Abstract
Background
As part of the growing lifestyle diversity in modern society, there is wide variation in the time of day individuals choose to exercise. Recent surveys in the US and Japan have reported that on weekdays, more people exercise in the evening, with fewer individuals exercising in the morning or afternoon. Exercise performed in the post-prandial state has little effect on accumulated fat oxidation over 24 h (24-h fat oxidation) when energy intake is matched to energy expenditure (energy-balanced condition). The present study explored the possibility that exercise increases 24-h fat oxidation only when performed in a post-absorptive state, i.e. before breakfast.
Methods
Indirect calorimetry using a metabolic chamber was performed in 10 young, non-obese men over 24 h. Subjects remained sedentary (control) or performed 60-min exercise before breakfast (morning), after lunch (afternoon), or after dinner (evening) at 50% of VO2max. All trials were designed to be energy balanced over 24 h. Time course of energy and substrate balance relative to the start of calorimetry were estimated from the differences between input (meal consumption) and output (oxidation).
Findings
Fat oxidation over 24 h was increased only when exercise was performed before breakfast (control, 456 ± 61; morning, 717 ± 64; afternoon, 446 ± 57; and evening, 432 ± 44 kcal/day). Fat oxidation over 24 h was negatively correlated with the magnitude of the transient deficit in energy and carbohydrate.
Interpretation
Under energy-balanced conditions, 24-h fat oxidation was increased by exercise only when performed before breakfast. Transient carbohydrate deficits, i.e., glycogen depletion, observed after morning exercise may have contributed to increased 24-h fat oxidation.
Keywords: 24 h fat oxidation, Metabolic chamber, Energy balance, Transient energy deficits
Highlights
-
•
Exercise performed before breakfast increases 24 h fat oxidation.
-
•
Exercise-induced transient energy deficit cues to increase 24 h fat oxidation.
-
•
Exercise in fed state doesn't increase 24 h fat oxidation in energy-balanced condition.
-
•
Urinary N2 excretion was not affected by time of the day when exercise was performed.
Indirect calorimetry using a metabolic chamber was performed in 10 young men over 24 h. Subjects remained sedentary or performed 60-min exercise before breakfast, after lunch or dinner at 50% of VO2max. All trials were designed to be energy balanced, i.e., intake and expenditure of energy over 24-h were matched. When exercise was performed after lunch or dinner, 24-h fat oxidation was similar to that of sedentary day, i.e. exercise didn't increase fat oxidation. Only when exercise was performed before breakfast, 24-h fat oxidation increased, and a significant transient energy deficit after morning exercise seems to stimulate 24-h fat oxidation.
1. Introduction
Obesity is defined as the abnormal accumulation of body fat and has become a major clinical burden worldwide (Swinburn et al., 2011). In order to prevent weight gain, the combination of reduced energy intake and increased physical activity has been recommended. Exercise guidelines (Garber et al., 2011, The Office for Lifestyle-Related Disease Control, 2006) and recommendations (World Health Organization, 2010) do not specifically comment on the most beneficial time of day for exercise. As part of the growing lifestyle diversity in modern society, there is wide variation in the time of day individuals choose to exercise. Recent surveys in the US and Japan have reported that on weekdays, most people exercise in the evening, with fewer individuals exercising in the morning or afternoon (Sports and Exercise, 2015, Anon., 2013). The skewed distribution of habitual exercise times toward the evening in the general population is in contrast with experimental conditions used in exercise studies, which are typically performed in the morning or afternoon and rarely in the evening.
To assess the effect of exercise on body fat balance, the measurement of accumulated fat oxidation over 24 h (24-h fat oxidation) is required. Persistent increases in fat oxidation during the post-exercise period require long-term calorimetry to evaluate the effect of exercise on body fat balance (Bielinski et al., 1985, Melanson et al., 2009a, Gaesser and Brooks, 1984, Henderson and Alderman, 2014). One of the earliest studies that reported 24-h fat oxidation was increased in response to exercise (Bielinski et al., 1985). However, the effect of exercise on 24-h fat oxidation is likely attributable to a state of negative energy balance on the day of exercise (Henderson and Alderman, 2014). If exercise is accompanied by an increase in energy intake to achieve energy balance, i.e., intake and expenditure of energy over 24 h are matched, 24-h fat oxidation remains similar to that observed on sedentary days (Melanson et al., 2009a, Melanson et al., 2009b, Dionne et al., 1999). Thus, exercise per se apparently has little effect on 24-h fat oxidation. Of note, the consensus among previous literature is derived from studies in which exercise was performed in the post-prandial state. Our previous studies indicate exercise increases 24-h fat oxidation if performed in a post-absorptive state. In experiments under energy-balanced conditions, greater 24-h fat oxidation is observed when exercise is performed before breakfast than that after breakfast (Shimada et al., 2013) or lunch (Iwayama et al., 2015). However, these studies did not include evaluations of the sedentary condition as a control.
The aim of the present study was to examine the effect of exercise performed before breakfast on 24 h fat oxidation compared to sedentary day. Accordingly, 24-h indirect calorimetry was performed on four occasions with a session of 60-min exercise performed before breakfast, after lunch, or after dinner. Identical measurements were performed on sedentary day. All experimental conditions were designed to be energy balanced over 24 h. In order to assess the mechanisms underlying a potential effect of exercise on 24-h fat oxidation, the association between exercise-induced transient energy deficits and 24-h fat oxidation was examined. The results of the present study contribute to our understanding of the effect of time of day on the beneficial effects of exercise on fat oxidation.
