Abstract
Background
In South Korea, chronic obstructive pulmonary disease (COPD) is one of the ten leading causes of death. COPD exacerbations are significantly associated with mortality in COPD patients. This study was conducted to investigate the epidemiology of COPD in South Korea, specifically the clinical characteristics of South Korean COPD patients, the COPD exacerbation rate and the risk factors associated with COPD exacerbations.
Methods
This study covers a 2-year interval. One year was data collected retrospectively and the second year was prospectively obtained data.
Results
A total of 1,114 subjects were enrolled in the study. These subjects were observed for a period of 1 year from the enrollment, and a total of 920 subjects completed the study. A total of 1,357 COPD exacerbations occurred in 711 subjects (63.8%) out of the total of 1,114 subjects during the study period of 2 years. Multivariate logistic regression results showed that if patients had had a pneumonia before the retrospective year of analysis, they had a 18 times greater chance of having an exacerbation during the prospective year when other variables were controlled. Also, the subjects who had a history of two or more exacerbations during the retrospective year were approximately 6 times more likely to experience the COPD exacerbation compared to those who did not.
Conclusions
This study examined the demographic and clinical characteristics of South Korean COPD patients and found that a history of pneumonia and two or more occurrences of exacerbation within 1 year was significantly associated with a higher rate of COPD exacerbation.
Keywords: Chronic obstructive pulmonary disease (COPD), South Korea, exacerbation, pneumonia, chronic bronchitis
Background
About 65 million people around the world find it distressingly difficult to breathe due to chronic obstructive pulmonary disease (COPD) (1). COPD is a major cause of morbidity and mortality worldwide (2) and is one of the ten leading causes of death in South Korea. The epidemiologic study carried out by the South Korean National Health and Nutritional Examination Survey (KNHANES) in 2008 showed that COPD prevalence in the population over the age of 40 was 19.4% among men and 7.9% among women, respectively (3).
COPD is characterized by persistent airflow limitation. As the disease progresses, there are a greater dependence on health care utilization, more frequent hospital admissions and higher costs (4). Also, several studies have indicated that exacerbations ought to be considered when examining the progression of COPD (5,6), since exacerbation data can be used to predict disease progression.
COPD exacerbation rates vary from study to study (7-9). For example, a study conducted in Latin America reported that the rate of exacerbations per year increased with disease severity from 0.13 exacerbations in Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1 to 0.87 in GOLD 2, 2.43 in GOLD 3 and 6.87 in GOLD 4. This is supported by another study which suggested that patients who have experienced more exacerbations will tend to likewise experience more frequent exacerbations in the future (10). The aim of this study was to investigate epidemiological data on COPD in South Korea to analyze the occurrence of COPD exacerbations and the risk factors associated with COPD exacerbations.
Methods
Study population
Patients eligible for this study were over 40 years of age and were diagnosed with COPD as defined by the GOLD criteria at least 1 year prior to enrollment, and had been assessed at the investigational site for at least 1 year. The exclusion criteria excluded patients who were currently involved in any other interventional studies and those diagnosed with cancer. All patients submitted their written informed consent. The study was approved by Institutional Review Board (IRB) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. This paper represents the results of our study covering a 24-month retrospective and prospective analysis.
Study design
This was a multicenter, retrospective and prospective, descriptive, epidemiological study, conducted as a ‘non-interventional study’ as defined in Directive 2001/20/EC. The study period included 6 months of enrollment, 12 months or more of retrospective analysis and 12 months of prospective analysis.
The starting point of the study was defined as the date of the first site visit; the endpoint of the study was defined as the last data collection point for the visit 2.
Data was collected on the COPD exacerbation events from a period of 1 year preceding the enrollment date and 1 more year following the enrollment and was also collected on the results of pulmonary function tests over the 2 years. Our analyses also included other data such as demographic information, medical history, COPD phenotype, COPD Assessment Test (CAT) score (11), comorbidity and COPD medication at enrollment.
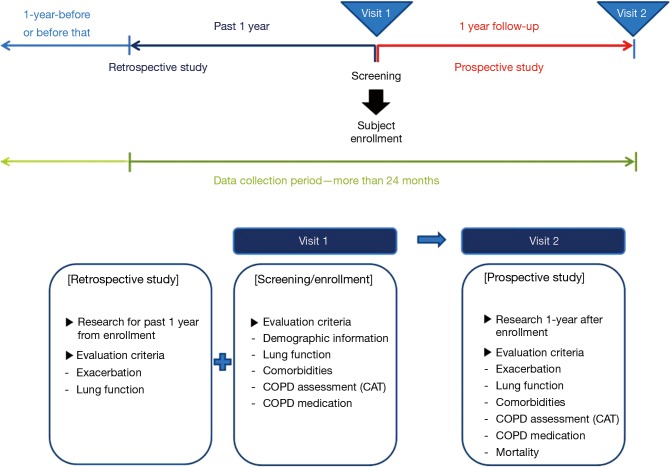
The pulmonary function test and medical history were investigated together to verify the correlation between the exacerbation rate and the physical condition of the patients. ‘Moderate exacerbation’ was defined as an event requiring treatment with a systemic corticosteroid and/or antibiotics, ‘severe exacerbation’ was an event requiring hospitalization, and ‘other exacerbation’ included visits to primary-care physicians or a change in the use of regular medication (12). In addition, CAT was performed at visit 2 to assess COPD status. The detail procedures followed in this study are presented in Figure 1.
