Abstract
Background
Abnormalities in membrane excitability and Na+ channel function are characteristic of amyotrophic lateral sclerosis (ALS). We aimed to examine the neuroprotective potential, safety and tolerability of the Na+ channel blocker and membrane stabiliser flecainide in ALS.
Methods
A double-blind, placebo-controlled, randomised clinical trial of flecainide (200 mg/day) for 32-weeks with a 12-week lead-in phase was conducted in participants with probable or definite ALS recruited from multiple Australian centres (ANZCT Registry number ACTRN12608000338369). Patients were reviewed by a cardiologist to rule out cardiac contraindications. Participants were randomly assigned (1:1) to flecainide or placebo using stratified permuted blocks by a central pharmacy. The primary outcome measure was the slope of decline of the ALS Functional Rating Scale-revised (ALS FRS-r) during the treatment period.
Findings
Between March 11, 2008 and July 1, 2010, 67 patients were screened, 54 of whom were randomly assigned to receive flecainide (26 patients) or placebo (28 patients). Four patients in the flecainide group and three patients in the placebo group withdrew from the study. One patient in the flecainide group died during the study, attributed to disease progression. Flecainide was generally well tolerated, with no serious adverse events reported in either group. There was no significant difference in the rate of decline in the primary outcome measure ALS-FRS-r between placebo and flecainide treated patients (Flecainide 0.65 [95% CI 0.49 to 0.98]; Placebo 0.81 [0.49 to 2.12] P = 0.50). However, the rate of decline of the neurophysiological index was significantly reduced in the flecainide group (Flecainide 0.06 [0.01 to 0.11]; Placebo 0.14 [0.09 to 0.19], P = 0.02). Placebo-treated patients demonstrated greater CMAP amplitude reduction during the course of the study in the subset of patients with a reduced baseline CMAP amplitude (Flecainide: − 15 ± 12%; Placebo − 59 ± 12%; P = 0.03). Flecainide-treated patients maintained stabilized peripheral axonal excitability over the study compared to placebo.
Interpretation
This pilot study indicated that flecainide was safe and potentially biologically effective in ALS. There was evidence that flecainide stabilized peripheral axonal membrane function in ALS. While the study was not powered to detect evidence of benefit of flecainide on ALS-FRS-r decline, further studies may demonstrate clinical efficacy of flecainide in ALS.
Keywords: Flecainide, Amyotrophic lateral sclerosis, Sodium channel, Neuroprotection
Highlights
-
•
To determine safety and neuroprotective potential, a double-blind, placebo-controlled, randomised trial of the Na+ channel blocking agent flecainide was conducted in ALS.
-
•
Flecainide was well tolerated, with no serious adverse events.
-
•
Although there was some evidence that flecainide stabilised peripheral axonal membrane function, the study was not powered to provide evidence of benefit on functional decline.
Changes in nerve excitability function occur in patients with amyotrophic lateral sclerosis (ALS). We conducted a double-blind, placebo-controlled, randomised clinical trial to examine the impact of a membrane/nerve stabilizer (flecainide) in ALS patients. Although there was some evidence that flecainide stabilised peripheral axonal membrane function in ALS, the study was not powered to find evidence that flecainide benefited patient function.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive and invariably fatal neurodegenerative disorder of motor neurons (Kiernan et al., 2011). There is currently no cure and a critical need to develop disease-modifying therapies. Multiple pathophysiological mechanisms underlie the development of ALS, including abnormalities in membrane excitability and ion channel function (Kiernan et al., 2011). Hyperexcitability and consequent excitotoxicity have been extensively documented in ALS and may be linked to the process of neurodegeneration (Mogyoros et al., 1998, Kuo et al., 2005, Kanai et al., 2006, Vucic and Kiernan, 2006, Vucic and Kiernan, 2010). Peripheral axonal excitability techniques demonstrated elevated persistent Na+ and reduced K+ conductances (Kanai et al., 2006, Vucic and Kiernan, 2006), a neurophysiological profile that would promote hyperexcitability, membrane instability and spontaneous activity. Increased persistent Na+ conductances and consequent hyperexcitability are also characteristic of ALS motor neurons from animal models (Kuo et al., 2005, Pieri et al., 2009, Quinlan et al., 2011).
Aberrant excitability and upregulation of persistent Na+ conductances have been postulated to lead to an increase in intracellular Na+ concentration, overwhelming the corrective effects of the Na+/K+ pump and the Na+/Ca2 + transporter, and resulting in motor neuron neurodegeneration via Ca2 +-mediated processes (Kapoor et al., 2003, Stys, 2005). Consequently, promotion of membrane stability, ion homeostasis and antagonism of persistent Na+ conductances may be neuroprotective in ALS. Riluzole, a neuroprotective agent that prolongs survival in ALS, partially blocks persistent Na+ conductances in ALS mouse models (Benoit and Escande, 1991, Urbani and Belluzzi, 2000, Kuo et al., 2005), relevant at clinical doses. Conversely, it has been suggested that axonal hyperexcitability may be an adaptive mechanism promoting neuroprotection in ALS. Specifically, enhancing neuronal hyperexcitability was reported to protect motor neurons from endoplasmic reticulum stress and subsequent degeneration (Saxena et al., 2013). Accordingly, it was postulated that targeting hyperexcitability via Na+ channel modulation as a therapeutic strategy may not be beneficial, perhaps producing adverse effects in motor neurons.
Flecainide is a well-characterized membrane stabilizer and Na+ channel blocking agent, particularly used in the context of cardiac medicine (Aliot et al., 2011). Studies in animal models have demonstrated that flecainide may exert neuroprotective benefits (Bechtold et al., 2005, Morsali et al., 2013). In light of this background, the aim of the present study was to examine the neuroprotective potential of flecainide and its impact on membrane excitability and function in ALS patients.
