Abstract
Various stem cell-based approaches for cardiac repair have achieved encouraging results in animal experiments, often leading to their rapid proceeding to clinical testing. However, freewheeling evolutionary developments of the stem cell theory might lead to dystopian scenarios where heterogeneous sources of therapeutic cells could promote mixed clinical outcomes in un-stratified patient populations. This review focuses on the lessons that should be learnt from the first generation of stem cell-based strategies and emphasizes the absolute requirement to better understand the basic mechanisms of stem cell biology and cardiogenesis. We will also discuss about the unexpected “big bang” in the stem cell theory, “blasting” the therapeutic cells to their unchallenged ability to release paracrine factors such as extracellular membrane vesicles. Paradoxically, the natural evolution of the stem cell theory for cardiac regeneration may end with the development of cell-free strategies with multiple cellular targets including cardiomyocytes but also other infiltrating or resident cardiac cells.
Keywords: Stem cells, Heart failure, Myocardial infarction, Cardiac regeneration, Inflammation
Highlights
-
•
Varied sources of therapeutic cells and low repair ability of the failing heart contribute to mixed results in clinical trials.
-
•
Consensus is still lacking concerning the appropriate type of therapeutic stem cells.
-
•
A clear understanding of cardiac development and adult cardiogenesis might increase the efficiency of regenerative therapies.
-
•
Delivery of stem cell-derived paracrine factor alone to the damaged heart may be sufficient to activate repair mechanisms.
Heart failure (HF) is a leading cause of mortality worldwide and a major problem of global health causing around 5% of the acute hospital admissions and accounting for around 10% of hospitalized patients in Europe and the United States. Importantly, the number of patients with HF is steadily increasing, as a consequence of an aging population and/or enlarging prevalence of cardiovascular risk factors such as diabetes (Gilbert and Krum, 2015) and improved survival rates after acute myocardial infarction (MI) putting a greater number of patients at risk of developing a late left ventricular dysfunction. Nevertheless, long-term survival has improved with recent medical therapies aiming at reducing cardiac overload and neurohumoral activation, as well as mineralocorticoid deregulation. Significant advances have also been achieved through surgical revascularization strategies including percutaneous coronary angioplasty and coronary artery bypass grafting. Current strategies for treating end-stage HF are based on replacing or supporting the failing heart by cardiac transplantation or left ventricular assist devices. However, more than 50% of HF patients die in 4 years after diagnosis and 40% of them perish or are readmitted to hospital within the first year. The poor prognosis of symptomatic HF is likely associated with the limited long-term efficacy of conventional therapeutic strategies on the underlying ongoing loss of cardiomyocytes, which is followed by the deleterious formation of a fibrotic scar in the failing heart.
Over the last decade, the classical paradigm that the human heart is a post-mitotic and terminally developed organ with no cell renewal capability has been undermined with the demonstration that cardiomyocyte turnover can occur in adult mammals, including humans (Sahara et al., 2015; Bergmann et al., 2009; Bergmann et al., 2015). However, such inherent capability of humans to regenerate myocardium with aging or after injury in adulthood is entirely insufficient to fully compensate for the loss of function associated with these conditions. Such statement confronts the scientific community with a unique and exciting challenge: can we enhance the regenerative capacity of cardiac tissue to abrogate adverse ventricular remodeling? Consistent with this, multiple different approaches have been developed to promote cardiomyocyte regeneration/proliferation in human injured hearts, including transplantation of autologous non-cardiac/cardiac somatic stem cells, injection of in vitro-derived cardiomyocytes, direct reprogramming of cardiac fibroblasts into cardiomyocytes in vivo, stimulation of dedifferentiation/proliferation of resident cardiomyocytes, and activation of endogenous cardiac progenitor cell populations. These therapeutic strategies, classified as either cell-based or cell-free, are currently being investigated for their cardiac repair potential and clinical application.
In particular, various cell-based approaches for cardiac repair have achieved encouraging results in animal experiments, often leading to their rapid proceeding to clinical testing. Although a multitude of clinical trials have been performed to date, their results remain ambiguous and no single-cell-based therapy for heart disease has been conclusively proven effective so far (Behfar et al., 2014). As a prototypic example of such controversy, two recent meta-analysis of cell-based therapy one in chronic HF (Fisher et al., 2015) and one in patients with acute MI (Gyongyosi et al., 2015) result in entirely different conclusions. In the meta-analysis of 31 randomized cell therapy trials in HF which included 1521 patients, exercise capacity, left ventricular ejection fraction and quality of life are improved in the treated patients (Fisher et al., 2015). In contrast, a second meta-analysis based on individual patient data reveals that cell therapy does not impact cardiac function and remodeling as well as the clinical outcome in patients with acute MI (Gyongyosi et al., 2015).
Such controversies prompt us to suggest that we need to step back in the natural evolution of the stem cell theory for therapeutic use and go “back to the trees” as claimed by the anti-progressive character from the famous novel of Roy Lewis (The Evolution Man). In other words, we need to go back to the root of stem cell biology and the concept of regenerative medicine. A clear understanding of stem cell biology and HF etiology may help researchers and clinicians in the field to provide definite evidences for stem cell efficacy in patients.
1. The Quest for the Ideal Source of Stem Cells With Regenerative or Cardiogenic Potential
There are a myriad of unresolved questions related to cell handling and preparation, repair ability of the failing heart (inflammatory status, timing of injection, endogenous cardiogenic and angiogenic potential), mode of cell delivery, clinical endpoints as well as methodologies used to assess those endpoints and this list is not exclusive.
Above all, there is no consensus on the basic question: which cell type to transplant, to improve efficacy and safety? The majority of trials used adult stem cells and mainly applied total bone marrow-derived mononuclear cells, bone marrow-derived marker selected cells or granulocyte-colony stimulating factor mobilized mononuclear cells (Silvestre et al., 2013). However, adult stem cells show a restrictive plasticity and more importantly most of the cardiovascular risk factors such as hypertension, diabetes, aging and active smoking have been shown to reduce the therapeutic potential of transplanted bone marrow-derived cells (Govaert et al., 2009; Ayala-Lugo et al., 2011; Ebrahimian et al., 2006; You et al., 2008; Roncalli et al., 2011). In addition, operating procedures to isolate these bone marrow derived cells are not standardized. Some of the trials used Ficoll-gradient, sedimentation or automated systems to isolate bone marrow cells. This may lead to profound heterogeneity in the therapeutic efficiency since cell isolation protocols have a major impact on the functional activity of medullary cells (Seeger et al., 2007). Consistent with this, bone marrow-derived cells stored in non-buffered saline supplemented with heparin, display reduction in their homing and functional activity even in animal models (Seeger et al., 2012). Other trials applied bone marrow-derived mesenchymal stem cells (Mathiasen et al., 2015;Hare et al., 2012; Heldman et al., 2014), bone marrow-derived mesenchymal stem cells exposed to a cardiogenic cocktail (Bartunek et al., 2013), skeletal myoblast-derived cells (Menasche et al., 2008) or adipose tissue-derived mesenchymal stem cells (ADSCs) (Perin et al., 2014; Houtgraaf et al., 2012). This latter source of therapeutic cells is also a good illustration of anticipated heterogeneity in future clinical trials. Adipose tissue-derived stem cells can be obtained from subcutaneous adipose tissues with the use of collagenase digestion. However, freshly isolated cells, also defined as the stroma vascular fraction (SVF), are known to be heterogeneous and contain hematopoietic cells and should be distinguished from ADSCs obtained after culture on plastic dishes. ADSCs, but not SVF, show a therapeutic effect in ischemic tissue in both mice and humans with critical limb ischemia (Planat-Benard et al., 2004; Lee et al., 2012b). In addition the source of fat has been shown to dictate human ADSCs reparative activity (Naftali-Shani et al., 2013).