Figure 1.
Study design. COPD, chronic obstructive pulmonary disease.
Statistical analysis
Descriptive data are reported in terms of mean ± standard deviations (SD) or the number of patients (percentages), as appropriate. For comparisons of patients’ characteristics, analysis of variance (ANOVA) (Kruskal-Wallis test, when appropriate) was used for continuous variables and Chi-square tests (Fisher’s exact test, when appropriate) were used for the categorical variables between patient groups by GOLD spirometry classification. The change of disposition for COPD exacerbations during the 2 years was carried out by Bowker’s test. Logistic regression was used to examine parameters potentially associated with COPD exacerbation occurrence. All variables in the multivariate regression model were considered as univariate logistic regression models for demographics or clinically meaningful variables such as forced expiratory volumes in 1 second (FEV1), CAT, body mass index (BMI), and phenotypes. The difference of dispositions between the enrollment and the prospective based on the GOLD revised 2011 were assessed by Bowker’s test. P values less than 0.05 were considered significant. No adjustments for multiple comparisons were made. All analyses were conducted using SAS software, version 9.2 [SAS® 9.2 (SAS Institute Inc., Cary, NC, USA) software].
Results
Participation status of study subjects
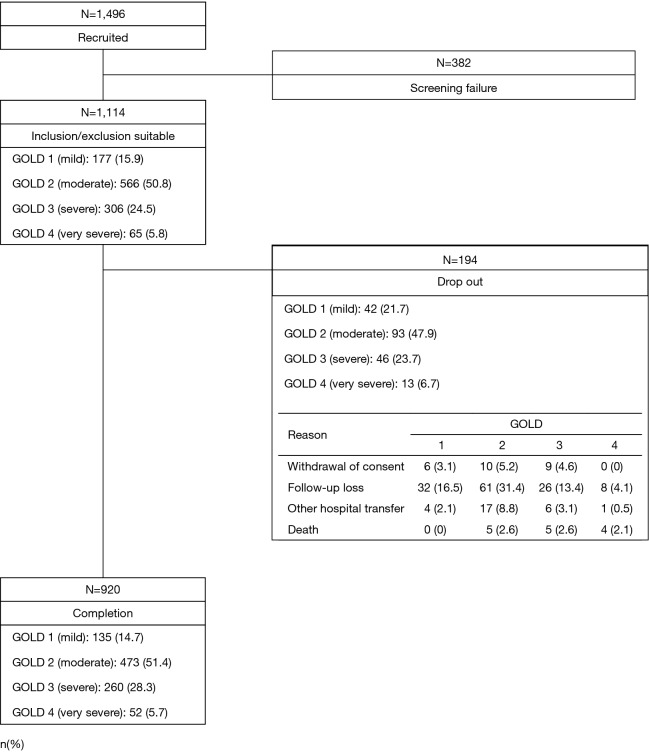
A total of 1,496 subjects at 46 participating institutions (listed up in Supplementary 1) in South Korea received screening tests, and 1,114 of these subjects who satisfied the inclusion/exclusion criteria were enrolled in the study. According to the GOLD spirometry classification, the 1,114 subjects consisted of 177 subjects (15.9%) in GOLD 1, 566 subjects (50.8%) in GOLD 2, 306 subjects (24.5%) in GOLD 3, and 65 subjects (5.8%) in GOLD 4. These subjects were placed under observation for a period of 1 year from the date of enrollment. A total of 920 subjects completed the study. As regards the subjects who were terminated early, their GOLD spirometry classifications were as follows: 42 subjects (21.7%) in GOLD 1, 93 subjects (47.9%) in GOLD 2, 46 subjects (23.7%) in GOLD 3, and 13 subjects (6.7%) in GOLD 4 (Figure 2). The reason for the early termination included withdrawal of consent, failure to participate in follow-up observations, and death. A total of 14 subjects died including 5 subjects (2.6%) in GOLD 2, 5 subjects (2.6%) in GOLD 3, and 4 subjects (2.1%) in GOLD 4. Among the 14 subjects, the deaths of 6 subjects were caused by COPD, and the rest of subjects had other reasons including three unknown cases.
Figure 2.
Patient’s disposition.
