Abstract
Structural rearrangements of chromosome 10 are frequently observed in glioblastoma multiforme and over 80 % of tumour samples archived in the catalogue of somatic mutations in cancer database had gene copy number loss for PI4K2A which encodes phosphatidylinositol 4-kinase type IIalpha. PI4K2A loss of heterozygosity mirrored that of PTEN, another enzyme that regulates phosphoinositide levels and also PIK3AP1, MINPP1, INPP5A and INPP5F. These results indicated a reduction in copy number for a set of phosphoinositide signalling genes that co-localise to chromosome 10q. This analysis was extended to a panel of phosphoinositide pathway genes on other chromosomes and revealed a number of previously unreported associations with glioblastoma multiforme. Of particular note were highly penetrant copy number losses for a group of X-linked phosphoinositide phosphatase genes OCRL, MTM1 and MTMR8; copy number amplifications for the chromosome 19 genes PIP5K1C, AKT2 and PIK3R2, and also for the phospholipase C genes PLCB1, PLCB4 and PLCG1 on chromosome 20. These mutations are likely to affect signalling and trafficking functions dependent on the PI(4,5)P2, PI(3,4,5)P3 and PI(3,5)P2 lipids as well as the inositol phosphates IP3, IP5 and IP6. Analysis of flanking genes with functionally unrelated products indicated that chromosomal instability as opposed to a phosphoinositide-specific process underlay this pattern of copy number variation. This in silico study suggests that in glioblastoma multiforme, karyotypic changes have the potential to cause multiple abnormalities in sets of genes involved in phosphoinositide metabolism and this may be important for understanding drug resistance and phosphoinositide pathway redundancy in the advanced disease state.
Keywords: PI 4-kinase, Cancer, Glioblastoma, Gene copy number
Introduction
Glioblastoma multiforme is the most common malignant brain tumour and accounts for the majority of deaths from this disease. A number of comprehensive recent publications have described the genetic changes associated with glioblastoma multiforme [1–13]. However, rather than assessing global genomic changes, this study concerns mutations likely to affect phosphoinositide metabolism and signalling. The initial focus herein is on phosphatidylinositol (PI) 4-kinase type IIα (PI4K2A), a 55 kDa constitutively membrane-associated enzyme which localises mainly to the trans-Golgi network (TGN) and late endosomes where it catalyses the formation of PI4P by D4 phosphorylation of its substrate lipid phosphatidylinositol [14]. PI4K2A is unique amongst the phosphoinositide kinases in that it is regulated by dual palmitoylation within its catalytic domain and by its targeting to cholesterol-rich membrane rafts where its activity is very sensitive to alterations to membrane sterol levels [15–22]. A number of intracellular trafficking roles have been described for this enzyme including the recruitment of the clathrin adapters AP-1 [23] and GGA [24] to TGN membranes and AP-3 to late endosomes [25]. More recent work has revealed the enzyme’s function in regulating Wnt receptor signalling [26, 27] and described its reciprocal regulation on endosomal membranes by the ubiquitin ligase itch [28]. In the central nervous system, PI4K2A is by far the most prevalent PI kinase activity and is most strongly expressed in cerebellar Purkinje cells and astrocytes [29]. Astrocytomas and subsequently glioblastoma multiforme usually develop from astrocytes, and this prompted us to investigate whether PI4K2A mutations are associated with this disease.
Several studies have demonstrated that PI4K2A controls the signalling and trafficking of cell surface receptors such as EGFR [30–32] and HER2 [33] which are known to stimulate oncogenic signalling. EGFR is often overexpressed in glioblastoma [12] and anti-EGFR therapies are a potential targeted therapy in this difficult-to-treat disease [4, 13, 34–40]. In addition to EGFR overexpression, loss of the tumour suppressor PTEN is a common feature in glioblastoma [1, 41–43]. PTEN is a phosphoinositide 5-phosphatase that in normal cells rapidly dephosphorylatesthe PI3K lipid product PI(3,4,5)P3 which is a potent stimulator of signalling via the Akt/mTOR pathway [44–47]. Deletion of PTEN results in sustained and elevated PI3K signalling and augmented cell proliferation [48]. Given that PI4KIIα has roles in regulating both EGFR and AKT signalling, an initial aim of this study was to ascertain if the PI4K2A gene is mutated in glioblastoma. Through analysis of publically accessible genomic data available via the COSMIC resource, we were able to establish that point mutations of PI4K2A were rare in glioblastoma but more than 80 % of samples from a cohort of 638 exhibited loss of PI4K2A heterozygosity; this level of copy number variation mirrored that of PTEN which also localises to chromosome 10p.
Methods
Genomic Analysis
The Catalogue of Somatic Mutations In Cancer (COSMIC) bioinformatics resource (http://www.sanger.ac.uk/cosmic) [49, 50] established and maintained by the Wellcome Trust Sanger Institute was used in order to identify mutations in the 638 individual tumour samples analysed by the Cancer Genome Atlas (TCGA). For these samples in the COSMIC database, gene copy number analysis was carried out using ASCAT (Allele-Specific Copy number Analysis of Tumours) algorithm software [51] available at http://heim.ifi.uio.no/bioinf/Projects/ASCAT. For this analysis, the average copy number for the genome is 1.90 and a reduction in total copy number to a value of 1.30 or less demarcates a loss, while a gain was set at copy number ≥ 3.
Protein Expression Analysis
The Human Protein Atlas [52, 53] (www.proteinatlas.org) online resource was utilised to investigate the expression of candidate proteins identified from the genomic analyses in immunohistochemically stained control cerebral cortex tissue and glioblastoma patient samples. The immunohistochemical data included here derives only from antibodies that also detected the correct size protein band on Western blots.
String Analysis
The String [54] Search Tool for the Retrieval of Interacting Genes/Proteins software (http://string-db.org) was used to visualise interactions and networks amongst the protein products from the phosphoinositide pathway genes identified as having the highest levels of copy number variation in glioblastoma.