2. Methods
2.1. Study Design and Participants
This trial was an investigator-initiated, double-blind, placebo-controlled and randomized clinical trial of flecainide treatment in ALS patients. Participants were recruited from multiple centres around Australia between March 2008 and July 2010 and all investigations were undertaken at a specialist ALS clinic.
Participants were eligible for inclusion in the trial if they were aged between 18 and 75 years, with a probable or definite diagnosis of ALS according to the revised El Escorial criteria (Brooks et al., 2000) and a disease duration of less than 5 years. Other inclusion criteria were a sniff nasal inspiratory pressure (SNIP) of greater than 50% predicted, normal cardiac rhythm and normal left ventricular function (determined by echocardiography) and ability to provide informed consent. Patients with a history of dementia or psychiatric illness, cardiac disease, significant impairment of hepatic or renal function or who were pregnant or lactating were excluded.
All patients provided written informed consent, with studies approved by the South Eastern Sydney and Illawarra Area Health Service Human Research Ethics Committee (Northern Sector). Approval was obtained from the Australian Therapeutic Goods Administration for the use of flecainide in the current trial. This study was registered with Australian and New Zealand Clinical Trials Registry under the number ACTRN12608000338369, and supported by the National Health and Medical Research Council of Australia (Project number #568743).
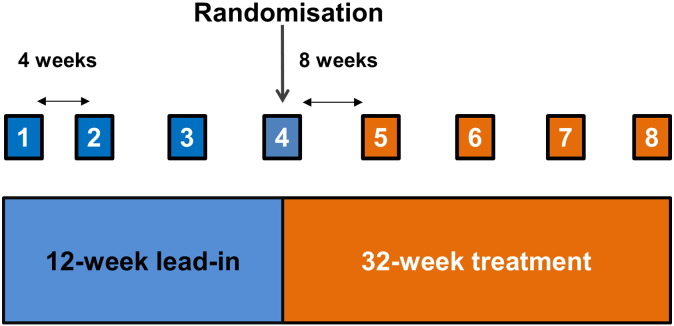
The trial was composed of a 12-week lead-in period comprised of three consecutive visits at 4 weekly intervals, enabling a calculation of the baseline rate of decline in ALSFRS-R to be determined (Fig. 1). During the lead-in period, all patients were formally reviewed by a cardiologist to rule out cardiac contraindications prior to receiving flecainide. All patients included in the study had normal cardiac rhythm and left ventricular function, determined by electrocardiography, without history of cardiac disease. All patients continued to receive riluzole (50 mg twice daily). Once randomized, patients entered the 32 week treatment period and were assessed every 8 weeks.
Fig. 1.
Trial design: Diagram demonstrating the 12-week lead-in period followed by randomization at the 4th visit (week 12), with the final visit occurring 32 weeks after randomization.
2.2. Randomisation and Masking
Participants were randomly assigned (1:1) to flecainide or placebo using permuted blocks stratified by disease onset site (bulbar or limb-onset). Randomization occurred after the non-treatment lead-in period at visit 4 (week 12). All randomizations were performed centrally by a specific clinical trials pharmacist in the Department of Pharmacy, Prince of Wales Hospital. Patients were initially prescribed flecainide 50 mg twice daily for one week and subsequently the dose was increased to 100 mg twice daily. Drug was supplied by Alphapharm Australia, while placebo was supplied by Stenlake Science and Nature, Australia. Drug and placebo were identical in appearance and were dispensed by the pharmacy at 8 weekly intervals during the treatment period. All investigators, assessors and evaluators remained blinded to treatment.
2.3. Primary Outcome
The primary outcome measure was the slope of decline of the ALS Functional Rating Scale-revised (ALS FRS-r) during the treatment period. The ALSFRS-r is a validated measure of functional impairment in ALS, with higher scores indicating better function (Range 0–48). The ALSFRS-r is divided into 4 subscores of 12 points each (bulbar, fine motor, gross motor and respiratory) (Cedarbaum et al., 1999).
2.4. Secondary Endpoints
Secondary endpoints included safety and tolerability, patient function (quality of life, grip and motor function, respiratory performance) and neurophysiological assessments as detailed below. At each study visit, any possible adverse events were documented. All patients underwent blood screening to assess liver, renal and haematological function at each 8 weekly review. Monitoring of adverse events was undertaken by an independent data safety committee which reviewed and analysed all data at the 6 and 12 month study timelines.
Health-related quality of life was assessed via the Short Form-36 (SF-36) questionnaire. Walking ability was assessed via the time in seconds to undertake a 6-m walk, with the fastest time recorded out of three attempts (Tiedemann et al., 2008). Grip strength was assessed by a Jaymar dynamometer (Asimow Engineering Co; Los Angeles, CA, USA) using the average of three trials for each hand. Respiratory function was assessed via forced vital capacity (FVC) and sniff nasal inspiratory pressure (SNIP). FVC was measured using a portable spirometer (MicroLab, CareFusion; Basingstoke, UK), using the best of three trials and reported as a percentage of the predicted value (Miller et al., 2005). SNIP was measured using the MicroRPM device (CareFusion; Basingstoke, UK) as a percentage of the predicted value by age and sex (Fitting et al., 1999, Cheah et al., 2009). The highest sniff pressure (cm H2O) from a minimum of 10 short sniffs through each nostril was recorded. SNIP was expressed as a percentage of the predicted value by age and sex, calculated using the formula previously derived by Fitting et al. (Fitting et al., 1999) and utilised as in Cheah et al. (Cheah et al., 2009).