Demographic information and clinical characteristics on subjects
Table 1 presents the demographic information and characteristics of the 1,114 subjects who participated in the study. Approximately 90% of the subjects were male, and the majority of the subjects were aged 60 or older and less than 80. The total CAT scores were found to be statistically different between the groups, as 13.4±7.3 in GOLD 1, 15.2±7.7 in GOLD 2, 19.5±8.1 in GOLD 3, and 25.2±7.4 in GOLD 4, indicating that the score increased as the pulmonary function worsened (P<0.001).There was no tendency for comorbidity to be correlated to a change in the spirometry classification. The majority of the subjects used a long acting muscarinic antagonist (LAMA) or a combination of inhaled corticosteroid (ICS) and long acting beta agonist (LABA). Also, a short acting beta 2 agonist (SABA) was administered pro re nata (PRN) in all subjects. As regards the frequency of use, LAMA was used on 106 subjects (59.9%) in GOLD 1, 444 subjects (78.4%) in GOLD 2, 264 subjects (86.3%) in GOLD 3, and 60 subjects (92.3%) in GOLD 4, which was more frequent than the use of other medications.
Table 1. Demographic and baseline clinical characteristics according to GOLD spirometry criteria.
| Characteristics | COPD GOLD |
||||
|---|---|---|---|---|---|
| GOLD 1 (n=177) (%) | GOLD 2 (n=566) (%) | GOLD 3 (n=306) (%) | GOLD 4 (n=65) (%) | Total (n=1,114) (%) | |
| Gender | |||||
| Male | 159 (89.8) | 514 (90.8) | 280 (91.5) | 62 (95.4) | 1,015 (91.1) |
| Female | 18 (10.2) | 52 (9.2) | 26 (8.5) | 3 (4.6) | 99 (8.9) |
| Age (yrs)† | |||||
| Age <60 | 15 (8.4) | 70 (12.4) | 48 (15.7) | 16 (24.6) | 149 (13.4) |
| 60≤ Age <70 | 52 (29.4) | 192 (33.9) | 117 (38.2) | 24 (36.9) | 385 (34.6) |
| 70≤ Age <80 | 90 (50.9) | 246 (43.5) | 129 (42.2) | 23 (35.4) | 488 (43.8) |
| Age ≥80 | 20 (11.3) | 58 (10.3) | 12 (3.9) | 2 (3.1) | 92 (8.3) |
| BMI | |||||
| BMI <18.5 | 13 (7.2) | 48 (8.5) | 54 (17.7) | 24 (36.9) | 139 (12.5) |
| 18.5≤ BMI <25 | 119 (67.2) | 393 (69.4) | 207 (67.7) | 36 (55.4) | 755 (67.8) |
| 25≤ BMI <30 | 43 (24.3) | 116 (20.5) | 43 (14.1) | 5 (7.7) | 207 (18.6) |
| BMI ≥ MI | 2 (1.1) | 9 (1.6) | 2 (0.7) | 0 (0) | 13 (1.2) |
| Smoking history | |||||
| Never-smokers | 13 (7.2) | 57 (10.1) | 30 (9.8) | 6 (9.2) | 106 (9.5) |
| Current smokers | 45 (25.4) | 126 (22.3) | 55 (18.0) | 11 (16.9) | 237 (21.3) |
| Former smokers | 119 (67.2) | 383 (67.7) | 221 (72.2) | 48 (73.9) | 771 (69.2) |
| COPD duration (yrs)† | |||||
| mean ± SD | 5.2±4.9 | 5.3±4.6 | 6.6±4.9 | 7.3±5.1 | 5.8±4.8 |
| COPD phenotype† | |||||
| Chronic bronchitis | 58 (32.7) | 163 (28.8) | 73 (23.9) | 6 (9.2) | 300 (26.9) |
| Emphysema | 63 (35.6) | 248 (43.8) | 151 (49.4) | 34 (52.3) | 496 (44.5) |
| Chronic bronchitis & emphysema | 56 (31.6) | 155 (27.4) | 82 (26.8) | 25 (38.5) | 318 (28.6) |
| Pulmonary function test‡ | |||||
| FEV1 (%)† | 83.2±15 | 62.1±12.2 | 41.7±10.9 | 28.4±11.8 | 59.4±20.1 |
| FVC (%)† | 101.3±17.3 | 87.7±17.2 | 72.6±17.6 | 62.8±19.6 | 86.2±20.8 |
| FEV1/FVC (%)† | 59.2±7.6 | 50.7±10.6 | 40.0±9.3 | 32.5±7.5 | 47.6±11.4 |
| COPD assessment test | |||||
| Total score | 13.4±7.3 | 15.2±7.7 | 19.5±8.1 | 25.2±7.4 | 16.7±8.3 |
| Cough | 1.5±1.3 | 1.7±1.3 | 1.9±1.3 | 2.5±1.5 | 1.8±1.3 |
| Phlegm (mucus) | 2±1.3 | 2.1±1.4 | 2.4±1.4 | 2.9±1.5 | 2.2±1.4 |
| Tight chest | 1.2±1.3 | 1.4±1.4 | 2.0±1.6 | 2.3±1.6 | 1.6±1.5 |
| Breathless | 2.8±1.5 | 3.3±1.3 | 4.1±1.0 | 4.7±0.6 | 3.5±1.4 |
| Activity | 1.1±1.3 | 1.5±1.5 | 2.3±1.7 | 3.3±1.6 | 1.8±1.7 |
| Confident | 1.1±1.5 | 1.5±1.6 | 2.4±1.7 | 3.6±1.5 | 1.8±1.7 |
| Sleep | 1.3±1.5 | 1.4±1.5 | 1.7±1.6 | 2.4±1.6 | 1.5±1.4 |
| Energy | 2.3±1.3 | 2.3±1.4 | 2.8±1.5 | 3.5±1.1 | 2.5±1.4 |