Although, there are many examples of therapies that appeared promising in animal studies, but which failed in the clinics, all lessons from experimental works should at least be considered before taking any definitive conclusions on this first generation of cell therapy. Very few studies attempt to rigorously compare the therapeutic potential of different sources of stem cells. Nevertheless, the best cardiac outcomes seem to be achieved by therapeutic cells obligated to a cardiomyocyte lineage. Hence, in experimental studies which have compared different cell types, cardiac-committed cells (c-kit+ or Sca-1+ cardiac stem cells, cardiospheres, induced pluripotent stem cell-derived cardiomyocytes) display greater therapeutic effects compared to those of cells not committed to a cardiac lineage such as bone marrow mononuclear cells, mesenchymal stem cells or skeletal myoblasts (Rossini et al., 2011; Oskouei et al., 2012; Li et al., 2012; Zheng et al., 2013; Citro et al., 2014). Of note, the superiority of cardiac-committed cells could be evidenced on the basis of various end points such as better engraftment, reduced extent of infarction and fibrosis, increase in angiogenesis, improvement of cardiac function and even mitigation of ventricular arrhythmias. In line with these observations, cardiac-committed cell therapies are being tested in the clinics using cardiosphere-derived cells obtained from a right ventricular biopsy (Makkar et al., 2012), c-kit+ cardiac progenitor cells grown from an intra-operatively harvested right appendage biopsy (Chugh et al., 2012; Bolli et al., 2013) and embryonic stem cell-derived cardiac progenitors (Menasche et al., 2015) which are currently tested in a pilot safety trial. Of interest, in a mouse model of acute MI, cardiosphere-derived cells isolated from HF patients led to the greatest therapeutic benefit with the highest left ventricular ejection fraction when compared to cells isolated from non-failing donors, suggesting that the overall efficacy of this stem cell approach is not necessarily dampened by the extent of the underlying left ventricular dysfunction (Cheng et al., 2014). Human-induced pluripotent stem cells are another potentially unlimited source for generation of cardiomyocytes (iPSC-CMs). However, current protocols for iPSC-CM derivation face several challenges, including variability in somatic cell sources and inconsistencies in cardiac differentiation efficiency. In addition, the overall therapeutic effect of the pluripotent stem cell-derived progeny may also depend on the degree of maturity of the stem/progenitor cell phenotype reflected by gene expression of cardiac transcription factors early in the differentiation process (Sanchez-Freire et al., 2014) whereas, unexpectedly, the extent of viability may not be as critical as usually thought. Namely, implantation, in a chronic infarction model, of engineered heart muscle constructed from human embryonic stem cell-derived cardiomyocytes led to high engraftment rates, long-term survival, and progressive maturation of human cardiomyocytes. With similar (and better than controls) functional outcomes regardless of the cells were viable or irradiated (i.e., nonviable), (Riegler, et al. 2015), which indirectly argues in favor of a paracrine mechanism of action (see below). Hence, standardized protocols to produce human stem cell-derived or directly reprogrammed cardiomyocytes or cardiac progenitors exhibiting “appropriate” maturation levels and engraftment are lacking.
More generally, our incomplete understanding of how the human heart develops and can regenerate, and of which intrinsic factor(s) account for the ultimate differences in regenerative capacities between lower vertebrate or neonatal mammalian hearts and adult mammalian hearts reduces the probability of success in our quest for the ideal source of stem cells for cardiac repair. For instance, cardiac progenitor cells are multipotent and give rise to cardiac endothelium, smooth muscle, and cardiomyocytes. A recent and very elegant study characterizes the cardiomyoblast intermediate that is committed to the cardiomyocyte fate. Such cardiomyoblasts express Hopx, which functions to coordinate local Bmp signals to inhibit the Wnt pathway, thus promoting cardiomyogenesis. The identification of this committed cardiomyoblast that retains a proliferative potential might be leveraged to increase the efficiency of cardiac regenerative therapies (Jain et al., 2015). Another illustration is the recent demonstration that epicardial cells feature a potent cardiogenic activity though the presence of follistatin-like 1 (Fstl1). Application of the human Fstl1 protein (FSTL1) via an epicardial patch stimulates cell cycle entry and division of pre-existing cardiomyocytes, improving cardiac function and survival in mouse and swine models of MI (Wei et al., 2015). Finally, exciting new evidences suggest that a specific subtype of c-kit positive cells such as the c-kit-positive/CD45 negative/tryptase negative cardiac stem cells are necessary and sufficient for myocyte regeneration, leading to complete cellular, anatomical, and functional myocardial recovery (Ellison et al., 2013). These data have rationalized the use of right atrium-derived c-kit+ progenitors in patients with both chronic left ventricular dysfunction (Chugh et al., 2012) and recent myocardial infarction (CARE-MI trial, currently ongoing). Interestingly, the ability of these cardiac progenitor cells to produce cardiomyocytes may depend on specific signals from the cardiac milieu. A high-resolution genetic lineage-tracing study in mice reveals that c-kit identifies multipotent progenitors of cardiac neural crest origin. The ability of these c-kit+-progenitors to differentiate into cardiomyocytes is governed by the activity of the bone morphogenetic protein pathway, a signaling pathway activated transiently during establishment of the cardiac crescent, and extinguished from the heart before cardiac neural crest progenitor invasion (Hatzistergos et al., 2015). Therefore, these findings likely resolve a long-standing controversy concerning the in situ myogenic potential of adult c-kit+ cardiac cells particularly in comparison to their neonatal counterparts (Zaruba et al., 2010; Jesty et al., 2012) and suggest that the homeostasis of the cardiac milieu regulates the cardiogenic potential of c-kit+ cardiac progenitor cells.
2. By the Way, How Does it Work?
To the best of our knowledge the worrisome answer to this question is: we still don't know. By analogy, it is difficult to picture any drug-based approach for HF without a precise knowledge of its active principle and the type of receptor it is supposed to interact with. However, it should be noted that despite the multiplicity of randomized clinical trials in patients with HF the additional benefit generated from the use of novel drugs remains very modest, with the possible exception of the recently tested drug that combines blockade of angiotensin receptors and neprilysin inhibition (Desai et al., 2015). We believe that one important reason for that stagnation is the focus on the optimization of therapies that pursue already treated pathophysiological processes, leading (at best) to incremental improvements in outcome. Hence, the inherent advantage of stem cell therapy may be their ability to shape non-redundant and critical disease-causing or aggravating pathways. Nevertheless, a clear understanding of stem cell-based mechanisms of action may lead to personalized medicine or, at least, to adaptation of standard operating procedures to the type of HF patients (for example, ischemic versus non-ischemic cardiomyopathy) and/or the stage of the disease.