The neurophysiological index was calculated for the ulnar nerve, recording compound muscle action potentials (CMAPs) from the abductor digiti minimi (ADM), according to previously reported techniques (Swash and de Carvalho, 2004). Peripheral motor axonal excitability studies were undertaken, utilising standardized threshold tracking protocols (Kiernan et al., 2000) and QTracW software (© Institute of Neurology, UK). The median nerve was stimulated at the wrist, recording CMAPs from the abductor pollicis brevis (APB) muscle. Cortical excitability studies were performed using threshold tracking transcranial magnetic stimulation (TMS) according to a previously described method (Vucic et al., 2006). Peripheral (N = 32) and cortical excitability studies (N = 22) were analysed at two time points — prior to randomization and at 32 weeks post-randomization.
2.5. Statistical Analysis
The primary analysis compared the rate of decline of ALSFRS-R between treatment and placebo arms, adjusted for patient-specific pre-treatment slope, using mixed models analysis where the intercept for each patient is random and the slopes are fixed effects, after determining that there were no treatment-by-time interactions. As bulbar and respiratory ALSFRS-R subscores did not decline significantly these variables were summarised by means rather than rate of decline. The sample size was calculated to be 100 patients to provide 80% power to detect a 25% change in rate of ALS FRS-r decline, accounting for 20% patient drop-out. Differences between means for peripheral and cortical excitability metrics were assessed using t-tests when normally distributed and Wilcoxon signed-rank tests when not normally distributed. Statistical analysis was performed in SAS 9.2 (USA) and SPSS (Version 22, IBM, USA). A two sided P value of ≤ 0.05 was considered significant.
3. Results
3.1. Study Participants
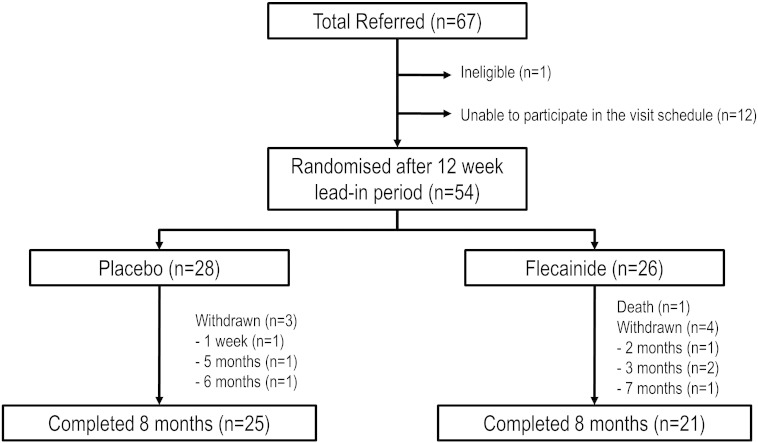
In total, 67 ALS patients were screened for inclusion in the study between March 2008 and July 2010 (Fig. 2). One patient was ineligible and 12 patients were unable to participate in the study visit schedule. 54 patients were randomized after the 12 week lead-in period and commenced on the assigned medication with 26 patients (54.2 ± 9.8 years; 16 males) allocated to the flecainide group and 28 to the placebo group (53.5 ± 10.6 years; 16 males; Fig. 2). Four patients from the flecainide group withdrew consent, one due to side-effects and three as they felt the medication was ineffective. Three patients from placebo group withdrew consent as they felt no benefit from treatment. One patient in the flecainide group died during the study, and this was attributed to ALS disease progression. The population for the intention-to-treat analysis was the total population included in the trial (N = 54).
Fig. 2.
Patient flow chart: Trial schematic, with the number of participants per treatment arm and number of patients completing the study.
Patients in the two groups exhibited similar demographic and clinical characteristics, as well as respiratory and neurophysiological features (Table 1). The median ALSFRS-r score in the entire cohort was 41 (range 36–42), corresponding to mild functional impairment. At the time of study entry, one patient from the placebo group was utilising non-invasive ventilation at night and no patients had a gastrostomy tube. In total, 13 of 54 patients had bulbar onset ALS (Table 1), with 38 of 41 limb onset patients reporting functional difficulties with upper limbs or demonstrating electrophysiological evidence of upper limb disease. The remaining 3 patients (2 flecainide; 1 placebo) did not demonstrate evidence of upper limb abnormalities at the time of the study. The mean serum flecainide level was 0.42 ± 0.03 mg/L (range 0.2–0.8 mg/L; measured at visit 7–8) in the flecainide-treated cohort, in line with the recorded therapeutic range for cardiac use of 0.2–1 mg/L (Tamargo et al., 2012). All placebo treated patients had a serum flecainide level of less than 0.2 mg/L.
Table 1.
Patient baseline demographic and clinical characteristics for flecainide and placebo cohorts. Results are expressed as number (%), mean ± standard deviation or median (Q1 - Q3).
| Flecainide (N = 26) | Placebo (N = 28) | |
|---|---|---|
| Age at baseline (years) | 54.2 ± 9.8 | 53.5 ± 10.6 |
| Male/female | 16/10 | 16/12 |
| Disease duration (months) | 23.8 (12.7–31.5) | 18.6 (12.7–23.5) |
| Weight (kg) | 75.0 ± 10.8 | 79.1 ± 14.5 |
| Bulbar-onset | 5 (19%) | 8 (29%) |
| Family history of disease | 5 (19%) | 3 (11%) |
| Grip strength, stronger hand (kg) | 21.5 (17.7–29.3) | 21.3 (13.1–33.4) |
| ALSFRS-r | 41.0 (38.0–42.0) | 40.0 (36.0–41.5) |
| Sniff nasal inspiratory pressure (% predicted) | 94.8 (55.8–113.4) | 76.7 (59.8–106.2) |
| Forced vital capacity (% predicted) | 84.6 ± 15.3 | 83.3 ± 16.1 |
| SF-36 physical component | 35.7 ± 10.7 | 34.4 ± 11.1 |
| SF-36 mental component | 54.7 ± 11.2 | 54.4 ± 8.0 |
| Neurophysiological index | 3.0 ± 1.2 | 2.7 ± 1.5 |
3.2. Clinical Efficacy
There was no significant difference in the rate of decline in ALSFRS-r slope during the lead-in phase between the placebo and the flecainide group (average slope in units per month [95% CI]: Flecainide 0.58 [0.27 to 0.88]; Placebo 0.49 [0.20 to 0.79]; P = 0.66). Following randomization, there remained no difference between the groups in the rate of decline in the ALSFRS-r slope, after adjusting for pre-treatment decline (Flecainide 0.65 [0.49 to 0.98]; Placebo 0.81 [0.49 to 2.12]; P = 0.50, Table 2) and accordingly insufficient evidence to suggest a difference in the primary outcome measure between flecainide and placebo groups. Importantly, flecainide therapy did not accelerate the rate of ALS FRS-r slope, thereby suggesting that flecainide-mediated Na+ channel blockade was not detrimental to disease progression.