2. Methods
2.1. Subject Characteristic
Ten young healthy men were recruited to the present study after providing written informed consent. This study was approved by the ethics committee of the University of Tsukuba. They were recreationally active but untrained individuals, who perform moderate exercise 2–4 days per week. The mean physical characteristics of included subjects were as follows: age, 23.8 ± 0.4 years; height, 173.2 ± 2.0 cm; body weight, 67.2 ± 2.8 kg; and body fat, 15.9 ± 0.9%. The mean maximal oxygen uptake (VO2max) was 50.8 ± 2.3 ml/kg/min. All subjects had no current medical conditions, and none were taking any medications at the time of study.
2.2. Pre-study Evaluation
The workload corresponding to 50% of the individual maximal VO2 was determined from an incremental exercise test on a bicycle ergometer (Aero bike 75XLIII, Combi, Tokyo, Japan) within a week before the first experiment (Shimada et al., 2013). All subjects performed an incremental exercise test which was comprised of submaximal and maximal tests. Initial workload of the submaximal test was 60 W and it was increased by 20 W every 4 min until ventilatory threshold was observed, ratings of Borg scale of perceived exertion reached 17 (very hard) or the workload reached 200 W. After a 5 min rest, subjects performed a maximal test that started from the last workload of the submaximal test, and it was increased by 10 W every min until exhaustion. The highest VO2 for consecutive 60 s during the maximal test was taken as subject's VO2 max. Respiratory gas analysis was continuously performed on a breath-by-breath basis using the computerized standard open circuit technique (AE-310 s, MINATO MEDICAL SCIENCE, Osaka, Japan).
2.3. Experimental Protocol
The present study had a randomized, repeated measures design comprised of four 24 h calorimetry trials with exercise sessions performed before breakfast (morning), after lunch (afternoon), after dinner (evening), or without an exercise session (control). A washout period of at least 1 week was inserted between each calorimetry, and all experiments were completed within 2 months. Subjects were asked to maintain their body weight throughout the study, with no significant difference in body weights observed between individual calorimetry trials (P > 0.1).
Subjects entered the metabolic chamber on the day prior to exercise sessions (day 1, 22:00). Once in the metabolic chamber, subjects slept for 7 h from 23:00 to 6:00. On day 2, three meals (breakfast at 8:00, lunch at 12:00, and dinner at 18:00) were provided, and subjects exercised at 50% of VO2max for 60 min beginning at 6:30 (morning), at 14:30 (afternoon), or at 20:30 (evening) using a bicycle ergometer or remained in a sedentary position (control). On day 3, subjects followed the same protocol as day 2 except for the time exercise sessions were performed and exited the chamber (6:30 for control and morning exercise trials, 14:30 for the afternoon exercise trial, and 20:30 for the evening exercise trial). Subjects were instructed to remain awake and maintain a sedentary position except when performed prescribed exercise sessions and to sleep only at times specified by the protocol. Twenty four-hour energy expenditure and nutrient oxidation were calculated from 6:00 on day 2 to 6:00 on day 3 and compared among the four experimental conditions.
Experimental meals were designed to achieve individual energy balance assuming a resting metabolic rate of 24.0 kcal/kg/day according to estimated energy requirements for Japanese individuals (Anon., 2010). Physical activity factor was assumed to be 1.75 (2780 ± 79 kcal/day) on day 1, 1.64 in trials with exercise sessions (2590 ± 50 kcal/day) or 1.27 in control trial (2027 ± 69 kcal/day) on day 2, and 1.27 on day 3. Expressed as percentages of total energy intake, experimental meals consisted of 15% protein, 25% fat, and 60% carbohydrate. The contributions of breakfast, lunch, and dinner to total 24-h energy intake were 33%, 33%, and 34%, respectively.
2.4. Measurements
Energy metabolism was measured using a room-size metabolic chamber (Fuji Medical Science, Chiba, Japan). The airtight chamber measured 2.00 × 3.45 × 2.10 m, with an internal volume of 14.49 m3. The chamber was furnished with a bed, desk, chair, toilet, and bicycle ergometer. The temperature and relative humidity of in-coming fresh air was controlled at 25.0 ± 0.5 °C and 55.0 ± 3.0%, respectively. Concentrations of oxygen (O2) and carbon dioxide (CO2) in out-going air were measured using an online process mass spectrometer (VG Prima δB, Thermo Electron, Winsford, UK). At every 5 min, O2 consumption (VO2) and CO2 production (VCO2) rates were calculated using an algorithm providing improved transient response (Tokuyama et al., 2009). Macronutrient oxidation and energy expenditure were calculated from VO2, VCO2, and urinary nitrogen excretion. Energy and nutrient balance relative to the start of 24-h calorimetry were estimated as the difference between input (meal consumption) and output (oxidation) (Shimada et al., 2013, Iwayama et al., 2015). For example, relative energy balance was defined as a function of time (t) from 6:00 on day 2.
Relative energy balance (t) = accumulated energy intake (t) − accumulated energy expenditure (t).
Non-exercise activity was estimated using a wrist watch-like device (ActiGraph, Ambulatory Monitoring, NY, USA) as the number of times the activity signal crossed the zero reference point per minute (Iwayama et al., 2015). No significant differences in non-exercise physical activity were observed among morning, afternoon, evening, and control trials (84 ± 7, 92 ± 8, 94 ± 8 and 81 ± 10 cpm, respectively; P > 0.6).