| Comorbidity | |||||
| Yes | 147 (83.5) | 426 (75.3) | 237 (77.5) | 46 (70.8) | 856 (76.8) |
| No | 29 (16.5) | 140 (24.7) | 69 (22.5) | 19 (29.2) | 257 (23.1) |
| Types of comorbidity | |||||
| Asthma | 29 (16.5) | 90 (15.9) | 57 (18.6) | 5 (7.7) | 181 (16.3) |
| Pneumonia | 16 (9.1) | 63 (11.1) | 46 (15.0) | 13 (20.0) | 138 (12.4) |
| Hypertension | 16 (9.1) | 62 (11) | 31 (10.1) | 8 (12.3) | 117 (10.5) |
| Benign prostatic hyperplasia | 31 (17.6) | 48 (8.5) | 19 (6.2) | 3 (4.6) | 101 (9.1) |
| Diabetes | 11 (6.3) | 24 (4.2) | 12 (3.9) | 3 (4.6) | 50 (4.5) |
| Osteoporosis | 4 (2.3) | 14 (2.5) | 14 (4.6) | 4 (6.2) | 36 (3.2) |
| Coronary artery diseases | 0 (0) | 22 (3.9) | 6 (2.0) | 1 (1.5) | 29 (2.6) |
| Atrial fibrillation | 3 (1.7) | 5 (0.9) | 6 (2.0) | 15 (23.1) | 29 (2.6) |
| Tuberculosis | 3 (1.7) | 10 (1.8) | 10 (3.3) | 2 (3.1) | 25 (2.2) |
| Depression | 5 (2.8) | 9 (1.6) | 6 (2.0) | 3 (4.6) | 23 (2.1) |
| Hyperlipidemia | 3 (1.7) | 11 (1.9) | 8 (2.6) | 1 (1.5) | 1 (1.5) |
| Cerebrovascular diseases | 5 (2.8) | 5 (0.9) | 2 (0.7) | 1 (1.5) | 13 (1.2) |
| Others | 3 (1.7) | 5 (0.9) | 6 (2.0) | 15 (23.1) | 29 (2.6) |
| COPD medication | |||||
| ICS | 3 (1.7) | 11 (1.9) | 11 (3.6) | 0 (0) | 25 (2.2) |
| LAMA | 106 (59.9) | 444 (78.4) | 264 (86.3) | 60 (92.3) | 874 (78.5) |
| LABA | 0 (0) | 1 (0.2) | 1 (0.3) | 0 (0) | 2 (0.2) |
| SABA | 19 (10.7) | 82 (14.5) | 79 (25.8) | 24 (36.9) | 204 (18.3) |
| SAMA | 0 (0) | 7 (1.2) | 3 (1.0) | 4 (6.2) | 14 (1.3) |
| ICS + LABA | 83 (46.9) | 352 (62.2) | 229 (74.8) | 59 (90.8) | 723 (64.9) |
Mean ± SD for COPD duration (yrs), pulmonary function test, COPD assessment test. Chi-square test for smoking history, COPD phenotype. ANOVA for COPD duration (yrs), COPD assessment test. Fisher’s exact test for gender, age (yrs), BMI. †, statistically significantunder the level of significant (5.0%; 2-sided); ‡, pulmonary function test was assessed at the prior year. COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; BMI, body mass index; FEV1, forced expiratory volumes in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; LAMA, long acting muscarinic antagonist; LABA, long acting beta agonist; SABA, short acting beta 2 agonist; SAMA, short acting muscarinic antagonist.
COPD exacerbation based on GOLD spirometry classification
Table 2 presents the characteristics of COPD exacerbation according to spirometry classification by either retrospective or prospective. In the retrospective analysis, the number of subjects who experienced a COPD exacerbation was statistically different between the groups, as 48 (27.1%) in GOLD 1, 175 (30.9%) in GOLD 2, 138 (45.1%) in GOLD 3 and 35 (53.8%) in GOLD 4 (P<0.001). In the prospective analysis, there were also statistical differences between the groups, as 30 subjects (22.2%) in GOLD 1, 144 subjects (30.4%) in GOLD 2, 112 subjects (43.1%) in GOLD 3 and 29 subjects (55.8%) in GOLD 4 (P<0.001). A total of 1,357 COPD exacerbations occurred in the study period of 2 years. Classified by severity, these included 369 “severe” exacerbations (27.2%) and 823 “moderate” exacerbations (60.6%). It was found that the greater the severity of COPD exacerbation, the more likely that the subject belonged to GOLD 4. In addition, the number of exacerbations per individual was found to be statistically different between the groups, as 1.5±1.1 in GOLD 1, 1.7±1.1 in GOLD 2, 2.0±1.7 in GOLD 3, and 2.6±2.5 in GOLD 4 in the retrospective analysis, showing that the average exacerbation increased as the pulmonary function deteriorated (P<0.001). This pattern was likewise confirmed in the prospective analysis: 1.5±1.1 in GOLD 1, 1.8±1.3 in GOLD 2, 2.2±1.8 in GOLD 3, and 2.7±2.7 in GOLD 4 (P<0.001).
