Abstract
Now, more and more complete video-assisted thoracoscopic surgery (cVATS) surgeons are capable of performing lobectomy by uniportal approach. However, concerns regarding the safety of uniportal procedures for complex cases such as neoadjuvant chemotherapy, bronchial sleeves or vascular reconstructions still remains. As experience with uniportal VATS has increased, its application toward more technically demanding operations has also expanded. This article describes a uniportal cVATS left upper lobectomy with partial pulmonary arterioplasty for lung cancer with calcified lymph nodes. In order to reduce the risk of bleeding, we looped the left main pulmonary artery and applied two-stage maneuvering for left upper lobe (LUL) bronchus, cut the bronchus at the distal end and close the stump using a stapler at the end, which are conducive to maximal safety.
Keywords: Complete video-assisted thoracoscopic surgery (cVATS), uniportal lobectomy, lung cancer, calcified lymph node, partial arterioplasty
Introduction
In the very recent several years, more and more complete video-assisted thoracoscopic surgery (cVATS) surgeons are capable of performing lobectomy by uniportal approach. Growing evidence suggest it is technically feasible and safe with immediate outcomes comparable to traditional approach following a transition period of stepwise instruction (1). However, concerns regarding the safety of uniportal procedures for complex cases still remains (1). Whether extension of indications to uniportal cVATS approach will compromise the short-term results and increase the conversion rate (1)? With the recent developments in VATS technology and acquired experience, incompleteness of interlobar fissures, pleural adhesions, preoperative chemotherapy, big size of lesion, and some cases of centrally located tumors are not supposed to be the contraindications for uniportal lobectomy for experienced and skilled VATS surgeons (2). In contrast, peribronchial and perivascular lymph node calcification may complicate and even preclude cVATS lobectomy (3,4). Here we present a uniportal cVATS left upper lobe (LUL) lobectomy with partial pulmonary arterioplasty for lung cancer with calcified lymph node.