Table 2.
Analysis of primary and secondary outcome measures. Intention-to treat analysis in 54 ALS patients. The primary outcome measure was the rate of decline in the Amyotrophic Lateral Sclerosis Functional Rating Scale-revised (ALS FRS-r) slope. Results are expressed as mean monthly rate of change (95% confidence interval).
| Outcome measure | Mean monthly rate of change (95% CI) |
P-value of treatment effect | |
|---|---|---|---|
| Flecainide | Placebo | ||
| ALS FRS-r | 0.65 (0.32–0.98) | 0.81 (0.49–1.12) | 0.50 |
| ALS FRS-r Fine Motor subscore | 0.19 (0.06–0.32) | 0.31 (0.18–0.43) | 0.19 |
| ALS FRS-r Gross Motor subscore | 0.21 (0.09–0.33) | 0.28 (0.16–0.39) | 0.39 |
| Sniff nasal inspiratory pressure (% predicted) | 1.90 (1.02–2.78) | 2.80 (1.99–3.6) | 0.13 |
| Forced vital capacity | 1.25 (0.58–1.92) | 1.64 (1.03–2.26) | 0.38 |
| 6-metre walking test | 0.26 (0.22–0.73) | 0.58 (0.13–1.04) | 0.32 |
| Strongest grip strength at baseline | 0.95 (0.50–1.4) | 1.06 (0.61–1.51) | 0.73 |
| SF-36 physical component summary | 0.64 (0.24–1.04) | 0.65 (0.28–1.01) | 0.98 |
| SF-36 mental component summary | 0.1 (0.43–0.64) | 0.36 (0.14–0.85) | 0.47 |
| Neurophysiological index | 0.06 (0.01–0.11) | 0.14 (0.09–0.19) | 0.02 |
Analysis of ALSFRS-r subscores also did not reveal any significant differences between treatment groups (Table 2), with the rate of decline of the ALSFRS-r subscores similar between treatment groups during the lead-in phase and after randomization. There was also no difference in rate of decline of ALSFRS-r slope between treatments for limb versus bulbar onset subgroups (P for interaction = 0.63).
3.3. Secondary Outcome Measures
3.3.1. Neurophysiological Measures
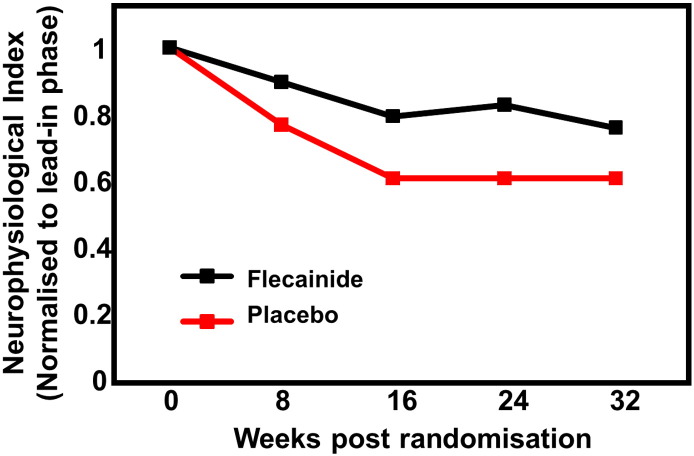
During the treatment phase, the decline in neurophysiological index was slowed in the flecainide-treated cohort compared to the placebo group (Fig. 3) adjusted for run-in decline. While during the lead-in phase there was no significant difference in the slope of decline of the neurophysiological index between the groups (Flecainide 0.13 [0.03–0.22]; Placebo 0.18 [0.08–0.27]; P = 0.36), the rate of decline of the neurophysiological index was significantly lower in the flecainide group than in placebo during the treatment phase (Flecainide 0.06 score points/month [0.01–0.11]; placebo 0.14 [0.09–0.19], P = 0.02, Table 2).
Fig. 3.
Neurophysiological index changes in flecainide and placebo treated patients: The rate of decline in the neurophysiological index was smaller for the flecainide group than the placebo-treated cohort.
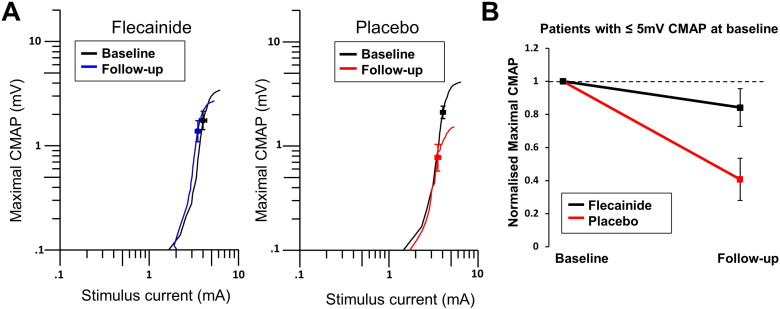
Peripheral axonal excitability remained relatively stable across the treatment phase in flecainide treated patients comparing baseline values to those after 32 ± 1 weeks of treatment, with threshold electrotonus parameters remaining stable, along with CMAP amplitude (Table 3). However, the placebo group demonstrated changes in a number of parameters (Table 3) including a reduction in maximal CMAP amplitudes (P = 0.01) and reductions in hyperpolarizing threshold electrotonus (All P < 0.01). In contrast, maximal CMAP was reduced by 39 ± 11% compared to baseline in the placebo-treated group and only by 14 ± 8% in the flecainide group, although this difference did not reach statistical significance (P = 0.09; Fig. 4). However there were differences in the extent of change over the 32 week treatment period between placebo and flecainide treated groups on a number of excitability parameters (Table 3; Hyperpolarising threshold electrotonus; Current threshold relationship All P < .01).
