Abstract
Neurotrophic factor and cAMP-dependent signaling promote the survival and neurite outgrowth of retinal ganglion cells (RGCs) after injury. However, the mechanisms conferring neuroprotection and neuroregeneration downstream to these signals are unclear. We now reveal that the scaffold protein muscle A-kinase anchoring protein-α (mAKAPα) is required for the survival and axon growth of cultured primary RGCs. Although genetic deletion of mAKAPα early in prenatal RGC development did not affect RGC survival into adulthood, nor promoted the death of RGCs in the uninjured adult retina, loss of mAKAPα in the adult increased RGC death after optic nerve crush. Importantly, mAKAPα was required for the neuroprotective effects of brain-derived neurotrophic factor and cyclic adenosine-monophosphate (cAMP) after injury. These results identify mAKAPα as a scaffold for signaling in the stressed neuron that is required for RGC neuroprotection after optic nerve injury.
Keywords: mAKAP, Retinal ganglion cells, Survival, Axon growth, Optic nerve injury
Graphical abstract
Highlights
-
•
mAKAPα is a stress-specific mediator of RGC survival.
-
•
mAKAP deletion does not affect RGC survival in development or in the uninjured adult retina.
-
•
mAKAP is downregulated after optic nerve injury, and its further deletion exacerbates RGC death.
-
•
mAKAP deletion suppresses the neuroprotective effects of cAMP and BDNF after injury.
After injury or in degenerative diseases, neurons of the central nervous system (CNS) fail to regenerate and often die partly due to a lack of pro-survival, trophic signaling. Better understanding of such signaling is important for the development of therapies that enhance survival and regeneration of neurons after injury. Here we identify a critical regulator of such signaling, mAKAPα, a scaffold protein that coordinates pro-survival signaling to enhance survival and regeneration in CNS neurons after injury. The neuroprotective role of mAKAPα will likely lead to further future insights into the detailed nature of survival signaling in adult neurons.
1. Introduction
Neuronal death following injury, including due to trauma or ischemia, remains an important source of long term disability with few adequate therapies. In the eye, blindness can result from the death of retinal ganglion cells (RGCs) that transmit visual information from the retina via the optic nerve to the lateral geniculate, pretectal, and superchiasmatic nuclei of the brain. Failure of central nervous system (CNS) neurons, including RGCs, to survive and regenerate their axons after injury results in part from a lack of adequate trophic signaling. RGC death can be delayed by application of exogenous neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), neurotrophin-4 (NT-4), fibroblast growth factor (FGF), and glial-derived neurotrophic factor (GDNF) (Mo et al., 2002, Unoki and Lavail, 1994, Wang et al., 2013). This trophic signaling can be enhanced by electrical stimulation or elevation of intracellular cyclic adenosine-monophosphate (cAMP) levels (Goldberg and Barres, 2000, Goldberg et al., 2002, Corredor et al., 2012). How cAMP potentiates the neuronal response to trophic factors is not well understood, although inhibiting cAMP-dependent protein kinase (PKA), an enzyme subject to tight spatial and temporal regulation in cells through its localization by the diverse family of “AKAP” scaffold proteins (Scott et al., 2013), blocks the positive effects of electrical activity and cAMP on neuronal survival and axon growth (Goldberg et al., 2002).
Muscle A-kinase anchoring protein (mAKAP; also known as AKAP6) is a 255 kDa scaffold protein localized to the nuclear envelope of neurons and striated myocytes and that binds PKA as well as a large number of signaling enzymes implicated in stress responses (Passariello et al., 2015). mAKAP also binds the cAMP target Epac1, adenylyl cyclases (types II and V), and the cAMP-specific phosphodiesterase 4D3. Because these enzymes participate in negative and positive feedback, cAMP levels are predicted to be tightly regulated around mAKAP “signalosomes,” providing local control over the phosphorylation of relevant PKA substrates. Differential PKA localization by scaffolds like mAKAP is likely to be especially important in cell types like neurons where subcellular domains, including cell bodies, axons and dendrites, are often separated across great distances. In neurons, other AKAPs such as AKAP79 function in synaptic signaling (Weisenhaus et al., 2010), but it is unknown whether any PKA scaffolds have a role in neuronal survival or axon growth. Neurons express the longer α isoform of mAKAP, mAKAPα, which contains an amino-terminal sequence with additional binding sites, e.g. for 3-phosphoinositide-dependent protein kinase-1 (PDK1) (Michel et al., 2005).
The function of neuronal mAKAPα has not been characterized, albeit deletion of the exon encoding the mAKAPα-specific N-terminus resulted in a failure-to-thrive phenotype (Michel et al., 2005). Besides binding enzymes related to cAMP signaling, mAKAPα also binds in neurons the mitogen-activated protein kinases MEK5 and ERK5 that are known to be important for neuronal survival in response to neurotrophins (Michel et al., 2005, Dodge-Kafka et al., 2005, Wang et al., 2006, Watson et al., 2001). Given that mAKAPα can coordinate cAMP and ERK5 signaling, we hypothesized that mAKAPα might be involved in survival or axon growth signaling in neurons, which require both of these signaling pathways to optimally promote these functions in RGCs(Goldberg et al., 2002). We now present evidence that mAKAPα is expressed in RGCs but discovered in fact that mAKAPα does not regulate RGC survival during normal development or in the absence of injury, but rather mediates stress-specific signaling of survival and axon growth in vitro and survival in vivo after optic nerve injury.
2. Materials & Methods
2.1. Animals
All procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the University of California San Diego and University of Miami Institutional Animal Care and Use Committees. All animals were randomly assigned to experimental groups and littermates were used as controls.
For in vitro experiments, litters of Sprague Dawley rats (Harlan Laboratories, Inc., Allen Park, MI) were used at postnatal day (P) 2–4 for isolation of RGCs. For in vivo experiments, 20 female and 20 male (2 months old) wildtype and mAKAPfl/fl C57BL/6 mice were used (Kritzer et al., 2014b). Cre recombination of the conditional mAKAP allele containing LoxP sites surrounding exon 9 results in exon 9 deletion, frame-shift and premature termination of translation within exon 10, 5′ of the exons encoding the docking sites for many relevant mAKAPα-binding partners, including nesprin-1α (conferring nuclear envelope localization), calcineurin, PDE4D3 and PKA (Li et al., 2010, PARE et al., 2005, Kapiloff et al., 1999). Note that no residual mAKAP N-terminal protein fragment was detected following knock-out (Fig. 1C and data not shown). A Math5-directed cre recombinase transgenic mouse was used to direct developmental mAKAPα knock-out in the retina (Yang et al., 2003, Tsien et al., 1996), while injection of AAV2 expressing both cre and GFP marker protein (AAV2-Cre) was used to direct selective, inducible knock-out in adult RGCs (Park et al., 2008). Heart extracts were obtained from mAKAPβ knock-out and control mice of the following genotypes following tamoxifen administration: Tg(Myh6-cre/Esr1*) / mAKAPfl/fl for knock-out and Tg(Myh6-cre/Esr1*) for control. All surgical procedures were performed under general anesthesia via intraperitoneal injection of ketamine (75 mg/kg) and xylazine (15 mg/kg). Mice also received subcutaneous injection of buprenorphine (0.03 mg/kg; Bedford Laboratories) as postoperative analgesic. Eye ointment containing erythromycin was applied to protect the cornea.
