Abstract
Background
While there is increasing evidence of altered brain connectivity in autism, the degree and direction of these alterations in connectivity and their uniqueness to autism has not been established. The aim of the present study was to compare connectivity in children with autism to that of typically developing controls and children with developmental delay without autism.
Methods
We assessed EEG spectral power, coherence, phase lag, Pearson and partial correlations, and epileptiform activity during the awake, slow wave sleep, and REM sleep states in 137 children aged 2 to 6 years with autism (n = 87), developmental delay without autism (n = 21), or typical development (n = 29).
Findings
We found that brain connectivity, as measured by coherence, phase lag, and Pearson and partial correlations distinguished children with autism from both neurotypical and developmentally delayed children. In general, children with autism had increased coherence which was most prominent during slow wave sleep.
Interpretation
Functional connectivity is distinctly different in children with autism compared to samples with typical development and developmental delay without autism. Differences in connectivity in autism are state and region related. In this study, children with autism were characterized by a dynamically evolving pattern of altered connectivity.
Keywords: Epilepsy, Power spectral analysis, Coherence, Pearson, Epileptiform, EEG
Highlights
-
•
We used EEG to examine the connectivity in young children in awake, rapid eye movement, and slow wave sleep (SWS) states.
-
•
Differences in coherence between the autism group and the other groups were maximal during SWS.
-
•
Sleep may be the most sensitive time to measure differences in neuro-development before they are observable in behavior.
Shared mechanisms underlie normal healthy sleep and normal brain development. Therefore, we suspect that differences in the way that the brain is behaving during sleep have the potential to tell us about what might be developing incorrectly in people with neurodevelopmental disorders. In addition, sleep evaluations of children allow us to filter out waking distractions, so that we are truly measuring the brain working offline. For instance, the differences in the way the brain was connected that we observed between our three study groups were by far the most notable during slow wave sleep. This finding highlights the importance of taking the state of the brain into account when commenting on the ‘connectedness’ of the brain as a potential biomarker for neurodevelopmental disorders. Future attempts to classify developmental disorders by using differences in connectivity must take into account brain state, whether awake or asleep as well as developmental stage.
1. Introduction
Autism Spectrum Disorder (ASD) is a group of complex neurodevelopmental disorders, characterized by deficits in social communication and interaction and restricted, repetitive, and stereotyped patterns of behavior. The symptoms are present from early childhood and are impairing to everyday functioning. Individuals with ASD have co-occurring intellectual disability, language disorder, and epilepsy at higher rates than the general population. A singular pathophysiological mechanism is unlikely to be responsible for the autistic phenotype. While genetics play an important role in ASD (Risch et al., 1999, Anney et al., 2010, Hallmayer et al., 2011, Pinto et al., 2014, Gaugler et al., 2014), environmental and other non-genetic factors can contribute to the development of autistic symptomatology (Hallmayer et al., 2011). A perplexing question is how such a disparate group of etiologies can result in a recognizably consistent phenotype of often devastating impairments in social communication and behavior.
Several sources document that the brains of individuals with ASD exhibit aberrant functional connectivity (Belmonte et al., 2004, Uddin et al., 2013, Kana et al., 2011, Casanova and Trippe, 2009, Muller et al., 2011, Dinstein et al., 2011), defined as the “temporal correlations between remote neurophysiological events,” (Friston, 2011) using MRI techniques (Pina-Camacho et al., 2012, Cheng et al., 2010). Many neuroimaging studies, using both functional MRI (fMRI) and diffusion tensor imaging (DTI) done during both task-specific (Koshino et al., 2005, Koshino et al., 2008, Just et al., 2007, Kana et al., 2006) and resting-state (idle) conditions (Cherkassky et al., 2006, Weng et al., 2010), have shown that individuals with ASD have reduced long-range (distant) brain connectivity when compared to people with neurotypical development. A separate set of studies have shown reduced short-range (local) connectivity (Kana et al., 2011, Koshino et al., 2005, Koshino et al., 2008, Just et al., 2007). Yet results from other studies have demonstrated increases in connectivity, both long-range (Cheng et al., 2010, Ben Bashat et al., 2007, Supekar et al., 2013) and short-range (Weng et al., 2010, Anderson et al., 2011, Khan et al., 2013, Lewis et al., 2013, Keown et al., 2013).
While MRI studies may offer anatomical insight about connectivity in autism, they lack temporal resolution. EEG and magnetoencephalography (MEG) studies provide a means of evaluating this parameter of functional connectivity. These coherence studies generally analyze the phase shift and amplitude ratio between two signals over time where consistency of the relationship, on a frequency by frequency basis, is interpreted as high coherence or evidence for functional connectivity. A number of studies have compared EEG coherence findings between ASD and typically developing control populations (Cantor et al., 1986, Murias et al., 2007, Coben et al., 2008, Lazarev et al., 2010, Isler et al., 2010, Barttfeld et al., 2011, Leveille et al., 2010, Duffy and Als, 2012). Similar to the MRI studies, there have been mixed results with some children with ASD demonstrating reduced coherences (Khan et al., 2013, Coben et al., 2008), while others have shown increases in coherences (Murias et al., 2007, Orekhova et al., 2014, Dominguez et al., 2013) or mixed patterns (Barttfeld et al., 2011, Leveille et al., 2010, Duffy and Als, 2012).
While it is not known why there are discrepancies in both the MRI and electrophysiology studies, the great variability in cohorts studied, age of subjects, state examined, patient number, and approach to data analysis likely all contribute to the lack of consensus. Another pressing question is whether the changes in connectivity identified in ASD are unique to the disorder or simply a reflection of impaired development. Here, we studied brain connectivity using electrophysiology data from a large cohort of children that had typical development (TYP), developmental delay (DD), or ASD during the awake, slow wave sleep (SWS), and rapid eye movement (REM) sleep state.