Table 2. Occurrence, status and frequency of COPD exacerbationon GOLD spirometry classification between retrospective and prospective.
| COPD exacerbation | Retrospective |
Prospective |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| GOLD 1 (n=177) | GOLD 2 (n=566) | GOLD 3 (n=306) | GOLD 4 (n=65) | GOLD 1 (n=135) | GOLD 2 (n=473) | GOLD 3 (n=260) | GOLD 4 (n=52) | ||
| Occurrence, n (%)* | |||||||||
| Yes | 48 (27.1) | 175 (30.9) | 138 (45.1) | 35 (53.8) | 30 (22.2) | 144 (30.4) | 112 (43.1) | 29 (55.8) | |
| No | 129 (72.9) | 391 (69.1) | 168 (54.9) | 30 (46.2) | 105 (77.8) | 329 (69.6) | 148 (56.9) | 23 (44.2) | |
| Severity of COPD exacerbation, n (%) | |||||||||
| Severe | 19 (26.8) | 62 (20.7) | 79 (28.7) | 35 (38.5) | 14 (31.8) | 65 (25.6) | 67 (27.4) | 28 (36.4) | |
| Moderate | 47 (66.2) | 203 (67.7) | 168 (61.1) | 45 (49.4) | 26 (59.1) | 155 (61.0) | 140 (57.1) | 39 (50.6) | |
| Other | 5 (7.0) | 35 (11.7) | 28 (10.2) | 11 (12.1) | 4 (9.1) | 34 (13.4) | 38 (15.5) | 10 (13.0) | |
| Total | 71 [100] | 300 [100] | 275 [100] | 91 [100] | 44 [100] | 254 [100] | 245 [100] | 77 [100] | |
| Frequency of COPD exacerbation, n (%)# | |||||||||
| Mean of frequency | 1.5±1.1 | 1.7±1.1 | 2.0±1.7 | 2.6±2.5 | 1.5±1.1 | 1.8±1.3 | 2.2±1.8 | 2.7±2.7 | |
| 1 | 35 (14.5) | 108 (44.8) | 82 (34.0) | 16 (6.6) | 24 (13.4) | 85 (47.5) | 57 (31.8) | 13 (7.3) | |
| ≥2 | 13 (8.4) | 67 (43.2) | 56 (36.1) | 19 (12.3) | 6 (4.4) | 59 (43.4) | 55 (40.4) | 16 (11.8) | |
Mean ± SD or n (%) for the statistics. *, Chi-square test; #, ANOVA. COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ANOVA, analysis of variance.
Disposition of COPD exacerbation
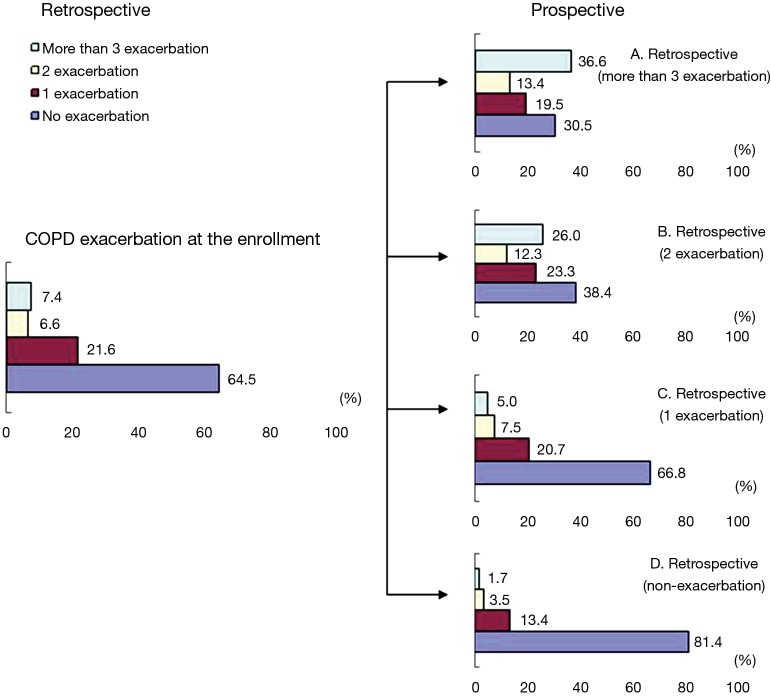
Table 3 presents the occurrence of COPD exacerbations in the entire period of 2 years. Among the subjects who had not experienced any exacerbations during the retrospective period, there were some who experienced exacerbation in the prospective: these included 96 subjects (13.4%) who had one exacerbation, 25 subjects (3.5%) who had two exacerbations, and 12 subjects (1.7%) who had three or more exacerbations. Among the subjects who had one exacerbation in the retrospective analysis, there were 18 subjects (7.5%) who had two exacerbations and 12 subjects (5.0%) who had three or more exacerbations in the prospective analysis. Among the subjects who had towed exacerbations in the retrospective analysis, 19 subjects (26.0%) were found to have experienced three or more exacerbations in the prospective. This data verifies that subjects who had a higher case of COPD exacerbation in the past have an increased probability of experiencing exacerbations. The changes in COPD exacerbation is presented in Figure 3.
