Abstract
Background
Assist/control (A/C) ventilation may induce delirium in patients with acute respiratory distress syndrome (ARDS). We conducted a trial to determine whether initial synchronized intermittent mandatory ventilation with pressure support (SIMV + PS) could improve clinical outcomes in these patients.
Methods
Intubated patients with moderate ARDS were enrolled and we compared SIMV + PS with A/C. Identical sedation, analgesia and ventilation strategies were performed. The co-primary outcomes were early (≤72 h) partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) and incidence of delirium. The secondary outcomes were all-cause in-hospital mortality, dosages of analgesics and sedatives, incidence of patient-ventilator asynchrony, and duration of mechanical ventilation and hospital stay.
Results
We screened 2,684 patients and 40 patients were enrolled in our study. In SIMV + PS, early (≤72 h) PaO2/FiO2 was greater improved than that at baseline and that in A/C (P<0.05) with lower positive end-expiratory pressure (PEEP) (8.7±3.0 vs. 10.3±3.2, P<0.001) and FiO2 (58%±18% vs. 67%±19%, P<0.001). We found more SIMV + PS success (defined as SIMV + PS successfully applied without switching to A/C) (100.0% vs. 16.7%, P<0.001), less male (46.3% vs. 85.7%, P=0.015) and pulmonary etiology of ARDS (53.8% vs. 92.9%, P=0.015), and lower PEEP (9.1±3.1 vs. 10.3±3.3, P=0.004) and FiO2 (58%±19% vs. 71%±19%, P<0.001) in survival patients. However, there were no significant differences in incidence of delirium and mortality, dosages of analgesics and sedatives, incidence of patient-ventilator asynchrony, duration of mechanical ventilation and hospital stay (P>0.05).
Conclusions
In patients with moderate ARDS, SIMV + PS can safely and effectively improve oxygenation, but does not decrease mortality, incidence of delirium and patient-ventilator asynchrony, dosages of analgesics and sedatives, and duration of mechanical ventilation and hospital stay.
Keywords: Respiratory distress syndrome, adult, respiration, artificial, delirium, mortality
Introduction
Analgesics and sedatives are important treatment components for mechanically ventilated patients to relieve pain and anxiety, decrease work of breathing and oxygen consumption, as well as improve patient-ventilator synchrony (1). However, these medications may have unpredictable effects with long-term use, such as delirium, which is a form of brain dysfunction characterized by an acute onset of disturbance in cognitive abilities with a fluctuating course over time (2). Studies have demonstrated that delirium is an independent predictor of mortality in mechanically ventilated patients (3). Daily interruption of sedative infusions is a strategy to minimize sedation, however, two large randomized controlled trials resulted in contrary conclusions in duration of mechanical ventilation and length of intensive care unit (ICU) stay (4,5).
Acute respiratory distress syndrome (ARDS) is a type of acute diffuse, inflammatory lung injury, which results in 16.1–33.3% of patients necessitating invasive mechanical ventilation (6,7), and the mortality rate as high as 35% (8-10). It has been reported that up to 70% of survivors of ARDS develop new cognitive, physical, and functional impairments during their critical illness (11-13). Assist/control (A/C) ventilation and synchronized intermittent mandatory ventilation with pressure support (SIMV + PS) are the most common modes of mechanical ventilation in ARDS (14). Ortiz and his colleagues conducted a secondary analysis of an observational study in which they compared 350 patients ventilated with SIMV + PS with 1,228 patients ventilated with A/C ventilation (15). They found that there was a trend toward a lower sedation in SIMV + PS, but the difference for in-hospital mortality was not significant after adjustment for propensity score (odds ratio 1.04; 95% confidence interval, 0.77–1.42; P=0.78). However, the causes of ventilation in their study are diverse, including ARDS, congestive heart failure, asthma, trauma, and neuromuscular disease. Therefore, the effects of SIMV + PS on clinical outcomes in patients with ARDS are still unknown.
Based on our hypothesis that, in moderate ARDS, SIMV + PS could improve oxygenation, decrease use of analgesics and sedatives and incidence of delirium, as well as in-hospital mortality, we conducted a randomized controlled trial to determine the role of SIMV + PS in these patients.
Methods
Patients were recruited form April through December 2014 at three ICUs with 80 beds, including emergency ICU, general ICU, and respiratory ICU, in West China Hospital, Sichuan University. The research ethics board approved the protocol, and written informed consent was obtained from the legal substitute decision makers for each patient. A complete description of the methods is available on the web of Chinese Clinical Trial Registry [http://www.chictr.org/cn/, registration number: ChiCTR-TRC-14004163; universal trial number (UTN): U1111-1152-2390].
Patients
Patients who were intubated and received invasive mechanical ventilation within 24 hours after enrollment were eligible if they met the criteria of moderate ARDS defined by the onset of new respiratory symptoms within a week, bilateral opacities on chest radiograph or computed tomography that could not fully explained by effusions, lobar/lung collapse, or nodules, and pulmonary edema not fully explained by cardiac failure or fluid overload, with 100 mmHg < partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ≤200 mmHg with positive end-expiratory pressure (PEEP) ≥5 cmH2O (16).
We excluded patients with pregnancy; age <18 years old; severe arrhythmia or acute myocardial ischemia; pneumothorax or mediastinal emphysema; intracranial hypertension; neuromuscular diseases that could impair spontaneous breathing; severe chronic obstructive pulmonary disease; severe multiple organs dysfunction (as defined by Marshall score ≥3); end-stage malignant carcinoma with an estimated 6-month mortality risk exceeding 50%; sickle cell disease; immunosuppression conditions, that is, neutrophil count less than 500/mm3 for more than 10 days, transplantation of allogeneic stem cells or solid organs, long-term use of corticosteroids (prednisone ≥0.3 mg/kg or other equivalent drugs for more than 3 weeks), or use of suppressors for T lymphocyte within the past 90 days; attending confounding trials within 30 days before enrollment; unwilling or refusing the use of full life support.