2.5. Statistical Analyses
Data in the main text and figures are presented as means ± SE. Mean values were compared using the Student's t-test between two trials. For multiple comparisons, one-way repeated measures analysis of variance, with post hoc pair-wise comparisons using the Bonferroni correction, was performed. Pooled data from our previous (Shimada et al., 2013, Iwayama et al., 2015) and the present study were used in regression analyses to evaluate the effect of transient energy and substrate deficits on 24-h fat oxidation. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS statistical software (Version 22, IBM Japan, Tokyo, Japan).
2.6. Funding
This study was supported in part by the Japan Society for the Promotion of Science (Grants-in-aid for Scientific Research B 25282215 and JSPS Fellows 26–3079) and Ministry of Education, Culture, Sports, Science and Technology, Japan (BAMIS Project 2010–2013). All funders had no role in study design, data collection, data analysis, interpretation and writing the manuscript.
3. Results
3.1. Energy Metabolism During Exercise
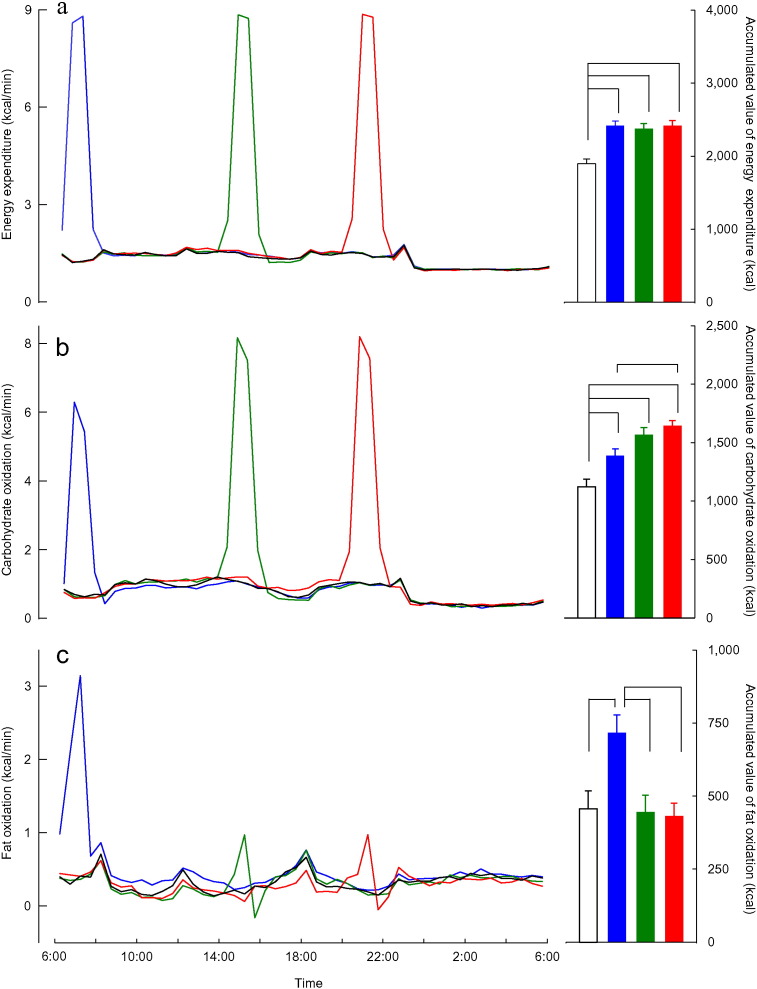
No significant differences in total energy expenditure during exercise sessions were observed between the three trials (morning, 525 ± 28; afternoon, 527 ± 27; evening, 529 ± 27 kcal/60 min; P > 0.5). Fat oxidation during exercise was higher during morning trials (158 ± 13 kcal/60 min) than during afternoon (42 ± 7 kcal/60 min) and evening trials (42 ± 5 kcal/60 min; P > 0.01; Fig. 1).
Fig. 1.
Energy expenditure and substrate oxidation.
Time courses (left, mean values plotted at 30-min interval) and accumulated values (right, kcal/24 h) of energy expenditure (a), carbohydrate oxidation (b), and fat oxidation (c) are shown for morning exercise ( ,
,  ), afternoon exercise (
), afternoon exercise ( ,
,  ), evening exercise (
), evening exercise ( ,
,  ), and control trials (—, □). Significantly different mean values are connected by lines (P < 0.01).
), and control trials (—, □). Significantly different mean values are connected by lines (P < 0.01).
3.2. Residual Effect of Exercise on Energy Metabolism
Close inspection of time courses of fat oxidation during the resting period during the morning (8:00–12:00) revealed a persistent increase in fat oxidation after morning exercise, with increased fat oxidation observed after morning exercise (98 ± 12 kcal/4 h) compared to that during control trials (64 ± 10 kcal/4 h; P < 0.05). Energy expenditure and substrate oxidation during sleep were indistinguishable among the four trials (P > 0.6), indicating no residual effect of exercise on energy metabolism at the end of 24-h calorimetry (day 3, 06:00).
3.3. Energy Metabolism Over 24-h
Significantly greater 24-h energy expenditure was observed during trials with exercise sessions than that during control trials. Significantly greater 24-h fat oxidation was observed during morning exercise trials (717 ± 64 kcal/day) than that during control trials (456 ± 61 kcal/day) and other exercise trials (afternoon, 446 ± 57; evening, 432 ± 44 kcal/day). In descending order, observed 24-h carbohydrate oxidation during trials were as follows: evening (1646 ± 43 kcal/day), afternoon (1567 ± 62 kcal/day), morning (1388 ± 58 kcal/day), and control trials (1123 ± 65 kcal/day). No significant differences in 24-h protein oxidation was observed among the four trials (morning, 311 ± 25; afternoon, 365 ± 28; evening, 340 ± 24 control; 317 ± 38, kcal/day; P > 0.4).