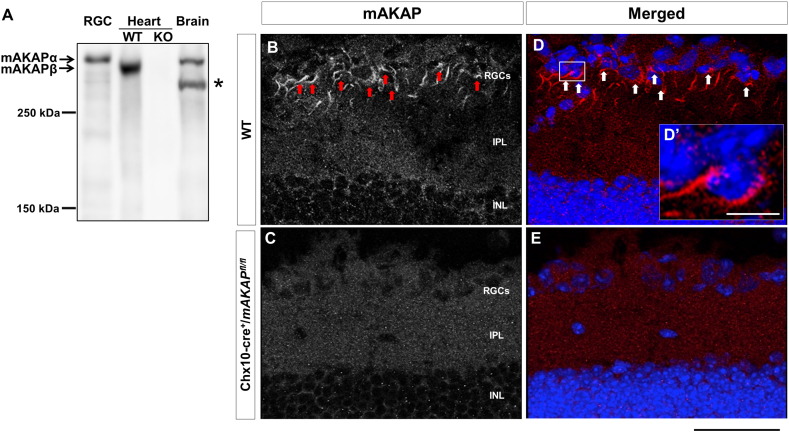
Fig. 1.
mAKAPα is expressed by RGCs. (A) Western blot demonstrated mAKAPα expression in postnatal day 3 rats RGCs and mice brain tissue. mAKAPβ was detected in control mouse (WT) heart tissue, but not in cardiac-specific knock-out (KO) mice. *Non-specific band inconsistently detected in brain. (B–E) Cryosections from both adult wild-type (WT) and Tg(Chx10-Cre);mAKAPfl/fl (mAKAP KO) mouse retinas were immunostained for mAKAP (red) and nuclei counterstained with DAPI (blue). Specific mAKAP-staining was detected in the ganglion cell layer of the WT retina (B and D, arrows) and potentially inner nuclear layer, but not in the Tg(Chx10-Cre);mAKAPfl/fl retinal sections (C and E). Higher magnification of the boxed region in D revealed that mAKAP expression is mainly perinuclear (D’). Scale bar in (B)–(E) represents 50 μm; Scale bar in (D’) represents 10 μm (N = 3).
2.2. Immunopanning of RGCs
RGCs were purified (> 99.5%) from postnatal (P2 to P4) Sprague–Dawley rats through sequential immunopanning, as previously described (Goldberg et al., 2002). RGCs were cultured on poly-D-lysine (PDL; 70 kDa, 10 μg/mL; Sigma, St. Louis, MO) and laminin (1 μg/mL; Invitrogen, Carlsbad, CA) in neurobasal (NB) serum-free defined medium containing insulin (5 μg/mL), sodium pyruvate (1 mM), L-glutamine (1 mM), triiodothyronine (T3; 40 ng/mL; Sigma), N-acetyl cysteine (NAC; 5 μg/mL; Sigma), B27 (1:50), BDNF (50 ng/ml), CNTF (10 ng/ml) and forskolin (5 mM) as described (Meyer-Franke et al., 1995).
2.3. Immunohistochemical Staining of mAKAP in Adult Mice Retina
2-month old mice were euthanized by 100% CO2 inhalation. Eyes were dissected and embedded in OCT for cryosection (10 μm) immediately. Sections were post-fixed in 4% PFA for 15 min and then washed 3 times in PBS. Retinal sections were blocked in 5% normal goat serum and 0.2% BSA in PBS for 30 min, then incubated for 1 h in the same buffer with FL100 rabbit antibody to mAKAP 245–340 (Li et al., 2013). After washing, retinal sections were incubated with Alexa 594-conjugated goat anti–rabbit secondary antibody (1:500; Invitrogen) for 1 h, before washing and mounting.
2.4. Western Blot Analysis
Protein extracts from acutely purified postnatal rat RGCs, adult mouse brain and heart lysed in 20 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% Triton, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM DTT, and protease inhibitors were quantified using the DC Protein Assay Kit II (Bio-Rad, California cat. 500–0112). Lysates were fractionated by SDS-PAGE and transferred to nitrocellulose membranes as previously described (Kapiloff et al., 1999, Yu et al., 2014). Western blots were developed using horseradish peroxidase-conjugated, donkey secondary antibodies, Supersignal West Chemiluminescent Substrates (Thermo Scientific) and a Fujifilm LAS-3000 imaging system.
2.5. RT-PCR
Total RNA was extracted from RGCs transfected with mAKAP siRNA or whole mouse retinas using a Qiagen RNeasy extraction kit (cat. 74104) and cDNA synthesis carried out using iScript reverse transcriptase (Bio-Rad). Quantitative PCR (qPCR) was performed on the CFX Connect Real-Time PCR System (Bio-Rad) using TaqMan Gene Expression Master Mix (Life Tech; Cat#4369016) with the following conditions: initial denaturation 95°C for 6 min followed by 35 cycles of denaturation (95°C for 45 s), annealing (58°C for 1 min) and extension (72°C for 1 min). HPRT expression was measured as a normalizer for each sample. Results were analyzed by the relative quantity (∆∆Ct) method, as previously described (Thellin et al., 1999, Jiang et al., 2012). The primers were as follows: HPRT (Life Tech; ID# Mm00446968_m1) and AKAP6 (ID#Mm01292745_m1).
2.6. RGC Survival and Neurite Growth Assays
Postnatal RGCs were electroporated immediately after isolation with control On-TARGETplus Non-targeting siRNA #1 (Thermofisher) or mAKAP ON-TARGETplus siRNA oligonucleotides (GAC GAA CCU UCC UUC CGA A UU) as previously described (Corredor et al., 2012). RGCs were then plated at low density (3000–5000 cells/well) on 48-well plates in growth media containing forskolin, BDNF and CNTF. To investigate the role of mAKAP in various neurotrophic signaling pathways we also cultured siRNA transfected RGCs in growth media containing either forskolin (5 μM) alone or forskolin with BDNF (50 ng/mL), CNTF (10 ng/mL), EGF (10 ng/mL), or FGF (20 ng/mL). For survival assays, RGCs were cultured for up to 3 days in growth media and then stained with live cell marker calcein (C3100MP, Life Technologies), dead cell marker sytox (S11368, Life Technologies), and Hoechst nuclear dye (H1399, Life Technologies). RGC survival was expressed as the ratio of total calcein-positive cells to calcein- and sytox-positive cells. For neurite outgrowth assays, RGCs were cultured in growth media for 3 days. After fixation in 4% PFA for 30 min and washing with PBS 3 times, RGCs were stained for βIII-tubulin and analyzed using ImageJ Neurite Tracer or by Cellomics automated microscopy to quantify neurite length. Over 300 cells per condition for each of three biological replicates were assayed in each experiment; a fourth experiment with unexplained low survival in all conditions was excluded from analysis.