Results and Discussion
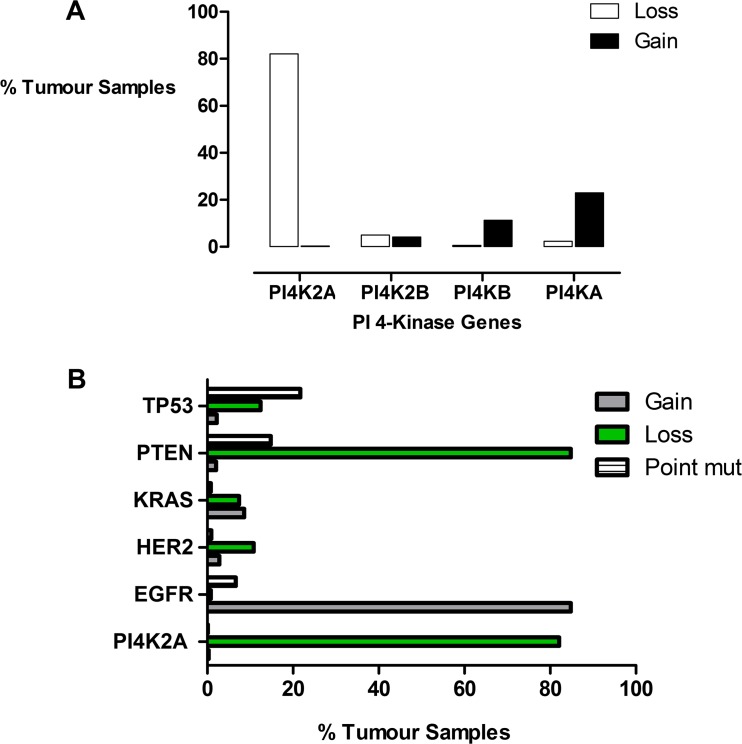
As a first step, we sought to determine if there was any evidence for PI 4-kinase mutations in glioblastoma. We used the COSMIC resource and genomic data from 638 different glioblastoma patient samples to investigate the mutational status of the 4 human PI 4-kinase genes, PI4KA, PI4KB, PI4K2A and PI4K2B. Point mutations giving rise to amino acid changes were rare for all four PI 4-kinase genes and were observed in less than 1 % of all the samples in the combined patient cohort. However, analysis of gene copy number variation revealed that in 82 % of cases, there was a decrease in PI4K2A copy number and this was not seen for any of the other PI 4-kinase genes (Fig. 1). The pattern of PI4K2A copy number variation was also compared with a panel of established oncogenes and tumour suppressors composed of PTEN, EGFR, HER2 and KRAS (Fig. 1). From this initial comparison, it became apparent that the level of PI4K2A gene copy number loss in glioblastoma mirrored that of a tumour suppressor such as PTEN as opposed to an oncogene such as EGFR that had an increased copy number. The loss of heterozgosity for PTEN and PI4K2A is biologically significant as these genes both localise to chromosome 10q and suggests that their copy number variation was due to a chromosome 10q deletion. For this to be the case, then the nearest neighbour genes to PI4K2A should also undergo a similar degree of gene copy number loss. To investigate this possibility, two genes immediately upstream and downstream of PI4K2A were identified. The upstream genes at the 10q24.2 locus were HOGA1 and MORN4 while the immediate downstream genes were AVPI1 and MARVELD1. Consistent with a copy number change caused by chromosomal loss, the pattern of copy number variation (0.2 % gain and 82 % loss) for each of these flanking genes in glioblastoma was almost identical to PI4K2A and PTEN. However, unlike PTEN and PI4K2A, there is no evidence for any of these flanking gene products being involved in receptor stimulated oncogenic signalling. These results also demonstrate that the copy number reduction of genes localised to chromosome 10 was not specific to the phosphoinositide pathway and that these changes could be explained by an overall loss of chromosome 10 heterozygosity.
Fig. 1.
a Gene copy number variation for the four human PI 4-kinase isozymes in glioblastoma multiforme. b Comparison of gene copy number variation for PI4K2A with established oncogenes. Data was derived from mining the COSMIC database
For a more complete analysis of genomic changes across the entire phosphoinositide pathway, a panel of genes encoding proteins involved in phosphoinositide metabolism, signalling and trafficking was investigated. Forty-five additional genes encoding phosphoinositide kinases, phosphatases, phospholipase C isozymes and also effector proteins such as the three Akt isoforms were included in this expanded analysis. Amongst the glioblastoma samples on COSMIC, copy number variation was frequently observed in this group of genes (Table 1). Copy number loss was most pronounced for phosphoinositide pathway genes localised to both arms of chromosome 10 and the X chromosome. For copy number amplifications, the greatest increases were seen for genes on chromosomes 19 and 20 followed by chromosomes 3 and 1. This rank ordering of chromosomes is in line with their known susceptibilities to undergo deletion or amplification in glioblastoma [42, 55–57]. Significantly, using this approach, it was possible to identify 17 new gene mutations, present in at least 10 % of the glioblastoma multiforme patient samples, with the potential to affect phosphoinositide metabolism and signalling.
Table 1.