Study design and randomization
The study was a prospective, single-centered, randomized controlled trial. Based on the computer-generated concealed randomization by SPSS 16.0 [Copyright (c) SPSS Inc. 1989–2007], patients were randomly assigned to receive mechanical ventilation with either SIMV + PS or A/C ventilation mode within 24 hours after intubation. After randomization, participants in each group were initialed by the corresponding ventilation mode, and identical ventilator and sedation strategies, as well as comprehensive intensive care were executed in both groups.
Ventilator procedures and analgesia and sedation strategies
“Lung protective ventilation” and “Open-lung approach” were performed in both study groups using Puritan Bennet 840 ventilators (Part No. 4-070088-00, Rev. F, October 2006) (9,10). Volume-controlled mode was used and target tidal volume (VT) was 6 mL/kg of predicted body weight with allowances for 4 to 8 mL/kg with plateau airway pressures not exceeding 30 cmH2O. Recruitment maneuver was performed 4 times per day by holding breath for 30 seconds at an airway pressure of 40 cmH2O and a FiO2 of 1.0, and after that PEEP was adjusted by FiO2 to meet the PaO2 ≥55 mmHg or oxygen saturation of pulse oximetry (SpO2) ≥88%. Ventilation rate was set to maintain the patients’ respiratory rate not exceeding 35 breaths per minute and preserve spontaneous breaths in patients with SIMV + PS. A pH goal between 7.30 and 7.45 in arterial blood gas analysis was achieved, and permissive hypercapnia was allowed with an arterial pH of not less than 7.15. We set the time or flow velocity of inspiration to keep the ratio of inspiration to expiration between 1:1 and 1:3, and the flow of 2 L/min to trigger the inspiration of ventilator.
SIMV + PS was switched to A/C, defined as SIMV + PS failure, if the patients presented progressive dyspnea with overt agitation or thoracoabdominal motion, or two consecutive arterial blood gas analyses indicated PaO2/FiO2 ≤100 mmHg. The weaning protocol was conduced according to current evidence-based guidelines including daily assessment of patients’ readiness to undergo the spontaneous breathing trial (17).
Identical analgesia and sedation strategies, supported by current recommendations, were implemented in both groups (2,18,19). On the basis of analgesia by fentanyl, we sedated the patients with midazolam and (or) propofol to meet the goal of Richmond Agitation-Sedation Scale (RASS) between −2 and −4. The initial intravenous bolus and the continuous infusion of fentanyl, midazolam, and propofol was 0.7–11.5 µg/kg, 0.03–0.3 mg/kg, 1–3 mg/kg, and 1–2 µg/kg/h, 1–2 mg/h, 5 µg/kg/min, respectively. Daily interruption of sedative infusions was conducted at 8:00 every morning to wake the patients using the standard criteria (18).
Data collection and outcome measures
Data on demographic and physiologic characteristics were recorded within 24 hours preceding randomization. Based on the CONSORT statement (www.consort-statement.org), three blinded independent researchers in the three ICUs separately recorded vital signs, ABG analysis, delirium incidence, as well as ventilator associated events after randomization at 2, 12, 24, 36, 48, 72 h, and on day 4 through day 28 in the pre-designed case report forms. We followed all patients up to 28 days or to the time of hospital discharge.
The co-primary end points were early (≤72 h) PaO2/FiO2 and incidence of delirium. Early PaO2/FiO2 was defined as the oxygenation within 72 hours after randomization and initiation of either SIMV + PS or A/C. We divided 6 time intervals within the first 72 hours, that is 2, 12, 24, 36, 48 and 72 h, and FiO2 provided by the ventilator and PaO2 detected by the arterial blood gas analysis were collected synchronously, thus calculating the oxygenation (PaO2/FiO2) for each time point. Delirium was defined as RASS of −3 to +4 and positive Confusion Assessment Method for Intensive Care Unit assessment (3,19). The secondary outcomes included all-cause in-hospital mortality, dosages of analgesics and sedatives, incidence of patient-ventilator asynchrony, duration of mechanical ventilation and hospital stay. Patients who were discharged to an alternative level of care facility were classified as alive at discharge. Patient-ventilator asynchrony was detected by visual inspection of the recordings using flow and airway pressure signals on ventilators (20). We measured five patterns of major asynchrony that were easily detected, including ineffective triggering, double-triggering, auto-triggering, short cycle, and prolonged cycle.
Statistical analysis
The primary and secondary outcomes were analyzed as intention-to-treat. An independent statistician, blinded to the randomization, conducted the statistical analysis.
Consecutive variables were reported as mean ± standard deviation, while categorical variables were reported as frequency and proportion. For primary and secondary outcomes, priori analyses by student’s t-test and Chi-square test or Fisher’s exact test were conducted correspondingly. Student’s t-test was applied to compare PaO2/FiO2 between each observational time point and baseline as well as between both treatment groups, so did in dosages of analgesics and sedatives. Chi-square test or Fisher’s exact test was used to compare incidence of delirium and patient-ventilator asynchrony, and mortality.
With the purpose of excluding the effects of ARDS etiologies on the clinical outcomes of SIMV + PS, we divided the participants into groups of pulmonary and extra-pulmonary etiology, and a post hoc analysis using student’s t-test was conduced to evaluate the primary and second outcomes. Similarly, we divided the enrolled patients into survivors and non-survivors as well as SIMV + PS success and failure to explore the potential factors associated with the mortality and the successful application of SIMV + PS in ventilated patients with moderate ARDS.
All analyses were performed using SPSS 16.0 [Copyright (c) SPSS Inc. 1989–2007], and all P values were two-sided with a significance level of α<0.05.
Results
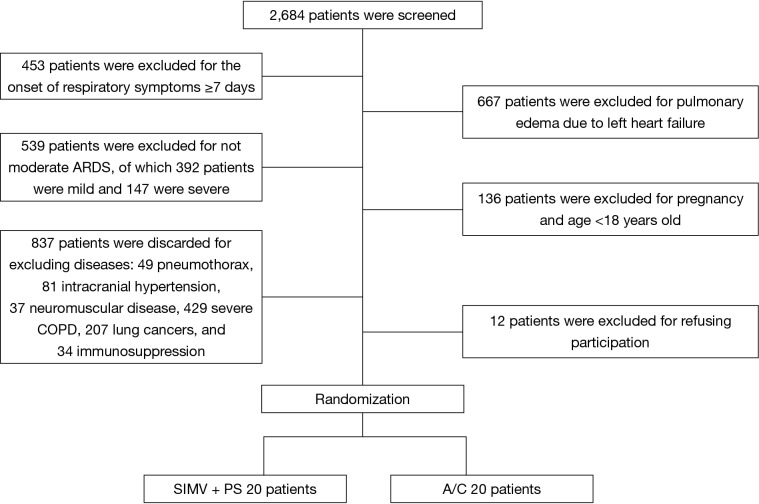
We screened 2,684 patients and finally enrolled 40 eligible patients with ARDS (20 patients in each study group) (Figure 1).