Table 3. Change of disposition for COPD exacerbation during the 2 years COPD exacerbation.
| Frequency of COPD exacerbation | Prospective (n, %) |
Total | P value | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | |||
| Retrospective | 0.765 | |||||
| 0 | 585 (81.4) | 96 (13.4) | 25 (3.5) | 12 (1.7) | 718 [100] | |
| 1 | 161 (66.8) | 50 (20.7) | 18 (7.5) | 12 (5.0) | 241 [100] | |
| 2 | 28 (38.4) | 17 (23.3) | 9 (12.3) | 19 (26.0) | 73 [100] | |
| ≥3 | 25 (30.5) | 16 (19.5) | 11 (13.4) | 30 (36.6) | 82 [100] | |
| Total | 799 (71.7) | 179 (16.1) | 63 (5.7) | 73 (6.6) | 1,114 [100] | |
Bowker test. COPD, chronic obstructive pulmonary disease.
Figure 3.
Change of COPD exacerbation from retrospective to prospective. COPD, chronic obstructive pulmonary disease.
Risk factors related to COPD exacerbation
Multivariate logistic regression analysis was performed to identify the parameters that affect the COPD exacerbations, and the results are shown in Table 4. First, subjects who had a history of pneumonia had a probability of COPD exacerbation 18.09 times higher than that of subjects who did not have a history of pneumonia (P<0.001). Also, the probability of exacerbation was 5.67 times greater for subjects who had two or more COPD exacerbations in the preceding year, compared to that of subjects who had less than two exacerbations (P<0.001). In addition, COPD exacerbation was also found to be affected by cases in which the total CAT score was 10 or higher and cases in which the FEV1 decreased.
Table 4. Risk factors associated with occurred COPD exacerbation.
| Risk factors | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| History of pneumonia (ref = no) | Yes | 18.09 | 8.86–36.94 | <0.001 |
| Total of CAT score (ref = CATSUM <10) | CATSUM ≥10 | 1.79 | 1.23–2.59 | 0.002 |
| FEV1 (%) | 0.98 | 0.97–0.99 | <0.001 | |
| COPD exacerbation (ref <2) | Exacerbation ≥2 | 5.67 | 3.56–9.03 | <0.001 |
Multivariate logistic regression model (P value <0.001). COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volumes in 1 second.
Classification according to the revised GOLD diagnostic criteria
The distribution of subjects, previously classified according to the 2010 GOLD (13), has been reclassified according to the new 2011 GOLD (14), which additionally includes CAT scores and the frequency of exacerbation. This newly organized data is presented in Table 5. The patients who showed no changes in symptoms or risks 1 year later in the prospective results were comprised of a total of 102 subjects (61.5%) from among those who had been classified as group A at the time of enrollment according to the revised 2011 GOLD classification, 238 subjects (64.7%) from among those who had been classified as group B, 15 subjects (38.5%) classified as group C, and 270 subjects (78.3%) classified as group D. As time progressed from enrollment to the time of the prospective results, there were subjects who became more symptomatic (CAT ≥10): these included 40 subjects (24.1%) who shifted from group A to group B and 11 subjects (28.2%) who shifted from group C to group D. Among the subjects who progressed from low risk to high risk (severe or very severe airflow limitation) as time progressed from enrollment to the time of the prospective results, there were 17 subjects (10.2%) who moved from group A to group C and 73 subjects (19.8%) who moved from group B to group D. Also, there were seven subjects (4.2%) who moved from group A to group D because both their symptoms and risks worsened.