2.7. Intravitreal Injection and Optic Nerve Crush
AAV2-Cre (2 μL) was injected intravitreally into adult wildtype and mAKAPfl/fl mice 2 weeks prior to optic nerve crush. Intravitreal injections were performed just posterior to the pars plana with a 31-gauge needle (Hamilton) connected to a 5 μL Hamilton syringe. Care was taken not to damage the lens. For nerve crush, the left optic nerve (ON) was exposed from outer canthus, and crushed for 5 s with a Dumont # 5 forceps (91150–20, F.S.T.) approximately 1.5 mm behind the globe. Care was taken to avoid damaging the blood supply to the retina. Immediately after optic nerve crush, separate cohorts of mice were treated with or without BDNF (5 μg) or cAMP analog CPT-cAMP (Sigma; 50 μM) in 3 μL PBS. Mice with any significant postoperative complications (e.g., retinal ischemia, cataract) were excluded from further analysis.
2.8. Retrograde Labeling of RGCs and Quantification of RGC Survival
Retrograde labeling with fluorogold (FG; fluorochrome) was performed 1 week before optic nerve crush for assay of RGC survival, as previously described (Chiu et al., 2008). In brief, the animals were anesthetized, and the skull exposed by a midline incision. Bilateral, 2-mm diameter craniotomies were placed 0.5 mm posterolateral to the sagittal and transverse sutures. A small piece of gelfoam (Gelfoam, USP) soaked in 4 μL 6% FG was then placed on the surface of the superior colliculus. The incision was then sutured closed.
One week after optic nerve crush, mice were euthanized by perfusion with 4% PFA. Retina flat mount was prepared as described before (Zhao et al., 2014). Briefly, the eyes were removed and postfixed with 4% PFA for 2 h at room temperature. Retinas were flat mounted in mounting medium (H-1000; Vector) on glass slides. Confocal images were acquired with a confocal laser scanning microscope (Leica DM 6000B; Leica Biosystems) and a × 40 magnification oil-immersion lens. The imaging and quantification were performed in a masked fashion. The methods used for these procedures were adapted from previous studies (Wang et al., 2013). Briefly, the retinas were divided into 4 quadrants and each quadrant was subdivided into 3 areas (central, middle, and peripheral), which were 1, 2, and 3 mm from the optic nerve head, respectively. One digital micrograph was randomly taken from each of the 12 fields. Thus, 12 images were quantified per retina. Fluorogold (FG)-positive cells were counted manually by one experienced investigator masked to experimental group and presented as cells/mm2 in each region. Microglia and macrophages, which may incorporate FG after phagocytosis of dying RGCs, were excluded based on morphological criteria as described before: RGCs appear as large, round cell bodies, no processes, almost homogenously labeled; microglia appear as small elongated cell bodies, branching processes, inhomogeneously labeled) (Biermann et al., 2011, Mansour-Robaey et al., 1994, Kikuchi et al., 2000). Whole retinal RGC density was estimated using the formula: (DC + 3DM + 5DP) / 9, where DC, DM, and DP represent densities of the central, middle, and peripheral regions, respectively. Each group contained at least 4 rats for obtaining mean densities.
2.9. Statistical Analysis
All cohort sample sizes were determined based on previous power calculations using variances determined in the prior publications noted above. All data were analyzed with Student's t-test or 1-way analysis of variance using SPSS version 20.0 (SPSS Inc) and graphed using Microsoft Office Excel 2010. All data are displayed as means of at least 3 independent experiments with standard error of the mean (SEM). Significance is represented with asterisks.
3. Results
3.1. mAKAPα is Expressed by RGCs
We have previously shown that mAKAP is expressed in the brain and striated muscle (Kapiloff et al., 1999). We now reveal that mAKAPα is present in the retina. mAKAPα was detected by immunoblot in extracts prepared from purified postnatal day 3 (P3) rat RGCs (Fig. 1A). A single band > 250 kDa was detected in RGC extracts similar to mobility to mAKAPα in brain. Cardiac mAKAPβ, which is identical to mAKAPα aa residues 245–2308 in the mouse due to alternative splicing, is 27 kDa smaller than the neuronal isoform (Michel et al., 2005) and migrated faster in SDS-PAGE (Fig. 1A). By immunohistochemistry, staining for mAKAPα was significant in the retinal ganglion cell layer (Fig. 1B and D, arrows) compared to control retinas from mAKAPfl/fl;Tg(Chx10-cre) mice lacking mAKAPα (Fig. 1C and E). Higher magnification images demonstrate a perinuclear patterning for RGC mAKAPα (Fig. 1D’), consistent with its localization at the nuclear envelope in myocytes and hippocampal neurons (Michel et al., 2005, PARE et al., 2005).
3.2. Altering mAKAP Expression Levels Decreases RGC Survival and Suppresses Neurite Extension In Vitro
Given the association of ERK5 and cAMP-signaling with mAKAP signalosomes (Michel et al., 2005, Dodge-Kafka et al., 2005), we hypothesized that the mAKAPα scaffold would be important for RGC survival and neurite extension after stroke and nerve injury. To begin testing this hypothesis, we cultured purified RGCs and either depleted mAKAPα by RNA interference (RNAi) or overexpressed the scaffold by transfection of a mAKAPα expression vector. Since we and others have previously shown that both cAMP elevation and neurotrophic factors strongly promote RGC survival and axonal extension in primary culture (Goldberg et al., 2002), RGCs were initially cultured in growth media containing BDNF, CNTF, and forskolin (to activate adenylyl cyclases and elevate intracellular cAMP levels) in this study. In vitro culture and transfection of RGCs induces stress, resulting in ongoing cell death even in neurotrophic factor-rich growth media, and serves as a useful approach to mimic in vivo injury. Two days after electroporation with mAKAP-specific siRNA, mAKAP mRNA expression was suppressed ~ 80% relative to control siRNA-treated cells (Fig. 2A). Notably, loss of mAKAP expression caused a significant decrease in RGC viability in culture (Fig. 2B and C), implying a role for mAKAPα signalosomes in neuronal survival. Controls at 2 h after transduction showed no significant difference between the groups (~ 60% survival for both control and mAKAP siRNA, data not shown), indicating that the effects of mAKAP RNAi were not merely a result of differential electroporation.