Table 3.
Peripheral axonal excitability findings. Axonal excitability parameters and functional assessment parameters in flecainide and placebo treated patients (N = 32) at baseline and final follow-up assessment. Data are presented as mean ± SEM or median (interquartile range).
| Flecainide |
Placebo |
|||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | P values | Baseline | Follow-up | P values | |
| Peak CMAP (mV) | 4.5 ± 0.7 | 3.8 ± 0.6 | 0.16 | 5.1 (3.8) | 1.3 (5.5) | 0.01 |
| Strength–duration time constant (ms) | 0.44 ± 0.02 | 0.44 ± 0.02 | 0.94 | 0.50 ± 0.03 | 0.48 ± 0.02 | 0.44 |
| Hyperpolarizing threshold electrotonus | ||||||
| 10–20 ms (%) | − 71.5 ± 1.0 | − 73.7 ± 1.5 | 0.25 | − 73.0 ± 0.9 | − 68.1 ± 1.5 | 0.002 |
| 20–40 ms (%) | − 88.7 ± 1.4 | − 91.9 ± 2.1 | 0.18 | − 91.8 ± 1.3 | − 84.7 ± 2.2 | 0.001 |
| 90–100 ms (%) | − 120 (37) | − 116 (26) | 0.22 | − 117.8 ± 4.1 | − 104.6 ± 3.7 | 0.007 |
| Current threshold relationship | ||||||
| Resting I/V slope (%) | 0.6 ± 0.02 | 0.6 ± 0.02 | 0.63 | 0.55 ± 0.02 | 0.61 ± 0.02 | 0.02 |
| Hyperpolarizing IV drift (%) | − 241 ± 14 | − 271 ± 15 | 0.002 | − 262 ± 11 | − 249 ± 14 | 0.24 |
Fig. 4.
Peripheral axonal excitability findings in flecainide treated patients versus placebo: A Stimulus response curve of stimulus current (mA) versus maximal CMAP (mV) between baseline and follow-up recordings in flecainide (left) and placebo (right) treated patients. B Decline in maximal CMAP normalized to baseline values in flecainide (black) and placebo (red) treated patients with less than or equal to 5 mV CMAP at the baseline recording.
Patients were subdivided into two groups — those with a preserved baseline maximal CMAP amplitude (> 5 mV) and those with a reduced CMAP amplitude (≤ 5 mV) at study baseline, as in prior studies (Kanai et al., 2006). In patients with a reduced CMAP amplitude at baseline (N = 16), placebo-treated patients demonstrated a significantly greater decline in CMAP amplitude (59 ± 12%) compared to flecainide-treated patients (15 ± 12%; P = 0.03; Fig. 4). This difference was not evident in flecainide and placebo-treated patients with a preserved CMAP at baseline (N = 16; flecainide 12.9 ± 12%; placebo 17.8 ± 15%; P = 0.80).
Threshold tracking TMS studies demonstrated that short interval intracortical inhibition (SICI) was not significantly modulated by flecainide during the treatment phase (Baseline SICI 2.8 ± 2.0%; After treatment SICI 2.4 ± 2.5%, P = 0.78) and remained reduced compared to normal values. Similarly, intracortical facilitation remained relatively stable (Baseline ICF -0.87 ± 1.8%; After treatment ICF − 2.7 ± 2.8; P = 0.65), with no differences evident following flecainide treatment.
3.3.2. Functional Outcome Measures
The rate of decline in functional outcome measures was not significantly different between flecainide and placebo groups for the 6-metre walking test (P = 0.32), grip strength (P = 0.73), physical quality of life (P = 0.98) or mental quality of life (P = 0.47; Table 2). Similarly, analysis of respiratory measures did not identify significant differences in the rate of decline in slope of forced vital capacity (P = 0.38, Table 2) and SNIP (P = 0.13, Table 2).
3.4. Safety and Tolerability
Flecainide was generally well tolerated. No serious adverse events were reported in either group. Importantly there were no severe cardiac adverse events recorded. Chest pain was reported by one flecainide-treated patient and two placebo-treated patients during the course of the trial. Palpitations were reported by two flecainide and two placebo-treated patients. Treatment was discontinued following cardiologist review in the flecainide-treated patient who experienced an episode of chest pain because physical disability prevented the undertaking of a cardiac stress test to rule out ischemic heart disease. A further flecainide-treated patient underwent cardiologist review due to an episode of dizziness to exclude arrhythmia and was continued on flecainide treatment.
The overall incidence of adverse events was similar between the groups (Table 4). 100% of flecainide patients and 88% of placebo treated patients reported an adverse event. None of these adverse events were directly attributable to flecainide. Adverse events reported in prior studies of flecainide in the cardiac setting have included dizziness, tremor, nausea and diarrhoea (Clementy et al., 1992). In the present study, dizziness was reported by 38% of flecainide-treated patients compared to 23% of placebo-treated patients, with nausea reported in 21% of flecainide-treated patients compared to 12% of placebo treated patients. Rates of tremor and diarrhoea were very similarly reported between flecainide and placebo-treated patients (Table 4). Excessive sleepiness was reported in 50% of flecainide-treated patients compared to 19% of placebo-treated patients.