Figure 1.
Study flow diagram. ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; SIMV, synchronized intermittent mandatory ventilation; PS, pressure support; A/C, assist/control.
Baseline characteristics
Baseline characteristics were summarized in Table 1. The mean age and proportion of male patients in SIMV + PS and A/C groups were 55.5 vs. 53.6 and 70.0% vs. 50.0%, respectively. There were no significant differences in acute physiology, age, chronic health evaluation II (APACHE II) (18.4±4.6 vs. 17.7±6.6, P=0.699) and Marshall (1.2±0.8 vs. 1.1±0.8, P=0.571) score, as well as baseline PaO2/FiO2 (137.5±26.8 vs. 124.6±22.6, P=0.107) between the two groups. We did not find significant differences in etiologies of ARDS and inflammatory measures such as white blood cell count (WBC), percentage of neutrophils, and procalcitonin.
Table 1. Baseline characteristics of patients in SIMV + PS and A/C.
| Characteristics | SIMV + PS (n=20) | A/C (n=20) |
|---|---|---|
| Sex (male, %) | 14 (70.0) | 10 (50.0) |
| Age (y) | 55.5±15.9 | 53.6±16.7 |
| Height (cm) | 161.2±5.1 | 163.2±5.0 |
| Weight (kg) | 60.4±8.1 | 62.7±7.9 |
| APACHE II | 18.4±4.6 | 17.7±6.6 |
| Marshall | 1.2±0.8 | 1.1±0.8 |
| RASS | −2.68±1.250 | −2.84±0.958 |
| VT (mL/kg) | 6.9±0.5 | 7.1±0.7 |
| T (°C) | 37.4±1.0 | 37.7±0.7 |
| RR (bpm) | 21.8±5.4 | 21.3±7.6 |
| HR (bpm) | 105.3±16.0 | 112.0±27.7 |
| SBP (mmHg) | 119.1±20.9 | 116.8±23.9 |
| Laboratory measures | ||
| pH | 7.38±0.07 | 7.39±0.10 |
| PaO2/FiO2 | 137.5±26.8 | 124.6±22.6 |
| PaCO2 (mmHg) | 40.5±10.0 | 41.1±13.0 |
| HCO3− (mmol/L) | 23.2±5.0 | 24.7±5.3 |
| Hb (g/L) | 98.5±21.0 | 115.0±18.4 |
| WBC (cell/L) | 17.13±8.22 | 14.18±6.76 |
| N (%) | 88.8±6.7 | 83.4±15.9 |
| ALB (g/L) | 27.0±5.3 | 28.7±4.1 |
| BUN (mmol/L) | 11.24±8.36 | 9.01±5.22 |
| Cr (umol/L) | 98.78±72.81 | 100.40±58.71 |
| Na+ (mmol/L) | 140.0±4.9 | 139.6±6.7 |
| K+ (mmol/L) | 4.2±0.7 | 3.9±0.4 |
| PCT (ng/mL) | 2.51±2.10 | 3.08±3.51 |
| Etiology | ||
| Pulmonary (%) | 14 (70.0) | 13 (65.0) |
| Pneumonia | 13 (65.0) | 11 (55.0) |
| Pulmonary contusion | 1 (5.0) | 1 (5.0) |
| Drowning | 0 (0.0) | 1 (5.0) |
| Extra-pulmonary (%) | 6 (30.0) | 7 (35.0) |
| SAP | 3 (15.0) | 6 (30.0) |
| Sepsis | 3 (15.0) | 1 (5.0) |
SIMV, synchronized intermittent mandatory ventilation; PS, pressure supportive; A/C, assist/control; APACHE II, acute physiology, age, chronic health evaluation; RASS, Richmond Agitation-Sedation Scale; VT, tidal volume; T, temperature; RR, respiratory rate; HR, heart rate; SBP, systolic blood pressure; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; PaCO2, arterial partial pressure of carbon dioxide; HCO3−, bicarbonate ion; Hb, hemoglobin; WBC, white blood cell count; N%, percentage of neutrophil; ALB, albumin; BUN, blood urea nitrogen; Cr, creatinine; Na+, sodium ion; K+, potassium ion; PCT, procalcitonin; SAP, severe acute pancreatitis.
Oxygenation
Figure 2 and Table 2 showed the early PaO2/FiO2 of patients in SIMV + PS and A/C groups. After intubation and receiving mechanical ventilation, the PaO2/FiO2 in two groups presented an increasing trend. In SIMV + PS group, the PaO2/FiO2 at each observational time point was significantly greater than that at baseline, while in A/C group, we did not find the similar change. In the same time, PaO2/FiO2 at each observational time point in SIMV + PSV group was significantly greater than that in A/C group. However, we found significantly lower PEEP (8.7±3.0 vs. 10.3±3.2, P<0.001) and FiO2 (58%±18% vs. 68%±20%, P<0.001) in SIMV + PS group in Table 3, but no significant differences in VT (7.0±0.5 vs. 6.9±0.8, P=0.489) and RASS score (−2.4±1.6 vs. −2.6±1.3, P=0.128) during the overall mechanical ventilation.
Figure 2.
