Abstract
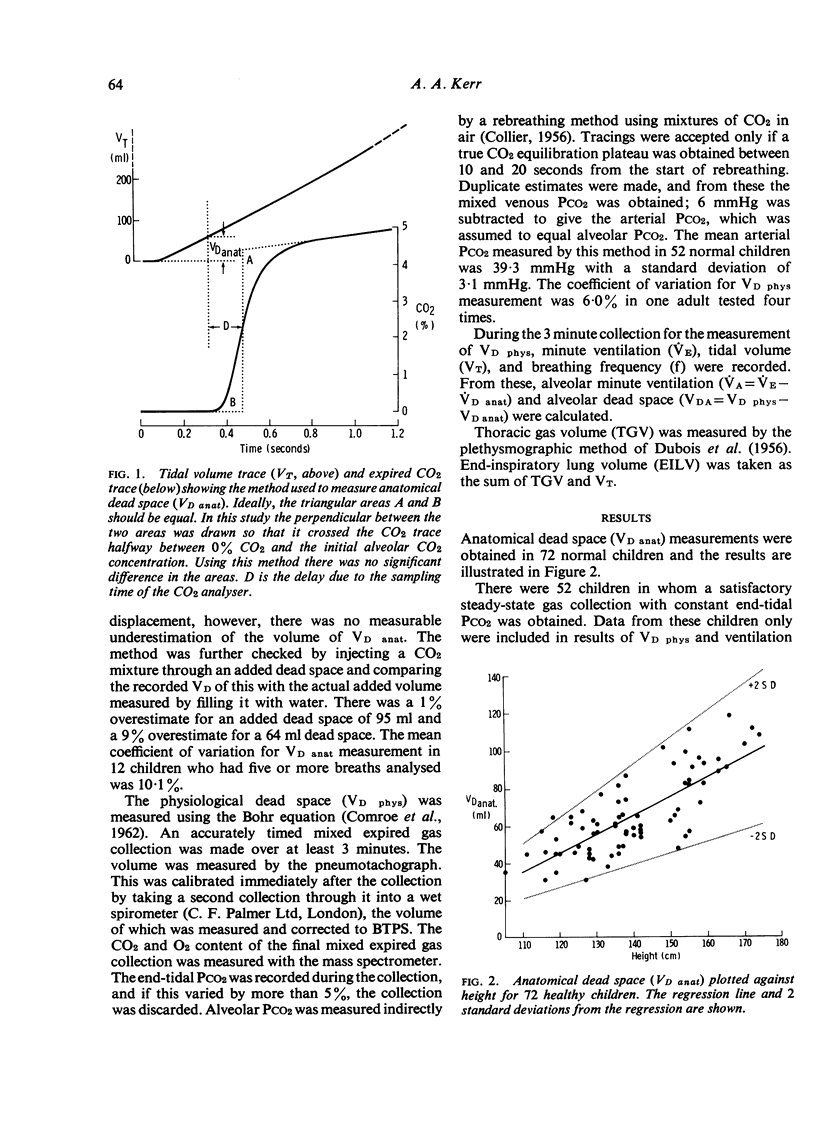
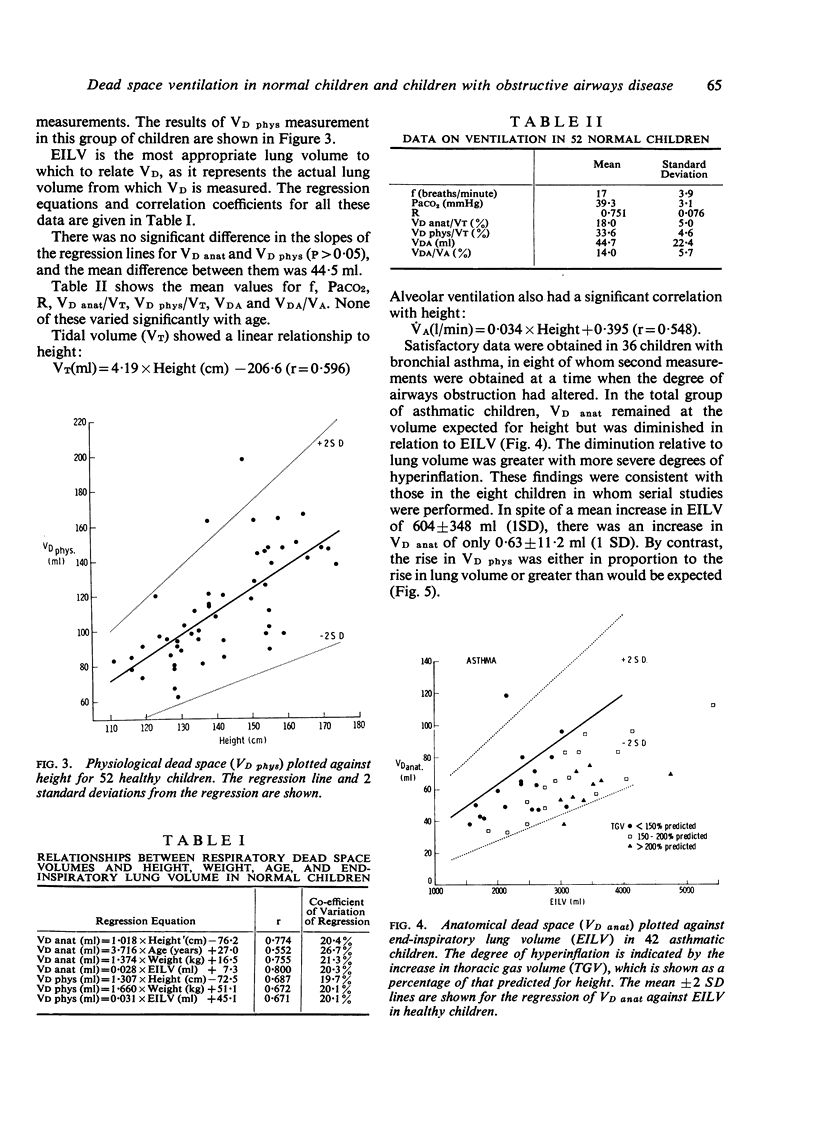
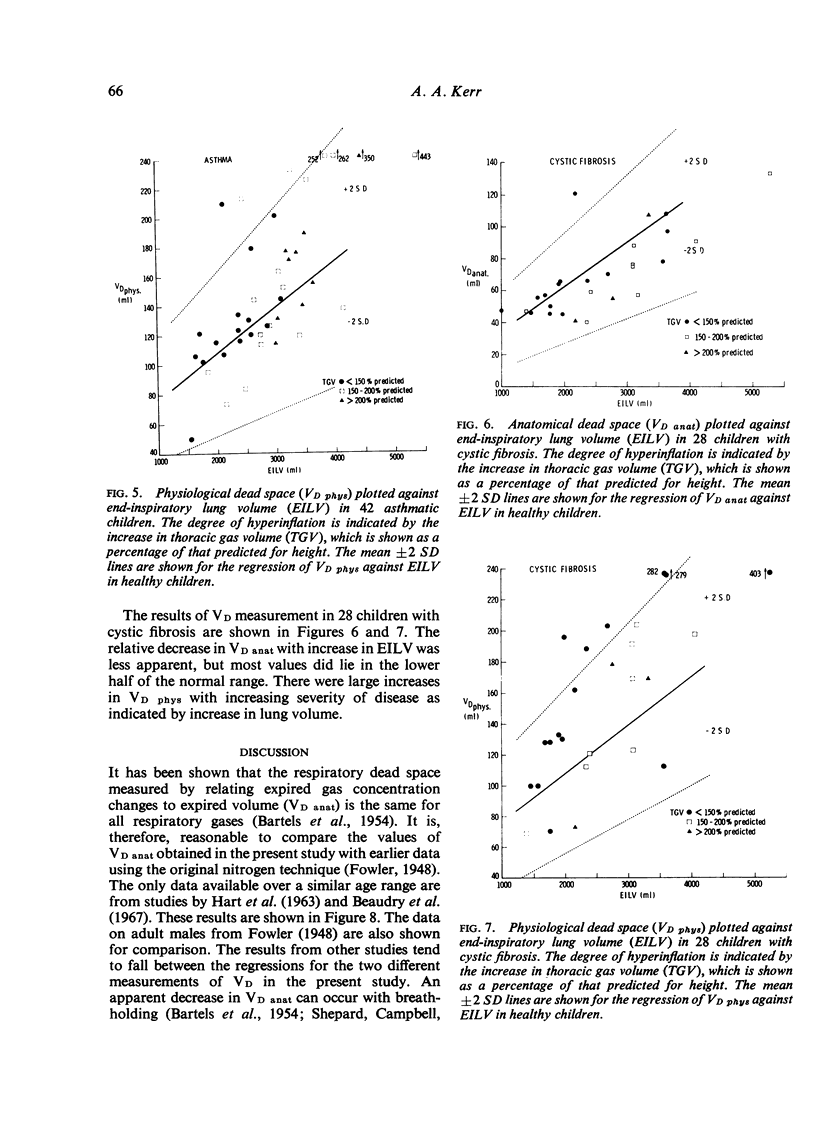
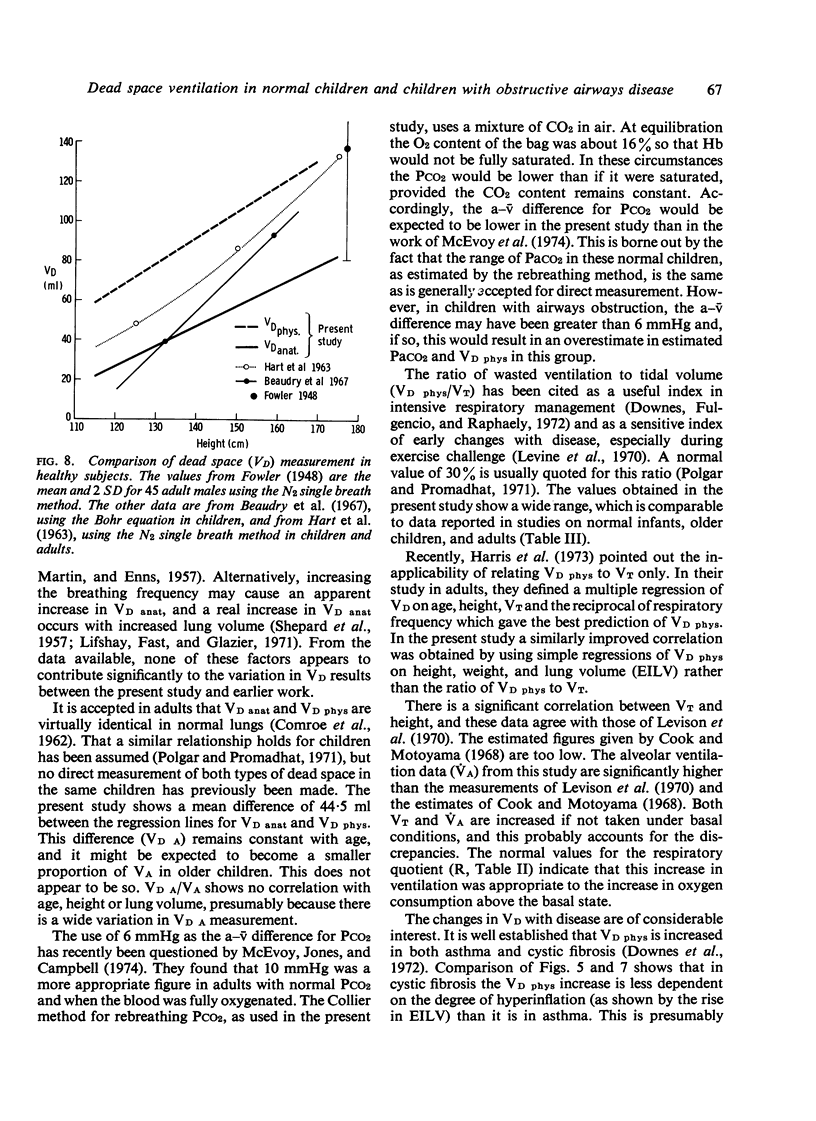
Anatomical dead space was measured in 72 normal children aged from 5 to 16 years, using the single breath method. There was a linear increase in this measurement with height, weight, and end-inspiratory lung volume. Physiological dead space was measured in 52 normal children using the Bohr equation and substituting a rebreathing PCO2 for alveolar PCO2. There was a parallel increase in this measurement with height, weight, and end-inspiratory lung volume. The difference between the two dead space measurements constitutes the alveolar dead space and was constant over the whole age range at 45 +/- 22 ml. The ratio of physiological dead space to tidal volume was 33-6 +/-4-6% and was unaltered by age or change in lung volume. The effect of airways obstruction on the dead space volumes was studied in 36 children with asthma and 28 with cystic fibrosis. Physiological dead space increased with increasing airways obstruction. Anatomical dead space remained constant in spite of marked increases in lung volume associated with the airways obstruction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTELS J., SEVERINGHAUS J. W., FORSTER R. E., BRISCOE W. A., BATES D. V. The respiratory dead space measured by single breath analysis of oxygen, carbon dioxide, nitrogen or helium. J Clin Invest. 