3.4. Relative Energy and Macronutrient Balance
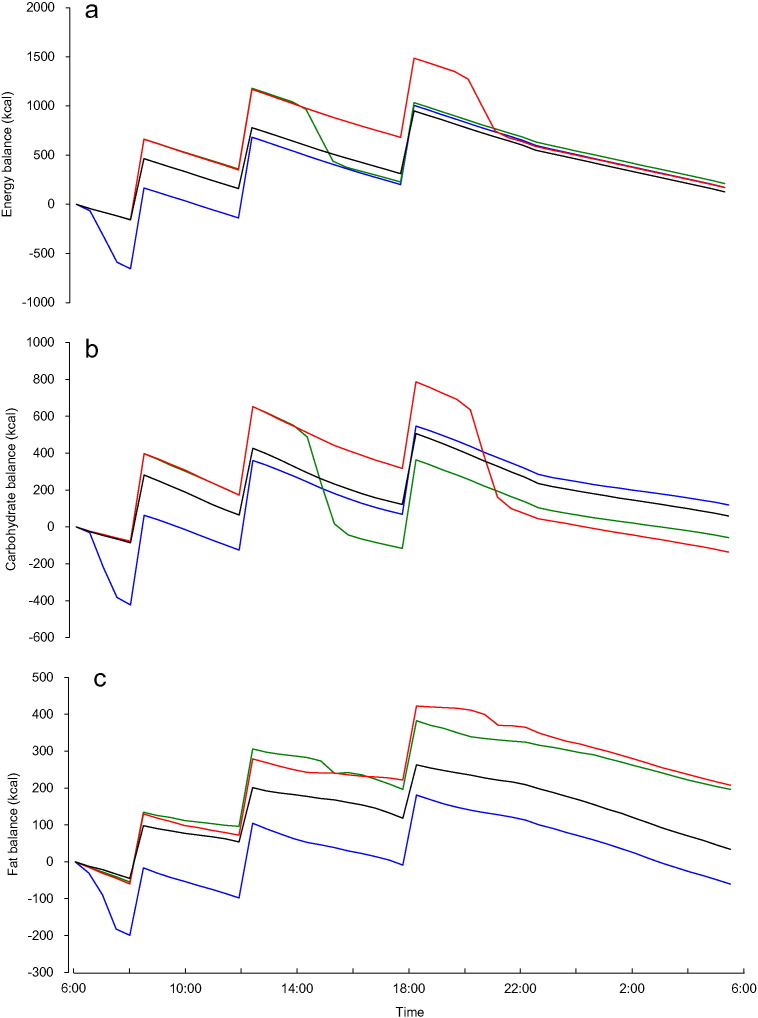
Relative energy balances for all trials converged to the same level at the end of study days as planned by experimental design, however, observed time courses differed among trials with transient energy deficits observed (Fig. 2). The lowest values of relative energy balance observed during time courses, i.e., greatest transient energy deficit, were greater in morning exercise trials than in other trials (morning, − 654 ± 30; afternoon, − 155 ± 6; evening, − 155 ± 6; control, − 155 ± 6, kcal; P < 0.01). Further, a negative correlation was observed between transient energy deficits and 24-h fat oxidation (r = − 0.59; P < 0.01). In relative carbohydrate balance time courses, the greatest transient deficits were observed after morning exercise (morning, − 422 ± 26; afternoon, − 156 ± 32; evening, − 77 ± 4; control, − 86 ± 7 kcal; P < 0.01). Further, the magnitude of the transient deficits in relative carbohydrate balance was found to be negatively correlated with 24-h fat oxidation (r = − 0.42; P < 0.01). The relative carbohydrate balances observed morning exercise trials eventually increased to levels higher than that of other trials at the end of study days, indicating transient carbohydrate deficits after morning exercise were supercompensated. The lowest relative fat balance values during morning exercise trials were lower than observed during other trials (morning, − 207 ± 16; afternoon, − 47 ± 8; evening, − 55 ± 6; control, − 44 ± 8 kcal; P < 0.01). Relative fat balances during morning exercise trials remained the lowest of all trials throughout the study, with lowest relative fat balance values found to be negatively correlated with 24-h fat oxidation (r = − 0.76; P < 0.01).
Fig. 2.
Time course of relative balances of energy, carbohydrate, and fat.
Mean values for relative energy balance (a), carbohydrate balance (b), and fat balance (c) are plotted at 30-min intervals for morning exercise ( ,
,  ), afternoon exercise (
), afternoon exercise ( ,
,  ), evening exercise (
), evening exercise ( ,
,  ), and control trials (—, □).
), and control trials (—, □).