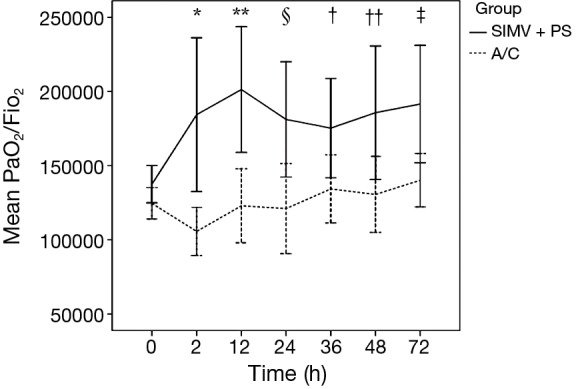
Early oxygenation (PaO2/FiO2) in SIMV + PS and A/C. Early (≤72 h) oxygenation (PaO2/FiO2) at each time point in SIMV + PS group and A/C group were compared. Solid line in black represented oxygenation (mean ± SD) in SIMV + PS group, while dashed line in black represented oxygenation (mean ± SD) in A/C group. SIMV + PS vs. A/C: *, 2 h, P=0.012; **, 12 h, P=0.002; §, 24 h, P=0.019; †, 36 h, P=0.038; ††, 48 h, P=0.042; ‡, 72 h, P=0.023. PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; SIMV, synchronized intermittent mandatory ventilation; PS, pressure supportive; A/C, assist/control.
Table 2. Early oxygenation (PaO2/FiO2) in SIMV + PS and A/C.
| Time | SIMV + PS (mmHg) | A/C (mmHg) | P |
|---|---|---|---|
| 0 h | 137.5±26.8 | 124.6±22.6 | 0.107 |
| 2 h | 184.4±93.6* | 105.7±24.2∫ | 0.012 |
| 12 h | 201.3±82.4** | 122.9±46.9∫∫ | 0.002 |
| 24 h | 181.2±78.1§ | 121.1±52.6∏ | 0.019 |
| 36 h | 175.3±65.2† | 134.3±46.2¢ | 0.038 |
| 48 h | 185.7±81.2†† | 130.6±40.3£ | 0.042 |
| 72 h | 191.5±76.9‡ | 140.2±32.4‖ | 0.023 |
SIMV + PS group: 0 h vs. *, 2 h, P=0.040; **, 12 h, P=0.002; §, 24 h, P=0.024; †, 36 h, P=0.023; ††, 48 h, P=0.018; ‡, 72 h, P=0.006. A/C group: 0 h vs. ∫, 2 h, P=0.038; ∫∫, 12 h, P=0.891; ∏, 24 h, P=0.792; ¢, 36 h, P=0.408; £, 48 h, P=0.591; ‖, 2 h, P=0.103. PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; SIMV, synchronized intermittent mandatory ventilation; PS, pressure supportive; A/C, assist/control.
Table 3. Dosages of sedatives, incidence of delirium and patient-ventilator asynchrony, and mortality in SIMV + PS and A/C.
| Variables | SIMV + PS (n=20) | A/C (n=20) | P |
|---|---|---|---|
| Ventilator parameters | |||
| VT (mL/kg) | 7.0±0.5 | 6.9±0.8 | 0.489 |
| PEEP (cmH2O) | 8.7±3.0 | 10.3±3.2 | <0.001 |
| FiO2 (%) | 58±18 | 68±20 | <0.001 |
| Dosages of analgesics and sedatives | |||
| RASS | 2.4±1.6 | 2.6±1.3 | 0.128 |
| Fentanyl (mg) | 11.6±7.2 | 14.6±12.3 | 0.344 |
| Midazolam (mg) | 637.5±626.6 | 1145.0±1281.0 | 0.120 |
| Propofol (mg) | 6161.5±5788.3 | 3979.0±4313.9 | 0.184 |
| Duration of MV (d) | 15.3±11.8 | 15.3±12.1 | 1.000 |
| Delerium (%) | 0 (0.0) | 4 (20.0) | 0.106 |
| Patient-ventilator asynchrony (events/person) | 1.3±1.8 | 1.9±2.8 | 0.460 |
| Patient-ventilator asynchrony/duration of MV | 0.11±0.13 | 0.16±0.25 | 0.414 |
| In-hospital deaths (%) | 6 (30.0) | 8 (40.0) | 0.507 |
| ICU stay (d) | 22.4±13.6 | 19.6±14.9 | 0.537 |
| In-hospital stay (d) | 32.3±20.6 | 28.2±22.3 | 0.554 |
| ICU cost (RMB) | 124,750.0±91,271.2 | 115,690.0±132,840.0 | 0.803 |
| In-hospital cost (RMB) | 149,030.0±110,496.0 | 129,000.0±134,046.0 | 0.609 |
SIMV, synchronized intermittent mandatory ventilation; PS, pressure supportive; A/C, assist/control; VT, tidal volume; PEEP, positive end-expiratory pressure; FiO2, fraction of inspired oxygen; RASS, Richmond Agitation-Sedation Scale; MV, mechanical ventilation; ICU, intensive care unit.
Delirium and second outcomes
No patient developed delirium in SIMV + PS group, while four patients developed delirium in A/C group, however, it did not show significant difference (Table 3). The dosages of fentanyl as well as midazolam and propofol were similar in both groups without significant differences (11.6±7.2 vs. 14.6±12.3, P=0.344; 637.5±626.6 vs. 1,145.0±1,281.0, P=0.120; 6,161.5±5,788.3 vs. 3,979.0±4,313.9, P=0.184; respectively). There was no significant difference in incidence of patient-ventilator asynchrony (1.3±1.8 vs. 1.9±2.8, P=0.460) even adjusted for the duration of mechanical ventilation (0.11±0.13 vs. 0.16±0.25, P=0.414). We did not find significant differences in duration of mechanical ventilation, as well as duration and cost of hospital stay.
Mortality
During our follow-up, 14 patients died and the overall mortality rate was 35%, of which 6 patients in SIMV + PS group while 8 patients in A/C group, but there was no significant difference (30.0% vs. 40.0%, P=0.507) (Table 3). In order to verify whether different causes of ARDS may affect the effects of ventilator modes on mortality, we divided the patients into groups of pulmonary etiology (n=27) and extra-pulmonary etiology (n=13). However, we did not find significant significance (11.1% vs. 53.8%, P=0.074; 0.0% vs. 14.3%, P=1.000; respectively) (Table 4).