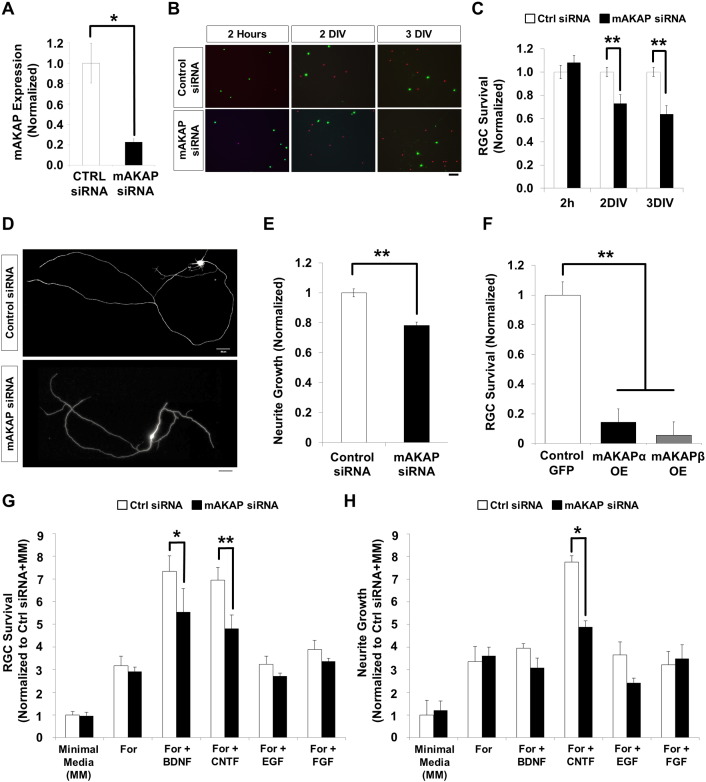
Fig. 2.
Requirement for mAKAP expression in RGC survival in vitro. (A) Quantitative PCR revealed that mAKAP mRNA expression was inhibited 80% 2 days after electroporation by mAKAP siRNA compared to control siRNA. (B) Purified primary RGCs electroporated with either control or mAKAP siRNA were cultured in growth media containing BDNF, CNTF and forskolin. Representative images of cells stained with calcein (live cell marker, green) and sytox (dead cell marker, red) at different time points after electroporation are shown. Scale bar represents 100 μm. (C) RGC survival normalized to the control siRNA-treated RGCs for the respective time point (N = 4). Two hours after electroporation, no difference in RGC survival was observed between control and mAKAP siRNA-treated RGCs. However, survival of mAKAP siRNA-treated RGCs after 2 and 3 days in vitro (DIV) was significantly lower than for control siRNA-treated RGCs. (D) Purified primary RGCs electroporated with either control or mAKAP siRNA were cultured in growth media containing BDNF, CNTF and forskolin for 3 days. Representative images of βIII tubulin immunostaining (neurite marker) are shown. Scale bar represents 50 μm. (E) Mean total neurite length per cell normalized to that for control siRNA (N = 4). Neurite extension was significantly longer in control cells than mAKAP siRNA treated cells. (F) Overexpression of mAKAPα and –β significantly reduced RGC survival compared to GFP transfected controls (N = 3). (G) Survival of RGCs electroporated with mAKAP or control siRNA and cultured in media with or without growth factors, normalized to control siRNA-treated RGCs cultured in media without growth factor (MM). Survival was reduced by mAKAP RNAi for cells cultured in forskolin with BDNF or CNTF, but not those cultured in forskolin alone or with EGF or FGF. Representative data from repeated experiments shown (N = 3). (H) Mean total neurite length of mAKAP and control siRNA-treated RGCs cultured in media with or without growth factors, normalized to control siRNA-treated RGCs cultured in media without growth factor (MM). Neurite growth was significantly inhibited by mAKAP RNAi only for RGCs cultured in forskolin and CNTF (N = 3). *P < 0.05, **P < 0.01; Student's t-test; error bars indicate standard error of mean.
Through the regulation of both common and distinct downstream signaling pathways, most neurotrophic factors that support RGC survival also promote RGC axon growth (Goldberg et al., 2002). We next tested whether mAKAPα expression was important for neurite extension in cultured RGCs. RGCs were transfected with either mAKAP-targeting or control siRNA and cultured in the presence of BDNF, CNTF and forskolin to promote neurite elongation. Three days after transfection, cells were fixed and stained to allow for quantitation of the total neurite length of the surviving RGCs. Following mAKAPα depletion, we found that mean total neurite length was significantly less compared to controls (Fig. 2D and E). These results indicate that mAKAP in RGCs plays an integral role in neurite outgrowth in response to neurotrophic factors and cAMP elevation.
Since mAKAP depletion decreased RGCs sensitivity to neurotrophins and cAMP, we hypothesized that overexpression of mAKAP scaffolds may enhance neurotrophic responsiveness and by extension improve survival and neurite outgrowth after an insult (i.e. transfection). Interestingly, overexpression of either mAKAPα or the muscle mAKAPβ isoform also led to significant cell death (Fig. 2F), and in the context of low survival little neurite growth was observed (data not shown). This result is consistent with the readily saturated mechanism for mAKAP peri-nuclear targeting by nesprin-1α (Pare et al., 2005) and the expected dominant negative effects of PKA displacement by introduction of anchoring disruptor peptides (Scott et al., 2013). Together, these data suggest that expression of mAKAPα at endogenous levels is required for RGC survival.
Given mAKAPα's function as a signalosome scaffold and its role in RGC survival and neurite growth, we tested whether the scaffold was required for the function of specific neurotrophins in vitro. We cultured purified RGCs electroporated with mAKAP or control siRNA in growth media containing either forskolin alone or in combination with 4 different families of neurotrophic factors (BDNF, CNTF, EGF, or FGF) and assayed cell survival and neurite growth. We found that mAKAPα knockdown significantly reduced survival in RGCs cultured in forskolin with BDNF or CNTF, but not in those cultured in forskolin alone or with EGF or FGF (Fig. 2G). Furthermore, silencing mAKAP expression in RGCs inhibited neurite outgrowth only in cells cultured in forskolin and CNTF (Fig. 2H). These results demonstrate that mAKAP is required in RGCs for the transduction of pro-survival signaling induced by BDNF and CNTF, and pro-growth signaling induced by CNTF, and support a broader, but potentially selective role for mAKAP signalosomes in neuroprotection.