Table 4.
Reported adverse events. Reported as a number of patients with at least one event of the particular type and percentage of all patients in each treatment group.
| Adverse event | Flecainide N = 24 | Placebo N = 26 |
|---|---|---|
| Dizziness | 9 (38%) | 6 (23%) |
| Tremor | 8 (33%) | 7 (27%) |
| Nausea | 5 (21%) | 3 (12%) |
| Diarrhoea | 4 (17%) | 4 (15%) |
| Chest pain | 1 (4%) | 2 (8%) |
| Palpitations | 2 (8%) | 2 (8%) |
| Coughing | 7 (29%) | 9 (35%) |
| Dry mouth | 12 (50%) | 10 (38%) |
| Difficulty sleeping | 8 (33%) | 9 (35%) |
| Excessive sleepiness | 12 (50%) | 5 (19%) |
| Joint pain | 10 (42%) | 8 (31%) |
| Muscle pain | 10 (42%) | 13 (50%) |
4. Discussion
This double-blind, placebo-controlled randomised study was designed to determine the neuroprotective potential of the Na+ channel blocker flecainide in patients with ALS. Flecainide did not produce significant adverse effects in ALS patients. While the study did not provide evidence of improvement in the primary outcome measure, the rate of decline in the ALS-FRS-r slope, there was also no evidence of an adverse effect mediated by Na+ channel blockade. There was no improvement in the clinical secondary outcome measures such as grip strength, 6-metre walk test or respiratory measures. However, the rate of decline in the slope of the neurophysiological index, a biomarker of lower motor neuron dysfunction, was significantly reduced in the flecainide group. Further, peripheral axonal excitability parameters demonstrated significant changes in placebo-treated patients but remained stable in flecainide-treated patients, suggesting that flecainide stabilised peripheral axonal function in ALS.
4.1. Flecainide as a Neuroprotective Strategy
Flecainide, a class Ic antiarrhythmic agent, is a strong blocker of persistent late Na+ currents produced by Nav1.5 channels in cardiac tissue, blocking Na+ channels in open conformation (Aliot et al., 2011). The rational for the potential of flecainide as a neuroprotective strategy in ALS is based on the deleterious consequences of disturbed membrane excitability on axonal function and integrity. Excessive excitability and accumulation of intra-axonal Na+ ions may potentially initiate a cascade, leading to axonal degeneration via reverse operation of the Na+/Ca2 + exchanger and excess Ca2 + entry (Stys, 2005). Blockade of sodium channels may promote neuroprotection by preventing the accumulation of Na+ in the axon. Accordingly, Na+ channel blockade has been trialed as a method of neuroprotection in a number of neurological disorders including multiple sclerosis, spinal cord injury and stroke (Schwartz and Fehlings, 2001). The only medication currently approved to treat ALS, riluzole, has also been demonstrated to inhibit voltage-gated Na+ currents, with effects on fast and inactivating currents, interacting with Nav1.6 sodium channel isoforms (Sierra Bello et al., 2012, Vucic et al., 2014), among other effects.
Recent studies have suggested that increased excitability may be adaptive, raising the possibility that modulation of excitability in ALS patients may have deleterious consequences for motor neuron integrity (Saxena et al., 2013). The present results do not suggest that treatment with a potent Na+ channel blocking agent, which would reduce axonal excitability, leads to enhanced peripheral neurodegeneration in ALS. Similarly, a recent trial of the Na+ channel blocker mexiletine in ALS did not demonstrate any decline in functional status associated with Na+ channel blockade (Shibuya et al., 2015). Further, there is in vitro evidence that Na+ channel modulation is neuroprotective in ALS, with neuronal hyperexcitability and subsequent motor neuron death prevented by Na+ channel blockers in spinal cord cultures (Fritz et al., 2013).
4.2. Neurophysiological Markers of Disease Burden in ALS
In the present study, measures of peripheral disease burden and membrane excitability demonstrated greater stability in flecainide-treated patients compared to the placebo cohort, suggesting a biological effect of flecainide at the level of the peripheral nerve. The neurophysiological index is a sensitive surrogate marker of ALS disease progression (Swash and de Carvalho, 2004). In previous ALS trials neurophysiological parameters demonstrated significant change over time, with the neurophysiological index declining 3.6% per month (de Carvalho et al., 2010) or 18.3% in 12 weeks (Cheah et al., 2011). Neurophysiological markers often decline more rapidly than clinical measures (Gordon et al., 2007), demonstrating greater responsiveness which may be appropriate for use in clinical trials. In the present study, flecainide-treated patients demonstrated a reduced decline in the neurophysiological index, compared to placebo-treated patients, suggesting relative preservation of lower motor neuron function.
Similarly, peripheral axonal excitability studies demonstrated changes in flecainide treated patients compared to placebo. Placebo-treated patients demonstrated a shift in threshold electrotonus and current/threshold relationship parameters over the 32-week treatment period, an excitability profile similar to that seen in ALS patients with more severe peripheral disease burden (Kanai et al., 2006). In contrast, flecainide-treated patients maintained relatively stable peripheral membrane excitability over 32 weeks. In the subset of patients with a reduced CMAP (≤ 5 mV) amplitude at study entry, patients treated with flecainide demonstrated significantly less reduction in CMAP amplitude than placebo-treated patients. Peripheral excitability abnormalities in ALS typically progress with time, although they are not linear and are dependent on clinical severity (Kanai et al., 2006), with variability reflecting diversity in disease onset and progression. Further, there were no specific Na+ channel related changes in the axonal excitability profile, suggest that rather than solely acting to modulate persistent Na+ current, flecainide may stabilise membrane function in ALS patients.