2.1. From Stem Cells to Biofactories
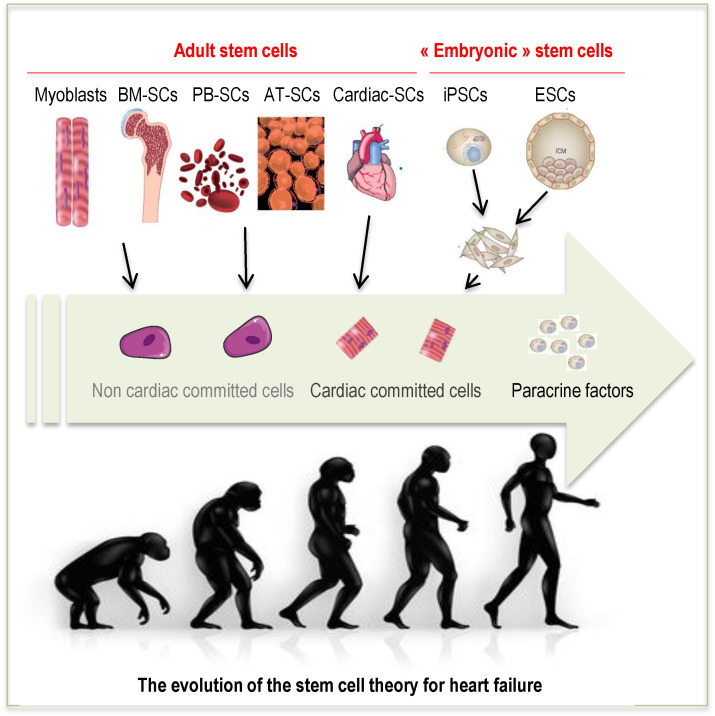
Initially, the objective of cell therapy was the integration of transplanted cells within the recipient myocardium with the assumption that their electrical coupling with host cardiomyocytes should translate into a mechanical contribution of the cellular graft to contractile function. However, the first generation of stem cell therapies used adult stem cells with limited ability to differentiate into cardiomyocytes even in vitro. As mentioned above, alternative sources of stem cells including pluripotent stem cells have been considered to leverage their intrinsic pluripotentiality and drive them towards a cardiac lineage. However, even for iPSC-CMs for example, an increased differentiation efficiency of cells derived from cardiac versus non-cardiac somatic sources does not contribute to improved functional outcomes after MI (Sanchez-Freire et al., 2014). In any case, it is then unlikely that a single even highly competent and plastic pluripotent cell can at each crossroad take the unique and correct decision required to become a cardiomyocyte, especially in an ischaemic or failing heart (Puceat, 2013). Over time, a major switch in the mechanistic archetype has occurred and therapeutic cells are now increasingly thought to primarily act as reservoirs of a wide array of bioactive entities that trigger endogenous repair pathways (Garbern and Lee, 2013) (Fig. 1). This statement is supported by the sharp discrepancy between the scarcity of sustained cell engraftment and the maintenance of a functional benefit, thereby making highly unlikely that the low amounts of detectable cells can account for the preservation of left ventricular function and geometry (Den Haan et al., 2012; Riegler et al., 2015). Consistent with this, while intramyocardially injected embryonic stem cell-derived cardiomyocytes have been shown to couple with host cardiomyocytes, such was not the case when the same cells were delivered under the form of an epicardial patch (Gerbin et al., 2015). Nevertheless, several studies have shown that cell-loaded patches with or without encapsulation were functionally more effective than injected cells, thereby supporting the idea that electro-mechanical integration is not a prerequisite for a successful outcome (Bellamy et al., 2015; Levit et al., 2013).
Fig. 1.
The evolution of the stem cell theory for heart failure. Initially, the objective of stem cell therapy was the integration of transplanted cells within the recipient myocardium with the assumption that their electrical coupling with host cardiomyocytes should translate into a mechanical contribution of the cellular graft to contractile function. The majority of trials used adult stem cells and mainly applied bone marrow- as well as peripheral blood-derived mononuclear cells or marker selected cells. Nevertheless, disregarded preclinical evidences suggest that the best cardiac outcomes seem to be achieved by therapeutic cells of cardiomyocyte lineage. This leads to the development of cardiac-committed cell therapies using cardiosphere-derived cells obtained from a right ventricular biopsy, c-kit-positive cardiac progenitor cells grown from an intra-operatively harvested right appendage biopsy and embryonic stem cell-derived cardiac progenitors. However, the recent “big bang” in the evolution of the stem cell theory suggests that therapeutic cells rather act as reservoirs of a wide array of bioactive entities that trigger multiple and synergic endogenous repair pathways. Abbreviations: BM: bone marrow, PB: peripheral blood; AT: adipose tissue; iPSCs: induced pluripotent stem cells; ESCs: embryonic stem cells; SCs: stem cells.
Assuming that the grafted cells primarily act as biofactories, the obvious question is whether their phenotype makes a difference, i.e., given the fact that multiple cell types secrete multiple factors, one can wonder whether cells could be easily exchangeable with, ultimately, similar outcome. However, as stated above, cardiac-committed cells display a higher therapeutic efficacy when compared to non-cardiac committed stem cells (Rossini et al., 2011; Oskouei et al., 2012; Li et al., 2012; Zheng et al., 2013; Citro et al. 2014). Furthermore, the better outcomes associated with these cardiac-committed cells also manifest in terms of paracrine factor production (Li et al., 2012) with a differential lineage-specific expression of some cytokines which might impact on the ultimate functional effects (Liu et al., 2014). In line with this reasoning, human embryonic stem cell-derived cardiac progenitors show an unchallenged ability to promote post-ischemic revascularization compared with adult stem cells such as BM-derived mesenchymal stem cells or cord-blood derived endothelial progenitor cells, despite a similar limited ability to incorporate into the targeted vascular network (Richart et al., 2014).
2.2. From Cardiac Differentiation to Cardiac Repair
This paracrine mechanism involves multiple complementary and likely non-exclusive pathways including stimulation of vascular growth and remodeling; attenuation of fibrosis; modulation of inflammation; control of differentiated cell survival and recruitment/activation of tissue-resident stem/progenitor cells. Altogether, these activated pathways are expected to synergistically improve tissue protection and preserve cardiac function. The next question pertains to the nature of the biomolecules released by transplanted cells.
2.2.1. Therapeutic Cells and Cardiac Resident Cells
Stem cells from different origins have been shown to release angiogenic and anti-apoptotic factors. In humans, bone marrow-derived cells secrete a cocktail of more than 25 factors and cytokines with proangiogenic activities. These secreted molecules include angiogenin, Vascular Endothelial Growth Factor (VEGF)-A, hepatocyte growth factor, and Fibroblast Growth Factor (FGF). Human and murine bone marrow-derived mesenchymal stem cells also release a broad range of factors with proangiogenic capacities including growth factors (VEGF, FGF-2, FGF-7, insulin growth factor (IGF), platelet-derived growth factor, placenta growth factor metalloproteases (MMP-1, MMP-2, MMP-9, t-PA) and factors involved in stem/progenitor cells mobilization and recruitment (Thymosine β4, SCF, G-CSF) (Gnecchi et al., 2005; Haynesworth et al., 1996; Kinnaird et al., 2004a; Kinnaird et al. 2004b). Accordingly, bone marrow-derived cells increase vascular density and local blood flow through tissues (Kamihata et al., 2001; Tse et al., 2007). The conditioned culture medium from cells of medullary origin also protects cardiomyocytes against the cell death induced by ischemia/reperfusion (Korf-Klingebiel et al., 2008). IGF-1 produced by mononuclear cells of medullary origin specifically inhibits the expression of the pre-microRNA (miR) and of the mature form of miRNA-34a. This miRNA strongly induces the apoptosis of cardiomyocytes. Indeed, the administration of mononuclear cells of medullary origin decreases the cardiac expression of miRNA-34a and cardiomyocyte apoptosis, and improves the function of the infarcted heart. These effects are totally abolished by simultaneous treatment with an antibody directed against IGF-1 (Iekushi et al., 2012). Similarly, transplantation of human iPSC-CMs into an acute mouse MI model has been shown to improve left ventricular function and attenuates cardiac remodeling, despite limited engraftment. Microfluidic single-cell profiling of this iPSC-derived cardiac progeny demonstrates that these cells release significant levels of proangiogenic and antiapoptotic factors in the ischemic microenvironment (Ong et al., 2015). However, if the vascular compartment is the target, we should keep in mind that most of the cardiovascular risk factors, abrogate the endogenous ability of affected cardiac tissue to promote vascular growth and remodeling (Silvestre et al., 2013). In addition, a marked variability in the degree of collateral development for a comparable severity of coronary artery obstructions is a common clinical observation (Werner et al., 2000) and may therefore discriminate responder versus non-responder patients and participate to the heterogeneity in the results of clinical trials.