Clinical data
A 66 years old male patient who complained of cough and chest pain for half one month was admitted to our hospital on December 24th 2014. Smoking index is equal to 900 (30 cigarette/day ×30 years). Preoperative computed tomography (CT) scan showed a 2.1 cm nodule in the LUL (Figure 1). Calcified lymph node was seen between the LUL bronchus and pulmonary artery and its branches (Figure 2). The patient underwent preoperative staging and cardiac and pulmonary function assessment. Positron emission tomography (PET)-scan indicates no signs of metastasis. Preoperative primary diagnosis: (I) LUL carcinoma; (II) chronic obstructive pulmonary disease (COPD). Under general anesthesia with double lumen tube, uniportal cVATS LUL lobectomy with partial pulmonary arterioplasty was performed on December 26th 2014 (Figure 3).
Figure 1.
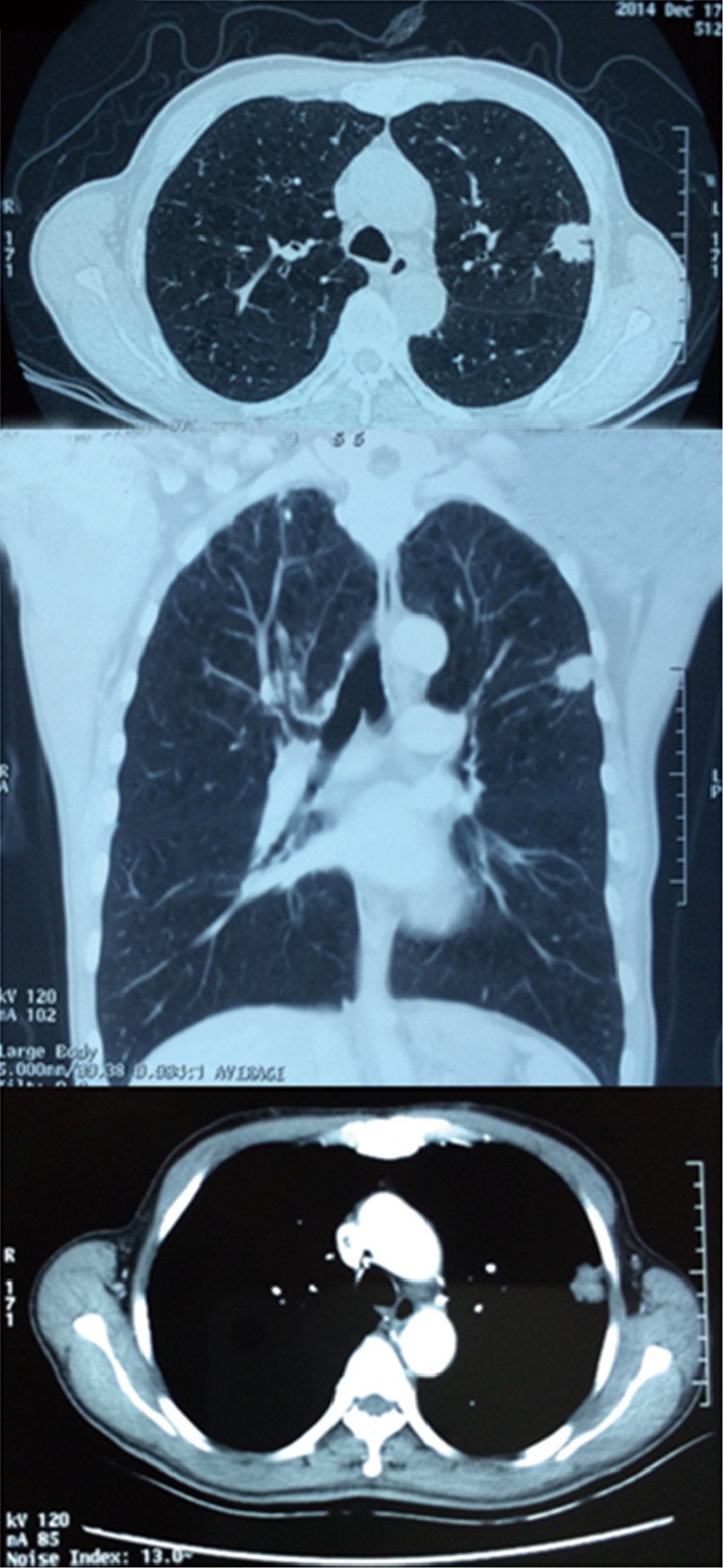
Preoperative CT showed a 2.1 cm nodule in the LUL. CT, computed tomography; LUL, left upper lobe.
Figure 2.
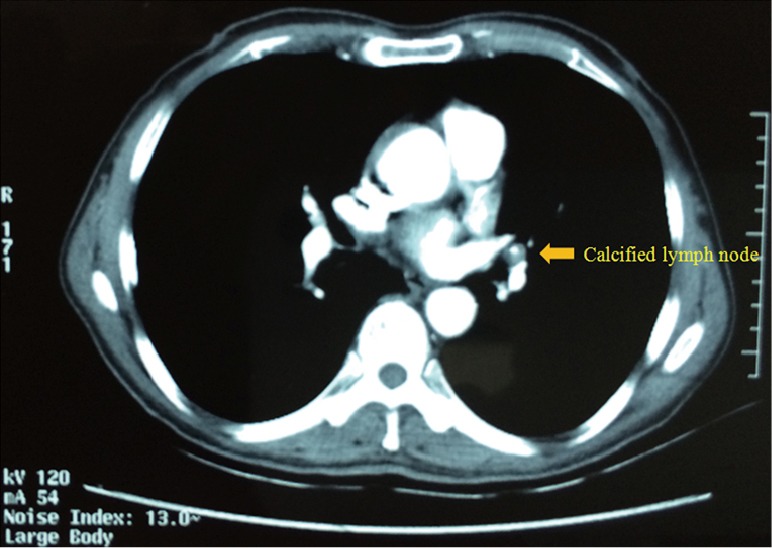
Calcified lymph node was seen between the LUL bronchus and pulmonary artery. LUL, left upper lobe.
Figure 3.

Uniportal cVATS lobectomy with partial pulmonary arterioplasty for lung cancer with calcified lymph node (5). cVATS, complete video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/740
Operative techniques
The endoscopic and double-jointed instruments used, placement and utilization of the single incision and different stance for the operating personnel and instruments are all identical to ever published method (6,7) except for some optimization strategy. (I) D’Amico forceps routinely used results in apparently increased dissection efficiency and safety. (II) Curved stapler (ENDO GIA, Tristapler, Covidien; FLEX, Johnson & Johnson) served as a “right angle forceps” while introduce the target vessels or bronchus by extensively increase the angle. (III) To locate the 3-4 cm single incision at the anterior axillary line via the tip of scapula, therefore the incision at the 4th or 5th intercostal space depends on the patient. The following are operative procedures step by step for this case.