A summary of the copy number changes in glioblastoma multiforme for a panel of genes involved in phosphoinositide signalling and trafficking
| Gene | Chromosome | Gain | Loss | Main effect on lipids | Previous report in glioma |
|---|---|---|---|---|---|
| INPP5B (1p34) | 1 | 8.80 | 2.70 | PI(4,5)P2 ↓ | No |
| PIK3R3 (1p36.2) | 1 | 8.80 | 2.20 | PI(3,4,5)P3 ↑ | Yes [88] |
| PI4KB (1q21) | 1 | 11.30 | 0.60 | PI4P ↑ | No |
| PIP5K1A (1q21.3) | 1 | 11.30 | 0.60 | PI(4,5)P2 ↑ | No |
| AKT3 (1q44) | 1 | 12.20 | 2.00 | – | Yes [89] |
| PIK3C2B (1q32) | 1 | 19.80 | 0.60 | PI(3,4)P2 ↑ | Yes [45, 90, 91] |
| INPP1 (2q32) | 2 | 2.50 | 3.80 | IP2 ↑ | No |
| INPP4A (2q11.2) | 2 | 4.50 | 1.60 | PI3P ↑ | No |
| PIKFYVE (2q34) | 2 | 3.50 | 2.40 | PI5P/PI(3,5)P2 ↑ | No |
| SACM1L (3p21.3) | 3 | 5.80 | 7.80 | PI4P ↑ | No |
| PIK3R4 (3q22.1) | 3 | 7.00 | 6.00 | PI3P ↑ | No |
| PIK3CB (3q22.3) | 3 | 6.90 | 5.20 | PI(3,4,5)P3 ↑ | Yes [92] |
| PIK3CA (3q26.3) | 3 | 11.80 | 3.50 | PI(3,4,5)P3 ↑ | Yes [93] |
| INPP4B (4q31.21) | 4 | 3.60 | 5.80 | PI(3,4)P2 ↑ | No |
| PIK3R1 (5q13.1) | 5 | 4.40 | 6.60 | PI(3,4,5)P3 ↓ | Yes [94] |
| FIG 4 (6q21) | 6 | 1.60 | 18.80 | PI(3,5)P2 ↑ | No |
| MTMR7 (8p22) | 8 | 6.30 | 8.00 | PI3P ↑ | No |
| PIP4K2A (10p12.2) | 10 | 2.20 | 77.60 | PI(4,5)P2 ↓ | No |
| MINPP1 (10q23) | 10 | 0.30 | 83.90 | IP6 ↑ | No |
| PTEN (10q23.3) | 10 | 2.00 | 84.80 | PI(3,4,5)P3 ↑ | Yes |
| PIK3AP1 (10q24.1) | 10 | 0.30 | 83.40 | PI(3,4,5)P3 ↓ | No |
| INPP5F (10q26.11) | 10 | 84.30 | PI(3,4,5)P3 ↑ | No | |
| INPP5A (10q26.3) | 10 | 83.40 | IP3 ↑ | Yes [66] | |
| PIK3C2A (11p15.5-p14) | 11 | 2.50 | 12.10 | PI3P/PI(3,4)P2 ↓ | No |
| PLCB3 (11q13) | 11 | 1.60 | 11.30 | PI(4,5)P2 ↑ | No |
| MTMR2 (11q22) | 11 | 2.70 | 9.70 | PI3P ↑ | No |
| PIK3C2G (12p.12) | 12 | 8.90 | 9.90 | PI(3,4,5)P3 ↑ or ↓ | Yes [95] |
| MTMR6 (13q12) | 13 | 1.60 | 26.50 | PI3P/PI(3,5)P2 ↑ | No |
| AKT1 (14q32.32) | 14 | 4.50 | 23.00 | – | Yes |
| PLCB2 (15q15) | 15 | 1.10 | 21.90 | PI(4,5)P2 ↑ | No |
| PLCG2 (16q24.1) | 16 | 3.10 | 11.60 | PI(4,5)P2 ↑ | No |
| PIK3R5 (17p13.1) | 17 | 2.70 | 10.70 | PI(3,4,5)P2 ↑ | No |
| PIP4K2B (17q12) | 17 | 2.80 | 10.70 | PI(4,5)P2 ↓ | No |
| MTMR4 (17q22–q23) | 17 | 6.40 | 5.50 | PI3P ↑ | No |
| PIK3C3 (18q12.3) | 19 | 4.90 | 7.50 | PI3P ↓ | No |
| PIP5K1C (19p13.3) | 19 | 30.20 | 5.00 | PI(4,5)P2 ↑ | No |
| PIP5K1B (19; 19 C1) | 19 | 8.00 | 9.70 | PI(4,5)P2 ↑ | No |
| AKT2 (19q13.1–q13.2) | 19 | 25.60 | 5.80 | – | Yes [89, 96] |
| PIK3R2 (19q13.2–q13.4) | 19 | 30.60 | 3.30 | PI(3,4,5)P3 ↑ | No |
| PLCB4 (20p12) | 20 | 31.80 | 2.40 | PI(4,5)P2 ↓ | No |
| PLCB1 (20p12) | 20 | 34.60 | 2.20 | PI(4,5)P2 ↓ | No |
| PLCG1 (20q12–q13.1) | 20 | 30.30 | 2.20 | PI(4,5)P2 ↓ | Yes[97] |
| PIK3IP1 (22q12.2) | 22 | 1.90 | 27.90 | PI(3,4,5)P3 ↑ | Yes [98] |
| MTMR8 (Xq11.2) | X | 5.60 | 65.70 | PI3P/PI(3,5)P2 ↑ | No |
| OCRL (Xq25) | X | 4.50 | 66.60 | PI(4,5)P2 ↑ | No |
| MTM1 (Xq28) | X | 4.50 | 66.00 | PI3P/PI(3,5)P2 ↑ | No |
The chromosomal localisation of each gene appears in brackets after its name. The “main effect on lipid” column refers to the predicted effect on either phosphoinositide lipid or inositol phosphate levels depending on whether gene copy number loss or gain predominates. The “previously reported in glioma” column indicates where there has been a previous publication demonstrating that a gene or gene product is involved in glioblastoma multiforme.
These results revealed that chromosomal instability affected particular sets of genes with the potential for chromosome-specific effects on phosphoinositide lipid levels metabolism (Table 1) with dyshomeostasis of PI(3,4,5)P3, PI(4,5)P2 and PI(3,5)P2 the most likely outcomes in glioblastoma multiforme. In the case of chromosome 10q loss, in addition to PI4K2A and PTEN, copy number losses in over 80 % of tumour samples were identified for four other phosphoinositide pathway genes. The effected genes were PIK3AP1 (10q24.1) also known as B-cell adaptor for phosphatidylinositol 3-kinase (BCAP), MINPP1 (10q23), INPP5F (10q26.11) and INPP5A (10q26.3). Two of these gene products, PTEN and INPP5F, are phosphoinositide 5-phosphatases and reductions in their enzymatic activities have the potential to induce sustained PI(3,4,5)P3 stimulation of the PI3K/mTOR pathway. However, unlike PTEN, INPP5F has not previously been considered as an oncogene in glioblastoma multiforme. Nevertheless, the potential decrease of PI3K lipid substrate brought about by the combination of PI4K2A loss and reduced PI3K activation due to diminished levels of the stimulatory protein PIK3AP1, could potentially off-set the effects of reduced PI(3,4,5)P3 degradation. Although this latter scenario is more speculative since there is no evidence yet for PI4K2A positively affecting PI(3,4,5)P3 generation [31] or for PIK3AP1 stimulating PI3K activity in the brain, even though this protein is a negative regulator of PI3K signalling and a potential tumour suppressor of cancers affecting other tissues [58–63].
Gene copy numbers for MINPP1 and INPP5A, two enzymes involved in the dephosphorylation of soluble inositol phosphates, were also reduced in the majority of glioblastoma multiforme patient samples. INPP5A [64–68] dephosphorylates the 5-position of the receptor-activated PLC product inositol (1,4,5)-tris-phosphate (IP3) which binds intracellular IP3 receptors to release Ca2+ stored in endoplasmic reticulum stores, while MINPP1 [69–72] is a 3-phosphatase that can hydrolyze multiple soluble inositol phosphate substrates including the downstream IP3 products inositol pentakisphosphate (IP5) and inositol hexakisphosphate (IP6). There is some evidence that IP6 can function asa possible alternative chemical energy supply to ATP with the potential to drive tumour growth under anaerobic conditions [71, 72]. In this way, loss of both chromosome 10 encoded inositol phosphatases has the potential to induce sustained and augmented signalling by receptors that activate phospholipase C. It is worth noting that with the exceptions of the established tumour suppressor PTEN and two reports mentioning INPP5A [64, 66], none of the other phosphoinositide pathway genes on chromosome 10 discussed here has hitherto been associated with glioblastoma multiforme.