While cortical hyperexcitability is a prominent feature in ALS, flecainide failed to exert significant modulating effects on the parameters of cortical excitability, unlike riluzole which produced a pseudonormalization of cortical excitability in ALS (Vucic et al., 2013). However, in the current study, all patients were on steady state riluzole treatment throughout, which may contribute to the lack of changes pre- and post-flecainide in cortical excitability. Further, while therapeutic dose levels for the cardiac setting were achieved in the present study, flecainide levels in cerebrospinal fluid may be lower than serum levels (Romain et al., 1999), suggesting that higher doses may be required to impact cortical excitability. Further studies with increased dosing ranges may be required to comment on clinical efficacy.
4.3. Safety and Efficacy
The present study has established the safety of flecainide in ALS, achieved via careful patient monitoring and preemptive cardiac screening. In appropriately selected patients, flecainide has a good safety profile, although should be avoided in patients with coronary artery or heart disease as it has been demonstrated to increase the risk of death in this cohort (Aliot et al., 2011). Flecainide must also be used with caution in patients with liver or kidney dysfunction. Critically all patients in this series were formally reviewed by a cardiologist prior to flecainide administration to rule out any cardiac or other contraindications. Patients in the flecainide group did not report significant adverse effects and there were no deaths attributed to flecainide use.
Although flecainide was tolerated and safe, there was no measureable benefit in the primary outcome measure nor in measures of respiratory function and motor function. The recruitment levels achieved in the study were not as high as anticipated, particularly in light of the variability between patients. While patients were recruited from multiple sites Australia-wide, the need for all assessments to be undertaken at a single site may have affected the number of patients able to participate. In addition to facilitate recruitment the study inclusion criteria allowed patients with disease duration of up to 5 years to be recruited. However, the neuroprotective effects of therapeutic agents may peak in the earlier stages of the disease, with a therapeutic window prior to irreversible motor neuron degeneration. A curvilinear rate of decline in ALS-FRS-r has also been reported, with the rates of decline being greatest during the first 18 months of symptom onset and during the terminal stages of illness (Gordon et al., 2007). Accordingly, these factors may contribute to the lack of significant modulation in ALS-FRS-r. However despite these limitations, the study identified significant biological activity of flecainide at the peripheral nerve level.
4.4. Conclusions
While this double-blind, randomised controlled trial of the Na+ channel blocker flecainide versus placebo did not identify any changes in the primary outcome measure, rate of decline in the ALS-FRS-r, flecainide was safe and no safety concerns were identified. Flecainide did not promote peripheral axonal degeneration but appeared to modulate axonal function in ALS patients, stabilizing axonal excitability and reducing decline in the neurophysiological index. These significant biological effects at the peripheral nerve level may warrant further investigation to determine the significance of reduced decline in the neurophysiological index and changes in peripheral axonal excitability. There are many challenges in conducting clinical trials in ALS cohorts, including patient recruitment and loss to follow-up. Accordingly, the present study was underpowered to make a clear statement regarding clinical efficacy of flecainide in this population. It remains to be determined the significance of these effects on survival and long term functional outcomes in ALS.
Funding
National Health and Medical Research Council of Australia (Project #568743). Additional support through the National Health and Medical Research Council of Australia Forefront program grant (#1037746) is gratefully acknowledged. JW was supported by a Bill Gole Fellowship of the Motor Neuron Disease Research Institute of Australia and SP is the recipient of a RG Menzies Foundation/National Health and Medical Research Council of Australia Training Fellowship (# 1016446). No funding sources were involved in any aspect of the study or the decision to submit for publication.
Author Contributions
BC, SV, MZ and MK designed the study. BC, JW, SP, CL and MZ collected the data. SP, SV, BC, CL, AK, KM, and MK and analysed and interpreted the data. All authors were involved in writing and revision of the manuscript and were responsible for final approval of the manuscript.
Conflict of Interest Statements
The authors declare no competing financial interests.
Acknowledgements
The authors would like to acknowledge the research participants and their families who participated in the study.
References
- Aliot E., Capucci A., Crijns H.J., Goette A., Tamargo J. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13:161–173. doi: 10.1093/europace/euq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold D.A., Yue X., Evans R.M., Davies M., Gregson N.A., Smith K.J. Axonal protection in experimental autoimmune neuritis by the sodium channel blocking agent flecainide. Brain. 2005;128:18–28. doi: 10.1093/brain/awh328. [DOI] [PubMed] [Google Scholar]
- Benoit E., Escande D. Riluzole specifically blocks inactivated Na+ channels in myelinated nerve fibre. Pflugers Arch. 1991;419:603–609. doi: 10.1007/BF00370302. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J.M., Stambler N., Malta E. The ALSFRS-R: a revised als functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J. Neurol. Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Cheah B.C., Boland R.A., Brodaty N.E. INSPIRATIonAL–INSPIRAtory muscle training in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2009;10:384–392. doi: 10.3109/17482960903082218. [DOI] [PubMed] [Google Scholar]
- Cheah B.C., Vucic S., Krishnan A.V., Boland R.A., Kiernan M.C. Neurophysiological index as a biomarker for als progression: validity of mixed effects models. Amyotroph. Lateral Scler. 2011;12:33–38. doi: 10.3109/17482968.2010.531742. [DOI] [PubMed] [Google Scholar]