1954 Jan;33(1):41–48. doi: 10.1172/JCI102868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudry P. H., Wise M. B., Seely J. E. Respiratory gas exchange at rest and during exercise in normal and asthmatic children. Am Rev Respir Dis. 1967 Feb;95(2):248–254. doi: 10.1164/arrd.1967.95.2.248. [DOI] [PubMed] [Google Scholar]
- COLLIER C. R. Determination of mixed venous CO2 tensions by rebreathing. J Appl Physiol. 1956 Jul;9(1):25–29. doi: 10.1152/jappl.1956.9.1.25. [DOI] [PubMed] [Google Scholar]
- COOK C. D., CHERRY R. B., O'BRIEN D., KARLBERG P., SMITH C. A. Studies of respiratory physiology in the newborn infant. I. Observations on normal premature and full-term infants. J Clin Invest. 1955 Jul;34(7 Pt 1):975–982. doi: 10.1172/JCI103165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS A. B., BOTELHO S. Y., BEDELL G. N., MARSHALL R., COMROE J. H., Jr A rapid plethysmographic method for measuring thoracic gas volume: a comparison with a nitrogen washout method for measuring functional residual capacity in normal subjects. J Clin Invest. 1956 Mar;35(3):322–326. doi: 10.1172/JCI103281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes J. J., Fulgencio T., Raphaely R. C. Acute respiratory failure in infants and children. Pediatr Clin North Am. 1972 May;19(2):423–445. doi: 10.1016/s0031-3955(16)32709-2. [DOI] [PubMed] [Google Scholar]