3.3. mAKAPα is Dispensable for RGC Survival During Development and in the Adult
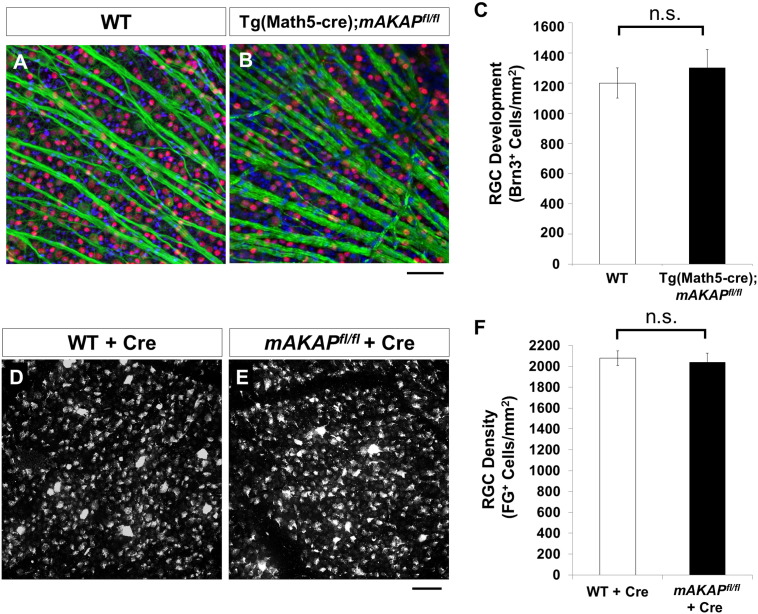
Because RGC survival and axon growth in vitro depended upon mAKAPα, we tested whether mAKAPα expression was important for RGC development in vivo. mAKAPα expression in retinal development was ablated by mating Tg(Math5-cre) driver mice, that express cre recombinase in the progenitors responsible for most postnatal RGCs (Brzezinski et al., 2012), to a floxed, conditional mAKAP allele we recently described (Kritzer et al., 2014b). Flat mounts of retinas from mAKAPfl/fl;Tg(Math5-cre) mice and littermate mAKAPfl/fl controls lacking cre (WT) were stained with Brn3 and TUJ1 to label RGC soma and axons, respectively (Fig. 3A and B). No difference in RGC numbers or their axon bundles in the optic nerve fiber layer were observed between mAKAPfl/fl;Tg(Math5-cre) and WT mice (Fig. 3C). These data suggest that mAKAPα is not necessary for the generation of normal numbers of RGCs during development or for proper intraretinal axon growth or guidance.
Fig. 3.
Retina-specific mAKAP knockout during development or when induced in the adult has no effect on RGC number. (A and B) Retinal flat mount from adult Tg(Math5-cre);mAKAPfl/fl knock-out mice and littermate mAKAPfl/fl controls lacking cre (WT) were stained with Brn3 (retinal ganglion cell marker, red), and βIII-tubulin (neurite marker, green) and counterstained with DAPI (nuclear marker, blue). Representative images of retinal segments are shown. Scale bar represents 50 μm. (C) RGC quantification demonstrated no difference in the number of Brn3-labeled RGCs between knock-out and control animals (n = 4 mice per group). (D and E) AAV2-Cre intravitreal injection of control WT and mAKAPfl/fl mice to knock out mAKAPα in the adult retina. RGCs were retrogradely labeled with fluorogold (FG). Representative images of retinal segments are shown. Scale bar represents 50 μm. (F) RGC quantification demonstrated a similar number of RGCs in both WT and mAKAPfl/fl animals two weeks after AAV2-cre application (n = 4 mice per group). n.s.: not significant; Student's t-test; error bars indicate standard error of mean.
Since we did not observe a deficit in retinal developmental in mAKAPα KO animals, we next asked whether mAKAPα knock-out in the mature retina would impair RGC survival. To induce RGC-selective conditional knock-out, AAV2-cre was injected intravitreally in adult mAKAPfl/fl mice. Retrograde labeling of RGCs with fluorogold (FG) was performed 1 week after virus injection. Two weeks after injection, the morphology of the FG labeled RGCs in mAKAP knockout mice was similar to those in control animals; neither phagocyte activation nor RGC scavenging was observed (Fig. 3D and E). No difference in number of FG-labeled RGCs was observed between mAKAPα-knockout and control AAV2-cre injected WT mice (Fig. 3F). Thus, persistent mAKAPα expression is not required for RGC survival in the unstressed adult retina.
3.4. mAKAPα Contributes to RGC Survival After Optic Nerve Injury
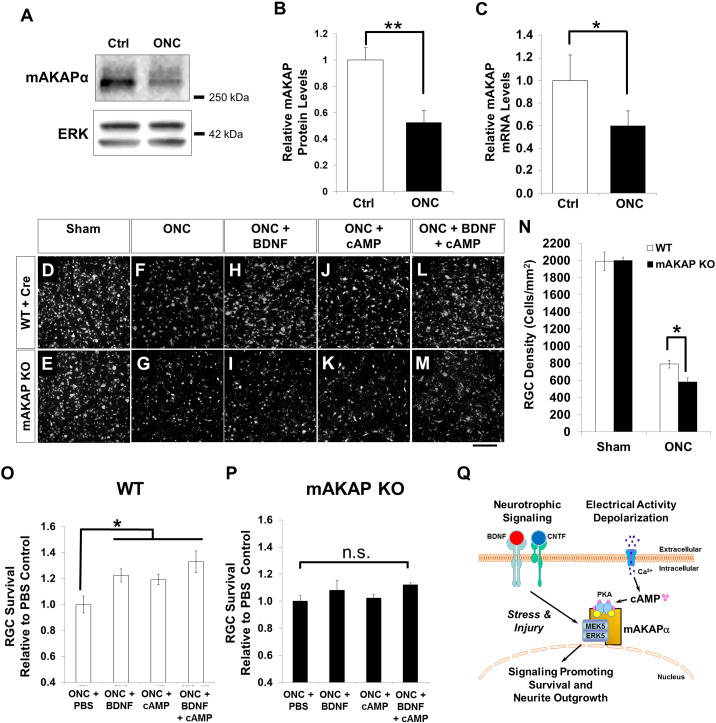
There are significant differences between the mechanisms conferring RGC survival during development and those conferring survival following injury in the adult (Huang et al., 2014). We considered that in contrast to the apparent unimportance of mAKAPα to RGC development, the results regarding mAKAPα obtained in vitro in “stressed” RGCs may have reflected a requirement for mAKAPα in vivo during stress conditions such as those that occur in optic nerve injury or stroke. To address this hypothesis, we assayed mAKAPα protein and mRNA levels after optic nerve crush, a model for adult nerve injury, and an experiment more readily interpretable than comparing protein levels in vivo to those in vitro after injury. We assayed mAKAPα protein levels in RGCs three days after optic nerve crush and found a significant reduction (~ 40–50%) compared to uninjured controls (Fig. 4A and B). Retinal mAKAP transcripts were similarly reduced (Fig. 4C). Note that the decrease in mAKAPα levels was not due to lower RGC numbers, as in this model, RGCs begin to die 3–5 days after injury, with apoptosis peaking 1–2 weeks later (data not shown and (Berkelaar et al., 1994)). Thus, loss of mAKAPα was associated with the induction of RGC death following trauma.
Fig. 4.