Another genomic alteration of note identified here that affected 65 % of the glioblastoma multiforme patient samples was copy number loss for a set of three phosphoinositide phosphatase genes associated with the X chromosome. These genes were MTMR8 (Xq11.2), OCRL (Xq25) and MTM1 (Xq28), and once again none of these phosphoinositide genes has previously been associated with glioblastoma multiforme (Table 1). Loss-of-function mutations in the protein products of each of these genes are known to cause different X-linked neurological diseases [73, 74] and endosomal trafficking defects that feature accumulations of their respective substrate lipids; PI3P and PI(3,5)P2 in the case of MTM1 [75–77] and MTMR8 [78], and PI(4,5)P2 for OCRL [79]. This trio of phosphatase genes has not been previously linked to cancer but in light of their proposed functions in endosomal trafficking [80–82] and autophagy [78, 83, 84], it is possible that loss of this gene combination results in profound defects in the intracellular trafficking and degradation of receptor tyrosine kinases [75] and, consequently, augmented pro-tumourigenic and proliferative signalling.
A number of potential gain-of-function mutations due to chromosomal amplifications were also identified in the patient tumour samples and these included several phosphoinositide pathway genes never before linked to cancers affecting the central nervous system (Table 1). The main loci that exhibited copy number gain were chromosomes 20 and 19 in approximately 30 % of the glioblastoma samples, and chromosome 1 in around 10 % of the patient cohort. Interestingly, while chromosomal loss impacted heavily on phosphoinositide phosphatases, thereby potentially augmenting signalling outputs, gene copy number changes associated with chromosomal amplification were also found to be biased towards potentiating oncogenic signalling (Table 1). However, for gene amplifications, the main changes were not in phosphoinositide phosphatase genes but more so in genes encoding phosphoinositide kinases such as PIP5K1C (19p13.3) and PIK3C2B (1q32), second messenger-generating enzymes such as the PLC isozymes PLCB4 (20p12), PLCB1 (20p12) and PLCG1 (20q12-q13.1) and phosphoinositide-dependent signalling proteins such as AKT2 (19q13.1-q13.2). These findings imply that chromosomal numerical aberrations that result in either gain or loss of gene copy number in glioblastoma multiforme can result in pro-oncogenic signalling and trafficking defects via the phosphoinositide pathway. This raises the possibility these altered phosphoinositide signalling outputs could be selected for on the basis that they confer a survival advantage during tumour progression. Further functional studies are required to investigate this hypothesis.
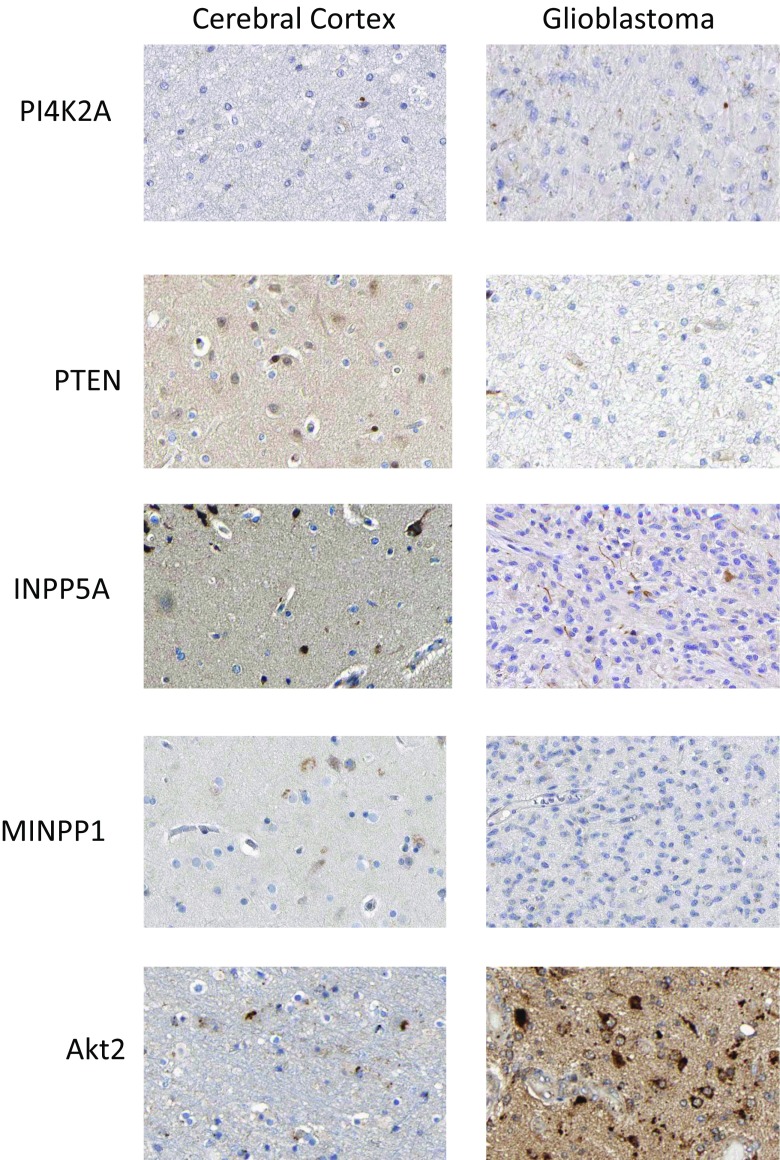
The Human Protein Atlas archive of immunohistochemically stained cancer tissues was interrogated to uncover if the gene copy number variation in the phosphoinositide pathway was reflected in altered protein expression (Fig. 2). For comparison purposes, immunohistochemically stained normal cerebral cortex tissue was included in the analyses. However, the cytological composition of the cerebral cortex differs greatly from that found in glioblastoma tumour samples, with the former containing a high density of neurons whereas the latter is composed almost entirely of tumour cells derived from astrocytes. Thus, given these underlying histological differences, it is difficult to directly compare patterns of expressed proteins between control and patient samples. Notwithstanding these caveats, it was possible to identify high-grade glioblastoma samples that exhibited patterns of protein expression that were consistent with the gene copy number variation results. More specifically, high levels of gene copy number loss were found for PTEN, PI4K2A, INPP5F and MINPP1 and there was concomitant reduced or absent expression of their cognate gene products in immunohistochemically stained glioblastoma tissues (Fig. 2). PI4K2A protein expression was not detected in 6/11 tumour samples; PTEN, was not detected in 10/12; INPP5A exhibited low to moderate staining in 3/12 diseased tissues and MINPP1 was not detected in 8/10 tumours. Conversely, for AKT2 which was subject to a copy number increase, the corresponding protein products were intensely stained in high-grade glioblastoma samples. These images are included to provide some evidence that the protein expression patterns predicted by the genomic analysis could be observed in an independent immunohistochemical study. It is very important to note that these results derive from malignancies where the karyotype has not been characterised. Therefore it is not possible to conclude thatthe observed protein expression patterns are a consequence of chromosomal numerical abnormalities and associated changes in gene copy number. Proof of such a functional association is beyond the scope of the current in silico study, and future studies will aim to simultaneously investigate the link between glioblastoma karyotype, gene copy number status and the expression levels of enzymes involved in phosphoinositide signalling and trafficking.