Dividing the major fissure
For LUL lobectomies, several methods were chosen to apply depending on the patient. (I) Fissureless technique; (II) via the major fissure first conducive to expose posterior branch artery, then to dissect and divide the superior-anterior hilar structure; (III) cut the LUL bronchus at the distal end and close the stump using a stapler at the end. We chose to first expose posterior branch artery which were engulfed by calcified lymph node. Endoscopic forceps for opening the sheath of artery and D’Amico forceps for blunt dissection is very helpful.
Dividing the superior pulmonary vein (SPV) and apical-anterior branch artery
For LUL lobectomies, adequate angle for stapler to introduce the vessels require more frequent to transect apical-anterior branch artery first followed by SPV. By extensively increase the angle of curved stapler and optimally chosen single incision, vice versa is not a hurdle.
Division of the anterior portion of major fissure
Fissure, especially the underdeveloped fissure is commonly divided by means of electrocautery, harmonic scalpel, stapler or a combination, each of which has its pros and cons. For major fissure, electrocautery, a more precise energy platform, is most commonly used in our institution as for division of intersegmental plane during segmentectomy by many Japanese VATS surgeons, which contributes to better preserving lung function, reduced cost from stapler used and prone to identifying more anatomical variation from arteries while not increase the likelihood of postoperative air leakage and bleeding.
To loop the left main pulmonary artery
Considering the potential arterial bleeding due to the calcified lymph node, there is need to loop the left main artery. After transecting the SPV, it is very convenient to do that in the left side. D’Amico forceps used herein results in apparently increased dissection efficiency and safety.
Two-stage maneuvering for LUL bronchus
Transecting the LUL bronchus followed by dividing the SPV and apical-anterior branch artery is the most common route during uniportal VATS procedure. In this case, calcified lymph node is exactly stuck between LUL bronchus, artery and its branches and left lower lobe bronchus. Catastrophic bleeding will come at any time when exerting an impelling force to pass through the posterior wall of the bronchus though to clamp the left main artery is on standby. We resort to the following strategy. First, using scissors or electrocautery to cut the LUL bronchus at the distal end; then perform the partial pulmonary arterioplasty for the lingual and posterior artery; finally, close the stump using a stapler after retrieval of the specimen.
Partial pulmonary arterioplasty for the lingual and posterior artery
At the level of side wall of main artery, partial pulmonary arterioplasty for the lingual and posterior artery was stapling. A branch basilar artery originating from lingual artery was simultaneously divided which should not affect the blood supply too much.
Dissecting the calcified lymph node to the distal end of bronchus
After the artery and its branches have been pushed away, the calcified lymph node was dissected to the distal end of bronchus. The lobe specimen was retrieved via a tissue collecting bag.
Close the bronchial stump at the basal level by a stapler
Two-stage maneuvering for LUL bronchus is a safe alternative to manage complex situation in which the insufficient gap between bronchus and artery exists. Moreover, conversion to open surgery or traditional VATS procedure is becoming increasing dispersible.
Mediastinal lymph node dissection, leakage testing, intercostals nerve blockade, insert drainage tube and incision closure seen in former article.
For the left subcarinal lymph node dissection, sharp and blunt approach, especially by D’Amico forceps, are jointly applied results in enough exposure for left subcaria region and the comparable en bloc lymphadenectomy effect under uniportal circumstances.
Discussion
Very recently, Diego Gonzalez-Rivas first described uniportal sleeve lobectomy and other complex resections including different types of bronchial and/or vascular reconstructions. He believed with gained experience the most complex cases can be performed in the same manner as with double or triple port approach, with excellent postoperative results (1).