3.5. Transient Energy Deficit as a Potential Mechanism to Increase 24-h Fat Oxidation
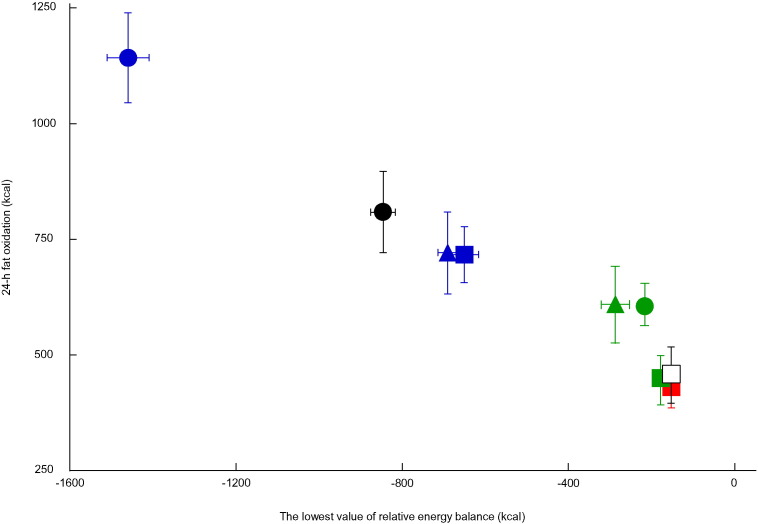
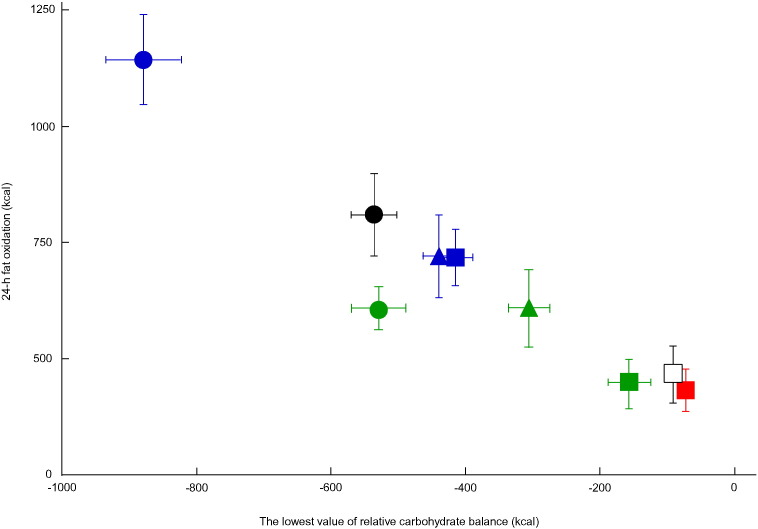
Combined data used for regression analysis are shown in Fig. 3, Fig. 4, Fig. 5. Lowest energy balance values were found to be negatively correlated with 24-h fat oxidation (r = − 0.71; P < 0.01). Similarly, lowest carbohydrate (r = − 0.51, P < 0.01) and fat balance values (r = − 0.87; P < 0.01) were found to be negatively correlated with 24-h fat oxidation.
Fig. 3.
Association between transient energy deficits and 24-h fat oxidation.
Mean ± SE of the lowest value of relative energy balance (kcal) and 24-h fat oxidation (kcal/24 h) for 9 groups from 3 different studies are plotted. Subjects in the present study completed 4 trials with exercise in the morning ( ), afternoon (
), afternoon ( ), and evening (
), and evening ( ) or without exercise (□). In our previous studies, 12 male subjects completed 2 trials of 60-min exercise session at 50% VO2max before breakfast (
) or without exercise (□). In our previous studies, 12 male subjects completed 2 trials of 60-min exercise session at 50% VO2max before breakfast ( ) or after breakfast (
) or after breakfast ( ) (Shimada et al., 2013), and 9 male subjects completed 3 trials of 100-min exercise sessions at 65% of VO2max before breakfast (
) (Shimada et al., 2013), and 9 male subjects completed 3 trials of 100-min exercise sessions at 65% of VO2max before breakfast ( ), after lunch (
), after lunch ( ), or two 50-min exercise sessions before breakfast and after lunch (●) (Iwayama et al., 2015). The correlation coefficient between lowest energy balance values and 24-h fat oxidation was − 0.71 (n = 91; P < 0.01).
), or two 50-min exercise sessions before breakfast and after lunch (●) (Iwayama et al., 2015). The correlation coefficient between lowest energy balance values and 24-h fat oxidation was − 0.71 (n = 91; P < 0.01).
Fig. 4.
Association between transient carbohydrate deficits and 24-h fat oxidation.
Mean ± SE of the lowest value of relative carbohydrate balance (kcal) and 24-h fat oxidation (kcal/24 h) for 9 groups from 3 different studies are plotted. Subjects in the present study completed 4 trials with exercise in the morning ( ), afternoon (
), afternoon ( ), and evening (
), and evening ( ) or without exercise (□). In our previous studies, 12 male subjects completed 2 trials of 60-min exercise session at 50% VO2max before breakfast (
) or without exercise (□). In our previous studies, 12 male subjects completed 2 trials of 60-min exercise session at 50% VO2max before breakfast ( ) or after breakfast (
) or after breakfast ( ) (Shimada et al., 2013), and 9 male subjects completed 3 trials of 100-min exercise sessions at 65% of VO2max before breakfast (
) (Shimada et al., 2013), and 9 male subjects completed 3 trials of 100-min exercise sessions at 65% of VO2max before breakfast ( ), after lunch (
), after lunch ( ), or two 50-min exercise sessions before breakfast and after lunch (●) (Iwayama et al., 2015). The correlation coefficient between lowest carbohydrate balance values and 24-h fat oxidation was − 0.51 (n = 91; P < 0.01).
), or two 50-min exercise sessions before breakfast and after lunch (●) (Iwayama et al., 2015). The correlation coefficient between lowest carbohydrate balance values and 24-h fat oxidation was − 0.51 (n = 91; P < 0.01).