- Clementy J., Dulhoste M.N., Laiter C., Deniov I., Dos Santos P. Flecainide acetate in the prevention of paroxysmal atrial fibrillation: a nine-month follow-up of more than 500 patients. Am. J. Cardiol. 1992:44A–49A. doi: 10.1016/0002-9149(92)91077-h. [DOI] [PubMed] [Google Scholar]
- de Carvalho M., Pinto S., Costa J., Evangelista T., Ohana B., Pinto A. A randomized, placebo-controlled trial of memantine for functional disability in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010;11:456–460. doi: 10.3109/17482968.2010.498521. [DOI] [PubMed] [Google Scholar]
- Fitting J.W., Paillex R., Hirt L., Aebischer P., Schluep M. Sniff nasal pressure: a sensitive respiratory test to assess progression of amyotrophic lateral sclerosis. Ann. Neurol. 1999;46:887–893. [PubMed] [Google Scholar]
- Fritz E., Izaurieta P., Weiss A. Mutant SOD1-expressing astrocytes release toxic factors that trigger motoneuron death by inducing hyperexcitability. J. Neurophysiol. 2013;109:2803–2814. doi: 10.1152/jn.00500.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P.H. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Kanai K., Kuwabara S., Misawa S. Altered axonal excitability properties in amyotrophic lateral sclerosis: impaired potassium channel function related to disease stage. Brain. 2006;129:953–962. doi: 10.1093/brain/awl024. [DOI] [PubMed] [Google Scholar]
- Kapoor R., Davies M., Blaker P.A., Hall S.M., Smith K.J. Blockers of sodium and calcium entry protect axons from nitric oxide-mediated degeneration. Ann. Neurol. 2003;53:174–180. doi: 10.1002/ana.10443. [DOI] [PubMed] [Google Scholar]
- Kiernan M.C., Burke D., Andersen K.V., Bostock H. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve. 2000;23:399–409. doi: 10.1002/(sici)1097-4598(200003)23:3<399::aid-mus12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kiernan M.C., Vucic S., Cheah B.C. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- Kuo J.J., Siddique T., Fu R., Heckman C.J. Increased persistent Na(+) current and its effect on excitability in motoneurones cultured from mutant sod1 mice. J. Physiol. 2005;563:843–854. doi: 10.1113/jphysiol.2004.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Mogyoros I., Kiernan M.C., Burke D., Bostock H. Strength–duration properties of sensory and motor axons in amyotrophic lateral sclerosis. Brain. 1998;121:851–859. doi: 10.1093/brain/121.5.851. [DOI] [PubMed] [Google Scholar]
- Morsali D., Bechtold D., Lee W. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain. 2013;136:1067–1082. doi: 10.1093/brain/awt041. [DOI] [PubMed] [Google Scholar]
- Pieri M., Carunchio I., Curcio L., Mercuri N.B., Zona C. Increased persistent sodium current determines cortical hyperexcitability in a genetic model of amyotrophic lateral sclerosis. Exp. Neurol. 2009;215:368–379. doi: 10.1016/j.expneurol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Quinlan K.A., Schuster J.E., Fu R., Siddique T., Heckman C.J. Altered postnatal maturation of electrical properties in spinal motoneurons in a mouse model of amyotrophic lateral sclerosis. J. Physiol. 2011;589:2245–2260. doi: 10.1113/jphysiol.2010.200659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romain N., Giroud C., Michaud K., Augsburger M., Mangin P. Fatal flecainide overdose. Forensic Sci. Int. 1999;106:115–123. doi: 10.1016/s0379-0738(99)00156-5. [DOI] [PubMed] [Google Scholar]
- Saxena S., Roselli F., Singh K. Neuroprotection through excitability and mTOR required in ALS motoneurons to delay disease and extend survival. Neuron. 2013;80:80–96. doi: 10.1016/j.neuron.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Schwartz G., Fehlings M.G. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J. Neurosurg. 2001;94:245–256. doi: 10.3171/spi.2001.94.2.0245. [DOI] [PubMed] [Google Scholar]
- Shibuya K., Misawa S., Kimura H. A single blind randomized controlled clinical trial of mexiletine in amyotrophic lateral sclerosis: efficacy and safety of sodium channel blocker phase II trial. Amyotroph. Lateral Scler. Frontotemporal Degener. 2015:1–6. doi: 10.3109/21678421.2015.1038277. (May 11) [DOI] [PubMed] [Google Scholar]
- Sierra Bello O., Gonzalez J., Capani F., Barreto G.E. In silico docking reveals possible riluzole binding sites on Nav1.6 sodium channel: implications for amyotrophic lateral sclerosis therapy. J. Theor. Biol. 2012;315:53–63. doi: 10.1016/j.jtbi.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Stys P.K. General mechanisms of axonal damage and its prevention. J. Neurol. Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Swash M., de Carvalho M. The neurophysiological index in ALS. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004;5(Suppl. 1):108–110. doi: 10.1080/17434470410020067. [DOI] [PubMed] [Google Scholar]
- Tamargo J., Capucci A., Mabo P. Safety of flecainide. Drug Saf. 2012;35:273–289. doi: 10.2165/11599950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tiedemann A., Shimada H., Sherrington C., Murray S., Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37:430–435. doi: 10.1093/ageing/afn100. [DOI] [PubMed] [Google Scholar]
- Urbani A., Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur. J. Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Vucic S., Kiernan M.C. Axonal excitability properties in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2006;117:1458–1466. doi: 10.1016/j.clinph.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Vucic S., Kiernan M.C. Upregulation of persistent sodium conductances in familial ALS. J. Neurol. Neurosurg. Psychiatry. 2010;81:222–227. doi: 10.1136/jnnp.2009.183079. [DOI] [PubMed] [Google Scholar]
- Vucic S., Howells J., Trevillion L., Kiernan M.C. Assessment of cortical excitability using threshold tracking techniques. Muscle Nerve. 2006;33:477–486. doi: 10.1002/mus.20481. [DOI] [PubMed] [Google Scholar]
- Vucic S., Lin C.S., Cheah B.C., Murray J., Menon P., Krishnan A.V., Kiernan M.C. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain. 2013;136:1361–1370. doi: 10.1093/brain/awt085. [DOI] [PubMed] [Google Scholar]
- Vucic D., Rothstein J.D., Kiernan M.C. Advances in treating amyotrophic lateral sclerosis: insights from pathophysiological studies. Trends Neurosci. 2014;37:433–442. doi: 10.1016/j.tins.2014.05.006. [DOI] [PubMed] [Google Scholar]