Table 5. Shift of the COPD subject by GOLD revised 2011.
| Prospective enrollment | Subject group (n, %) |
Total [n, %] | P value | |||
|---|---|---|---|---|---|---|
| GOLD A | GOLD B | GOLD C | GOLD D | |||
| GOLD A | 102 (61.5) | 40 (24.1) | 17 (10.2) | 7 (4.2) | 166 [100] | 0.003 |
| GOLD B | 49 (13.3) | 238 (64.7) | 8 (2.2) | 73 (19.8) | 368 [100] | |
| GOLD C | 10 (25.6) | 3 (7.7) | 15 (38.5) | 11 (28.2) | 39 [100] | |
| GOLD D | 10 (2.9) | 41 (11.9) | 24 (6.9) | 270 (78.3) | 345 [100] | |
| Total | 171(18.6) | 322 (35.1) | 64 (7.0) | 361 (39.3) | 918 [100] | |
Bowker test. Withdrawals are 196. COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Discussion
We enrolled 1,114 subjects from within South Korea and investigated the clinical characteristics of these South Korean COPD patients, their COPD exacerbation rate and the risk factors associated with COPD exacerbations. A total of 1,357 COPD exacerbations occurred in the study period.
When patients were grouped based on GOLD spirometry classifications, the exacerbation rate increased with disease severity, as has been demonstrated in Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study (15).
Patients were divided into groups based on the frequency of exacerbation during the retrospective study, and among the subjects who had not experienced any exacerbations during the retrospective period, 18.6% were found to have had one or more COPD exacerbations in the prospective observation period. In contrast, among the subjects who had two exacerbations in the retrospective analysis, 61.6% of subjects were found to have experienced one or more exacerbations in the prospective period. This data verifies that subjects who had a higher case of COPD exacerbation in the past have an increased probability of experiencing exacerbations.
In the multivariate logistic regression analysis, the association of exacerbation status was 5.67 times higher among subjects who had two or more exacerbations than among subjects who had less than two exacerbations. On the whole, this result corroborates the ECLIPSE study, which concluded that the single best predictor of exacerbations is a history of exacerbations (15).
The exacerbation rate was 18.09 times greater among patients who had experienced pneumonia in the retrospective analysis than among those who did not have pneumonia. This indicated that among COPD patients, history of pneumonia is an important predictor of the risk of COPD exacerbation. In the UK National COPD Resources and Outcomes Project of 2008, COPD exacerbations were associated with worse outcomes among patients with a history of pneumonia. In patient mortality was 11% and 7% and 90-day mortality was 17% and 13% for pneumonia and non-pneumonia patients, respectively (P<0.001) (16). In addition, the exacerbation rate was 1.79 times greater among cases in which the total CAT score was 10 or higher in the retrospective analysis than among those in which the score was less than 10. This suggested that a total CAT score of 10 or higher is associated with COPD exacerbations.
The distribution of subjects has been reclassified according to the new 2011 GOLD criteria, which additionally includes CAT scores and frequency of exacerbation. As time progressed from enrollment to the prospective results, 156 subjects (17.0%) became more symptomatic and/or high risk, but 137 subjects (15.0%) became less symptomatic and/or low risk. A further study will be required to investigate this difference.
The strengths of this study are that it included a large sample size, used relatively broad inclusion criteria and the used observational data which better reflected the actual current epidemiological situation in South Korea. Even though this non-interventional study succeeded in generating a large body of data, there were limitations inherent in the study design, in regards to potential bias, the study’s effects and the lack of a control group. Nevertheless, our results suggested that the possibility of exacerbation occurrence was higher among COPD patients who had history of pneumonia, a high CAT score and two or more exacerbations than among subjects who did not have these features. It must be acknowledged, however, that these findings will need to be established more clearly by an extended study capable of confirming the effect of the frequency, management and treatment of pneumonia on COPD exacerbation occurrence.
Conclusions
In conclusion, the COPD exacerbation rate was higher among the patients who had a history of pneumonia or a high rate of COPD exacerbation in the preceding period of 1 year. This is the first large-scale study to reveal the risk factors of COPD exacerbation in South Korea. Further clinical trials will be conducted to measure the relationship between other variables and the reduction in exacerbations for meaningful sub-populations of COPD patients.
Acknowledgements
This study was sponsored by Takeda Pharmaceuticals Korea Co., Ltd. Editorial assistance as medical writer was provided by YooYoung Shin, Seoul CRO, Korea, supported by Takeda Pharmaceuticals Korea Co. Ltd. Forty six participating institutions are listed up in the Supplementary 1.
