA values of these two methods are rather close each other, thus validating the default methodologies. At any water content, the activation barriers (EA) for transformation from canonical to zwitterionic structures are rather low, and accordingly the transformation to zwitterionic structures in gas phase will not be kinetically hindered. It is thus assumed that the zwitterionic distribution at a given water content (n ≥ 0) are determined principally by their thermodynamic stabilities.
Fig. 5.
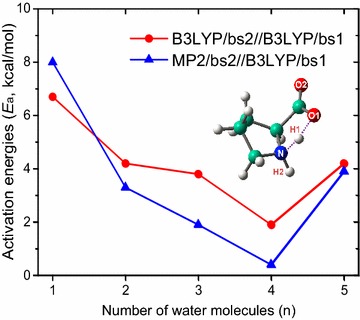
Activation energies (E A) for the transformation to zwitterions (P C nW I) in presence of water molecules (n = 1–5) calculated at different levels of theory. Two reaction paths can lead to P C 4W I (Table 2), and the path with the lower activation barrier is given here
Conclusions
Conformational analyses are performed for interacted structures of proline conformers (PA, PB and PC) and water with a wide range of contents (n = 1–5). Five water molecules are enough to fill up the first shell of proline functional sites (carboxylic and amido). The conformational preferences of proline are altered by gradual increase of water contents, and intermolecular H-bonds play a crucial role in deciding their relative stabilities.
It is interesting to find that one water molecule has already rendered zwitterionic proline to be geometrically stable with formation of two strong intermolecular H-bonds. Unlike the canonical conformers, the relative stabilities of zwitterionic proline improve monotonously and pronouncedly with gradual increase of water contents. Zwitterionic proline is energetically preferential over canonical structures at n = 4 and conformationally predominant at n = 5; in these cases, rather complex H-bonding networks are constructed between water and proline. Zwitterionic stabilities of different amino acids differ substantially and increase in the order of glycine <proline <arginine.
At a given water content, the activation barriers for transformation from canonical to zwitterionic conformers are significantly dependent on reaction paths; nonetheless, the activation barriers are rather low for all water contents. It is thus demonstrated that the zwitterionic distribution, at any water content (n ≥ 0), will be determined mainly by the thermodynamic rather than kinetic factor.
Author’s contributions
GY performed the experiments, analyzed the data and drafted the manuscript. LJZ and YC helped analyze the data and contributed in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (21473137) and Fourth Excellent Talents Program of Higher Education in Chongqing (2014-03) and Fundamental Research Funds for the Central Colleges (SWU113049 and XDJK2014C106).
Competing interests
The authors declare that they have no competing interests.
Additional file
10.1186/s40064-015-1661-8 Relative stabilities for different proline conformers with presence of 0, 1, 2, 3, 4 and 5 water molecules (Tables S1–S5) as well as structures of proline conformers with presence of 3, 4 and 5 water molecules (Figures S1–S8) and transition states for the conformational transformation from canonical to zwitterionic proline with presence of 1, 2, 3, 4, 5 water molecules (Figures S9–S13).
Contributor Information
Gang Yang, Phone: 086-023-68251545, Email: theobiochem@gmail.com.
Lijun Zhou, Email: 455206647@qq.com.
Yang Chen, Email: 929022258@qq.com.
References
- Ai HQ, Bu YX, Han KL. Glycine-Zn+/Zn2+ and their hydrates: on the number of water molecules necessary to stabilize the switterionic glycine-Zn+/Zn2+ over the nonzwitterionic ones. J Chem Phys. 2003;118:10973–10985. doi: 10.1063/1.1575192. [DOI] [Google Scholar]
- Ajitha MJ, Suresh CH. A higher energy conformer of (S)-proline is the active catalyst in intermolecular aldol reaction: evidence from DFT calculations. J Mol Catal A: Chem. 2011;345:37–43. doi: 10.1016/j.molcata.2011.05.016. [DOI] [Google Scholar]
- Alln WD, Czinki E, Csásázr AG. Molecular structure of proline. Chem Eur J. 2004;10:4512–4517. doi: 10.1002/chem.200400112. [DOI] [PubMed] [Google Scholar]