- Harris E. A., Hunter M. E., Seelye E. R., Vedder M., Whitlock R. M. Prediction of the physiological dead-space in resting normal subjects. Clin Sci Mol Med. 1973 Sep;45(3):375–386. doi: 10.1042/cs0450375. [DOI] [PubMed] [Google Scholar]
- Levine G., Housley E., MacLeod P., Macklem P. T. Gas exchange abnormalities in mild bronchitis and asymptomatic asthma. N Engl J Med. 1970 Jun 4;282(23):1277–1282. doi: 10.1056/NEJM197006042822301. [DOI] [PubMed] [Google Scholar]
- Levison H., Featherby E. A., Weng T. R. Arterial blood gases, alveolar-arterial oxygen difference, and physiologic dead space in children and young adults. Am Rev Respir Dis. 1970 Jun;101(6):972–974. doi: 10.1164/arrd.1970.101.6.972. [DOI] [PubMed] [Google Scholar]
- Lifshay A., Fast C. W., Glazier J. B. Effects of changes in respiratory pattern on physiological dead space. J Appl Physiol. 1971 Sep;31(3):478–483. doi: 10.1152/jappl.1971.31.3.478. [DOI] [PubMed] [Google Scholar]
- McEvoy J. D., Jones N. L., Campbell E. J. Mixed venous and arterial Pco2. Br Med J. 1974 Dec 21;4(5946):687–690. doi: 10.1136/bmj.4.5946.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellemgaard K. The alveolar-arterial oxygen difference: its size and components in normal man. Acta Physiol Scand. 1966 May;67(1):10–20. doi: 10.1111/j.1748-1716.1966.tb03281.x. [DOI] [PubMed] [Google Scholar]