2. Methods
2.1. Study Design and Participants
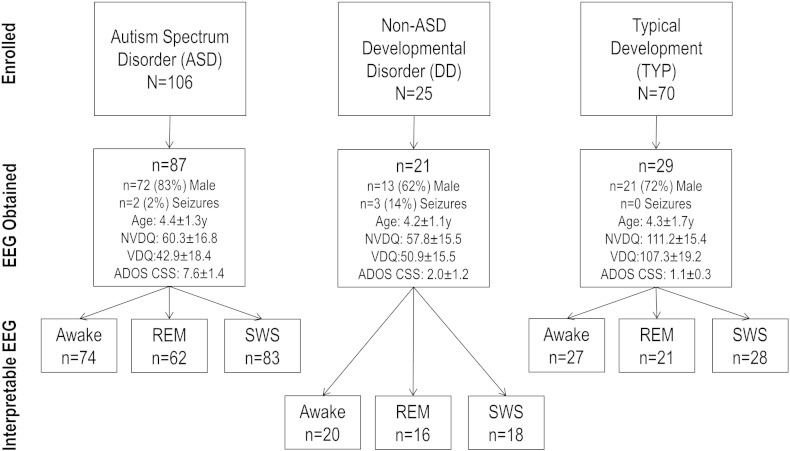
The study sample consisted of participants in an NIH natural history study of ASD approved by the National Institutes of Health Combined Neurosciences Institutional Review Board. The total study sample consisted of 201 children aged 2 to 6 years with ASD (n = 106), DD (n = 25), and TYP (n = 70); EEG was obtained from 137 (68%) children (see Fig. 1).
Fig. 1.
Patient disposition and sample characteristics. ASD = autism spectrum disorder. DD = non-ASD developmental disorder. TYP = typically developing. NVDQ = nonverbal developmental quotient. VDQ = verbal developmental quotient. Sample sizes for ADOS CSS are ASD = 86, DD = 20, TYP = 20.
Consent was obtained from the parent or guardian of each participant. Recruitment for the ASD group was based on parental concern about ASD. Children were enrolled in the ASD group if they met the DSM-IV-TR (American Psychiatric Association, 2000) criteria for autistic disorder based on research-reliable administrations of the Autism Diagnostic Interview-Revised (ADI-R; or a Toddler version) (Rutter et al., 2003), the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000), and clinical judgment. Cognitive ability was assessed using a developmental quotient (ratio IQ) from either the Mullen Scales of Early Learning (Mullen, 1995) or the Differential Abilities Scales (Elliott, 2007), depending on the child's level of function. Children in the DD group were specifically recruited to serve as an IQ-matched comparison group, and were targeted based on history of language, cognitive, or general developmental delay. Children were enrolled in the DD group if developmental quotient scores were more than 1·5 standard deviations below the population mean and if ASD was ruled out through clinical assessment and administration of the ADI-R and ADOS. TYP controls were enrolled if there was no history of developmental delay, no members of the immediate family were diagnosed with ASD, full-scale IQ was no more than 1·5 standard deviations below the population mean, and if ADOS and clinical assessment revealed no concerns about ASD.
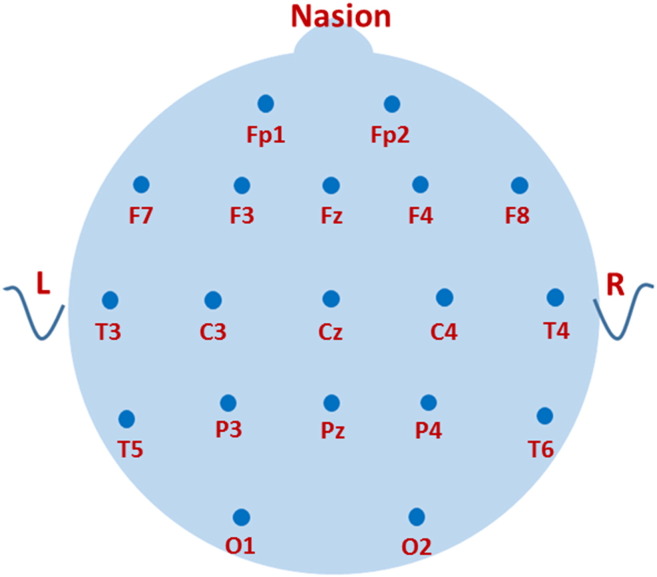
Digital EEGs were recorded during the fully awake, drowsy, and sleep states using the 10–20 System of Electrode placement (Fig. 2). Ten minute segments of awake, SWS, and REM sleep were selected for analysis. Sections were selected in which artifact was minimal. Portions of records with suppression bursts or ictal events were excluded. Wakefulness was assessed by evaluation of eye blinks and technologist observations. If there was a concern about possible drowsiness, the epoch was not evaluated. Studies were excluded if there was insufficient alert awake recording, excessive artifact or technical difficulties with the recording. The final number of interpretable EEGs for each state is listed in Fig. 1.
Fig. 2.
Electrode placement.
2.2. Procedures
Analysis was performed masked to participant diagnosis using Neuroguide software (Applied Neuroscience, Inc., St. Petersburg, Florida) using a linked-ear montage. Please see supplementary materials for derivations of frequencies, amplitude asymmetries, EEG spectral analysis and coherences and functional connectivity.
2.3. Statistical Analyses
2.3.1. Empirically Derived Subgroups Based on Functional Connectivity
This set of analyses was performed in the R programming language (Team, 2015). To assess functional connectivity, we computed voltage correlations and partial correlations between each electrode pair for each recording session. For each session, the complete set of correlations/partial correlations forms a matrix that can be conceptualized as a network between probe pairs. To investigate the differences between the ASD and the TYP and DD groups, all networks were combined into a single matrix (the data matrix) as follows: correlation matrices of one type, either partial or Pearson, were linearized by converting the top triangle to a vector. These vectors were concatenated into a single matrix in which each column represents a single network derived from a patient recording session, and each row holds the correlation for a single pair of electrodes over all patients and sessions. We performed hierarchical clustering on the data matrix to determine the intrinsic groupings of networks within the study population.
To further investigate the ability of correlation networks to classify children with autism and to extract defining structural features from these networks, we used support vector machines (SVM) to find a hyperplane separating two classes of points in high-dimensional space. Once the best separating hyperplane was found, support vectors were used to calculate a vector “w” perpendicular to the separating hyperplane. To determine the success of the SVM model in classifying the data, we divided the data set into a training set and a testing set. We used 50% of patients in the training set and 50% in the testing set. We randomly assigned patients to either the training or testing set 100 times and asked how well the model classifies patients in the testing set (two-fold cross-validation). The stratification of the patient groups (ASD, DD, and TYP) was preserved in the training and testing groups. To find differences between the classes that persist over all training and testing sessions, we averaged “w” across all iterations. We kept only values that were consistently positive or negative across all 100 trials. All others were fixed at zero.
Two-fold cross-validation allows us to measure both the accuracy and robustness of the SVM. Accuracy is the fraction of correct classifications in the testing data and measures how well the model generalizes to unseen data. We trained and tested on both halves of the data used and we average the accuracies for both halves. Robustness is defined as the Pearson correlation between the “w” vectors for the models trained on each half of the data. Robustness measures how similar the models are that are trained on different halves of the data.