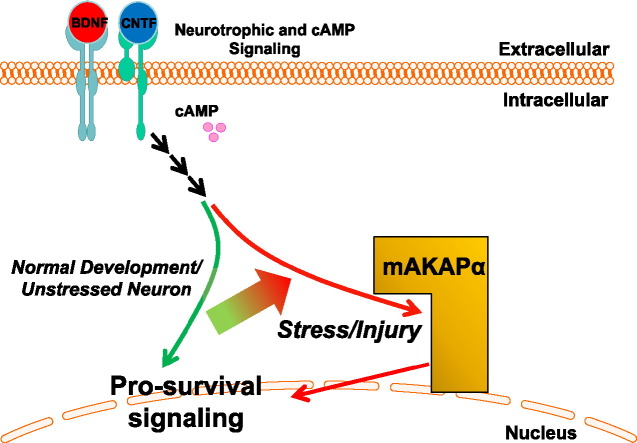
Loss of mAKAP exacerbates RGC cell death after optic nerve injury. (A) Western blot for mAKAP and ERK1/2 protein in uninjured RGCs and 3 days after optic nerve crush (ONC). (B) Quantitation of protein expression revealed a 40–50% reduction in RGC mAKAPα levels compared to uncrushed controls (N = 3). (C) Quantitative PCR demonstrating a similar reduction in retinal mAKAPα mRNA expression 3 days after crush injury (n = 3). (D–P) Induced mAKAP knockout (KO) in the adult suppresses RGC survival after optic nerve injury. AAV2-cre was injected intravitreally in mice 2 weeks prior to optic nerve crush. RGCs were retrogradely labeled with fluorogold 1 week prior to optic nerve injury. (D–M) Representative images of flat-mounted retinal segments in different conditions as marked. Scale bar represents 100 μm. (N–P) Quantification of RGC survival. A similar number of RGCs was observed in WT and mAKAP KO mice (mAKAPfl/fl animals with AAV2-cre injection) following sham optic nerve injury (exposed optic nerve, without crush). By one week after optic nerve crush, however, significantly fewer fluorogold-labeled RGCs were observed in mAKAP knockout animals than control animals (N). Application of BDNF and cAMP significantly increased RGC survival after optic nerve injury in WT animals (O), but had no effect on mAKAP KO mice (P) (n = 4 mice per group). *P < 0.05, **P < 0.01; one-way analysis of variance; error bars indicate standard error of mean. (Q) Model for mAKAPα-dependent signaling in stressed RGCs. mAKAPα can organize signalosomes that contain signaling molecules such as ERK5 and PKA that are responsive to upstream neurotrophin and electrical signals and that we propose will induce gene expression promoting both RGC survival and neurite outgrowth following injury.
We next asked whether mAKAPα reduction is causally related to RGC death following trauma by removing any residual RGC mAKAPα expression via mAKAP gene deletion. We found that after optic nerve crush, the survival of RGCs in AAV2-cre injected mAKAPfl/fl mice was significantly less than that in AAV2-cre injected wild-type controls (Fig. 4F, G and N). We and others have previously reported that BDNF and cAMP improve RGC survival following nerve injury (Mo et al., 2002, Goldberg et al., 2002, Corredor et al., 2012). As before, intravitreal injection of BDNF and/or the cAMP analog CPT-cAMP significantly increased RGC survival in AAV2-cre injected wild-type animals after optic nerve crush (Fig. 4H, J, L and O). Remarkably, however, neither BDNF, nor CPT-cAMP, nor their combination was able to promote RGC survival after crush injury in AAV2-cre injected mAKAPfl/fl mice (Fig. 4I, K, M and P). Together, these results show that mAKAPα is required for both endogenous signaling and signaling induced by exogenous BDNF and cAMP that promote RGC survival after optic nerve injury.
4. Discussion
Here we document a stress-specific neurotrophic factor signaling pathway in CNS neurons, identifying the physiologic function for mAKAPα in regulation of RGC survival and neurite growth. We show that mAKAPα is expressed in RGCs in the mature retina, and using RNA interference and conditional gene deletion, that mAKAPα supports the survival and neurite extension of primary neurons in vitro and the survival of RGCs in vivo after optic nerve injury.
The central observation of our study is that mAKAPα is required for pro-survival signaling in RGCs after optic nerve injury in the adult, but not during normal development or in uninjured RGCs. This difference implies that divergent signaling pathways control neuronal survival during development and following injury. These results are reminiscent of those recently found following mAKAPβ knock-out in the cardiac myocyte wherein mAKAPβ was dispensable in the developing heart, but required for the induction of pathological cardiac remodeling following pressure overload or catecholamine infusion (Kritzer et al., 2014a). Taken together, these data suggest that mAKAP signalosomes play critical roles in the regulation of intracellular signaling pathways invoked in disease or injury.
Interruption of target-derived neurotrophic support is a key factor diminishing RGC survival following axon injury (Yip and So, 2000, Shen et al., 1999, Moore and Goldberg, 2010). Following optic nerve crush, trophic survival signaling can be induced by exogenous BNDF or cAMP (Shen et al., 1999), but here we find that this signaling was effective in promoting survival only in RGCs expressing mAKAPα. We found endogenous mAKAPα expression dropped significantly in RGCs after optic nerve crush, contemporaneously with the induction of RGC death. Complete loss of mAKAPα via gene deletion further exacerbated RGC loss. In addition, any enhancement of RGC survival by exogenous cAMP and/or BDNF after injury depended upon the residual (50–60%) mAKAPα normally expressed after optic nerve crush-mediated protein down-regulation. mAKAPα overexpression was also deleterious to cultured RGCs, confirming that overexpression of binding proteins for critical signaling proteins are often disruptive to signaling pathways (Scott et al., 2013). Together our data imply that the organization of perinuclear signalosomes by the mAKAPα scaffold is limiting for the transduction of cAMP and neurotrophic factor signaling required for RGC survival following trauma or insult.
Neurotrophic factors regulate RGC survival and neurite growth through both common and distinct downstream signaling pathways (Goldberg et al., 2002). For example, MAPK/ERK and PI3K/AKT signaling pathways are involved in BDNF induced neuronal survival and axonal growth (Nakazawa et al., 2002) whereas CNTF elicits its biological actions through JAK/STAT3, PI3K/AKT, and MAPK/ERK signaling pathways (Goldberg et al., 2002, Ip and Yancopoulos, 1996, Peterson et al., 2000, Dolcet et al., 2001, Kaur et al., 2002). The mechanisms how mAKAPα scaffolding contributes to neurotrophic dependent- and/or independent-RGC survival and axon growth will need to be elucidated in future studies. As indicated above, mAKAPα can target both ERK5 and PKA to the neuronal nuclear envelope (Michel et al., 2005). MAPK signaling is critical for the survival effects of neurotrophic factors in RGCs (Goldberg et al., 2002). In RGCs and other neurons, ERK5 mediates neurotrophin-dependent (including BDNF), retrograde pro-survival signaling that is initiated in the axon (Watson et al., 2001, Pazyra-Murphy et al., 2009, Wang et al., 2006, Van Oterendorp et al., 2014). cAMP also potentiates CNS neuronal survival and axon growth in response to neurotrophic factors (Goldberg et al., 2002). The underlying mechanism for this potentiation remains obscure, but likely involves crosstalk between activity-induced, Ca2 +-dependent signaling pathways and neurotrophic factor-induced MAPK signaling pathways (Steinberg and Brunton, 2001). mAKAPα provides a potential platform for such crosstalk, since it binds machinery critical to both cAMP and MAPK signaling (Dodge-Kafka et al., 2005). Both ERK5 and its upstream activator, MEK5, indirectly bind mAKAP through the phosphodiesterase PDE4D3 (Dodge-Kafka et al., 2005). Interestingly, ERK5 phosphorylation of PDE4D3 has been shown to inhibit local cAMP degradation, increasing PKA activity (Dodge-Kafka and Kapiloff, 2006). Thus, at mAKAPα signalosomes cAMP and MAPK signaling can synergistically oppose retrograde cell death after axon injury (Fig. 4Q). How different signaling pathways are involved, and balanced in the mAKAPα complex for particular function (neuronal survival and/or neurite growth) under different conditions are of great interest and will need to be further elucidated in future studies.