Stimulation of endogenous cardioblasts by exogenous cells can also mediate the therapeutic regeneration of injured myocardium. This concept was nicely explored in an elegant study in which cell therapy experiments were carried out in mice constitutively producing the green fluorescent protein (GFP) exclusively in the cardiomyocytes following a pulse of 4-OH-tamoxifen. The MI dilutes the pool of cardiomyocytes expressing GFP, revealing substitution with GFP-negative progenitors. The administration of c-kit+ cells derived from bone marrow decreased further the number of GFP+ cardiomyocytes. This effect was not associated with transdifferentiation of c-kit+ cells into cardiomyocytes or with cell fusion phenomena; it was associated with a stimulation of endogenous cardiogenic progenitor activity (Loffredo et al., 2011). Similarly, cell therapy with cardiosphere-derived cells amplifies innate cardioblast-mediated tissue regeneration, in part through the secretion of CXCL12 by transplanted cells. Genetically labeled isolated cardioblasts express cardiac transcription factors and sarcomeric proteins, exhibit spontaneous contractions, and form mature cardiomyocytes in vivo after injection into unlabeled recipient hearts (Malliaras et al., 2014).
2.2.2. Therapeutic Cells and Cardiac Infiltrating Cells
Transplanted stem cells have also been shown to modulate the number and activation mode of inflammatory cells populating the cardiac tissue. Bone marrow-derived cells increase IL-1 and TNF-α in cardiac tissue, three and seven days, respectively, after their administration (Kamihata et al., 2001). Other cytokines have been identified among the many factors secreted by cells of medullary origin including the chemokines CCL-2, CCL-23, CCL-24, CXCL-6, CXCL-12 and CXCL-13, as well as IL-10 (Haynesworth et al., 1996; Korf-Klingebiel et al., 2008). Furthermore, the intramyocardial injection of IL-10 deficient BM derived cells does not rescue the defective cardiac function after MI. The IL-10-dependent improvement provided by transplanted cells was not caused by reduced infarct size, neutrophil infiltration, or capillary density, but rather was associated with decreased T lymphocyte accumulation, reactive hypertrophy, and myocardial collagen deposition (Burchfield et al., 2008). Cardiac homeostasis and inflammatory cells are intertwined and emerging evidences suggest that, notably, monocytes and/or macrophages may provide the necessary signals to drive cardiogenesis. During the inflammatory reaction, two sequential phases defined by the expression of Ly6C on monocytes can be identified in the infarcted myocardium (Nahrendorf et al., 2007; Nahrendorf and Swirski, 2013). Many of these monocytes may either die or exit the cardiac tissue whereas surviving monocytes populating the ischemic milieu may acquire a macrophage phenotype with M1-like and/or M2-like activation mode associated with specific functions in the resolution of inflammation, tissue repair and remodeling (Sica and Mantovani, 2012). Recent works also indicate that the adult heart expands distinct populations of macrophages including subsets of resident macrophages of embryonic origin, with opposite roles in inflammation and cardiac recovery (Lavine et al., 2014; Epelman et al., 2014a; Epelman et al., 2014b; Heidt et al., 2014). Interestingly, using a cell-depletion model, it has been shown that heart regeneration and neoangiogenesis following MI depends on neonatal macrophages. Neonates depleted of macrophages were unable to regenerate their cardiac tissue and formed fibrotic scars, resulting in reduced cardiac function. Immunophenotyping and gene expression profiling of cardiac macrophages from regenerating and non-regenerating hearts indicated that regenerative macrophages have a unique polarization phenotype and secrete numerous soluble factors that may facilitate the formation of new myocardium (Aurora et al., 2014). Bone marrow-derived cells (activated by LPS or TNF-alpha) reprogram macrophages by releasing prostaglandin E(2) through activation of EP2 and EP4 receptors and stimulation of IL-10 secretion (Nemeth et al., 2009). In keeping with these observations, cardiosphere-derived cells reduced the number of CD68 + macrophages but polarized an effector macrophage population within the heart with distinctive cardioprotective phenotype. Conversely, the systemic depletion of macrophages with clodronate abolished cardiosphere-derived cells-mediated cardioprotection (De Couto et al., 2015). Finally, IL-13 has been recently identified as a major regulator of cardiomyocyte differentiation and proliferation as well as cardiac regeneration in the mouse model (O'meara et al., 2015). Whereas numerous studies focus on innate immune cells, the role of the adaptive immune system should also be considered especially since adaptive immunity has been shown to regulate post-natal organogenesis and is known to control cardiac remodeling after acute MI (Plaks et al., 2015; Zouggari et al., 2013; Hofmann and Frantz, 2015).
This intrinsic cross talk between inflammatory cells and stem cells may participate to the heterogeneity of the clinical results. First, the origin and nature of stem cells may control their ability to modulate inflammation and second the microenvironment of the cardiac target tissue will certainly impact the inflammatory reaction. Hence, the time of delivery or the type of HF patients likely dictates the overall effect of therapeutic cells on the inflammatory response and subsequently on cardiac function and remodeling.
However, regardless the mechanisms of action a precise characterization of the cell-released factors purportedly accounting for their benefits still remains elusive.
2.3. From Cell to Cell-Free Therapies
There is an increasing body of evidence that these factors could be clustered in extracellular membrane vesicles. Extracellular membrane vesicles (EVs), including exosomes and microparticles (MP) have been detected in all biological fluids and conditioned cell culture media and appear to be secreted by all cell types. These vesicles are bounded by lipid bilayer membranes and do not have nuclei. They contain mRNA, miRNA, proteins, lipid rafts, and other bioactive molecules. They are coated with surface markers, some of which are specific to the vesicle sub-type. Exosomes and MP differ in their size and biogenesis; exosomes are 30–150 μm in size and are formed within the late endosome, while MP are 100–1000 μm and are shed from the cell membrane. These vesicles may contain key determinants necessary to maintain stem cell properties and their quantitative reduction or loss may result in cellular differentiation or phenotypic changes (Bauer et al., 2011). Alternatively, they cargo a wide array of biomolecules, including microRNAs, proteins, lipids and genetic material that they can transfer to target cells to influence important differentiated cell functions and even regulate other stem cells in their respective niches. Hence, MVs from pluripotent embryonic stem cells are able to reprogram hematopoietic progenitors and revert them to a more primitive state (Ratajczak et al., 2007). Their role as mediators of the effects of cell therapy is demonstrated by their reparative effects in different disease states (Leroyer et al., 2009) including MI (Barile et al., 2014; Khan et al., 2015) and by the observation that EV administration can largely recapitulate the benefits of transplanted cells. Interestingly, EVs-mediated transfer of biomolecules to target cells occurs within a relatively short time frame which would be consistent with the protective effects of transplanted cells despite their fast clearance from the transplanted tissue (Yuan et al., 2009). Consistent with this, bone marrow cell extracts prepared by subjecting BM-derived cells to three freeze-thaw cycles followed by microcentrifugation reduce infarct size and improve cardiac function to a similar extends, as intact cells (Yeghiazarians et al., 2009). Likewise, irradiated embryonic stem cells provide a significant improvement in cardiac function after MI (Burt et al., 2012) and, as mentioned above, irradiation of embryonic stem cell-derived cardiomyocytes did not prevent them to yield a functional outcome similar to that of their viable counterparts (Riegler et al., 2015). So far, similar beneficial roles have been attributed to MVs derived from mesenchymal stem cells (Lee et al., 2012a), induced pluripotent stem cells (Bobis-Wozowicz et al., 2015) and cardiosphere-derived cells (Tseliou et al., 2015) but the nature of the optimal parent cell still remains to be defined. Notably, EVs contains a multitude of miRNAs, known to regulate endogenous repair (Seeger et al., 2013), and some of them playing a pivotal role in tissue protection induced by stem cell-derived EVs (Khan et al., 2015; Gray et al., 2015). Nevertheless, the beneficial effects of EVs likely rely on the interplay of the multitude of factors that they cargo and it might actually be therapeutically counterproductive to deconstruct the vesicular content. It is likely that because of the number of biologically active compounds that they can release, these vesicles feature advantages over the delivery of defined factors that only target a single signaling pathway.