2.3.2. Other Analyses
The remainder of the analyses consisted of between-group comparison of all electrophysiological measures (power [absolute, relative, and ratios], spectral coherence/Pearson correlations, phase lag, and amplitude asymmetry). Coherence values were subjected to Fisher-transformation for statistical analysis. A series of general linear models, controlling for mean-centered age, was used to evaluate differences between the ASD and DD and the ASD and TYP groups. SAS/STAT Version 9·3 was used for all analyses. The SAS/STAT MULTTEST procedure was used to calculate false discovery rate (FDR (Benjamini and Hochberg, 1995)) adjusted p-values. Given the exploratory nature of these analyses, we do not use a strict cutoff for statistical significance.
2.4. Funding
This research was supported by the National Institute of Mental Health Intramural Research Program.
3. Results
3.1. Coherence and Phase lag
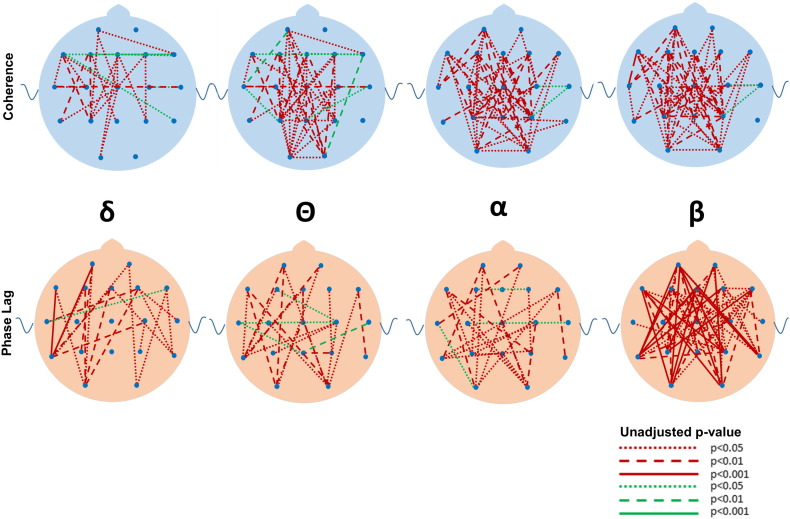
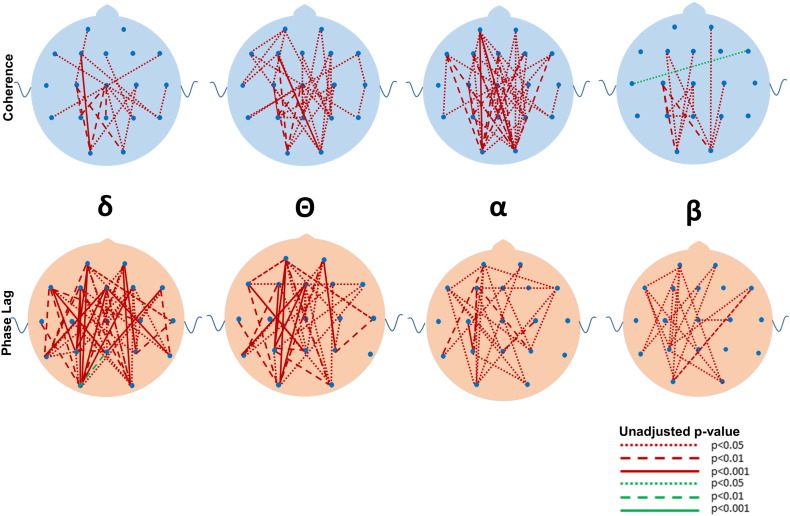
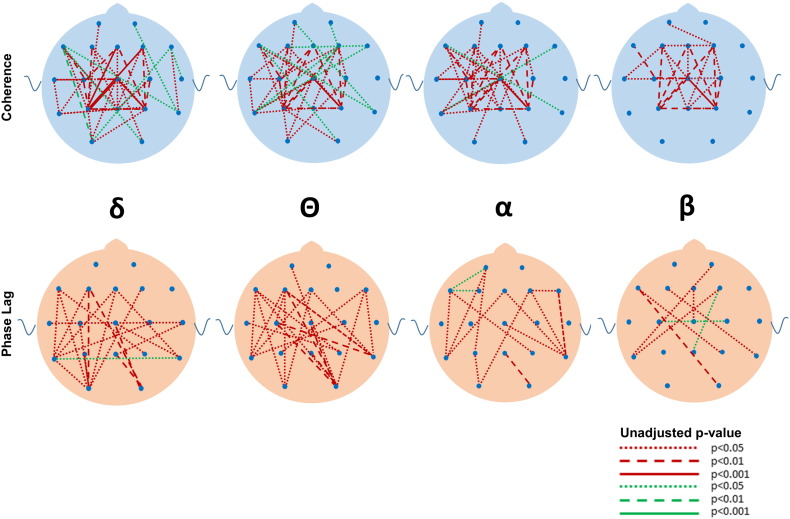
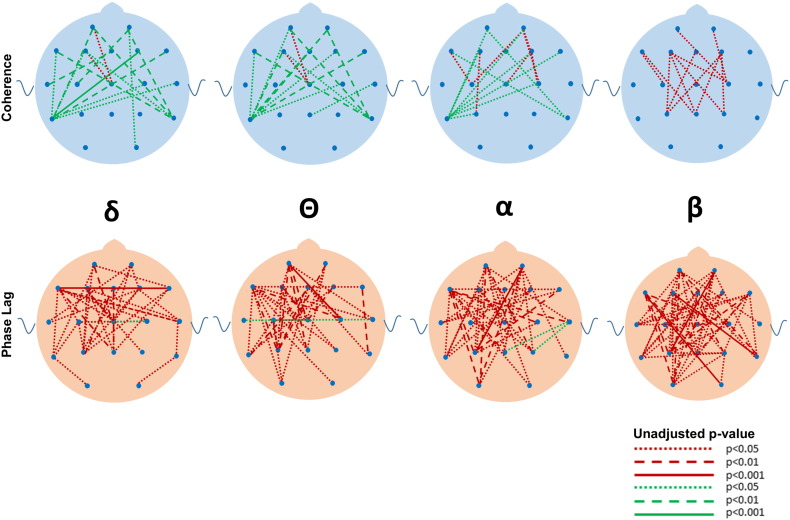
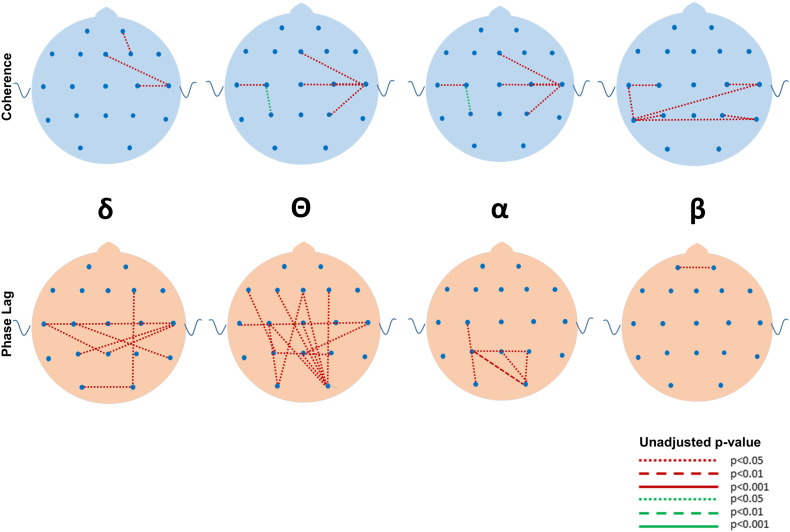
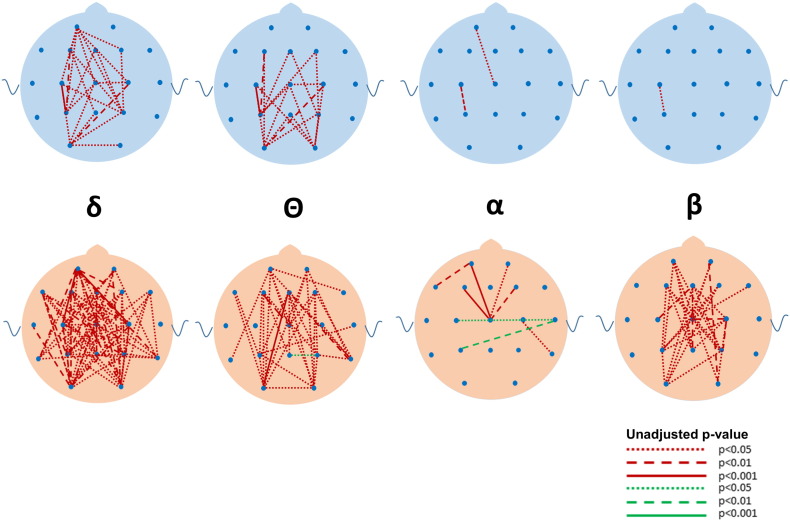
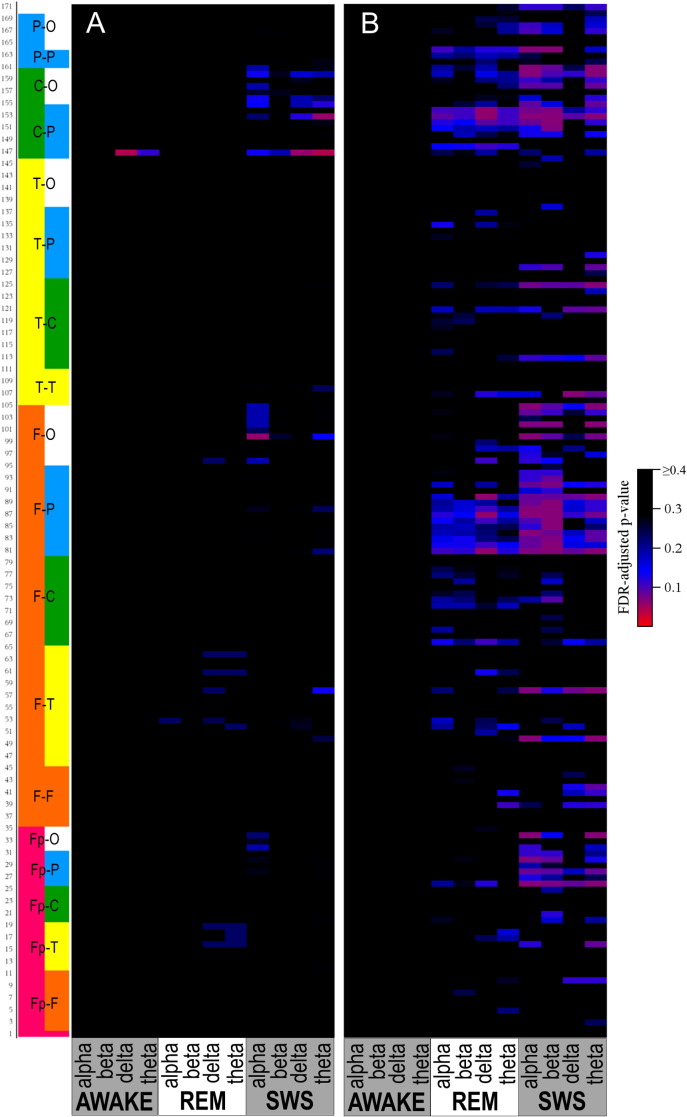
Although many differences between the ASD and DD and TYP groups were observed in mean coherence (see Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10 for uncorrected p-values), a few remained after FDR-correction (Fig. 3, Supplementary Tables S1 and S2). Most striking was the increased coherence observed in ASD relative to TYP, almost exclusively during SWS, concentrated in the frontal–parietal pairs. No differences in coherence were observed between ASD and TYP during the awake state. Few differences in coherence between ASD and DD remained following correction, in any state. See Fig. 3
Fig. 5.
Uncorrected differences in coherence (top row) and phase lag (bottom row) between ASD and TYP groups during slow wave sleep in the four major frequencies. Red and green lines represent p values. Red lines indicate that the ASD group has greater coherences and reduced phase lag than the TYP group whereas green lines indicate lower coherences or greater phase lag compared to the TYP group. Note the marked increased in coherences and reductions in phase lag in the ASD compared to the TYP group. Coding for p values as described in Fig. 2.
Fig. 6.
Uncorrected differences in coherence (top row) and phase lag (bottom row) between ASD and DD groups during slow wave sleep in the four major frequencies. Note the marked increased in coherences and reductions in phase lag in the ASD compared to the DD group. Coding for p values as described in Fig. 2.
Fig. 7.