- Balta B, Aviyente V. Solvent Effects on Glycine II. Water-assisted Tautomerization. J Comput Chem. 2004;15:690–703. doi: 10.1002/jcc.10422. [DOI] [PubMed] [Google Scholar]
- Constantino E, Rodriguez-Santiago L, Sodupe M, Tortajada J. Interaction of Co+ and Co2+ with Glycine. A Theoretical Study. J Phys Chem A. 2005;109:224–230. doi: 10.1021/jp047590y. [DOI] [PubMed] [Google Scholar]
- Corradini D, Strekalova EG, Stanley HE, Gallo P. Microscopic mechanism of protein cryopreservation in an aqueous solution with trehalose. Sci Rep. 2013;3:1218. doi: 10.1038/srep01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral L, Mό O, Yáňez M, Moran D, Radom L, Salpin JY, Tortajada J. An experimental and theoretical investigation of gas-phase reactions of Ca2+ with glycine. Chem Eur J. 2006;12:6787–6796. doi: 10.1002/chem.200600127. [DOI] [PubMed] [Google Scholar]
- Császár AG. Conformers of gaseous glycine. J Am Chem Soc. 1992;114:9568–9575. doi: 10.1021/ja00050a041. [DOI] [Google Scholar]
- Eszter C, Czinki E, Csásázr AG. Conformers of gaseous proline. Chem Eur J. 2003;9:1008–1019. doi: 10.1002/chem.200390103. [DOI] [PubMed] [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Cheeseman MAJR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2013) Gaussian 09, Revision A.9. Gaussian, Inc., Wallingford
- Gutowski M, Skurski P, Simons J. Dipole-bound anions of glycine based on the zwitterion and neutral structures. J Am Chem Soc. 2000;122:10159–10162. doi: 10.1021/ja001658f. [DOI] [Google Scholar]
- Hoyau S, Ohanessian G. Interaction of alkali metal cations (Li+-Cs+) with glycine in the gas phase: a theoretical study. Chem Eur J. 1998;4:1561–1569. doi: 10.1002/(SICI)1521-3765(19980807)4:8<1561::AID-CHEM1561>3.0.CO;2-Z. [DOI] [Google Scholar]
- Hu CH, Shen M, Schaefer HF., III Glycine conformational analysis. J Am Chem Soc. 1995;115:2923–2929. doi: 10.1021/ja00060a046. [DOI] [Google Scholar]
- Hwang TK, Eom GY, Choi MS, Jang SW, Kim JY, Lee S. Microsolvation of lysine by water: computational study of stabilized zwitterion. J Phys Chem B. 2011;115:10147–10153. doi: 10.1021/jp202850s. [DOI] [PubMed] [Google Scholar]
- Im S, Jang SW, Lee S, Lee Y, Kim B. Arginine zwitterion is more stable than the canonical form when solvated by a water molecule. J Phys Chem A. 2008;112:9767–9770. doi: 10.1021/jp801933y. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Gordon MS. On the number of water molecules necessary to stabilize the glycine zwitterion. J Am Chem Soc. 1995;117:8159–8170. doi: 10.1021/ja00136a013. [DOI] [Google Scholar]
- Kapitán J, Baumruk V, Kopecký V, Jr, Pohl R, Bouř P. Proline zwitterion dynamics in solution, glass and crystalline state. J Am Chem Soc. 2006;128:13451–13462. doi: 10.1021/ja062958l. [DOI] [PubMed] [Google Scholar]
- Kass SR. Zwitterion-dianion complexes and anion-anion clusters with negative dissociation energies. J Am Chem Soc. 2005;127:13098–13099. doi: 10.1021/ja053391w. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee Y, Lee S. Effects of microsolvation on the relative stability of zwitterionic vs canonical proline. Chem Phys Lett. 2014;608:177–185. doi: 10.1016/j.cplett.2014.05.105. [DOI] [Google Scholar]
- Kokpol SU, Doungdee PB, Hannongbua SV, Rode BM, Imtrakul LJP. Ab initio study of the hydration of the glycine zwitterion. J Chem Soc Faraday Trans. 1988;2(84):1789–1792. doi: 10.1039/f29888401789. [DOI] [Google Scholar]
- Levy Y, Onuchic JN. Water and proteins: a love-hate relationship. Proc Natl Acad Sci USA. 2004;101:3325–3326. doi: 10.1073/pnas.0400157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Zhong ZJ, Wu HZ. DFT and MP2 investigations of l-proline and its hydrated complexes. J Mol Model. 2011;17:2623–2630. doi: 10.1007/s00894-011-0957-z. [DOI] [PubMed] [Google Scholar]
- Rand RP. Probing the role of water in protein conformation and function. Philos Trans R Soc Lond B Biol Sci. 2004;359:1277–1284. doi: 10.1098/rstb.2004.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KN, Gauld JW, Boyd RJ. Density functional study of the proline-catalyzed direct aldol reaction. J Phys Chem A. 2002;106:5155–5159. doi: 10.1021/jp020079p. [DOI] [Google Scholar]