Table 4. Subanalysis of SIMV + PS and A/C in pulmonary and extra-pulmonary ARDS.
| Variables | Pulmonary |
P | Extra-pulmonary |
P | ||
|---|---|---|---|---|---|---|
| SIMV + PS (n=14) | A/C (n=13) | SIMV + PS (n=6) | A/C (n=7) | |||
| Dosages of analgesics and sedatives | ||||||
| Fentanyl (mg) | 11.6±7.2 | 11.5±10.9 | 0.315 | 11.4±7.7 | 20.5±13.4 | 0.173 |
| Midazolam (mg) | 550.0±628.5 | 868.5±1,142.0 | 0.248 | 841.7±627.2 | 1,659.0±1,454.3 | 0.230 |
| Propofol (mg) | 7,396.4±6,344.5 | 4,896.2±4,255.5 | 0.101 | 3,280.0±2,902.4 | 2,275.7±4,183.8 | 0.631 |
| Duration of MV (d) | 14.9±11.4 | 13.2±10.3 | 0.897 | 16.2±13.7 | 19.0±15.1 | 0.732 |
| Delerium (%) | 0 (0.0) | 3 (23.1) | 0.098 | 0 (0.0) | 1 (14.3) | 1.000 |
| Patient-ventilator asynchrony (events/person) | 1.1±1.3 | 1.3±2.3 | 0.339 | 1.7±2.7 | 2.9±3.6 | 0.517 |
| Patient-ventilator asynchrony/duration of MV | 0.12±0.13 | 0.10±0.18 | 0.545 | 0.09±0.15 | 0.26±0.33 | 0.264 |
| In-hospital deaths (%) | 1 (11.1) | 7 (53.8) | 0.074 | 0 (0.0) | 1 (14.3) | 1.000 |
| ICU stay (d) | 17.4±10.5 | 16.3±15.0 | 0.346 | 34.2±13.2 | 25.7±13.6 | 0.281 |
| In-hospital stay (d) | 25.0±15.6 | 20.9±16.8 | 0.788 | 49.2±22.1 | 41.7±26.0 | 0.593 |
| ICU cost (RMB) | 88,059.0±62,243.1 | 72,492.0±62,498.3 | 0.659 | 210,350.0±94,989.0 | 195,910.0±191,096.0 | 0.870 |
| In-hospital cost (RMB) | 102,790.0±59,560.1 | 81,244.0±65,386.9 | 0.673 | 256,910.0±131,226.0 | 217,700.0±185,010.0 | 0.673 |
SIMV, synchronized intermittent mandatory ventilation; PS, pressure supportive; A/C, assist/control; ARDS, acute respiratory distress syndrome; MV, mechanical ventilation; ICU, intensive care unit.
We further divided the enrolled patients into groups of survivors (n=26) and non-survivors (n=14) to investigate the factors associated with mortality in patients with moderate ARDS. We found that, in survivor group, there were more patients with SIMV + PS success (100.0% vs. 16.7%, P<0.001), fewer patients in male (46.3% vs. 85.7%, P=0.015), less patients with pulmonary etiology of ARDS (53.8% vs. 92.9%, P=0.015), and lower PEEP (9.1±3.1 vs. 10.3±3.3, P=0.004) and FiO2 (58%±19% vs. 71%±19%, P<0.001) during mechanical ventilation compared with non-survivor group (Table 5).
Table 5. Baseline characteristics of patients in survivors and non-survivors.
| Characteristics | Survivors (n=26) | Non-survivors (n=14) | P |
|---|---|---|---|
| Ventilator mode (SIMV, %) | 14 (53.8) | 6 (42.9) | 0.507 |
| SIMV success (%) | 14 (100.0) | 1 (16.7) | <0.001 |
| Sex (male, %) | 12 (46.3) | 12 (85.7) | 0.015 |
| Age (y) | 51.8±16.2 | 59.6±15.4 | 0.149 |
| ARDS etiology (pulmonary, %) | 14 (53.8) | 13 (92.9) | 0.015 |
| APACHE II | 17.5±5.2 | 18.9±6.4 | 0.450 |
| Marshall | 1.0±0.8 | 1.4±0.9 | 0.087 |
| VT (mL/kg) | 7.0±0.7 | 7.0±0.5 | 0.761 |
| PEEP (cmH2O) | 9.1±3.1 | 10.3±3.3 | 0.004 |
| FiO2 (%) | 58±19 | 71±19 | <0.001 |
| PaO2/FiO2 | 135.1±27.6 | 123.5±19.2 | 0.172 |
| pH | 7.40±0.09 | 7.36±0.07 | 0.210 |
| PaCO2 (mmHg) | 38.9±9.1 | 44.1±14.7 | 0.176 |
| Hb (g/L) | 104.5±22.2 | 110.9±19.2 | 0.367 |
| N (%) | 88.3±6.6 | 82.0±18.6 | 0.123 |
| ALB (g/L) | 27.6±4.7 | 28.3±5.1 | 0.665 |
| PCT (ng/mL) | 3.43±3.08 | 11.47±1.92 | 0.173 |
| Delerium (%) | 4 (15.4) | 0 (0.0) | 0.278 |
| Patient-ventilator asynchrony/duration of MV | 0.18±0.22 | 0.06±0.12 | 0.068 |
SIMV, synchronized intermittent mandatory ventilation; ARDS, acute respiratory distress syndrome; APACHE II, acute physiology, age, chronic health evaluation; VT, tidal volume; PEEP, positive end-expiratory pressure; FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen; PaCO2, arterial partial pressure of carbon dioxide; MV, mechanical ventilation; Hb, hemoglobin; N%, percentage of neutrophil; ALB, albumin; PCT, procalcitonin.
Factors associated with SIMV + PS success
The result, in which SIMV + PS success was related to fewer deaths in moderate ARDS, directed our further investigation of factors associated with SIMV + PS success. Patients in SIMV + PS group were divided into SIMV + PS success (n=15) and failure (n=5) groups. Baseline characteristics of the patients in two groups were summarized in Table 6. APACHE II and Marshall score, baseline PaO2/FiO2, hemoglobin, WBC, albumin, and procalcitonin were not significantly different in the two groups. Compared with SIMV + PS failure, the dosages of analgesics and sedatives, incidence of delirium and patient-ventilator asynchrony, as well as duration and cost of hospital stay in SIMV + PS success did not show any significant differences. However, we found that the age (49.7±13.7 vs. 72.6±7.4, P=0.002) was significantly older and the mortality rate (6.7% vs. 100.0%, P<0.001) was significantly higher in SIMV + PS failure group than that in SIMV + PS success group.