Uncorrected differences in coherence (top row) and phase lag (bottom row) between ASD and TYP groups during REM in the four major frequencies. Note the marked increased in coherences and reductions in phase lag in the ASD compared to the TYP group. Coding for p values as described in Fig. 2.
Fig. 8.
Uncorrected differences in coherence (top row) and phase lag (bottom row) between ASD and DD groups during REM in the four major frequencies. Note the reductions in coherences in the β, α, and Θ bandwidths and reductions in phase lag in the ASD compared to the DD group. Coding for p values as described in Fig. 2.
Fig. 9.
Uncorrected differences in coherence (top row) and phase lag (bottom row) between ASD and TYP groups during the waking state in the four major frequencies. Note the minimal differences between the groups at most frequencies during the awake state. Coding for p values as described in Fig. 2.
Fig. 10.
Uncorrected differences in coherence (top row) and phase lag (bottom row) between ASD and DD groups during the waking state in the four major frequencies. Note the increased in coherences and reductions in phase lag in the ASD versus the DD group at the δ and Θ frequencies. Coding for p values as described in Fig. 2.
Fig. 3.
Heatmap of FDR-adjusted p-values for comparison of coherence in ASD versus DD (Panel A) and ASD versus TYP (Panel B). Pairs are alternately numbered; number corresponds to order in Supplementary Tables S1 and S2. Pairs are grouped by region: Fp = Frontopolar, F = frontal, T = temporal, C = central, and O = occipital.
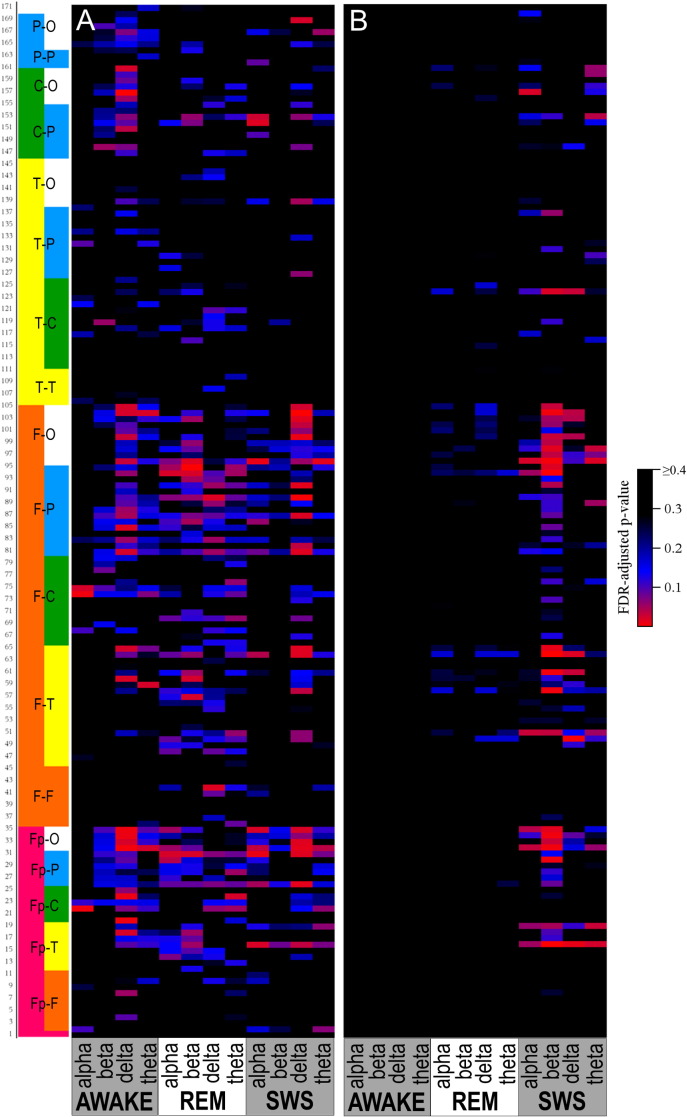
Fig. 4.
Heatmap of FDR-adjusted p-values for comparison of phase lag in ASD versus DD (Panel A) and ASD versus TYP (Panel B). Pairs are alternately numbered; number corresponds to order in Supplementary Tables S3 and S4. Pairs are grouped by region: Fp = Frontopolar, F = frontal, T = temporal, C = central, and O = occipital.
Following FDR correction, differences in phase lag between ASD and TYP (ASD < TYP) were observed almost exclusively during SWS, most notably in long-distance pairs (Fig. 4; see Supplementary Tables). Differences between ASD and DD (ASD < DD) were more common, but the pattern was diffuse across states, bands, and electrode locations.
3.2. Spectral Power
No differences were seen in FFT absolute or relative power or power ratios between the children with ASD and DD or TYP. No consistent differences were seen in amplitude asymmetry between the ASD and TYP or ASD and DD groups in any of the states.
3.3. Epileptiform Activity
The rate of subjects with interictal spikes did not differ among groups; nine (10%) ASD, two (10%) DD, and two (7%) of the TYP group. Table 1 lists the type of epileptiform activity observed in the three groups.
Table 1.
Epileptiform activity.
| Awake (spikes/min) | Drowsiness/stage II (spikes/min) | SWS (spikes/min) | ||
|---|---|---|---|---|
| ASD (n = 9) | C4 spikes | 0·1 | 8·1 | 10·4 |
| O1 spikes | 5·9 | 10·2 | 0·1 | |
| C3/C4 spikes | 2·9 | 19·4 | 20·8 | |
| C3 spikes | 11·7 | 13·1 | 8·8 | |
| T6/P4 spikes | 3·0 | 3·0 | 1·7 | |
| Gen. polyspikes | 2·4 | 3·0 | 2·6 | |
| C4 spikes | 0·6 | 8·0 | 0·4 | |
| Bifrontal spike/wave | 0·0 | 6·0 | 6·0 | |
| O2 spikes | 5·7 | 7·3 | 12·3 | |
| DD (n = 2) | T3/T5 spikes | 12·5 | 56·0 | 56·0 |
| Right spike/wave | 2·1 | 0·7 | 0·2 | |
| TYP (n = 2) | P4 spikes | 3·2 | 2·5 | 0·0 |
| C4 spikes | 1·7 | 4·2 | 6·0 |
Note: ASD = autism spectrum disorder. DD = non-ASD developmental disorder. TYP = typically developing.