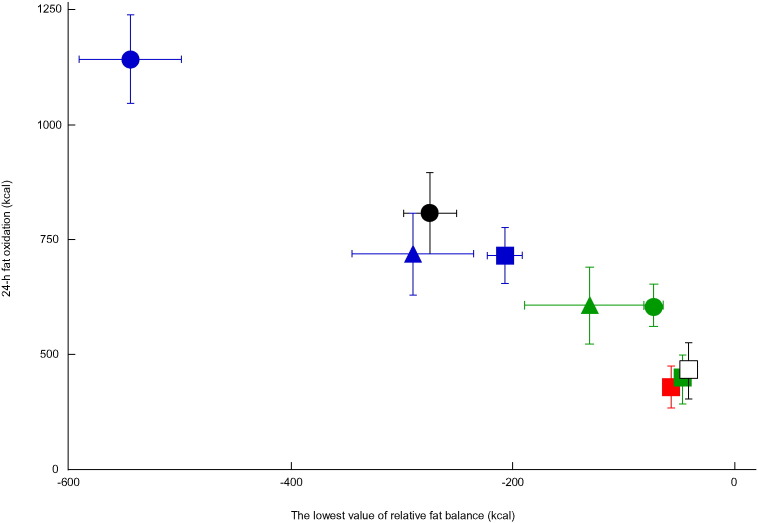
Fig. 5.
Association between transient fat deficits and 24-h fat oxidation.
Mean ± SE of the lowest value of relative fat balance (kcal) and 24-h fat oxidation (kcal/24 h) for 9 groups from 3 different studies are plotted. Subjects in the present study completed 4 trials with exercise in the morning ( ), afternoon (
), afternoon ( ), and evening (
), and evening ( ) or without exercise (□). In our previous studies, 12 male subjects completed 2 trials of 60-min exercise session at 50% VO2max before breakfast (
) or without exercise (□). In our previous studies, 12 male subjects completed 2 trials of 60-min exercise session at 50% VO2max before breakfast ( ) or after breakfast (
) or after breakfast ( ) (Shimada et al., 2013), and 9 male subjects completed 3 trials of 100-min exercise sessions at 65% of VO2max before breakfast (
) (Shimada et al., 2013), and 9 male subjects completed 3 trials of 100-min exercise sessions at 65% of VO2max before breakfast ( ), after lunch (
), after lunch ( ), or two 50-min exercise sessions before breakfast and after lunch (●) (Iwayama et al., 2015). The correlation coefficient between lowest fat balance values and 24-h fat oxidation was − 0.87 (n = 91; P < 0.01).
), or two 50-min exercise sessions before breakfast and after lunch (●) (Iwayama et al., 2015). The correlation coefficient between lowest fat balance values and 24-h fat oxidation was − 0.87 (n = 91; P < 0.01).
4. Discussion
Our main finding was that exercise increased 24-h fat oxidation only when performed after overnight fasting—the time of day when body energy content reaches its nadir. Exercise performed prior to breakfast further decreased relative energy balance inducing a transient energy deficit, the magnitude of which was found to be negatively correlated with 24-h fat oxidation.
Of the macronutrients stored in the body, the pool size of carbohydrate is the smallest, and metabolic responses to changes in carbohydrate storage are more sensitive than those for fat and protein (Flatt, 1988). Glycogen storage reached its nadir after overnight fasting and was further reduced by early morning exercise. Carbohydrate depletion after morning exercise in the present study was roughly equivalent to 18% of whole-body glycogen storage, assuming whole-body glycogen stores in the post-absorptive state are approximately 2300 kcal (Hargreaves, 2000). The magnitude of transient carbohydrate deficit, i.e., the lowest value of relative carbohydrate balance, was found to be negatively correlated with 24-h fat oxidation. Recent studies have provided evidence supporting a potential molecular link between glycogen depletion and activation of fat oxidation (Philp et al., 2012, Izumida et al., 2013). Tissue glycogen functions not only as carbohydrate reserve but also as molecular sensor capable of activating signaling pathways in response to exercise including the nuclear translocation of AMPK and up-regulation of genes responsible for fat oxidation (Philp et al., 2012). Analysis of combined data sets further strengthened the observed association between transient energy and carbohydrate deficits and 24-h fat oxidation. Thus, transient energy deficits, particularly with regards to carbohydrate, appear to contribute to increased 24-h fat oxidation, even when energy intake and expenditure is matched over 24-h periods.
One of metabolic characteristics of subjects predisposed to obesity is a decreased 24-h fat-to-carbohydrate oxidation ratio compared with normal-weight subjects, and training-induced increases in fat oxidation over 24-h have been shown to be a predictor of exercise-induced weight loss (Zurlo et al., 1990, Barwell et al., 2009). The findings of the present study indicate exercise before breakfast, a common practice among athletes and recreational runners, may have greater efficacy in reducing body fat.
Recent developments in chronobiology have revealed that the effects of interventions, such as drug administration and nutrient intake, are dependent on the time of days they are applied. Accordingly, the effect of exercise may also vary depending on when it is performed and remains a popular topic among the lay press. The exercise protocol adopted in the present study is consistent with current guidelines and recommendations and is expected have beneficial effects on weight maintenance (Garber et al., 2011, The Office for Lifestyle-Related Disease Control, 2006, World Health Organization, 2010), however, exercise performed in a post-prandial condition was found to have little effect on 24-h fat oxidation. If exercise were a pill to burn body fat, it would be effective only when taken before breakfast or together with restricted food consumption.