Gs Wang and colleagues began to perform biportal cVATS lobectomy since 1998, uniportal cVATS lobectomy since June, 2012. To date, more than 500 cases of uniportal lobectomy, segmentectomy, pneumonectomy, mediastinal tumor, lung volume reduction surgery and other procedures have been accomplished by this team (6,7). We shared the learning curve with Diego from anterior minithoracotomy, biportal approach, to uniportal approach. Live surgery events and experimental courses in Coruña, Spain and Shanghai, China hosted by Diego and occasional and repeated exchanges with foreign and domestic VATS masters greatly flattened and shortened our learning curve. With the recent developments in VATS technology, enhancement of the surgical instruments and acquired experience, incompleteness of interlobar fissures, pleural adhesions, preoperative chemotherapy, big size of lesion, and some centrally located tumor are not supposed to be the contraindications in our center. We completed a case of sleeve segmentectomy for an 86-year-old man with carcinoid tumor in the superior bronchus in the left lower lobe on 1st September, 2014. Lobectomy with systematic lymph node dissection and excision of anterior mediastinal tumor concurrently in semi lateral decubitus position and xiphoid uniportal approach are also accomplished. We agree on previous experience in uniportsl cVATS is necessary to perform these advanced cases with success.
However, peribronchial and perivascular lymph node calcification may complicate and even preclude cVATS lobectomy (3,4). In a study examining unplanned conversion for VATS lobectomy by Park and colleagues, 41% of conversions were due to hilar nodal anthracofibrosis and hilar adhesions. When the authors retrospectively reviewed the CT scans, hilar calcifications were seen in 71% of these patients. Our data and experiences strongly support this statement. Calcified lymphadenopathy involving the branch cause exactly same risk or solutions as direct invasions by tumor itself of the arterial branch or the truck. Even sometimes it is much more harmful due to obscuring the vessel from visualization and making it difficult to differentiate the layers on the vessel and also extremely dangerous as the traction for the calcified node can tear the pulmonary artery.
But each of “technical contraindication” has its own way to solve (2). Ryoichi Nakanishi and colleagues report their initial experience of traditional thoracoscopic lobectomy with the partial removal and reconstruction of the pulmonary artery caused by direct invasions of the artery or its branch or calcified lymphadenopathy involving the branch (8). In uniportal circumstances, we advised several notes to follow. First, to assure the safety and reliability in the performance of a resection and reconstruction of the artery, gain proximal and distal control of the artery is necessitated or at least on standby. Then, to reduce the suture by needle holder as much as possible, it is better using stapler because in these particular cases, partial removal and reconstruction of the pulmonary artery is frequently enough. To achieve sufficient access or gap for the stapler, another technical issue would also cooperate which we used herein called two-stage maneuvering for bronchus.
In conclusion, we believed uniportal cVATS lobectomy with partial pulmonary arterioplasty for lung cancer with calcified lymph node is feasible when all associated technical issues are properly addressed, even though hilar calcifications can make thoracoscopic dissection of the hilum technically challenging and increases the risk of vascular injury. I would like to emphasize one more points, conversion should be regarded as a means of completing resections in a traditional manner rather than as a surgical failure if you feel unsafe to continue with uniportal cVATS lobectomy.
Acknowledgements
The authors thank all the staff of the Collaborative Group of Small-Sized Lung Cancer (CSSLC) in Shenzhen People’s Hospital.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pischik VG. Technical difficulties and extending the indications for VATS lobectomy. J Thorac Dis 2014;6:S623-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [DOI] [PubMed] [Google Scholar]
- 4.Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011;35:590-5. [DOI] [PubMed] [Google Scholar]
- 5.Wang GS, Wang J, Rao ZP, et al. Uniportal cVATS lobectomy with partial pulmonary arterioplasty for lung cancer with calcified lymph node. Asvide 2015;2:162. Available online: http://www.asvide.com/articles/740 [DOI] [PMC free article] [PubMed]
- 6.Wang GS, Wang Z, Wang J, et al. Biportal complete video-assisted thoracoscopic lobectomy and systematic lymphadenectomy. J Thorac Dis 2013;5:875-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GS, Wang Z, Wang J, et al. Uniportal complete video-assisted thoracoscopic lobectomy with systematic lymphadenectomy. J Thorac Dis 2014;6:1011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakanishi R, Yamashita T, Oka S. Initial experience of video-assisted thoracic surgery lobectomy with partial removal of the pulmonary artery. Interact Cardiovasc Thorac Surg 2008;7:996-1000. [DOI] [PubMed] [Google Scholar]