Table 6. Baseline characteristics of patients in SIMV + PS success and failure.
| Characteristics | SIMV + PS success (n=15) | SIMV + PS failure (n=5) | P |
|---|---|---|---|
| Sex (male, %) | 9 (60.0) | 5 (35.7) | 0.260 |
| Age (y) | 49.7±13.7 | 72.6±7.4 | 0.002 |
| APACHE II | 17.6±3.6 | 20.6±7.0 | 0.219 |
| Marshall | 1.1±0.9 | 1.6±0.5 | 0.224 |
| Laboratory measures | |||
| pH | 7.39±0.08 | 7.36±0.05 | 0.476 |
| PaO2/FiO2 | 142.4±27.3 | 123.0±21.6 | 0.169 |
| PaCO2 (mmHg) | 38.0±7.1 | 47.9±14.5 | 0.053 |
| HCO3− (mmol/L) | 22.1±4.6 | 25.8±5.5 | 0.167 |
| Hb (g/L) | 95.5±22.9 | 107.4±11.2 | 0.286 |
| WBC (cell/L) | 18.0±8.9 | 14.4±5.6 | 0.405 |
| N (%) | 87.9±7.4 | 91.6±3.5 | 0.302 |
| ALB (g/L) | 27.9±5.1 | 24.5±5.8 | 0.226 |
| PCT (ng/mL) | 2.41±1.94 | 2.86±3.51 | 0.809 |
| Delerium (%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Etiology | |||
| Pulmonary (%) | 9 (60.0) | 5 (100.0) | 0.260 |
| Extra-pulmonary (%) | 6 (40.0) | 0 (0.0) | 0.260 |
| Dosages of analgesics and sedatives | |||
| Fentanyl (mg) | 10.0±5.5 | 16.3±10.0 | 0.088 |
| Midazolam (mg) | 598.0±626.4 | 756.0±684.4 | 0.638 |
| Propofol (mg) | 5,595.3±4,205.6 | 7,860.0±9,614.1 | 0.464 |
| Duration of MV (d) | 13.3±9.2 | 21.2±17.5 | 0.201 |
| Patient-ventilator asynchrony (events/person) | 1.5±1.8 | 0.8±1.8 | 0.476 |
| Patient-ventilator asynchrony/duration of MV | 0.12±0.12 | 0.07±0.15 | 0.422 |
| In-hospital deaths (%) | 1 (6.7) | 5 (100.0) | <0.001 |
| ICU stay (d) | 22.8±12.7 | 21.2±17.5 | 0.826 |
| In-hospital stay (d) | 34.3±19.9 | 26.0±23.6 | 0.448 |
| ICU cost (RMB) | 126,160.0±92,537.4 | 120,520.0±97,819.4 | 0.909 |
| In-hospital cost (RMB) | 154,710.0±118,388.0 | 131,980.0±91,948.6 | 0.702 |
SIMV, synchronized intermittent mandatory ventilation; PS, pressure supportive; APACHE II, acute physiology, age, chronic health evaluation; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; PaCO2, arterial partial pressure of carbon dioxide; HCO3−, bicarbonate ion; Hb, hemoglobin; WBC, white blood cell count; N%, percentage of neutrophil; ALB, albumin; PCT, procalcitonin; MV, mechanical ventilation; ICU, intensive care unit.
Figure 3 and Table 7 showed the early PaO2/FiO2 of patients in SIMV + PS success and failure. We found that, in SIMV + PS success group, the PaO2/FiO2 improvement at 2 and 36 h was significantly greater than that in SIMV + PS failure group, and, at each time point, the PaO2/FiO2 was higher than that at baseline (0 h). In A/C group, however, we did not find any significant differences.
Figure 3.
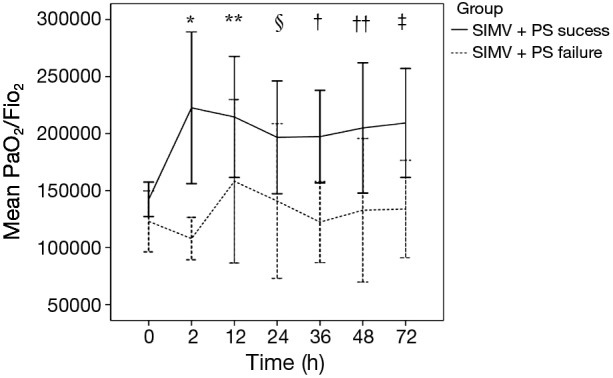
Early oxygenation (PaO2/FiO2) in SIMV + PS success and failure. Early (≤72 h) oxygenation (PaO2/FiO2) at each time point in groups of SIMV + PS success and failure were compared. Solid line in black represented oxygenation (mean ± SD) in SIMV + PS success group, while dashed line in black represented oxygenation (mean ± SD) in SIMV + PS failure group. SIMV + PS success vs. failure: *, 2 h, P=0.018; **, 12 h, P=0.243; §, 24 h, P=0.182; †, 36 h, P=0.025; ††, 48 h, P=0.132; ‡, 72 h, P=0.086. PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; SIMV, synchronized intermittent mandatory ventilation; PS, pressure supportive.
Table 7. Early oxygenation (PaO2/FiO2) in SIMV + PS success and failure.
| Time | SIMV + PS success (mmHg) | SIMV + PS failure (mmHg) | P |
|---|---|---|---|
| 0 h | 142.4±27.3 | 123.0±21.6 | 0.169 |
| 2 h | 222.6±93.0* | 107.9±15.0∫ | 0.018 |
| 12 h | 214.6±87.9** | 158.2±45.0∫∫ | 0.243 |
| 24 h | 196.7±81.9§ | 140.8±54.6∏ | 0.182 |
| 36 h | 197.4±63.9† | 122.4±28.6¢ | 0.025 |
| 48 h | 205.0±85.1†† | 132.8±39.6£ | 0.132 |
| 72 h | 209.3±79.1‡ | 133.8±26.9‖ | 0.086 |
SIMV + PS success group: 0 h vs. *, 2 h, P=0.004; **, 12 h, P=0.006; §, 24 h, P=0.023; †, 36 h, P=0.006; ††, 48 h, P=0.013; ‡, 72 h, P=0.005. SIMV + PS failure group: 0 h vs. ∫, 2 h, P=0.234; ∫∫, 12 h, P=0.164; ∏, 24 h, P=0.517; ¢, 36 h, P=0.970; £, 48 h, P=0.649; ‖, 72 h, P=0.522. PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; SIMV, synchronized intermittent mandatory ventilation; PS, pressure supportive.