3.4. Pearson and Partial Voltage Correlations
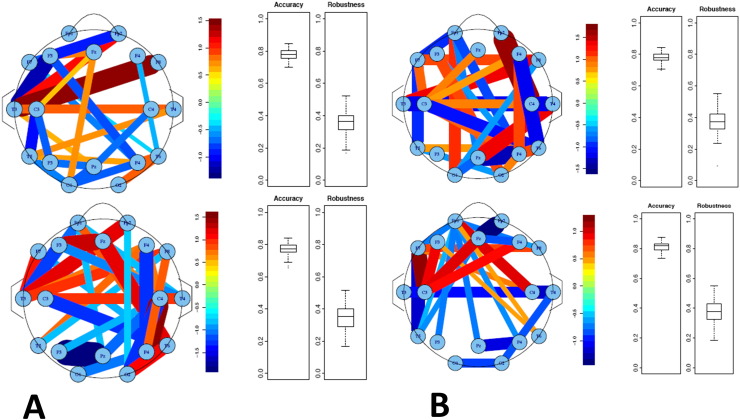
The hierarchial clustering algorithm failed to differentiate the three groups of patients and was discarded. However, using the support vector machines (SVM) model, the ASD children clearly separated from both the TYP and DD groups using both Pearson and partial correlations with a high degree of accuracy (Fig. 11). Two-fold cross-validation was used to test the accuracy and robustness of the SVM model. For SVM models trained on random halves of the cohort, the median testing accuracy was greater than 0.75, indicating that the models captured structure that generalized to the rest of the cohort. For random guessing using the proportions of ASD, DD, and TYP subjects, the expected accuracy was 0.52 for the ASD vs. TYP comparison and 0.69 for the ASD vs. DD. The null expected accuracy of the ASD vs. DD comparison was high due to the imbalance of study populations. The SVM model, however, improved over random guessing in all cases and achieves greater than 0.8 median testing accuracy for the partial correlations (Fig. 11b). Using either Pearson or partial correlations, the robustness of the model was low indicating that while group differences were observed in mean functional connectivity, individual patient differences within each group were high.
Fig. 11.
Pearson and partial correlations of ASD versus TYP group (A) and ASD versus DD group (B). Pearson correlations are on top while partial correlations are on bottom. The data demonstrates that there are clear differences between groups with a high degree of accuracy. The pattern is mixed with both increased and decreased correlations between groups. A red line means that the correlation between these two electrodes was significantly higher in the ASD than in the TYP or DD group. The blue line reflects a significantly low correlation, i.e. the ASD group has a lower correlation that the TYP or DD group.
4. Discussion
Our study reports on a cohort of young children with ASD who show evidence of altered functional connectivity as measured by several patterns of oscillatory activity. In comparison to the TYP subjects, the children with ASD had increased coherence at multiple electrode pairs and at multiple bandwidths, and phase lag was reduced in comparison to both DD and TYP. Consistent with these observed differences in the crude comparisons at each pair and bandwidth, the supervised learning model was successful in separating the three groups on the basis of both Pearson and partial correlations. However, the robustness of the model was low, reflecting the high degree of individual differences within each group.
Whereas EEG coherence is a measure of the consistency of phase differences over space and computes “phase synchrony” or “phase stability” between spatially distant generators, phase lag represents the phase difference between EEG signals. On a frequency-by-frequency basis, EEG spectral coherence represents the consistency of the phase difference between two EEG signals when compared over time whereas phase lag measures the actual differences in phase. In practice, high coherence values are taken as a measure of strong connectivity between the brain regions that produce the compared EEG signals (Srinivasan et al., 2007).
As in this study, decreased EEG phase lag is often associated with high coherence indicating that in children with ASD there are highly coherent networks with an unvarying relationship among phases in different brain regions, but also a network with a remarkably high and rigid coupling of phase across brain regions. While on average, the brains of the children with ASD appeared to be overly coherent, there were a few regions with reduced coherences when compared to the other groups of children. The most marked differences between the ASD cohort and the other groups were observed during SWS sleep, with minimal differences between groups recorded during the wake state.
The role of sleep in the proper maturation of the developing brain is an area of current intense interest, with the contribution of state-specific processes to synaptic refinement just beginning to be understood. The vast majority of ASD coherence studies are not performed during sleep, and taken as a whole, show very mixed results. Some of the waking evaluations used EEG or magnetoencephalography (Coben et al., 2008) and demonstrated reduced coherences (Khan et al., 2013, Coben et al., 2008), with other studies reporting increases (Murias et al., 2007, Orekhova et al., 2014, Dominguez et al., 2013) or mixed patterns (Barttfeld et al., 2011, Duffy and Als, 2012). In the MRI literature, the consistent pattern emerging across several studies is that while intrinsic functional connectivity in adolescents and adults with autism is generally reduced compared with age-matched controls, functional connectivity in younger children with ASD appears to be increased (Uddin et al., 2013, Nomi and Uddin, 2015). Kitzbichler et al. took an elegant approach to the apparent discrepancies of over-connectivity versus under-connectivity in a study of ASD versus control (ages 6–21 years), examining both MEG and MRI in the resting state in each subject. The authors concluded that the true relationship is more complicated with the major differences being mediated by both region and frequency examined (Kitzbichler et al., 2015). Our current study adds to the complexity surrounding the search for electrophysiologic biomarker signatures of aberrant neurodevelopment by positing that in addition to age, bandwidth and region, brain state matters enormously to any measurements of differences in coherence in the developing brain. Sleep is a protected time for brain maturation and changes that are detected only during sleep may provide an early window affording valuable information about the rapid and dynamic changes that must take place to build normal functional relationships. The two major factors that drove the nature of connectivity abnormalities in ASD were the mediating frequency band and whether the network included frontal nodes. These factors determined whether clustering and integration were increased or decreased in cortical resting state networks in ASD.
Our recent review of the literature revealed only two studies that evaluated coherence in ASD in the sleeping brain, and together, the studies included more adults than children. In 2010, Leveille et al. (Leveille et al., 2010) compared coherence during REM sleep between nine adults with ASD and 13 typically developing adults. The authors found no differences in interhemispheric coherence patterns, however, they did show increased intrahemispheric coherence in the left visual cortex involving both short- and long-range connections and reductions in coherence in the right frontal lobe. In contrast, a 2010 study by Lazar et al. (Lazar et al., 2010) recorded 18 children and young adults with Asperger's disorder and normal IQ and compared them to 13 children and young adults without ASD or intellectual disability in non-REM sleep. As Leveille's group did, this study also reported no difference in interhemispheric coherence patterns, but reported a comparative reduction in intrahemispheric coherence patterns in the right, fronto-central area in those with ASD. These are very small studies and direct comparison is made difficult by the differences in the presence or absence of intellectual disabilities and in sleep states examined.