Supplementary 1 Study sites and investigators
| Centre No. | Name of study sites | Principle Investigators |
|---|---|---|
| 01 | Seoul St. Mary’s Hospital, The Catholic University of Korea | YoungKyoon Kim |
| 02 | St. Paul’s Hospital, The Catholic University of Korea | SangHaak Lee |
| 03 | Yeouido St. Mary’s Hospital, The Catholic University of Korea | HyoungKyu Yoon |
| 04 | Incheon St. Mary’s Hospital, The Catholic University of Korea | JoongHyun Ahn |
| 05 | Kyung Hee University Hospital at Gangdong | JeeHong Yoo |
| 06 | GangNeung Asan Hospital | BockHyun Jung |
| 07 | Konkuk University Medical Center | KwangHa Yoo |
| 08 | Konyang University Hospital | MoonJun Na |
| 09 | Kyungpook National University Hospital | JaeYong Park |
| 10 | Gyeongsang National University Hospital | JongDeog Lee |
| 11 | Kyung Hee University Medical Center | MyungJae Park |
| 12 | Keimyung University Dongsan Medical Center | ChiYoung Jung |
| 13 | Korea University Guro Hospital | JaeJeong Shim |
| 14 | Korea University Anam Hospital | SangYeub Lee |
| 15 | Daegu Catholic University Medical Center | KyungChan Kim |
| 16 | Daegu Fatima Hospital | YeonJae Kim |
| 17 | Dongguk University Gyeongju Hospital | HyeSook Choi |
| 18 | Dongguk University Ilsan Hospital | GunMin Park |
| 19 | Maryknoll Medical Center | IkSu Choi |
| 20 | Seoul National University Bundang Hospital | Choon-Taek Lee |
| 21 | Samsung Medical Center | HoJoong Kim |
| 22 | Seoul National University Hospital | ChulGyu Yoo |
| 23 | Seoul Veterans Hospital | YongHo Roh |
| 24 | Asan Medical Center | SangDo Lee |
| 25 | St. Carollo Hospital | DongRyeol Chae |
| 26 | Soonchunhyang University Bucheon Hospital | DoJin Kim |
| 27 | Soonchunhyang University Seoul Hospital | Soo-Taek Uh |
| 28 | Soonchunhyang University Cheonan Hospital | KiHyun Seo |
| 29 | Wonju Severance Christian Hospital | SukJoong Yong |
| 30 | Gangnam Severance Hospital | HyungJung Kim |
| 31 | Severance Hospital | YoungSam Kim |
| 32 | Yeungnam University Medical Center | KwanHo Lee |
| 33 | Ulsan University Hospital | SeungWon Ra |
| 34 | Wonkwang University School of Medicine | HakRyul Kim |
| 35 | Wonkwang University Sanbon Hospital | HuiJung Kim |
| 37 | Ewha Womans University Medical Center | JungHyun Jang |
| 38 | Inje University Sanggye Paik Hospital | SooJeon Choi |
| 39 | Chonnam National University Hospital | SungChul Lim |
| 40 | Chonbuk National University Hospital | YongChul Lee |
| 41 | Chung-ang University Hospital | InWon Park |
| 42 | Chungnam National University Hospital | SungSoo Jung |
| 43 | Hallym University Kangdong Sacred Heart Hospital | YongBum Park |
| 44 | Hallym University Sacred Heart Hospital | Ki-Suck Jung |
| 45 | Hallym University Chuncheon Sacred Heart Hospital | ChangYoul Lee |
| 46 | Hanyang University Medical Center | HoJoo Yoon |
| 47 | Kyungpook National University Hospital | Jaehee Lee |
The center No. 36, Eulji General Hospital was discontinued.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.World Health Organization. World Health Report 2000: health systems: improving performance. Geneva, Switzerland, 2000.
- 2.Ko FW, Lim TK, Hancox RJ, et al. Year in review 2013: Chronic obstructive pulmonary disease, asthma and airway biology. Respirology 2014;19:438-47. [DOI] [PubMed] [Google Scholar]
- 3.Yoo KH, Kim YS, Sheen SS, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology 2011;16:659-65. [DOI] [PubMed] [Google Scholar]
- 4.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care 2003;48:1204-13; discussion 1213-5. [PubMed] [Google Scholar]
- 6.Arostegui I, Esteban C, García-Gutierrez S, et al. Subtypes of patients experiencing exacerbations of COPD and associations with outcomes. PLoS One 2014;9:e98580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roede BM, Bindels PJ, Brouwer HJ, et al. Antibiotics and steroids for exacerbations of COPD in primary care: compliance with Dutch guidelines. Br J Gen Pract 2006;56:662-5. [PMC free article] [PubMed] [Google Scholar]
- 8.de Oca MM, Tálamo C, Halbert RJ, et al. Frequency of self-reported COPD exacerbation and airflow obstruction in five Latin American cities: the Proyecto Latinoamericano de Investigacion en Obstruccion Pulmonar (PLATINO) study. Chest 2009;136:71-8. [DOI] [PubMed] [Google Scholar]
- 9.Langsetmo L, Platt RW, Ernst P, et al. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med 2008;177:396-401. [DOI] [PubMed] [Google Scholar]
- 10.Suissa S, Dell'Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012;67:957-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones PW. COPD assessment test --rationale, development, validation and performance. COPD 2013;10:269-71. [DOI] [PubMed] [Google Scholar]
- 12.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl 2003;41:46s-53s. [DOI] [PubMed] [Google Scholar]
- 13.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (updated 2010). Available online: http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf
- 14.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (updated 2011). Available online: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf
- 15.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. [DOI] [PubMed] [Google Scholar]
- 16.Roberts CM, Buckingham RJ, Pursey NA, et al. (Clinical Effectiveness and Evaluation unit, Royal College of Physicians of London). The National Chronic Obstructive Pulmonary Disease Resources and Outcomes Project (NCROP) Final Report. Royal College of Physicians of London, British Thoracic Society and British Lung Foundation; 2009 May.