Other mAKAP binding partners have been reported to promote the survival and axon growth of CNS neurons, including for example, the Ca2 +/calmodulin-dependent phosphatase calcineurin and the transcription factor MEF2 (Passariello et al., 2015, Akhtar et al., 2012). The unique mAKAPα N-terminal domain not present in mAKAPβ can directly bind PDK1 (Michel et al., 2005). PDK1 binding to mAKAPα can contribute to the activation of p90 ribosomal S6 kinase (RSK) that promotes neuronal survival in response to neurotrophic factors by both transcription-dependent and -independent mechanisms. For example, by direct phosphorylation RSK inactivates BAD, a pro-apoptotic protein family member, and activates transcription factor cAMP response element-binding protein (CREB) (Bonni et al., 1999).
In conclusion, we have identified mAKAPα expression in RGCs and demonstrated a requirement for mAKAPα in neuronal survival and neurite growth in cell culture and after optic nerve injury in vivo. Future studies will identify the mAKAPα-orchestrated signaling modules relevant to these processes and may suggest targets for neuroprotection after acute injury or in neurodegenerative disease.
Author Contributions
Yan Wang, Evan G. Cameron performed in vitro RGC survival and neurite growth assays and collected data. Yan Wang, Tu Nguyen and Rahul Lodhavia performed in vivo experiments and collected data. Evan G. Cameron, Jinliang Li, Travis L Stiles and Michael D. Kritzer performed Western blots and RT-PCRs. Yan Wang and Jonathan Hertz performed immunostaining. Jeffrey L. Goldberg, Michael S. Kapiloff, Yan Wang and Evan G. Cameron designed the study, analyzed data and wrote the paper. All authors discussed the results and commented on the manuscript. The authors declare that they have no conflicts of interest.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We gratefully acknowledge funding from the NEI (R01-EY022129 to MSK and JLG; P30-EY022589 to UCSD), and an unrestricted grant from Research to Prevent Blindness, Inc.
Contributor Information
Michael S. Kapiloff, Email: mkapiloff@med.miami.edu.
Jeffrey L. Goldberg, Email: jlgoldbe@stanford.edu.
References
- Akhtar M.W., Kim M.S., Adachi M., Morris M.J., Qi X., Richardson J.A., Bassel-Duby R., Olson E.N., Kavalali E.T., Monteggia L.M. In vivo analysis of MEF2 transcription factors in synapse regulation and neuronal survival. PLoS ONE. 2012;7:e34863. doi: 10.1371/journal.pone.0034863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelaar M., Clarke D.B., Wang Y.C., Bray G.M., Aguayo A.J. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J. Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann J., Lagreze W.A., Schallner N., Schwer C.I., Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol. Vis. 2011;17:1275–1286. [PMC free article] [PubMed] [Google Scholar]
- Bonni A., Brunet A., West A.E., Datta S.R., Takasu M.A., Greenberg M.E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Brzezinski J.A.T., Prasov L., Glaser T. Math5 defines the ganglion cell competence state in a subpopulation of retinal progenitor cells exiting the cell cycle. Dev. Biol. 2012;365:395–413. doi: 10.1016/j.ydbio.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu K., Lau W.M., Yeung S.C., Chang R.C., So K.F. J. Vis. Exp.; 2008. Retrograde Labeling of Retinal Ganglion Cells by Application of Fluoro-Gold on the Surface of Superior Colliculus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredor R.G., Trakhtenberg E.F., Pita-Thomas W., Jin X., Hu Y., Goldberg J.L. Soluble adenylyl cyclase activity is necessary for retinal ganglion cell survival and axon growth. J. Neurosci. 2012;32:7734–7744. doi: 10.1523/JNEUROSCI.5288-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge-Kafka K.L., Kapiloff M.S. The mAKAP signaling complex: integration of cAMP, calcium, and MAP kinase signaling pathways. Eur. J. Cell Biol. 2006;85:593–602. doi: 10.1016/j.ejcb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Dodge-Kafka K.L., Soughayer J., Pare G.C., Carlisle Michel J.J., Langeberg L.K., Kapiloff M.S., Scott J.D. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcet X., Soler R.M., Gould T.W., Egea J., Oppenheim R.W., Comella J.X. Cytokines promote motoneuron survival through the Janus kinase-dependent activation of the phosphatidylinositol 3-kinase pathway. Mol. Cell. Neurosci. 2001;18:619–631. doi: 10.1006/mcne.2001.1058. [DOI] [PubMed] [Google Scholar]
- Goldberg J.L., Barres B.A. The relationship between neuronal survival and regeneration. Annu. Rev. Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- Goldberg J.L., Espinosa J.S., Xu Y., Davidson N., Kovacs G.T., Barres B.A. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Huang L., Hu F., Xie X., Harder J., Fernandes K., Zeng X.Y., Libby R., Gan L. Pou4f1 and pou4f2 are dispensable for the long-term survival of adult retinal ganglion cells in mice. PLoS ONE. 2014;9:e94173. doi: 10.1371/journal.pone.0094173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip N.Y., Yancopoulos G.D. The neurotrophins and CNTF: two families of collaborative neurotrophic factors. Annu. Rev. Neurosci. 1996;19:491–515. doi: 10.1146/annurev.ne.19.030196.002423. [DOI] [PubMed] [Google Scholar]
- Jiang T., Chang Q., Zhao Z., Yan S., Wang L., Cai J., Xu G. Melatonin-mediated cytoprotection against hyperglycemic injury in Muller cells. PLoS ONE. 2012;7:e50661. doi: 10.1371/journal.pone.0050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapiloff M.S., Schillace R.V., Westphal A.M., Scott J.D. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J. Cell Sci. 1999;112(Pt 16):2725–2736. doi: 10.1242/jcs.112.16.2725. [DOI] [PubMed] [Google Scholar]
- Kaur N., Wohlhueter A.L., Halvorsen S.W. Activation and inactivation of signal transducers and activators of transcription by ciliary neurotrophic factor in neuroblastoma cells. Cell. Signal. 2002;14:419–429. doi: 10.1016/s0898-6568(01)00280-7. [DOI] [PubMed] [Google Scholar]