Functional connectivity may vary with age and therefore, any evaluation of brain activity patterns as potential biomarkers must be mindful that the developmental timing of the evaluation is likely a critical factor in the interpretation of differences. Although there have been a few small, longitudinal EEG studies in typical children (Kurth et al., 2013, Tarokh et al., 2014) reporting region-specific increases in sleep coherence across development, there are no longitudinal ASD coherence studies for comparison. Such evaluations would inform on developmental trajectories and perhaps provide insight into the timing for optimal intervention. We do not comment on age-related changes in coherence or phase lag in this cross-sectional study, as our forthcoming report on the longitudinal data from this sample will better address this question.
The evaluation of coherence during sleep as a valuable and informative exercise is undeniable. The sleep EEG not only reflects the maturation of the brain (Kurth et al., 2013) but also allows for examination of dynamic neural networks in the absence of external stimuli. In addition, new insight into the function of sleep states makes it imperative that we not overlook the brain's activity during sleep in neurodevelopmental disorders. While the exact function of sleep is unknown, the strongest evidence from human and animal experimental studies suggests that sleep's major role is to regulate brain plasticity (Wang et al., 2011). SWS is implicated in learning and memory throughout the lifespan and a prevailing hypothesis has been that SWS is essential for the consolidation of memories temporarily laid down during waking hours. However, recent work on REM dependent neuroplasticity in the cat visual cortex (Dumoulin et al., 2015) suggests a similar role for REM sleep in the proper maturation of the sensory cortices in the developing brain. Given that there are both age- and state-dependent differential effects of sleep on neuronal responses and processes, further evaluation of sleep coherence during critical windows of neuromaturation is clearly important to our understanding of neurodevelopmental disorders.
We also found that voltage correlation using Pearson or partial correlation coefficients could distinguish groups of children with ASD from groups of TYP and DD children. Voltage correlation is a measure of the co-modulation of the amplitude envelopes (i.e., power) of oscillations in two brain areas and is also referred to as “power-to-power correlation” or “amplitude–amplitude coupling.” It is important to note that while both coherences and the voltage correlations provide information about functional connectivity, they provide distinctly different information. The voltage correlations are measures of the degree of association between amplitudes of the EEG across sites and does not calculate phase nor does it involve the measurement of the consistency of phase relationships seen with coherence. The functional significance of amplitude correlation is less clear than the mechanistic interpretation of phase coherence. However, taken together, this data indicate that brain connectivity in ASD is distinctly different on average from children with other developmental disabilities or typical development.
Finally, we note that children with autism are frequently reported to have epileptiform activity, in the form of spikes, sharp waves, and spike-and-wave discharges on their EEG (Hashimoto et al., 2001, Hughes and Melyn, 2005, Parmeggiani et al., 2007, Spence and Schneider, 2009). In this study, the rate of epileptiform activity was low. The low incidence of epileptiform activity in the ASD group suggests that it was not a contributing factor in our findings.
5. Limitations
In this study, only the ASD and DD groups are matched on NVDQ. Thus, we were unable to compare our TYP group to high-functioning children with ASD. We describe observed differences in functional connectivity as measured by spontaneous electroencephalographic discharges between this ASD cohort with ID and other developmental groups by region and state. While we do review some of the differing parameters in the extant literature (differences in IQ and age of participants), this is not an exhaustive review nor do we propose a unifying theory of aberrant neurodevelopment from this analysis. The relationship of functional coherence to age and behavior is still very much an open question and we look forward to addressing it more fully in our forthcoming analysis of the longitudinal data from this sample.
Authors' Contributions
Drs. Buckley, Thurm, and Swedo were responsible for the design and implementation of the study, as well as all aspects of manuscript preparation. Drs. Scott, Tyler, Mahoney, Mr. Burroughs, and Dr. Holmes contributed to data analysis, data interpretation, and manuscript preparation. Dr. Farmer contributed to data analysis and manuscript preparation. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Conflicts of Interest
The authors have no conflicts of interest to report.
Acknowledgments
This research was supported by the Intramural Program of the National Institute of Mental Health. The views expressed in this paper do not necessarily represent the views of the NIMH, NIH, HHS, or the United States Government. Protocol number 06-M-0102 and NCT00298246.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.11.004.
Appendix A. Supplementary data
Supplementary tables.
Supplementary material.
References
- American Psychiatric Association . American Psychiatric Publishing; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders, DSM-IV-TR. [Google Scholar]
- Anderson J.S., Nielsen J.A., Froehlich A.L. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134(Pt 12):3742–3754. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anney R., Klei L., Pinto D. A genome-wide scan for common alleles affecting risk for autism. Hum. Mol. Genet. 2010;19(20):4072–4082. doi: 10.1093/hmg/ddq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P., Wicker B., Cukier S., Navarta S., Lew S., Sigman M. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia. 2011;49(2):254–263. doi: 10.1016/j.neuropsychologia.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Belmonte M.K., Cook E.H., Jr., Anderson G.M. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol. Psychiatry. 2004;9(7):646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D., Kronfeld-Duenias V., Zachor D.A. Accelerated maturation of white matter in young children with autism: a high b value DWI study. NeuroImage. 2007;37(1):40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995:289–300. [Google Scholar]
- Cantor D.S., Thatcher R.W., Hrybyk M., Kaye H. Computerized EEG analyses of autistic children. J. Autism Dev. Disord. 1986;16(2):169–187. doi: 10.1007/BF01531728. [DOI] [PubMed] [Google Scholar]