- Remko M, Rode BM. Catalyzed peptide bond formation in the gas phase. Phys Chem Chem Phys. 2001;3:4667–4673. doi: 10.1039/b105623a. [DOI] [Google Scholar]
- Remko M, Rode BM. Effect of metal ions (Li+, Na+, K+, Mg2+, Ca2+, Ni2+, Cu2+, and Zn2+) and water coordination on the structure of glycine and zwitterionic glycine. J Phys Chem A. 2006;110:1960–1967. doi: 10.1021/jp054119b. [DOI] [PubMed] [Google Scholar]
- Rimola A, Corno M, Zicovich-Wilson CM, Ugliengo P. Ab initio modeling of protein/biomaterial interactions: glycine adsorption at hydroxyapatite surfaces. J Am Chem Soc. 2008;130:16181–16183. doi: 10.1021/ja806520d. [DOI] [PubMed] [Google Scholar]
- Rimola A, Costa D, Sodupe M, Lambert JF, Ugliengo P. Silica surface features and their role in the adsorption of biomolecules: computational modeling and experiments. Chem Rev. 2013;113:42160–44313. doi: 10.1021/cr3003054. [DOI] [PubMed] [Google Scholar]
- Snoek LC, Kroemery RT, Simons JPA. Spectroscopic and computational exploration of tryptophan-water cluster structures in the gas phase. Phys Chem Chem Phys. 2002;4:2130–2139. doi: 10.1039/b200059h. [DOI] [Google Scholar]
- Teeter MM. Water-protein interactions: theory and experiment. Annu Rev Biophys Biophys Chem. 1991;20:577–600. doi: 10.1146/annurev.bb.20.060191.003045. [DOI] [PubMed] [Google Scholar]
- Tian SX, Li HB, Yang JL. Monoanion BH4− can stabilize zwitterionic glycine with dihydrogen bonds. Chemphyschem. 2009;10:1435–1437. doi: 10.1002/cphc.200900158. [DOI] [PubMed] [Google Scholar]
- Timasheff SN. Protein-solvent interactions and protein conformation. Acc Chem Res. 1970;3:62–68. doi: 10.1021/ar50026a004. [DOI] [Google Scholar]
- Wu RH, McMahon TB. Stabilization of the zwitterionic structure of proline by an alkylammonium ion in the gas phase. Angew Chem Int Ed Engl. 2007;46:3668–3671. doi: 10.1002/anie.200605174. [DOI] [PubMed] [Google Scholar]
- Yamabe S, Ono N, Tsuchida N. Molecular interactions between glycine and h2o affording the zwitterion. J Phys Chem A. 2003;107:7915–7922. doi: 10.1021/jp030495p. [DOI] [Google Scholar]
- Yang G, Zhou LJ. Zwitterionic versus canonical amino acids over the various defects in zeolites: a two-layer ONIOM calculation. Sci Rep. 2014;4:6594. doi: 10.1038/srep06594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Zu YG, Liu CB, Fu YJ, Zhou LJ. Stabilization of amino acid zwitterions with varieties of anionic species: the intrinsic mechanism. J Phys Chem B. 2008;112:7104–7110. doi: 10.1021/jp710394f. [DOI] [PubMed] [Google Scholar]
- Yang G, Zu YG, Fu YJ, Zhou LJ, Zhu RX, Liu CB. Assembly and stabilization of multi-amino acid zwitterions by the zn(ii) ion: a computational exploration. J Phys Chem B. 2009;113:4899–4906. doi: 10.1021/jp808741c. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Wu XM, Zhou LJ, Yang G. A Proline-based neuraminidase inhibitor: DFT studies on the zwitterion conformation, stability and formation. Int J Mol Sci. 2009;10:3918–3930. doi: 10.3390/ijms10093918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Yang ZW, Zhou LJ, Zhu RX, Liu CB. A revisit to proline-catalyzed aldol reaction: interactions with acetone and catalytic mechanisms. J Mol Catal A: Chem. 2010;316:112–117. doi: 10.1016/j.molcata.2009.10.008. [DOI] [Google Scholar]
- Yang G, Zhu C, Zhou LJ. Stabilization of zwitterionic proline by DMSO. Int J Quantum Chem. 2015;115:1746–1752. doi: 10.1002/qua.25012. [DOI] [Google Scholar]
- Yu D, Rauk A, Armstrong DA. Radicals and ions of glycine: an ab initio study of the structures and gas-phase thermochemistry. J Am Chem Soc. 1995;117:1789–1996. doi: 10.1021/ja00111a017. [DOI] [Google Scholar]
- Zhang K, Chung-Phillips A. Conformers of gaseous protonated glycine. J Comput Chem. 1998;19:1862–1876. doi: 10.1002/(SICI)1096-987X(199812)19:16<1862::AID-JCC7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]