Discussion
ARDS is an acute onset of lung injury caused by heterogeneous diseases, which is still a critical clinical problem with greatly high mortality. Invasive mechanical ventilation is an important life support for patients with ARDS, however, it can also induce lung injuries to the patients (9). Maunder and Slutsky reported that the high inspiratory airway pressure suggested the presence of excessive distension or “stretch” of the aerated lung, and the disruption of pulmonary epithelium and endothelium released inflammatory mediators which increased lung inflammation and caused injury to other organs (21,22). “Lung protective ventilation” and “open-lung approach” were introduced to avoid the further lung injury by mechanical ventilation.
Although ventilation strategies of “lung protective ventilation” and “open-lung approach” have been demonstrated to improve hypoxemia and decrease mortality (8-10), but in a prospective cohort study, Hsieh and his colleagues found more delirium in patients with ARDS (23). They enrolled 564 ICU patients and divided them into non-intubated (n=198), intubated without ARDS (n=318) and intubated with ARDS (n=48), and the result showed that intubated patients with ARDS had the highest prevalence of delirium (73% vs. 7% vs. 0.5%, P<0.001) compared with intubated without ARDS and non-intubated patients, and after adjustment for delirium and persistent coma, the association between ARDS and in-hospital mortality was reduced (odds ratio 5.63, P=0.009), which suggested that delirium was associated with mortality in patients with ARDS. Moreover, other studies proposed that overdose of sedatives during ICU may induce delirium, which we suspected may be caused by the intolerance and patient-ventilator asynchrony due to the non-physiological low VT and high PEEP (3,24,25).
Sydow and his colleagues conducted a trial in patients with ARDS using volume-controlled inverse ratio ventilation (VC-IRV) and airway pressure release ventilation (APRV), and they found that patients breathing during APRV have lower sedation (26), that is, different ventilation modes may resulted in different usage of sedatives, which was also demonstrated by Ortiz in his observational study comparing SIMV + PS with A/C (15). Therefore, a hypothetic theory was proposed that SIMV + PS may decrease mortality in patients with ARDS by reducing the usage of the sedatives and decreasing the incidence of delirium. To our knowledge, however, randomized controlled studies concerning this issue are still scarce, and our study pioneered in this area for the first time.
Our study result showed a trend, although without significant difference, toward a lower dosages of analgesics and sedatives and less incidence of delirium and patient-ventilator asynchrony in patients receiving SIMV + PS, which was contrary to the result reported by Robinson (27), but in accordance with that demonstrated by Ortiz (15). The different outcomes concluded in these studies may due to the variable patients enrolled. In the study conducted by Robinson, they identified all traumatically injured patients, while in Ortiz’s study, the reasons for mechanical ventilation included chronic obstructive pulmonary disease, asthma, neuromuscular disease, postoperative, sepsis, as well as congestive heart failure, but the percentage of ARDS covered only about 2%.
Oxygenation improvement is an important factor to evaluate the efficacy of mechanical ventilation in patients with ARDS. In our study, the PaO2/FiO2 in SIMV + PS group at each time point (2, 12, 24, 36, 48, and 72 h) was significantly greater than that in A/C group and that at baseline (0 h) (Figure 2 and Table 2), which implied that SIMV + PS could improve oxygenation earlier and more effectively than A/C in patients with moderate ARDS. Meanwhile, our study also showed that, in SIMV + PS group, PEEP and FiO2 were significantly lower than that in A/C group, which demonstrated that the mechanisms of SIMV + PS in improving oxygenation might not be due to PEEP. Spontaneous breaths may play a key role, which are greatly preserved in SIMV + PS. During spontaneous inspiration, the negative pressure in pleural space combined with the PEEP and PS provided by SIMV + PS could better improve the lung compliance than the mechanical inspiration with only PEEP and inspiratory pressure (PI) in A/C, and, moreover, negative pressure induced by spontaneous inspiration can attract more venous blood returning back to the thoracic cavity and result in reducing ventilation/perfusion mismatch, especially the dead space ventilation.
Variable factors have been demonstrated to be associated with mortality in patients with ARDS, such as oxygenation level (8-10), delirium (23), etiology inducing ARDS (28,29), age and APACHE II score (30,31). Compared with A/C, our study showed that SIMV + PS could better improve oxygenation, but could not significantly decrease mortality in patients with ARDS (Tables 2,3), which we think is offset by no significant difference in incidence of delirium found in our study. At the meantime, our study suggested that female patients with extra-pulmonary etiology of ARDS who succeeded in using SIMV + PS with lower PEEP and FiO2 had better clinical outcomes, but we did not find significant differences in baseline PaO2/FiO2, pH, partial pressure of arterial carbon dioxide (PaCO2), APACHE II score and age between survivors and non-survivors (Table 5). As analyzed in our previous study, in addition to the pathophysiological change of ARDS, there may be direct damages to the lung parenchyma and interstitium in pulmonary etiology of ARDS, which result in difficulties in improving oxygenation (32). However, we could not conclude that the factors mentioned above are independent due to our limited study sample without multivariate logistic regression analysis.
As shown in our study, five in six patients died in SIMV + PS group that failed and switched to A/C, and SIMV + PS success was associated with better outcomes. Therefore, further investigation of factors predicting SIMV + PS success is important. We divided the patients receiving SIMV + PS into success and failure, and the result showed that, compared with SIMV + PS failure, the age was younger and the early oxygenation was better improved in SIMV + PS success (Tables 6,7). Therefore, patients with young age and early oxygenation improvement could be associated with more success in using SIMV + PS, thus we recommend SIMV + PS not be initially implemented in older patients with moderate ARDS, and early closely monitoring arterial blood gas analyses once SIMV + PS is used.