- Casanova M., Trippe J. Radial cytoarchitecture and patterns of cortical connectivity in autism. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009;364(1522):1433–1436. doi: 10.1098/rstb.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Chou K.H., Chen I.Y., Fan Y.T., Decety J., Lin C.P. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. NeuroImage. 2010;50(3):873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cherkassky V.L., Kana R.K., Keller T.A., Just M.A. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Coben R., Clarke A.R., Hudspeth W., Barry R.J. EEG power and coherence in autistic spectrum disorder. Clin. Neurophysiol. 2008;119(5):1002–1009. doi: 10.1016/j.clinph.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Dinstein I., Pierce K., Eyler L. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70(6):1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez L.G., Stieben J., JLP V., Shanker S. The imaginary part of coherency in autism: differences in cortical functional connectivity in preschool children. PLoS One. 2013:8(10). doi: 10.1371/journal.pone.0075941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy F.H., Als H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls — a large case control study. BMC Med. 2012;10:64. doi: 10.1186/1741-7015-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin M.C., Aton S.J., Watson A.J., Renouard L., Coleman T., Frank M.G. Extracellular signal-regulated kinase (ERK) activity during sleep consolidates cortical plasticity in vivo. Cereb. Cortex. 2015;25(2):507–515. doi: 10.1093/cercor/bht250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott C.D. Second Edition. The Psychological Corporation; San Antonio, TX: 2007. Differential Ability Scales. [Google Scholar]
- Friston K.J. Functional and effective connectivity: a review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Gaugler T., Klei L., Sanders S.J. Most genetic risk for autism resides with common variation. Nat. Genet. 2014;46(8):881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J., Cleveland S., Torres A. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Sasaki M., Sugai K., Hanaoka S., Fukumizu M., Kato T. Paroxysmal discharges on EEG in young autistic patients are frequent in frontal regions. J. Med. Investig. 2001;48(3–4):175–180. [PubMed] [Google Scholar]
- Hughes J.R., Melyn M. EEG and seizures in autistic children and adolescents: further findings with therapeutic implications. Clin. EEG Neurosci. 2005;36(1):15–20. doi: 10.1177/155005940503600105. [DOI] [PubMed] [Google Scholar]
- Isler J.R., Martien K.M., Grieve P.G., Stark R.I., Herbert M.R. Reduced functional connectivity in visual evoked potentials in children with autism spectrum disorder. Clin. Neurophysiol. 2010;121(12):2035–2043. doi: 10.1016/j.clinph.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Kana R.K., Minshew N.J. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb. Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Libero L.E., Moore M.S. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys. Life Rev. 2011;8(4):410–437. doi: 10.1016/j.plrev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Keown C.L., Shih P., Nair A., Peterson N., Mulvey M.E., Muller R.A. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep. 2013;5(3):567–572. doi: 10.1016/j.celrep.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Gramfort A., Shetty N.R. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc. Natl. Acad. Sci. U. S. A. 2013;110(8):3107–3112. doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzbichler M.G., Khan S., Ganesan S. Altered development and multifaceted band-specific abnormalities of resting state networks in autism. Biol. Psychiatry. 2015;77(9):794–804. doi: 10.1016/j.biopsych.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H., Carpenter P.A., Minshew N.J., Cherkassky V.L., Keller T.A., Just M.A. Functional connectivity in an FMRI working memory task in high-functioning autism. NeuroImage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Koshino H., Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. FMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb. Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S., Achermann P., Rusterholz T., Lebourgeois M.K. Development of brain EEG connectivity across early childhood: does sleep play a role? Brain Sci. 2013;3(4):1445–1460. doi: 10.3390/brainsci3041445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar A.S., Lazar Z.I., Biro A. Reduced fronto-cortical brain connectivity during NREM sleep in Asperger syndrome: an EEG spectral and phase coherence study. Clin. Neurophysiol. 2010;121(11):1844–1854. doi: 10.1016/j.clinph.2010.03.054. [DOI] [PubMed] [Google Scholar]
- Lazarev V.V., Pontes A., Mitrofanov A.A., de Azevedo L.C. Interhemispheric asymmetry in EEG photic driving coherence in childhood autism. Clin. Neurophysiol. 2010;121(2):145–152. doi: 10.1016/j.clinph.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Leveille C., Barbeau E.B., Bolduc C. Enhanced connectivity between visual cortex and other regions of the brain in autism: a REM sleep EEG coherence study. Autism Res. 2010;3(5):280–285. doi: 10.1002/aur.155. [DOI] [PubMed] [Google Scholar]
- Lewis J.D., Theilmann R.J., Townsend J., Evans A.C. Network efficiency in autism spectrum disorder and its relation to brain overgrowth. Front. Hum. Neurosci. 2013;7:845. doi: 10.3389/fnhum.2013.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Mullen E.M. American Guidance Service, Inc.; xCircle Pines, MN: 1995. Mullen Scales of Early Learning. [Google Scholar]
- Muller R.A., Shih P., Keehn B., Deyoe J.R., Leyden K.M., Shukla D.K. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb. Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M., Webb S.J., Greenson J., Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol. Psychiatry. 2007;62(3):270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi J.S., Uddin L.Q. Developmental changes in large-scale network connectivity in autism. NeuroImage Clin. 2015;7:732–741. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova E.V., Elsabbagh M., Jones E.J. EEG hyper-connectivity in high-risk infants is associated with later autism. J. Neurodev. Disord. 2014;6(1):40. doi: 10.1186/1866-1955-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmeggiani A., Posar A., Antolini C., Scaduto M.C., Santucci M., Giovanardi-Rossi P. Epilepsy in patients with pervasive developmental disorder not otherwise specified. J. Child Neurol. 2007;22(10):1198–1203. doi: 10.1177/0883073807306265. [DOI] [PubMed] [Google Scholar]
- Pina-Camacho L., Villero S., Fraguas D. Autism spectrum disorder: does neuroimaging support the DSM-5 proposal for a symptom dyad? A systematic review of functional magnetic resonance imaging and diffusion tensor imaging studies. J. Autism Dev. Disord. 2012;42(7):1326–1341. doi: 10.1007/s10803-011-1360-4. [DOI] [PubMed] [Google Scholar]
- Pinto D., Delaby E., Merico D. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 2014;94(5):677–694. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N., Spiker D., Lotspeich L. A genomic screen of autism: evidence for a multilocus etiology. Am. J. Hum. Genet. 1999;65(2):493–507. doi: 10.1086/302497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., LeCouteur A., Lord C. Western Psychological Services; Los Angeles, CA: 2003. Autism Diagnostic Interview-Revised (ADI-R) [Google Scholar]
- Spence S.J., Schneider M.T. The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr. Res. 2009;65(6):599–606. doi: 10.1203/01.pdr.0000352115.41382.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R., Winter W.R., Ding J., Nunez P.L. EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J. Neurosci. Methods. 2007;166(1):41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Khouzam A. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013;5(3):738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L., Carskadon M.A., Achermann P. Early adolescent cognitive gains are marked by increased sleep EEG coherence. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: a language and environment for statistical computing. [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Grone B., Colas D., Appelbaum L., Mourrain P. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends Neurosci. 2011;34(9):452–463. doi: 10.1016/j.tins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S.J., Wiggins J.L., Peltier S.J. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.
Supplementary material.