Fig. 2.
Expression of phosphoinositide pathway proteins in healthy cerebral cortex tissue and glioblastoma multiforme sections. Images were obtained by immunohistochemical staining of paraffin-embedded tissue samples using isoform-specific antibodies directed against the protein products of PI4K2A, PTEN, INPP5A, MINPP1 and AKT2. Antibody binding appears as brown/black staining on a background of blue haematoxylin counterstain. All images are from the Human Protein Atlas
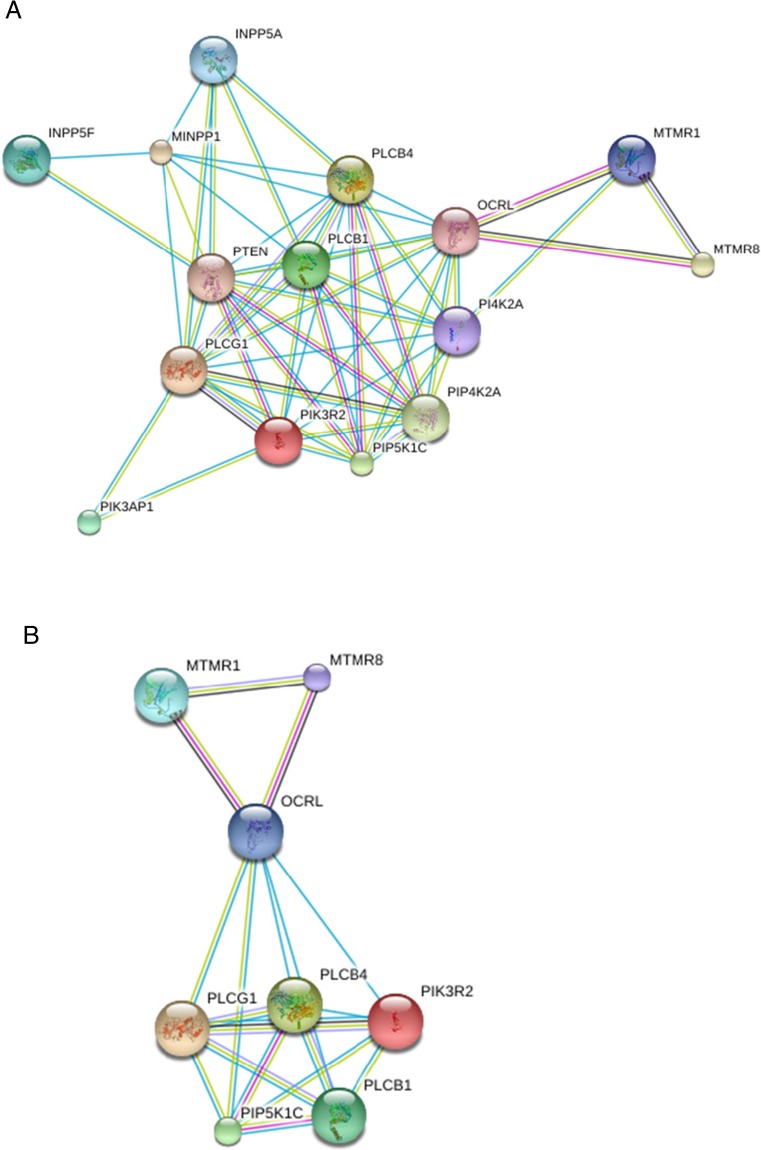
The functional relationships between the most commonly affected phosphoinositide pathway gene products, defined as copy number variation in ≥30 % of samples, were assessed using freely available STRING software (Fig. 3). Using this approach it was established that all of the identified gene products are highly connected at the protein interaction level. Hence, a deletion or amplification of any one element in this putative pathway has the potential to reset the entire tumour-specific network of phosphoinositide-metabolising enzymes. To illustrate this scenario, STRING analysis is also shown for a tumour cell with a homozygous chromosome 10p deletion in order to show how the phosphoinositide metabolic network in glioblastoma could reorganise when a subset of gene products are lost through a gross karyotypic change. The majority of the affected gene products identified are involved in lipid dephosphorylation and hydrolysis, indicating that in glioblastoma, changes to phosphoinositide metabolism occur mainly via defective catabolism as opposed to altered synthesis. However, the degree to which the balance between synthesis and degradation is deregulated depends very much on the pattern of chromosomal instability in each tumour, thus leading to different permutations of phosphoinositide signalling and trafficking outputs contingent on which subset of genes is affected. These findings have implications for our understanding of drug resistance and target selection, particularly when it comes to chemotherapeutics aimed at inhibiting the PI3K/AKT/mTOR pathway [85–87]. Rather than focusing on a single enzyme, a more productive approach in cancer therapeutics could be to map the overall landscape of phosphoinositide pathway mutations present and to consider how these relate to the chromosomal structural abnormalities prevalent in aggressive cancers such as glioblastoma multiforme. This strategy will enable the identification of functionally redundant and alternative biochemical pathways to drug resistance that result from gene copy number variation and tumour-specific chromosomal aberrations.
Fig. 3.
a STRING analysis illustrating the protein interaction network for the phosphoinositide pathway enzymes most commonly affected by gene copy number variation in glioblastoma multiforme. b Diagram illustrating how the protein interaction network would reset following a chromosome 10 deletion
Acknowledgments
The author acknowledges support from the Royal Free Charity and helpful comments from Shane Minogue and David Brown.