Limitations of our trial include low sample size, our inability to analyze the oxygenation as well as the rate of successful weaning and reintubation in the later term. In this preliminary trial, based on the mortality rate reported in SIMV + PS and A/C (30% vs. 40%), we assumed a decrease of 10% of mortality in SIMV + PS, a relative risk reduction of 20%, 80% power (1-β) and a 2-sided t-test at a significance level of α<0.05, and the target sample size was calculated to be 860 patients, which will be completed in our ongoing future work.
Conclusions
In summary, for selected patients with moderate ARDS, SIMV + PS better and earlier improve oxygenation in patients with moderate ARDS with lower PEEP and FiO2 compared with A/C, but can not decrease the incidence of delirium and patient-ventilator asynchrony, the dosages of analgesics and sedatives, duration of mechanical ventilation and hospital stay. SIMV + PS success, female, extra-pulmonary etiologies of ARDS, and lower PEEP and FiO2 during mechanical ventilation are associated with better outcomes, and old age and delayed oxygenation improvement are associated with SIMV + PS failure, while young age and early oxygenation improvement are associated with high SIMV + PS success.
Acknowledgements
We thank Professor Dongtao Lin (College of Foreign Languages, Sichuan University), who is specialized in biomedical writing and editing, for copyediting this manuscript.
Funding: This study was supported by grant #2013SZ0001 from Sichuan Science and Technology Agency.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Brush DR, Kress JP. Sedation and analgesia for the mechanically ventilated patient. Clin Chest Med 2009;30:131-41. [DOI] [PubMed] [Google Scholar]
- 2.Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med 2012;185:486-97. [DOI] [PubMed] [Google Scholar]
- 3.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291:1753-62. [DOI] [PubMed] [Google Scholar]
- 4.Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471-7. [DOI] [PubMed] [Google Scholar]
- 5.Mehta S, Burry L, Cook D, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA 2012;308:1985-92. [DOI] [PubMed] [Google Scholar]
- 6.Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345-55. [DOI] [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 2004;30:51-61. [DOI] [PubMed] [Google Scholar]
- 8.Amato MB, Barbas CS, Medeiros DM, et al. Beneficial effects of the "open lung approach" with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med 1995;152:1835-46. [DOI] [PubMed] [Google Scholar]
- 9.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. [DOI] [PubMed] [Google Scholar]
- 10.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 2012;185:1307-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Needham DM, Dinglas VD, Bienvenu OJ, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ 2013;346:f1532. [DOI] [PMC free article] [PubMed]
- 13.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005;171:340-7. [DOI] [PubMed] [Google Scholar]
- 14.Thompson BT, Hayden D, Matthay MA, et al. Clinicians' approaches to mechanical ventilation in acute lung injury and ARDS. Chest 2001;120:1622-7. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz G, Frutos-Vivar F, Ferguson ND, et al. Outcomes of patients ventilated with synchronized intermittent mandatory ventilation with pressure support: a comparative propensity score study. Chest 2010;137:1265-77. [DOI] [PubMed] [Google Scholar]
- 16.ARDS Definition Task Force , Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: The berlin definition. JAMA 2012;307:2526-33. [DOI] [PubMed] [Google Scholar]
- 17.MacIntyre NR, Cook DJ, Ely EW, Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001;120:375S-95S. [DOI] [PubMed] [Google Scholar]
- 18.Kress JP, O’Connor MF, Pohlman AS, et al. Sedation of critically ill patients during mechanical ventialtion. A comparison of propofol and midazolam. Am J Respir Crit Care Med 1996;153:1012-8. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Inouye SK, Benard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703-10. [DOI] [PubMed] [Google Scholar]
- 20.Thille AW, Rodriguez P, Cabello B, et al. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 2006;32:1515-22. [DOI] [PubMed] [Google Scholar]
- 21.Maunder RJ, Shuman WP, McHugh JW, et al. Preservation of normal lung regions in the adult respiratory distress syndrome: analysis by computed tomography. JAMA 1986;255:2463-5. [PubMed] [Google Scholar]
- 22.Slutsky AS. Lung injury caused by mechanical ventilation. Chest 1999;116:9S-15S. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh SJ, Soto GJ, Hope AA, et al. The association between acute respiratory distress syndrome, delirium, and in-hospital mortality in intensive care unit patients. Am J Respir Crit Care Med 2015;191:71-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010;38:1513-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, et al. Long term outcome after delirium in the intensive care unit. J Clin Nurs 2009;18:3349-57. [DOI] [PubMed] [Google Scholar]
- 26.Sydow M, Burchardi H, Ephraim E, et al. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994;149:1550-6. [DOI] [PubMed] [Google Scholar]
- 27.Robinson BR, Blakeman TC, Toth P, et al. Patient-ventilator asynchrony in a traumatically injured population. Respir Care 2013;58:1847-55. [DOI] [PubMed] [Google Scholar]
- 28.Shaver CM, Bastarache JA. Clinical and Biological Heterogeneity in Acute Respiratory Distress Syndrome: Direct Versus Indirect Lung Injury. Clin Chest Med 2014;35:639-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dushianthan A, Grocott MP, Postle AD, et al. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J 2011;87:612-22. [DOI] [PubMed] [Google Scholar]
- 30.Villar J, Fernández RL, Ambrós A, et al. A Clinical Classification of the Acute Respiratory Distress Syndrome for Predicting Outcome and Guiding Medical Therapy. Crit Care Med 2015;43:346-53. [DOI] [PubMed] [Google Scholar]
- 31.Singh G, Gladdy G, Chandy TT, et al. Incidence and outcome of acute lung injury and acute respiratory distress syndrome in the surgical intensive care unit. Indian J Crit Care Med 2014;18:659-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J, Wang MY, Zhu H, et al. Can non-invasive positive pressure ventilation prevent endotracheal intubation in acute lung injury/acute respiratory distress syndrome? A meta-analysis. Respirology 2014;19:1149-57. [DOI] [PubMed] [Google Scholar]