Our observation that exercise performed in a post-prandial condition had little effect on 24-h fat oxidation corroborates previous studies performed under energy-balanced conditions (Melanson et al., 2009a, Melanson et al., 2009b, Dionne et al., 1999). It is well known that fat oxidation during exercise is significantly reduced by carbohydrate consumption prior to the exercise (Coyle et al., 1985, Achten and Jeukendrup, 2004). In addition, increases in fat oxidation during exercise may be offset during the post-exercise period, as indicated by previous studies (Melanson et al., 2009a, Melanson et al., 2009b, Dionne et al., 1999). Furthermore, increased energy intake in trials with exercise sessions appeared to suppress fat oxidation during the resting period. Fat oxidation during the daytime (8:00–20:00), when subjects in evening exercise trials had consumed 563 ± 45 more calories at breakfast, lunch and dinner than in control trials in which participants had yet to perform exercise, was lower in evening exercise trials (170 ± 19 kcal/12 h) compared control trials (213 ± 32 kcal/12 h; P = 0.11), although this difference did not reach statistical significance. There is a large body of evidence supporting an effect of low intensity endurance exercise on stimulating fat oxidation, however, cautious interpretation is required when evaluating the translational potential of these findings. First, these findings are predominantly derived from experiments after overnight fasting to eliminate the effect of nutrient intake prior to exercise. Second, these studies did not account for changes in energy metabolism during the post-exercise period. Some studies continued to measure fat oxidation after the cessation of exercise, but the following-up observations after exercise were often intermittent and generally short (Dumortier et al., 2005, Kuo et al., 2005).
When evaluating the translational potential of the present study, the following limitations should be considered. Some previous studies report that exercise in glycogen-depleted state stimulates protein catabolism (Blomstrand and Saltin, 1999). However, accumulated protein oxidation over 24-h were not significantly different among 4 trials in the present study. Exercise after an overnight fast reduced whole-body glycogen content by ~ 18%, but didn't depleted glycogen. The findings of the present study are unable to be extrapolated to provide information regarding the long-term effects of post-absorptive exercise on reducing body fat. A single session of exercise performed before breakfast led to increased fat oxidation while carbohydrate oxidation was reduced under energy-balanced condition. However, the positive carbohydrate balance would eventually be counterbalanced by autoregulatory increases carbohydrate oxidation and suppression of fat oxidation (Shetty et al., 1994). Alternatively, increased glycogen storage under free-living conditions may decrease the magnitude of subsequent energy intake as carbohydrate balance is a strong predictor of subsequent ad libitum food intake (Pannacciulli et al., 2007). Beneficial long-term effects of exercise in the carbohydrate-depleted state on glucose tolerance, insulin sensitivity, endurance capacity, and expression of skeletal muscle genes responsible for fat oxidation have been reported (Van Proeyen et al., 2010, Hansen et al., 2005, Yeo et al., 2008). However, the long-term effects of exercise performed in a post-absorptive state on body fat content remain to be elucidated. Furthermore, potential sex differences in fat oxidation during and after exercise warrant further studies including female subjects (Henderson et al., 2007).
In conclusion, 24-h fat oxidation was increased by exercise only when performed before breakfast under energy-balanced conditions. Significant exercise-induced transient energy deficits, which are more likely when exercise is performed before breakfast, serve as a cue to increase 24-h fat oxidation.
Author contributions
K.I. and R Kurihara are contributed equally to the study as chief author. K.I., R Kurihara, Y.N., R Kawabuchi, I.P., K.T. did the conception and design of research; K.I., R Kurihara, R Kawabuchi, I.P. performed experiments; K.I. prepared figures; K.I. and K.T. drafted manuscript; M.K. analyzed data; H.O. and M.K. developed the statistical analysis plans; Y.N., M.S. and K.T. edited and revised manuscript; K.T. approved final version of manuscript.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- Achten J., Jeukendrup A.E. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20:716–727. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Anon. Ministry of Health Labour and Welfare of Japan; Tokyo: 2010. Dietary Reference Intakes for Japanese. [Google Scholar]
- Anon. Statistics Bureau, Ministry of Internal Affairs and Communications; Japan, Tokyo: 2013. Survey on Time Use and Leisure Activities. [Google Scholar]
- Barwell N.D., Malkova D., Leggate M., Gill J.M. Individual responsiveness to exercise-induced fat loss is associated with change in resting substrate utilization. Metabolism. 2009;58:1320–1328. doi: 10.1016/j.metabol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinski R., Schutz Y., Jéquier E. Energy metabolism during the postexercise recovery in man. Am. J. Clin. Nutr. 1985;42:69–82. doi: 10.1093/ajcn/42.1.69. [DOI] [PubMed] [Google Scholar]
- Blomstrand E., Saltin B. Effect of muscle glycogen on glucose, lactate and amino acid metabolism during exercise and recovery in human subjects. J. Physiol. 1999;514:293–302. doi: 10.1111/j.1469-7793.1999.293af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle E., Coggan A., Hemmert M., Lowe R., Walters T. Substrate usage during prolonged exercise following a preexercise meal. J. Appl. Physiol. 1985;59:429. doi: 10.1152/jappl.1985.59.2.429. [DOI] [PubMed] [Google Scholar]