Conflict of Interest
The author declares no conflict of interest.
Abbreviations
- IP3
Inositol(1,4,5)-tris-phosphate (IP3)
- PI
Phosphatidylinositol
- PI3K
Phosphoinositide 3-kinase
- TGN
trans-Golgi network
References
- 1.Kim H, et al. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci U S A. 2010;107(5):2183–2188. doi: 10.1073/pnas.0909896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YW, et al. Identification of prognostic gene signatures of glioblastoma: a study based on TCGA data analysis. Neuro-Oncol. 2013;15(7):829–839. doi: 10.1093/neuonc/not024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research, N Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frattini V, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45(10):1141–1149. doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Y, et al. Network analysis of genomic alteration profiles reveals co-altered functional modules and driver genes for glioblastoma. Mol Biosyst. 2013;9(3):467–477. doi: 10.1039/c2mb25528f. [DOI] [PubMed] [Google Scholar]
- 6.Nigro JM, et al. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005;65(5):1678–1686. doi: 10.1158/0008-5472.CAN-04-2921. [DOI] [PubMed] [Google Scholar]
- 7.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SK, et al. A survey of glioblastoma genomic amplifications and deletions. J Neurooncol. 2010;96(2):169–179. doi: 10.1007/s11060-009-9959-4. [DOI] [PubMed] [Google Scholar]
- 9.Reifenberger, G., et al., Molecular characterization of long-term survivors of glioblastoma using genome- and transcriptome-wide profiling. Int J Cancer, 2014 [DOI] [PubMed]
- 10.Roversi G, et al. Identification of novel genomic markers related to progression to glioblastoma through genomic profiling of 25 primary glioma cell lines. Oncogene. 2006;25(10):1571–1583. doi: 10.1038/sj.onc.1209177. [DOI] [PubMed] [Google Scholar]
- 11.Sintupisut N, Liu PL, Yeang CH. An integrative characterization of recurrent molecular aberrations in glioblastoma genomes. Nucleic Acids Res. 2013;41(19):8803–8821. doi: 10.1093/nar/gkt656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sottoriva A, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhaak RG, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton EL, Minogue S, Waugh MG. Mammalian phosphatidylinositol 4-kinases as modulators of membrane trafficking and lipid signaling networks. Prog Lipid Res. 2013;52(3):294–304. doi: 10.1016/j.plipres.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waugh MG. Raft-like membranes from the trans-Golgi network and endosomal compartments. Nat Protoc. 2013;8(12):2429–2439. doi: 10.1038/nprot.2013.148. [DOI] [PubMed] [Google Scholar]
- 16.Waugh MG, et al. Detergent-free isolation and characterization of cholesterol-rich membrane domains from trans-Golgi network vesicles. J Lipid Res. 2011;52(3):582–589. doi: 10.1194/jlr.D012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waugh MG, et al. Localization of a highly active pool of type II phosphatidylinositol 4-kinase in a p97/valosin-containing-protein-rich fraction of the endoplasmic reticulum. Biochem J. 2003;373(Pt 1):57–63. doi: 10.1042/bj20030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waugh MG, et al. Identification and characterization of differentially active pools of type IIalpha phosphatidylinositol 4-kinase activity in unstimulated A431 cells. Biochem J. 2003;376(Pt 2):497–503. doi: 10.1042/bj20031212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waugh MG, et al. Lipid and peptide control of phosphatidylinositol 4-kinase IIalpha activity on Golgi-endosomal rafts. J Biol Chem. 2006;281(7):3757–3763. doi: 10.1074/jbc.M506527200. [DOI] [PubMed] [Google Scholar]
- 20.Minogue S, et al. Relationship between phosphatidylinositol 4-phosphate synthesis, membrane organization, and lateral diffusion of PI4KIIalpha at the trans-Golgi network. J Lipid Res. 2010;51(8):2314–2324. doi: 10.1194/jlr.M005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barylko B, et al. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J Biol Chem. 2001;276(11):7705–7708. doi: 10.1074/jbc.C000861200. [DOI] [PubMed] [Google Scholar]
- 22.Barylko B, et al. Palmitoylation controls the catalytic activity and subcellular distribution of phosphatidylinositol 4-kinase II{alpha} J Biol Chem. 2009;284(15):9994–10003. doi: 10.1074/jbc.M900724200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YJ, et al. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114(3):299–310. doi: 10.1016/S0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, et al. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol Biol Cell. 2007;18(7):2646–2655. doi: 10.1091/mbc.E06-10-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craige B, Salazar G, Faundez V (2008) Phosphatidylinositol-4-kinase type IIalpha contains an AP-3 sorting motif and a kinase domain that are both required for endosome traffic. Mol Biol Cell [DOI] [PMC free article] [PubMed]
- 26.Pan W, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321(5894):1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Y, et al. Regulation of phosphatidylinositol kinases and metabolism by Wnt3a and Dvl. J Biol Chem. 2009;284(34):22544–22548. doi: 10.1074/jbc.M109.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mossinger J, et al. (2012) Phosphatidylinositol 4-kinase IIalpha function at endosomes is regulated by the ubiquitin ligase Itch. EMBO Rep [DOI] [PMC free article] [PubMed]
- 29.Simons JP, et al. Loss of phosphatidylinositol 4-kinase 2alpha activity causes late onset degeneration of spinal cord axons. Proc Natl Acad Sci U S A. 2009;106(28):11535–11539. doi: 10.1073/pnas.0903011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minogue S, et al. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J Cell Sci. 2006;119(Pt 3):571–581. doi: 10.1242/jcs.02752. [DOI] [PubMed] [Google Scholar]
- 31.Chu KM, et al. Differential effects of the phosphatidylinositol 4-kinases, PI4KIIalpha and PI4KIIIbeta, on Akt activation and apoptosis. Cell Death Dis. 2010;1:e106. doi: 10.1038/cddis.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, et al. Dual inhibition of EGFR at protein and activity level via combinatorial blocking of PI4KIIalpha as anti-tumor strategy. Protein Cell. 2014;5(6):457–468. doi: 10.1007/s13238-014-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, et al. PI4KIIalpha is a novel regulator of tumor growth by its action on angiogenesis and HIF-1alpha regulation. Oncogene. 2010;29(17):2550–2559. doi: 10.1038/onc.2010.14. [DOI] [PubMed] [Google Scholar]