- NELSON N. M., PROD'HOM L. S., CHERRY R. B., LIPSITZ P. J., SMITH C. A. Pulmonary function in the newborn infant. I. Methods: ventilation and gaseous metabolism. Pediatrics. 1962 Dec;30:963–974. [PubMed] [Google Scholar]
- Olsen C. R., Stevens A. E., McIlroy M. B. Rigidity of tracheae and bronchi during muscular constriction. J Appl Physiol. 1967 Jul;23(1):27–34. doi: 10.1152/jappl.1967.23.1.27. [DOI] [PubMed] [Google Scholar]
- RADFORD E. P., Jr Ventilation standards for use in artificial respiration. J Appl Physiol. 1955 Jan;7(4):451–460. doi: 10.1152/jappl.1955.7.4.451. [DOI] [PubMed] [Google Scholar]
- RAINE J. M., BISHOP J. M. A-a difference in O2 tension and physiological dead space in normal man. J Appl Physiol. 1963 Mar;18:284–288. doi: 10.1152/jappl.1963.18.2.284. [DOI] [PubMed] [Google Scholar]
- SHEPARD R. H., CAMPBELL E. J., MARTIN H. B., ENNS T. Factors affecting the pulmonary dead space as determined by single breath analysis. J Appl Physiol. 1957 Sep;11(2):241–244. doi: 10.1152/jappl.1957.11.2.241. [DOI] [PubMed] [Google Scholar]
- YOUNG A. C. Dead space at rest and during exercise. J Appl Physiol. 1955 Jul;8(1):91–94. doi: 10.1152/jappl.1955.8.1.91. [DOI] [PubMed] [Google Scholar]