- Dionne I., Van Vugt S., Tremblay A. Postexercise macronutrient oxidation: a factor dependent on postexercise macronutrient intake. Am. J. Clin. Nutr. 1999;69:927–930. doi: 10.1093/ajcn/69.5.927. [DOI] [PubMed] [Google Scholar]
- Dumortier M., Thoni G., Brun J.F. Substrate oxidation during exercise: impact of time interval from the last meal in obese women. Int. J. Obes. (Lond) 2005;29:966–974. doi: 10.1038/sj.ijo.0802991. [DOI] [PubMed] [Google Scholar]
- Flatt J.P. Importance of nutrient balance in body weight regulation. Diabetes Metab. Rev. 1988;4:571–581. doi: 10.1002/dmr.5610040603. [DOI] [PubMed] [Google Scholar]
- Gaesser C.A., Brooks G.A. Metabolic basis of post-exercise oxygen consumption: a review. Med. Sci. Sports Exerc. 1984;16:29–43. [PubMed] [Google Scholar]
- Garber C.E., Blissmer B., Deschenes M.R. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Hansen A.K., Fischer C.P., Plomgaard P., Andersen J.L., Saltin B., Pedersen B.K. Skeletal muscle adaptation: training twice every second day vs. training daily. J. Appl. Physiol. 2005;98:93–99. doi: 10.1152/japplphysiol.00163.2004. [DOI] [PubMed] [Google Scholar]
- Hargreaves M. Carbohydrate metabolism and exercise. In: Garrett W.E., Kirkendall D.T., editors. Exercise and Sport Science. 2000. pp. 3–8. [Google Scholar]
- Henderson G.C., Alderman B.L. Determinants of resting lipid oxidation in response to a prior bout of endurance exercise. J. Appl. Physiol. 2014;116:95–103. doi: 10.1152/japplphysiol.00956.2013. [DOI] [PubMed] [Google Scholar]
- Henderson G.C., Fattor J.A., Michael A., Horning M.A. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J. Physiol. 2007;584:963–981. doi: 10.1113/jphysiol.2007.137331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwayama K., Kawabuchi R., Park I. Transient energy deficit induced by exercise increases 24-h fat oxidation in young trained men. J. Appl. Physiol. 2015;118:80–85. doi: 10.1152/japplphysiol.00697.2014. [DOI] [PubMed] [Google Scholar]
- Izumida Y., Yahagi N., Takeuchi Y. Glycogen shortage during fasting triggers liver–brain–adipose neurocircuitry to facilitate fat utilization. Nat. Commun. 2013;4:2316. doi: 10.1038/ncomms3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.C., Fattor J.A., Henderson G.C. Lipid oxidation in fit young adults during postexercise recovery. J. Appl. Physiol. 2005;99:349–356. doi: 10.1152/japplphysiol.00997.2004. [DOI] [PubMed] [Google Scholar]
- Melanson E.L., MacLean P.S., Hill J.O. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc. Sport Sci. Rev. 2009;37:93–101. doi: 10.1097/JES.0b013e31819c2f0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson E.L., Gozansky W.S., Barry D.W., MacLean P.S., Grunwald G.K., Hill J.O. When energy balance is maintained, exercise does not induce negative fat balance in lean sedentary, obese sedentary, or lean endurance-trained individuals. J. Appl. Physiol. 2009;107:1847–1856. doi: 10.1152/japplphysiol.00958.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N., Salbe A.D., Ortega E., Venti C.A., Bogardus C., Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am. J. Clin. Nutr. 2007;86:625–632. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp A., Hargreaves M., Baar K. More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am. J. Physiol. 2012;302:E1343–E1351. doi: 10.1152/ajpendo.00004.2012. [DOI] [PubMed] [Google Scholar]
- Shetty P.S., Prentice A.M., Goldberg G.R. Alterations in fuel selection and voluntary food intake in response to isoenergetic manipulation of glycogen stores in humans. Am. J. Clin. Nutr. 1994;60:534–543. doi: 10.1093/ajcn/60.4.534. [DOI] [PubMed] [Google Scholar]
- Shimada K., Yamamoto Y., Iwayama K. Effect of exercise performed before or after breakfast on 24-h fat oxidation. Metabolism. 2013;62:793–800. doi: 10.1016/j.metabol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Sports and Exercise, Bureau of Labor Statistics, United States Department of Labor 2015. http://www.bls.gov/spotlight/2008/sports/ Available from. Accessed 2 August.
- Swinburn B.A., Sacks G., Hall K.D. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- The Office for Lifestyle-Related Disease Control, General Affairs Division, Health Service Bureau, Ministry of Health, Labour and Welfare of Japan . 2006. Exercise and Physical Activity Reference for Health Promotion 2006 (EPAR2006): Physical Activity, Exercise, and Physical Fitness. [Google Scholar]
- Tokuyama K., Ogata H., Katayose Y., Satoh M. Algorithm for transient response of whole body indirect calorimeter: deconvolution with a regularization parameter. J. Appl. Physiol. 2009;106:640–650. doi: 10.1152/japplphysiol.90718.2008. [DOI] [PubMed] [Google Scholar]
- Van Proeyen K., Szlufcik K., Nielens H. Training in the fasted state improves glucose tolerance during fat-rich diet. J. Physiol. 2010;21:4289–4302. doi: 10.1113/jphysiol.2010.196493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; 2010. Global Recommendations on Physical Activity for Health. [PubMed] [Google Scholar]
- Yeo W.K., Paton C.D., Garnham A.P., Burke L.M., Carey A.L., Hawley J.A. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J. Appl. Physiol. 2008;105:1462–1470. doi: 10.1152/japplphysiol.90882.2008. [DOI] [PubMed] [Google Scholar]
- Zurlo F., Lillioja S., Esposito-Del P.A. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am. J. Physiol. 1990;259 doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]