Confirmation that the use of EVs alone could successfully substitute for cells would have major clinically relevant advantages with regard to standard operating procedures, streamlining of the regulatory path and final costs. Nevertheless, specific attention should be paid to the phenotype and the mode of isolation/culture/purification of the parent cells. Genesis and release of EVs are indeed a very dynamic processes and this may lead to heterogeneity in their biological activity and thus in their therapeutic potential. Ultimately, the characterization of their most active forms (i.e. exosomes versus MPs) and/or ingredients may offer the perspective of possible synthesis of biomimetic compounds.
3. Conclusion
Lessons from cell-based therapies reveal that the next generation of therapeutic approaches needs to better fit the patient presentation and the nature of the therapeutic product. First, a more systematic stratification of patients is required to delineate acute versus chronic disease, to identify responders and non-responders and to take into account co-morbidities to improve the response of the failing heart and better shape future clinical trials. Second, emerging evidences suggest that rather than transplanting cells that directly engraft and differentiate/proliferate in the host tissue, delivering paracrine factors alone to the damaged heart may be sufficient to activate repair mechanisms. Whereas the initial autologous stem cell-based cardiac regeneration therapy led to a rather rapid translation into early-phase clinical trials, the evolution of the stem cell theory may ultimately lead to a more thoughtful development of cell-free strategies with multiple targets including cardiomyocytes but also other infiltrating or resident cardiac cells.
4. Outstanding Questions
The proficient development of the next generation of stem cell-based therapy for HF will depend on the ability of the scientific community to address three major existential questions. The first question concerns the cell type: the best outcomes seem to be achieved by therapeutic cells phenocopying targeted cells into the affected territory. This argues in favor of the use of cardiac-committed cells among which the pluripotent stem cell-derived cardiac progeny is particularly attractive. However, the optimal stage at which these cells should be grafted remains a critical but unsettled issue. Transplantation of mature, fully differentiated ESC-derived cardiomyocytes has been shown functionally effective to induce remuscularization of infarcted myocardium (Chong et al., 2014) but, at the opposite, early progenitors feature the possible advantages of a higher resistance to the hypoxic milieu they are transplanted in and a greater plasticity in response to local cues allowing the generation of both contractile and vascular cells, which, in turn, might enhance graft survival. Since the use of pluripotent stem cells now allows to tightly control the sequential steps of the cardiac differentiation pathway, head-to-head comparisons of early progenitors versus more mature cardiomyocytes are eagerly required to define the optimal maturation level promoting both the highest engraftment and the most effective therapeutic effect. The second question relates to the mechanisms of action. The “big bang” in the evolution of the stem cell theory leads to the unexpected assumption that therapeutic cells primarily act as biofactories. This implies to focus on early cell retention, rather than on sustained cell survival, allowing the therapeutic cells to deliver sufficient amounts of factors underpinning their action. Biomaterials are here critical adjuncts to optimize this residency time. The paracrine hypothesis also gives more flexibility for using allogeneic cells in that targeting an only transient engraftment requires to delay, and no longer to avoid, rejection, thereby simplifying immunomodulation regimens. The third question deals with manufacturing since a broad dissemination of cardiac cell therapy requires the development of automated systems with highly reproducible therapeutic products. The development of cell-free strategies may lead to production process, similar to that of a pharmaceutical compound, facilitating an expended standardized clinical use. This also emphasizes the interest of using allogeneic cells as a biosource for these therapeutic paracrine factors (possibly clustered in extracellular vesicles) because of their suitability for industrially-relevant and cost-effective scale-up and quality control procedures. Regardless of the final product to be used (cells or their secretome), streamlining the production process is mandatory to make this therapy cost-effective and allow to target the large number of patients most likely to benefit from it, i.e., those who have exhausted conventional therapies of heart failure but have not yet reached the end stage of their disease, in which case cardiac replacement becomes the only option.
5. Search Strategy and Selection Criteria
Data from this review were identified by searches of PubMed and references from relevant articles using the search terms “Heart failure”, “Myocardial infarction”, “Stem cells”, “Bone marrow”, “Cardiac regeneration”, “Paracrine”, “Inflammation”, “Exosomes”, and “Microparticles”. Only articles published in English were included. Abstracts and reports from meetings were excluded.
Author Contribution
JS Silvestre : literature search and writing.
P Menasché : literature search and writing.
Funding/Acknowledgements
This work was supported by public funds from the French National Institute for Health and Medical Research (Inserm), University Paris Descartes and the LabEx REVIVE, as well as by charity funds from the Fondation de France (FDF/2014-00047970), the LeDucq Foundation (SHAPEHEART network), the Association Française contre les Myopathies (ANR-12-RPIB-0015), the Foundation for Medical Research (DEQ20120323734) and the French National Research Agency (ANR-13-BSV1-0015-0). The above mentioned funding sources did not participate to the writing or the decision to submit the present manuscript.
Contributor Information
Jean-Sébastien Silvestre, Email: jean-sebastien.silvestre@inserm.fr.
Philippe Menasché, Email: philippe.menasche@aphp.fr.
References
- Aurora A.B., Porrello E.R., Tan W., Mahmoud A.I., Hill J.A., Bassel-Duby R., Sadek H.A., Olson E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Lugo A., Tavares A.M., Paz A.H., Alegretti A., Miquelito L., Bock H., Giugliani R., Clausell N., Cirne-Lima E., Rohde L.E. Age-dependent availability and functionality of bone marrow stem cells in an experimental model of acute and chronic myocardial infarction. Cell Transplant. 2011;20:407–419. doi: 10.3727/096368909X519283. [DOI] [PubMed] [Google Scholar]
- Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L.M., Torre T., Siclari F., Moccetti T., Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- Bartunek J., Behfar A., Dolatabadi D., Vanderheyden M., Ostojic M., Dens J., El Nakadi B., Banovic M., Beleslin B., Vrolix M., Legrand V., Vrints C., Vanoverschelde J.L., Crespo-Diaz R., Homsy C., Tendera M., Waldman S., Wijns W., Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- Bauer N., Wilsch-Brauninger M., Karbanova J., Fonseca A.V., Strauss D., Freund D., Thiele C., Huttner W.B., Bornhauser M., Corbeil D. Haematopoietic stem cell differentiation promotes the release of prominin-1/CD133-containing membrane vesicles—a role of the endocytic–exocytic pathway. EMBO Mol. Med. 2011;3:398–409. doi: 10.1002/emmm.201100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behfar A., Crespo-Diaz R., Terzic A., Gersh B.J. Cell therapy for cardiac repair—lessons from clinical trials. Nat. Rev. Cardiol. 2014;11:232–246. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- Bellamy V., Vanneaux V., Bel A., Nemetalla H., Emmanuelle Boitard S., Farouz Y., Joanne P., Perier M.C., Robidel E., Mandet C., Hagege A., Bruneval P., Larghero J., Agbulut O., Menasche P. Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J. Heart Lung Transplant. 2015;34:1198–1207. doi: 10.1016/j.healun.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Bergmann O., Bhardwaj R.D., Bernard S., Zdunek S., Barnabe-Heider F., Walsh S., Zupicich J., Alkass K., Buchholz B.A., Druid H., Jovinge S., Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O., Zdunek S., Felker A., Salehpour M., Alkass K., Bernard S., Sjostrom S.L., Szewczykowska M., Jackowska T., Dos Remedios C., Malm T., Andra M., Jashari R., Nyengaard J.R., Possnert G., Jovinge S., Druid H., Frisen J. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Bobis-Wozowicz S., Kmiotek K., Sekula M., Kedracka-Krok S., Kamycka E., Adamiak M., Jankowska U., Madetko-Talowska A., Sarna M., Bik-Multanowski M., Kolcz J., Boruczkowski D., Madeja Z., Dawn B., Zuba-Surma E.K. Human induced pluripotent stem cell-derived microvesicles transmit RNAs and proteins to recipient mature heart cells modulating cell fate and behavior. Stem Cells. 2015;33:2748–2761. doi: 10.1002/stem.2078. [DOI] [PubMed] [Google Scholar]
- Bolli R., Tang X.L., Sanganalmath S.K., Rimoldi O., Mosna F., Abdel-Latif A., Jneid H., Rota M., Leri A., Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield J.S., Iwasaki M., Koyanagi M., Urbich C., Rosenthal N., Zeiher A.M., Dimmeler S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ. Res. 2008;103:203–211. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- Burt R.K., Chen Y.H., Verda L., Lucena C., Navale S., Johnson J., Han X., Lomasney J., Baker J.M., Ngai K.L., Kino A., Carr J., Kajstura J., Anversa P. Mitotically inactivated embryonic stem cells can be used as an in vivo feeder layer to nurse damaged myocardium after acute myocardial infarction: a preclinical study. Circ. Res. 2012;111:1286–1296. doi: 10.1161/CIRCRESAHA.111.262584. [DOI] [PubMed] [Google Scholar]
- Cheng K., Malliaras K., Smith R.R., Shen D., Sun B., Blusztajn A., Xie Y., Ibrahim A., Aminzadeh M.A., Liu W., Li T.S., De Robertis M.A., Marban L., Czer L.S., Trento A., Marban E. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart. Fail. 2014;2:49–61. doi: 10.1016/j.jchf.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J., Gantz J.A., Fugate J.A., Muskheli V., Gough G.M., Vogel K.W., Astley C.A., Hotchkiss C.E., Baldessari A., Pabon L., Reinecke H., Gill E.A., Nelson V., Kiem H.P., Laflamme M.A., Murry C.E. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh A.R., Beache G.M., Loughran J.H., Mewton N., Elmore J.B., Kajstura J., Pappas P., Tatooles A., Stoddard M.F., Lima J.A., Slaughter M.S., Anversa P., Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citro L., Naidu S., Hassan F., Kuppusamy M.L., Kuppusamy P., Angelos M.G., Khan M. Comparison of human induced pluripotent stem-cell derived cardiomyocytes with human mesenchymal stem cells following acute myocardial infarction. PLoS One. 2014;9 doi: 10.1371/journal.pone.0116281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Couto G., Liu W., Tseliou E., Sun B., Makkar N., Kanazawa H., Arditi M., Marban E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J. Clin. Invest. 2015;125:3147–3162. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Haan M.C., Grauss R.W., Smits A.M., Winter E.M., Van Tuyn J., Pijnappels D.A., Steendijk P., Gittenberger-DE GROOT A.C., VAN DER LAARSE A., Fibbe W.E., DE VRIES A.A., Schalij M.J., Doevendans P.A., Goumans M.J., Atsma D.E. Cardiomyogenic differentiation-independent improvement of cardiac function by human cardiomyocyte progenitor cell injection in ischaemic mouse hearts. J. Cell. Mol. Med. 2012;16:1508–1521. doi: 10.1111/j.1582-4934.2011.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A.S., Mcmurray J.J., Packer M., Swedberg K., Rouleau J.L., Chen F., Gong J., Rizkala A.R., Brahimi A., Claggett B., Finn P.V., Hartley L.H., Liu J., Lefkowitz M., Shi V., Zile M.R., Solomon S.D. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur. Heart J. 2015;36:1990–1997. doi: 10.1093/eurheartj/ehv186. [DOI] [PubMed] [Google Scholar]
- Ebrahimian T.G., Heymes C., You D., Blanc-Brude O., Mees B., Waeckel L., Duriez M., Vilar J., Brandes R.P., Levy B.I., Shah A.M., Silvestre J.S. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison G.M., Vicinanza C., Smith A.J., Aquila I., Leone A., Waring C.D., Henning B.J., Stirparo G.G., Papait R., Scarfo M., Agosti V., Viglietto G., Condorelli G., Indolfi C., Ottolenghi S., Torella D., Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Epelman S., Lavine K.J., Beaudin A.E., Sojka D.K., Carrero J.A., Calderon B., Brija T., Gautier E.L., Ivanov S., Satpathy A.T., Schilling J.D., Schwendener R., Sergin I., Razani B., Forsberg E.C., Yokoyama W.M., Unanue E.R., Colonna M., Randolph G.J., Mann D.L. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S., Lavine K.J., Randolph G.J. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S.A., Doree C., Mathur A., Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ. Res. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- Garbern J.C., Lee R.T. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbin K.A., Yang X., Murry C.E., Coulombe K.L. Enhanced electrical integration of engineered human myocardium via intramyocardial versus epicardial delivery in infarcted rat hearts. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R.E., Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385:2107–2117. doi: 10.1016/S0140-6736(14)61402-1. [DOI] [PubMed] [Google Scholar]
- Gnecchi M., He H., Liang O.D., Melo L.G., Morello F., MU H., Noiseux N., Zhang L., Pratt R.E., Ingwall J.S., Dzau V.J. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Govaert J.A., Swijnenburg R.J., Schrepfer S., Xie X., Van Der Bogt K.E., Hoyt G., Stein W., Ransohoff K.J., Robbins R.C., Wu J.C. Poor functional recovery after transplantation of diabetic bone marrow stem cells in ischemic myocardium. J. Heart Lung Transplant. 2009;28(1158–1165) doi: 10.1016/j.healun.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W.D., French K.M., Ghosh-Choudhary S., Maxwell J.T., Brown M.E., Platt M.O., Searles C.D., Davis M.E. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ. Res. 2015;116:255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyongyosi M., Wojakowski W., Lemarchand P., Lunde K., Tendera M., Bartunek J., Marban E., Assmus B., Henry T.D., Traverse J.H., Moye L.A., Surder D., Corti R., Huikuri H., Miettinen J., Wohrle J., Obradovic S., Roncalli J., Malliaras K., Pokushalov E., Romanov A., Kastrup J., Bergmann M.W., Atsma D.E., Diederichsen A., Edes I., Benedek I., Benedek T., Pejkov H., Nyolczas N., Pavo N., Bergler-Klein J., Pavo I.J., Sylven C., Berti S., Navarese E.P., Maurer G., Investigators A. Meta-analysis of cell-based cardiac studies (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 2015;116:1346–1360. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J.M., Fishman J.E., Gerstenblith G., Difede Velazquez D.L., Zambrano J.P., Suncion V.Y., Tracy M., Ghersin E., Johnston P.V., Brinker J.A., Breton E., Davis-sproul J., Schulman I.H., Byrnes J., Mendizabal A.M., Lowery M.H., Rouy D., Altman P., Wong Po Foo C., Ruiz P., Amador A., Da Silva J., Mcniece I.K., Heldman A.W., George R., Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzistergos K.E., Takeuchi L.M., Saur D., Seidler B., Dymecki S.M., Mai J.J., White I.A., Balkan W., Kanashiro-Takeuchi R.M., Schally A.V., Hare J.M. Proc Natl Acad Sci U S A; 2015. cKit + Cardiac Progenitors of Neural Crest Origin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynesworth S.E., Baber M.A., Caplan A.I. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J. Cell. Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Heidt T., Courties G., Dutta P., Sager H.B., Sebas M., Iwamoto Y., Sun Y., Da Silva N., Panizzi P., Van Der Laan A.M., Swirski F.K., Weissleder R., Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ. Res. 2014;115:284–295. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman A.W., Difede D.L., Fishman J.E., Zambrano J.P., Trachtenberg B.H., Karantalis V., Mushtaq M., Williams A.R., Suncion V.Y., Mcniece I.K., Ghersin E., Soto V., Lopera G., Miki R., Willens H., Hendel R., Mitrani R., Pattany P., Feigenbaum G., Oskouei B., Byrnes J., Lowery M.H., Sierra J., Pujol M.V., Delgado C., Gonzalez P.J., Rodriguez J.E., Bagno L.L., Rouy D., Altman P., Foo C.W., Da Silva J., Anderson E., Schwarz R., Mendizabal A., Hare J.M. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann U., Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ. Res. 2015;116:354–367. doi: 10.1161/CIRCRESAHA.116.304072. [DOI] [PubMed] [Google Scholar]
- Houtgraaf J.H., Den Dekker W.K., Van Dalen B.M., Springeling T., De Jong R., Van Geuns R.J., Geleijnse M.L., Fernandez-Aviles F., Zijlsta F., Serruys P.W., Duckers H.J. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012;59:539–540. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- Iekushi K., Seeger F., Assmus B., Zeiher A.M., Dimmeler S. Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction. Circulation. 2012;125(1765–73):S1–S7. doi: 10.1161/CIRCULATIONAHA.111.079699. [DOI] [PubMed] [Google Scholar]
- Jain R., LI D., Gupta M., Manderfield L.J., Ifkovits J.L., Wang Q., Liu F., Liu Y., Poleshko A., Padmanabhan A., Raum J.C., LI L., Morrisey E.E., LU M.M., Won K.J., Epstein J.A. HEART DEVELOPMENT. Integration of Bmp and Wnt signaling by Hopx specifies commitment of cardiomyoblasts. Science. 2015;348 doi: 10.1126/science.aaa6071. (aaa6071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesty S.A., Steffey M.A., Lee F.K., Breitbach M., Hesse M., Reining S., Lee J.C., Doran R.M., Nikitin A.Y., Fleischmann B.K., Kotlikoff M.I. c-kit + precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. U. S. A. 2012;109:13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihata H., Matsubara H., Nishiue T., Fujiyama S., Tsutsumi Y., Ozono R., Masaki H., Mori Y., Iba O., Tateishi E., Kosaki A., Shintani S., Murohara T., Imaizumi T., Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- Khan M., Nickoloff E., Abramova T., Johnson J., Verma S.K., Krishnamurthy P., Mackie A.R., Vaughan E., Garikipati V.N., Benedict C., Ramirez V., Lambers E., Ito A., Gao E., Misener S., Luongo T., Elrod J., Qin G., Houser S.R., Koch W.J., Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird T., Stabile E., Burnett M.S., Epstein S.E. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ. Res. 2004;95:354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- Kinnaird T., Stabile E., Burnett M.S., Shou M., Lee C.W., Barr S., Fuchs S., Epstein S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- Korf-Klingebiel M., Kempf T., Sauer T., Brinkmann E., Fischer P., Meyer G.P., Ganser A., Drexler H., Wollert K.C. Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur. Heart J. 2008;29:2851–2858. doi: 10.1093/eurheartj/ehn456. [DOI] [PubMed] [Google Scholar]
- Lavine K.J., Epelman S., Uchida K., Weber K.J., Nichols C.G., Schilling J.D., Ornitz D.M., Randolph G.J., Mann D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Mitsialis S.A., Aslam M., Vitali S.H., Vergadi E., Konstantinou G., Sdrimas K., Fernandez-Gonzalez A., Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.C., AN S.G., Lee H.W., Park J.S., Cha K.S., Hong T.J., Park J.H., Lee S.Y., Kim S.P., Kim Y.D., Chung S.W., Bae Y.C., Shin Y.B., Kim J.I., Jung J.S. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ. J. 2012;76:1750–1760. doi: 10.1253/circj.cj-11-1135. [DOI] [PubMed] [Google Scholar]
- Leroyer A.S., Ebrahimian T.G., Cochain C., Recalde A., Blanc-Brude O., Mees B., Vilar J., Tedgui A., Levy B.I., Chimini G., Boulanger C.M., Silvestre J.S. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–2817. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- Levit R.D., Landazuri N., Phelps E.A., Brown M.E., Garcia A.J., Davis M.E., Joseph G., Long R., Safley S.A., Suever J.D., Lyle A.N., Weber C.J., Taylor W.R. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.S., Cheng K., Malliaras K., Smith R.R., Zhang Y., Sun B., Matsushita N., Blusztajn A., Terrovitis J., Kusuoka H., Marban L., Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.L., Nagai T., Tokunaga M., Iwanaga K., Matsuura K., Takahashi T., Kanda M., Kondo N., Naito A.T., Komuro I., Kobayashi Y. Anti-inflammatory peptides from cardiac progenitors ameliorate dysfunction after myocardial infarction. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo F.S., Steinhauser M.L., Gannon J., Lee R.T. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar R.R., Smith R.R., Cheng K., Malliaras K., Thomson L.E., Berman D., Czer L.S., Marban L., Mendizabal A., Johnston P.V., Russell S.D., Schuleri K.H., Lardo A.C., Gerstenblith G., Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K., Ibrahim A., Tseliou E., Liu W., Sun B., Middleton R.C., Seinfeld J., Wang L., Sharifi B.G., Marban E. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol Med. 2014;6:760–777. doi: 10.1002/emmm.201303626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiasen A.B., Qayyum A.A., Jorgensen E., Helqvist S., Fischer-Nielsen A., Kofoed K.F., Haack-Sorensen M., Ekblond A., Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur. Heart J. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- Menasche P., Alfieri O., Janssens S., Mckenna W., Reichenspurner H., Trinquart L., Vilquin J.T., Marolleau J.P., Seymour B., Larghero J., Lake S., Chatellier G., Solomon S., Desnos M., Hagege A.A. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- Menasche P., Vanneaux V., Hagege A., Bel A., Cholley B., Cacciapuoti I., Parouchev A., Benhamouda N., Tachdjian G., Tosca L., Trouvin J.H., Fabreguettes J.R., Bellamy V., Guillemain R., Suberbielle Boissel C., Tartour E., Desnos M., Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- Naftali-Shani N., Itzhaki-Alfia A., Landa-Rouben N., Kain D., Holbova R., Adutler-Lieber S., Molotski N., Asher E., Grupper A., Millet E., Tessone A., Winkler E., Kastrup J., Feinberg M.S., Zipori D., Pevsner-Fischer M., Raanani E., Leor J. The origin of human mesenchymal stromal cells dictates their reparative properties. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M., Swirski F.K. Monocyte and macrophage heterogeneity in the heart. Circ. Res. 2013;112:1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M., Swirski F.K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J.L., Libby P., Weissleder R., Pittet M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M., Hu X., Jelinek I., Star R.A., Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'meara C.C., Wamstad J.A., Gladstone R.A., Fomovsky G.M., Butty V.L., Shrikumar A., Gannon J.B., Boyer L.A., Lee R.T. Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration. Circ. Res. 2015;116:804–815. doi: 10.1161/CIRCRESAHA.116.304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.G., Huber B.C., Hee LEE W., Kodo K., Ebert A.D., MA Y., Nguyen P.K., Diecke S., Chen W.Y., WU J.C. Microfluidic single-cell analysis of transplanted human induced pluripotent stem cell-derived cardiomyocytes after acute myocardial infarction. Circulation. 