- 34.Brennan CW, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrasco-Garcia E, Saceda M, Martinez-Lacaci I. Role of receptor tyrosine kinases and their ligands in glioblastoma. Cell. 2014;3(2):199–235. doi: 10.3390/cells3020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francis JM, et al. (2014) EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov [DOI] [PMC free article] [PubMed]
- 37.Kalman B, et al. Epidermal growth factor receptor as a therapeutic target in glioblastoma. Neuromol Med. 2013;15(2):420–434. doi: 10.1007/s12017-013-8229-y. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, et al. Diverse mechanisms of somatic structural variations in human cancer genomes. Cell. 2013;153(4):919–929. doi: 10.1016/j.cell.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, et al. A mechanism for the upregulation of EGF receptor levels in glioblastomas. Cell Rep. 2013;3(6):2008–2020. doi: 10.1016/j.celrep.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenton TR, et al. Resistance to EGF receptor inhibitors in glioblastoma mediated by phosphorylation of the PTEN tumor suppressor at tyrosine 240. Proc Natl Acad Sci U S A. 2012;109(35):14164–14169. doi: 10.1073/pnas.1211962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appin CL, Brat DJ. Molecular genetics of gliomas. Cancer J. 2014;20(1):66–72. doi: 10.1097/PPO.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 42.Arslantas A, et al. The importance of genomic copy number changes in the prognosis of glioblastoma multiforme. Neurosurg Rev. 2004;27(1):58–64. doi: 10.1007/s10143-003-0279-4. [DOI] [PubMed] [Google Scholar]
- 43.Wang SI, et al. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 1997;57(19):4183–4186. [PubMed] [Google Scholar]
- 44.Lino MM, Merlo A. PI3Kinase signaling in glioblastoma. J Neurooncol. 2011;103(3):417–427. doi: 10.1007/s11060-010-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knobbe CB, Reifenberger G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3'-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003;13(4):507–518. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 47.Luchman HA, et al. (2014) Dual mTORC1/2 blockade inhibits glioblastoma brain tumor initiating cells in vitro and in vivo and synergizes with temozolomide to increase orthotopic xenograft survival. Clin Cancer Res [DOI] [PubMed]
- 48.Fraser MM, et al. PTEN loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64(21):7773–7779. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 49.Forbes SA et al. (2008) The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum Genet. Chapter 10: p. Unit 10 11 [DOI] [PMC free article] [PubMed]
- 50.Forbes SA, et al. COSMIC: mining complete cancer genomes in the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2011;39(Database issue):D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Loo P, et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci U S A. 2010;107(39):16910–16915. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ponten F, et al. The human protein atlas as a proteomic resource for biomarker discovery. J Intern Med. 2011;270(5):428–446. doi: 10.1111/j.1365-2796.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- 53.Uhlen M, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol. 2010;28(12):1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 54.Jensen LJ, et al. STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37(Database issue):D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardina PJ et al (2008) Ploidy status and copy number aberrations in primary glioblastomas defined by integrated analysis of allelic ratios, signal ratios and loss of heterozygosity using 500 K SNP Mapping Arrays. BMC Genomics 9:489 [DOI] [PMC free article] [PubMed]
- 56.Jesionek-Kupnicka D, et al. Association of loss of heterozygosity with shorter survival in primary glioblastoma patients. Pol J Pathol. 2013;64(4):268–275. doi: 10.5114/pjp.2013.39335. [DOI] [PubMed] [Google Scholar]
- 57.Mao X, Hamoudi RA. Molecular and cytogenetic analysis of glioblastoma multiforme. Cancer Genet Cytogenet. 2000;122(2):87–92. doi: 10.1016/S0165-4608(00)00278-8. [DOI] [PubMed] [Google Scholar]
- 58.Guerrero-Preston R, et al. Key tumor suppressor genes inactivated by “greater promoter” methylation and somatic mutations in head and neck cancer. Epigenetics. 2014;9(7):1031–1046. doi: 10.4161/epi.29025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koutros S, et al. Pooled analysis of phosphatidylinositol 3-kinase pathway variants and risk of prostate cancer. Cancer Res. 2010;70(6):2389–2396. doi: 10.1158/0008-5472.CAN-09-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Safavi S et al. (2014) Novel gene targets detected by genomic profiling in a consecutive series of 126 adult acute lymphoblastic leukemia cases. Haematologica [DOI] [PMC free article] [PubMed]
- 61.Ni M, et al. B-cell adaptor for PI3K (BCAP) negatively regulates toll-like receptor signaling through activation of PI3K. Proc Natl Acad Sci U S A. 2012;109(1):267–272. doi: 10.1073/pnas.1111957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okada T, et al. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13(6):817–827. doi: 10.1016/S1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- 63.Troutman TD, et al. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci U S A. 2012;109(1):273–278. doi: 10.1073/pnas.1118579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bassi C, et al. Transcriptional changes in U343 MG—a glioblastoma cell line exposed to ionizing radiation. Hum Exp Toxicol. 2008;27(12):919–929. doi: 10.1177/0960327108102045. [DOI] [PubMed] [Google Scholar]
- 65.Kasumu AW, et al. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci. 2012;32(37):12786–12796. doi: 10.1523/JNEUROSCI.1643-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milinkovic V, et al. Identification of novel genetic alterations in samples of malignant glioma patients. PLoS One. 2013;8(12):e82108. doi: 10.1371/journal.pone.0082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekulic A, et al. Loss of inositol polyphosphate 5-phosphatase is an early event in development of cutaneous squamous cell carcinoma. Cancer Prev Res (Phila) 2010;3(10):1277–1283. doi: 10.1158/1940-6207.CAPR-10-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Windhorst S, et al. Inositol-1,4,5-trisphosphate 3-kinase A regulates dendritic morphology and shapes synaptic Ca2+ transients. Cell Signal. 2012;24(3):750–757. doi: 10.1016/j.cellsig.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Chi H, et al. Targeted deletion of MINPP1 provides new insight into the activity of multiple inositol polyphosphate phosphatase in vivo. Mol Cell Biol. 2000;20(17):6496–6507. doi: 10.1128/MCB.20.17.6496-6507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gimm O, et al. Somatic mutation and germline variants of MINPP1, a phosphatase gene located in proximity to PTEN on 10q23.3, in follicular thyroid carcinomas. J Clin Endocrinol Metab. 2001;86(4):1801–1805. doi: 10.1210/jcem.86.4.7419. [DOI] [PubMed] [Google Scholar]