- Kikuchi M., Tenneti L., Lipton S.A. Role of p38 mitogen-activated protein kinase in axotomy-induced apoptosis of rat retinal ganglion cells. J. Neurosci. 2000;20:5037–5044. doi: 10.1523/JNEUROSCI.20-13-05037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer M.D., Li J., Passariello C.L., Gayanilo M., Thakur H., Dayan J., Dodge-Kafka K., Kapiloff M.S. The scaffold protein mAKAPbeta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ. Heart Fail. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer M.D., Li J., Passariello C.L., Gayanilo M., Thakur H., Dayan J., Dodge-Kafka K., Kapiloff M.S. The scaffold protein muscle A-kinase anchoring protein beta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ. Heart Fail. 2014;7:663–672. doi: 10.1161/CIRCHEARTFAILURE.114.001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kritzer M.D., Michel J.J., Le A., Thakur H., Gayanilo M., Passariello C.L., Negro A., Danial J.B., Oskouei B., Sanders M., Hare J.M., Hanauer A., Dodge-Kafka K., Kapiloff M.S. Anchored p90 ribosomal S6 kinase 3 is required for cardiac myocyte hypertrophy. Circ. Res. 2013;112:128–139. doi: 10.1161/CIRCRESAHA.112.276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Negro A., Lopez J., Bauman A.L., Henson E., Dodge-Kafka K., Kapiloff M.S. The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J. Mol. Cell. Cardiol. 2010;48:387–394. doi: 10.1016/j.yjmcc.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour-Robaey S., Clarke D.B., Wang Y.C., Bray G.M., Aguayo A.J. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A., Kaplan M.R., Pfrieger F.W., Barres B.A. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Michel J.J., Townley I.K., Dodge-Kafka K.L., Zhang F., Kapiloff M.S., Scott J.D. Spatial restriction of PDK1 activation cascades by anchoring to mAKAPalpha. Mol. Cell. 2005;20:661–672. doi: 10.1016/j.molcel.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Mo X., Yokoyama A., Oshitari T., Negishi H., Dezawa M., Mizota A., Adachi-Usami E. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Invest. Ophthalmol. Vis. Sci. 2002;43:2401–2405. [PubMed] [Google Scholar]
- Moore D.L., Goldberg J.L. Four steps to optic nerve regeneration. J. Neuroophthalmol. 2010;30:347–360. doi: 10.1097/WNO.0b013e3181e755af. [DOI] [PubMed] [Google Scholar]
- Nakazawa T., Tamai M., Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest. Ophthalmol. Vis. Sci. 2002;43:3319–3326. [PubMed] [Google Scholar]
- PARE G.C., EASLICK J.L., MISLOW J.M., MCNALLY E.M., KAPILOFF M.S. Nesprin-1alpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp. Cell Res. 2005;303:388–399. doi: 10.1016/j.yexcr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Park K.K., Liu K., Hu Y., Smith P.D., Wang C., Cai B., Xu B., Connolly L., Kramvis I., Sahin M., He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passariello C.L., Li J., Dodge-Kafka K., Kapiloff M.S. mAKAP-A master scaffold for cardiac remodeling. J. Cardiovasc. Pharmacol. 2015;65:218–225. doi: 10.1097/FJC.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazyra-Murphy M.F., Hans A., Courchesne S.L., Karch C., Cosker K.E., Heerssen H.M., Watson F.L., Kim T., Greenberg M.E., Segal R.A. A retrograde neuronal survival response: target-derived neurotrophins regulate MEF2D and bcl-w. J. Neurosci. 2009;29:6700–6709. doi: 10.1523/JNEUROSCI.0233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson W.M., Wang Q., Tzekova R., Wiegand S.J. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J. Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.D., Dessauer C.W., tasken K. Creating order from chaos: cellular regulation by kinase anchoring. Annu. Rev. Pharmacol. Toxicol. 2013;53:187–210. doi: 10.1146/annurev-pharmtox-011112-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Wiemelt A.P., Mcmorris F.A., Barres B.A. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron. 1999;23:285–295. doi: 10.1016/s0896-6273(00)80780-1. [DOI] [PubMed] [Google Scholar]
- Steinberg S.F., Brunton L.L. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu. Rev. Pharmacol. Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- Thellin O., Zorzi W., Lakaye B., De Borman B., Coumans B., Hennen G., Grisar T., Igout A., Heinen E. Housekeeping genes as internal standards: use and limits. J. Biotechnol. 1999;75:291–295. doi: 10.1016/s0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Tsien J.Z., Chen D.F., Gerber D., Tom C., Mercer E.H., Anderson D.J., Mayford M., KANDEL E.R., Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Unoki K., Lavail M.M. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest. Ophthalmol. Vis. Sci. 1994;35:907–915. [PubMed] [Google Scholar]
- Van Oterendorp C., Sgouris S., Schallner N., Biermann J., Lagreze W.A. Retrograde neurotrophic signaling in rat retinal ganglion cells is transmitted via the ERK5 but not the ERK1/2 pathway. Invest. Ophthalmol. Vis. Sci. 2014;55:658–665. doi: 10.1167/iovs.13-12985. [DOI] [PubMed] [Google Scholar]
- Wang Y., Brown D.P.J.R., Duan Y., Kong W., Watson B.D., Goldberg J.L. A novel rodent model of posterior ischemic optic neuropathy. JAMA Ophthalmol. 2013;131:194–204. doi: 10.1001/2013.jamaophthalmol.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Su B., Xia Z. Brain-derived neurotrophic factor activates ERK5 in cortical neurons via a Rap1-MEKK2 signaling cascade. J. Biol. Chem. 2006;281:35965–35974. doi: 10.1074/jbc.M605503200. [DOI] [PubMed] [Google Scholar]
- Watson F.L., Heerssen H.M., Bhattacharyya A., Klesse L., Lin M.Z., Segal R.A. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- Weisenhaus M., Allen M.L., Yang L., Lu Y., Nichols C.B., Su T., Hell J.W., Mcknight G.S. Mutations in AKAP5 disrupt dendritic signaling complexes and lead to electrophysiological and behavioral phenotypes in mice. PLoS ONE. 2010;5:e10325. doi: 10.1371/journal.pone.0010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Ding K., Pan L., Deng M., Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev. Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Yip H.K., So K.F. Axonal regeneration of retinal ganglion cells: effect of trophic factors. Prog. Retin. Eye Res. 2000;19:559–575. doi: 10.1016/s1350-9462(00)00009-4. [DOI] [PubMed] [Google Scholar]
- Yu B., Xu P., Zhao Z., Cai J., Sternberg P., Chen Y. Subcellular distribution and activity of mechanistic target of rapamycin in aged retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2014;55:8638–8650. doi: 10.1167/iovs.14-14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Xu P., Jie Z., Zuo Y., Yu B., Soong L., Sun J., Chen Y., Cai J. Gammadelta T cells as a major source of IL-17 production during age-dependent RPE degeneration. Invest. Ophthalmol. Vis. Sci. 2014;55:6580–6589. doi: 10.1167/iovs.14-15166. [DOI] [PMC free article] [PubMed] [Google Scholar]