2015;132:762–771. doi: 10.1161/CIRCULATIONAHA.114.015231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouei B.N., Lamirault G., Joseph C., Treuer A.V., Landa S., Da Silva J., Hatzistergos K., Dauer M., Balkan W., Mcniece I., Hare J.M. Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Transl. Med. 2012;1:116–124. doi: 10.5966/sctm.2011-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin E.C., Sanz-Ruiz R., Sanchez P.L., Lasso J., Perez-Cano R., Alonso-Farto J.C., Perez-David E., Fernandez-Santos M.E., Serruys P.W., Duckers H.J., Kastrup J., Chamuleau S., Zheng Y., Silva G.V., Willerson J.T., Fernandez-Aviles F. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: the PRECISE trial. Am. Heart J. 2014;168(88–95) doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Plaks V., Boldajipour B., Linnemann J.R., Nguyen N.H., Kersten K., Wolf Y., Casbon A.J., Kong N., Van Den Bijgaart R.J., Sheppard D., Melton A.C., Krummel M.F., Werb Z. Adaptive immune regulation of mammary postnatal organogenesis. Dev. Cell. 2015;34:493–504. doi: 10.1016/j.devcel.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planat-Benard V., Silvestre J.S., Cousin B., Andre M., Nibbelink M., Tamarat R., Clergue M., Manneville C., Saillan-Barreau C., Duriez M., Tedgui A., Levy B., Penicaud L., Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Puceat M. Could a pluripotent stem cell give rise to a high yield of a single cell lineage: a myocardial cell? Curr. Opin. Genet. Dev. 2013;23:498–499. doi: 10.1016/j.gde.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Ratajczak M.Z., Machalinski B., Wojakowski W., Ratajczak J., Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- Richart A., Loyer X., Neri T., Howangyin K., Guerin C.L., Ngkelo A., Bakker W., Zlatanova I., Rouanet M., Vilar J., Levy B., Rothenberg M., Mallat Z., Puceat M., Silvestre J.S. MicroRNA-21 coordinates human multipotent cardiovascular progenitors therapeutic potential. Stem Cells. 2014;32:2908–2922. doi: 10.1002/stem.1789. [DOI] [PubMed] [Google Scholar]
- Riegler J., Tiburcy M., Ebert A., Tzatzalos E., Raaz U., Abilez O.J., Shen Q., Kooreman N.G., Neofytou E., Chen V.C., Wang M., Meyer T., Tsao P.S., Connolly A.J., Couture L.A., Gold J.D., Zimmermann W.H., Wu J.C. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 2015;117:720–730. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncalli J., Mouquet F., Piot C., Trochu J.N., Le Corvoisier P., Neuder Y., Le Tourneau T., Agostini D., Gaxotte V., Sportouch C., Galinier M., Crochet D., Teiger E., Richard M.J., Polge A.S., Beregi J.P., Manrique A., Carrie D., Susen S., Klein B., Parini A., Lamirault G., Croisille P., Rouard H., Bourin P., Nguyen J.M., Delasalle B., Vanzetto G., Van Belle E., Lemarchand P. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. Eur. Heart J. 2011;32:1748–1757. doi: 10.1093/eurheartj/ehq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A., Frati C., Lagrasta C., Graiani G., Scopece A., Cavalli S., Musso E., Baccarin M., Di Segni M., Fagnoni F., Germani A., Quaini E., Mayr M., Xu Q., Barbuti A., Difrancesco D., Pompilio G., Quaini F., Gaetano C., Capogrossi M.C. Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc. Res. 2011;89:650–660. doi: 10.1093/cvr/cvq290. [DOI] [PubMed] [Google Scholar]
- Sahara M., Santoro F., Chien K.R. Programming and reprogramming a human heart cell. EMBO J. 2015;34:710–738. doi: 10.15252/embj.201490563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Freire V., Lee A.S., Hu S., Abilez O.J., Liang P., Lan F., Huber B.C., Ong S.G., Hong W.X., Huang M., WU J.C. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J. Am. Coll. Cardiol. 2014;64:436–448. doi: 10.1016/j.jacc.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger F.H., Rasper T., Fischer A., Muhly-Reinholz M., Hergenreider E., Leistner D.M., Sommer K., Manavski Y., Henschler R., Chavakis E., Assmus B., Zeiher A.M., Dimmeler S. Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardiovascular repair. Circ. Res. 2012;111:854–862. doi: 10.1161/CIRCRESAHA.112.265678. [DOI] [PubMed] [Google Scholar]
- Seeger F.H., Tonn T., Krzossok N., Zeiher A.M., Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur. Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- Seeger F.H., Zeiher A.M., Dimmeler S. MicroRNAs in stem cell function and regenerative therapy of the heart. Arterioscler. Thromb. Vasc. Biol. 2013;33:1739–1746. doi: 10.1161/ATVBAHA.113.300138. [DOI] [PubMed] [Google Scholar]
- Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre J.S., Smadja D.M., Levy B.I. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol. Rev. 2013;93:1743–1802. doi: 10.1152/physrev.00006.2013. [DOI] [PubMed] [Google Scholar]
- Tse H.F., Siu C.W., Zhu S.G., Songyan L., Zhang Q.Y., Lai W.H., Kwong Y.L., Nicholls J., Lau C.P. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. Eur. J. Heart Fail. 2007;9:747–753. doi: 10.1016/j.ejheart.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tseliou E., Fouad J., Reich H., Slipczuk L., De Couto G., Aminzadeh M., Middleton R., Valle J., Weixin L., Marban E. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J. Am. Coll. Cardiol. 2015;66:599–611. doi: 10.1016/j.jacc.2015.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K., Serpooshan V., Hurtado C., Diez-Cunado M., Zhao M., Maruyama S., Zhu W., Fajardo G., Noseda M., Nakamura K., Tian X., Liu Q., Wang A., Matsuura Y., Bushway P., Cai W., Savchenko A., Mahmoudi M., Schneider M.D., Van Den Hoff M.J., Butte M.J., Yang P.C., Walsh K., Zhou B., Bernstein D., Mercola M., Ruiz-Lozano P. 2015. Epicardial FSTL1 Reconstitution Regenerates the Adult Mammalian Heart. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner G.S., Richartz B.M., Gastmann O., Ferrari M., Figulla H.R. Immediate changes of collateral function after successful recanalization of chronic total coronary occlusions. Circulation. 2000;102:2959–2965. doi: 10.1161/01.cir.102.24.2959. [DOI] [PubMed] [Google Scholar]
- Yeghiazarians Y., Zhang Y., Prasad M., Shih H., Saini S.A., Takagawa J., Sievers R.E., Wong M.L., Kapasi N.K., Mirsky R., Koskenvuo J., Minasi P., YE J., Viswanathan M.N., Angeli F.S., Boyle A.J., Springer M.L., Grossman W. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol. Ther. 2009;17:1250–1256. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You D., Cochain C., Loinard C., Vilar J., Mees B., Duriez M., Levy B.I., Silvestre J.S. Hypertension impairs postnatal vasculogenesis: role of antihypertensive agents. Hypertension. 2008;51:1537–1544. doi: 10.1161/HYPERTENSIONAHA.107.109066. [DOI] [PubMed] [Google Scholar]
- Yuan A., Farber E.L., Rapoport A.L., Tejada D., Deniskin R., Akhmedov N.B., Farber D.B. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaruba M.M., Soonpaa M., Reuter S., Field L.J. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.X., Weng Y.L., Zhou C.Q., Wen Z.Z., Huang H., Wu W., Wang J.F., Wang T. Comparison of cardiac stem cells and mesenchymal stem cells transplantation on the cardiac electrophysiology in rats with myocardial infarction. Stem Cell Rev. 2013;9:339–349. doi: 10.1007/s12015-012-9367-6. [DOI] [PubMed] [Google Scholar]
- Zouggari Y., Ait-Oufella H., Bonnin P., Simon T., Sage A.P., Guerin C., Vilar J., Caligiuri G., Tsiantoulas D., Laurans L., Dumeau E., Kotti S., Bruneval P., Charo I.F., Binder C.J., Danchin N., Tedgui A., Tedder T.F., Silvestre J.S., Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]