- 71.Windhorst S, et al. Tumour cells can employ extracellular Ins(1,2,3,4,5,6)P(6) and multiple inositol-polyphosphate phosphatase 1 (MINPP1) dephosphorylation to improve their proliferation. Biochem J. 2013;450(1):115–125. doi: 10.1042/BJ20121524. [DOI] [PubMed] [Google Scholar]
- 72.Wundenberg T, et al. Discovery of InsP6-kinases as InsP6-dephosphorylating enzymes provides a new mechanism of cytosolic InsP6 degradation driven by the cellular ATP/ADP ratio. Biochem J. 2014;462(1):173–184. doi: 10.1042/BJ20130992. [DOI] [PubMed] [Google Scholar]
- 73.Clayton EL, Minogue S, Waugh MG. Phosphatidylinositol 4-kinases and PI4P metabolism in the nervous system: roles in psychiatric and neurological diseases. Mol Neurobiol. 2013;47(1):361–372. doi: 10.1007/s12035-012-8358-6. [DOI] [PubMed] [Google Scholar]
- 74.Billcliff PG, Lowe M. Inositol lipid phosphatases in membrane trafficking and human disease. Biochem J. 2014;461(2):159–175. doi: 10.1042/BJ20140361. [DOI] [PubMed] [Google Scholar]
- 75.Cao C, et al. Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol Biol Cell. 2008;19(8):3334–3346. doi: 10.1091/mbc.E08-04-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schaletzky J, et al. Phosphatidylinositol-5-phosphate activation and conserved substrate specificity of the myotubularin phosphatidylinositol 3-phosphatases. Curr Biol. 2003;13(6):504–509. doi: 10.1016/S0960-9822(03)00132-5. [DOI] [PubMed] [Google Scholar]
- 77.Blondeau F, et al. Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway. Hum Mol Genet. 2000;9(15):2223–2229. doi: 10.1093/oxfordjournals.hmg.a018913. [DOI] [PubMed] [Google Scholar]
- 78.Zou J, et al. Myotubularin-related protein (MTMR) 9 determines the enzymatic activity, substrate specificity, and role in autophagy of MTMR8. Proc Natl Acad Sci U S A. 2012;109(24):9539–9544. doi: 10.1073/pnas.1207021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vicinanza M, et al. OCRL controls trafficking through early endosomes via PtdIns4,5P(2)-dependent regulation of endosomal actin. EMBO J. 2011;30(24):4970–4985. doi: 10.1038/emboj.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nandez R, et al. A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. Elife. 2014;3:e02975. doi: 10.7554/eLife.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mehta ZB, Pietka G, Lowe M. The cellular and physiological functions of the Lowe syndrome protein OCRL1. Traffic. 2014;15(5):471–487. doi: 10.1111/tra.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choudhury R, et al. Differential clathrin binding and subcellular localization of OCRL1 splice isoforms. J Biol Chem. 2009;284(15):9965–9973. doi: 10.1074/jbc.M807442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Qusairi L, et al. Lack of myotubularin (MTM1) leads to muscle hypotrophy through unbalanced regulation of the autophagy and ubiquitin-proteasome pathways. FASEB J. 2013;27(8):3384–3394. doi: 10.1096/fj.12-220947. [DOI] [PubMed] [Google Scholar]
- 84.Fetalvero KM, et al. Defective autophagy and mTORC1 signaling in myotubularin null mice. Mol Cell Biol. 2013;33(1):98–110. doi: 10.1128/MCB.01075-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Juric D, et al. (2014) Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature [DOI] [PMC free article] [PubMed]
- 86.Markman B, Tao JJ, Scaltriti M. PI3K pathway inhibitors: better not left alone. Curr Pharm Des. 2013;19(5):895–906. doi: 10.2174/138161213804547213. [DOI] [PubMed] [Google Scholar]
- 87.Fan QW, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9(5):341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soroceanu L, et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A. 2007;104(9):3466–3471. doi: 10.1073/pnas.0611271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Endersby R, et al. Nonredundant functions for Akt isoforms in astrocyte growth and gliomagenesis in an orthotopic transplantation model. Cancer Res. 2011;71(12):4106–4116. doi: 10.1158/0008-5472.CAN-10-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nobusawa S, et al. Intratumoral patterns of genomic imbalance in glioblastomas. Brain Pathol. 2010;20(5):936–944. doi: 10.1111/j.1750-3639.2010.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riemenschneider MJ, Knobbe CB, Reifenberger G. Refined mapping of 1q32 amplicons in malignant gliomas confirms MDM4 as the main amplification target. Int J Cancer. 2003;104(6):752–757. doi: 10.1002/ijc.11023. [DOI] [PubMed] [Google Scholar]
- 92.Chen H, et al. PTEN restoration and PIK3CB knockdown synergistically suppress glioblastoma growth in vitro and in xenografts. J Neurooncol. 2011;104(1):155–167. doi: 10.1007/s11060-010-0492-2. [DOI] [PubMed] [Google Scholar]
- 93.Hui AB, et al. Detection of multiple gene amplifications in glioblastoma multiforme using array-based comparative genomic hybridization. Lab Investig. 2001;81(5):717–723. doi: 10.1038/labinvest.3780280. [DOI] [PubMed] [Google Scholar]
- 94.Mizoguchi M, et al. Genetic alterations of phosphoinositide 3-kinase subunit genes in human glioblastomas. Brain Pathol. 2004;14(4):372–377. doi: 10.1111/j.1750-3639.2004.tb00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dellinger AE, Nixon AB, Pang H. Integrative pathway analysis using graph-based learning with applications to TCGA colon and ovarian data. Cancer Informat. 2014;13(Suppl 4):1–9. doi: 10.4137/CIN.S13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang G, Kang C, Pu P. Increased expression of Akt2 and activity of PI3K and cell proliferation with the ascending of tumor grade of human gliomas. Clin Neurol Neurosurg. 2010;112(4):324–327. doi: 10.1016/j.clineuro.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Serao NV, et al. Cell cycle and aging, morphogenesis, and response to stimuli genes are individualized biomarkers of glioblastoma progression and survival. BMC Med Genomics. 2011;4:49. doi: 10.1186/1755-8794-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jamshidi N, et al. Illuminating radiogenomic characteristics of glioblastoma multiforme through integration of MR imaging, messenger RNA expression, and DNA copy number variation. Radiology. 2014;270(1):1–2. doi: 10.1148/radiol.13130078. [DOI] [PMC free article] [PubMed] [Google Scholar]