Abstract
Mice deficient in intestinal alkaline phosphatase (IAP) develop type 2 diabetes mellitus (T2DM). We hypothesized that a high level of IAP might be protective against T2DM in humans. We determined IAP levels in the stools of 202 diabetic patients and 445 healthy non-diabetic control people. We found that compared to controls, T2DM patients have approx. 50% less IAP (mean +/− SEM: 67.4 +/− 3.2 vs 35.3 +/− 2.5 U/g stool, respectively; p < 0.000001) indicating a protective role of IAP against T2DM. Multiple logistic regression analyses showed an independent association between the IAP level and diabetes status. With each 25 U/g decrease in stool IAP, there is a 35% increased risk of diabetes. The study revealed that obese people with high IAP (approx. 65 U/g stool) do not develop T2DM. Approx. 65% of the healthy population have < 65.0 U/g stool IAP, and predictably, these people might have ‘the incipient metabolic syndrome’, including ‘incipient diabetes’, and might develop T2DM and other metabolic disorders in the near future. In conclusion, high IAP levels appear to be protective against diabetes irrespective of obesity, and a ‘temporal IAP profile’ might be a valuable tool for predicting ‘the incipient metabolic syndrome’, including ‘incipient diabetes’.
Abbreviations: AP, alkaline phosphatase; BMI, body mass index; FPG, fasting plasma glucose; IAP, intestinal alkaline phosphatase; IAP-KO, IAP knockout; L-Arg, L-homoarginine; l-Phe, l-phenylalanine; LPS, lipopolysaccharides; TNAP, tissue non-specific alkaline phosphatase
Keywords: Fatty liver, Glucose intolerance, Hypertension, Incipient diabetes, Insulin resistance, The metabolic syndrome, Obesity
Graphical abstract
Highlights
-
•
Diabetes patients have very low levels of intestinal alkaline phosphatase (IAP) in their stool.
-
•
Obese people with high IAP do not develop diabetes.
-
•
A low level of IAP in a healthy person is indicative of having the incipient (latent) metabolic syndrome (diabetes).
-
•
Approximately 65% of the healthy population have the incipient metabolic syndrome including incipient diabetes.
-
•
‘Temporal IAP profiling’ should diagnose the incipient metabolic syndrome (diabetes).
Currently, diabetes is diagnosed by high blood sugar, for example having fasting plasma glucose (FPG) > 7.0 mmol/l. High FPG is associated only with already established diabetes, and therefore FPG cannot predict years in advance whether a person have incipient (latent) diabetes. This study shows that diabetes patients have very low levels of the gut enzyme intestinal alkaline phosphatase (IAP) in their stool indicating a protective role of IAP against diabetes. Even obese people do not develop diabetes if their IAP levels are high. Approx. 65% of healthy people have low IAP suggesting that these people have the incipient (latent) metabolic syndrome including incipient diabetes, and are very vulnerable to develop diabetes and other metabolic disorders in the near future. The study suggests that regular monitoring of stool IAP (temporal IAP profiling) might be very helpful to diagnose ‘incipient’ diabetes, the metabolic syndrome and other metabolic disorders.
1. Introduction
Type 2 diabetes mellitus (T2DM) is characterized by hyperglycemia, and it is a major global health problem that affects nearly 5.3% population of the world with devastating consequences in the context of healthcare cost, morbidity and mortality. T2DM causes long-term damage, dysfunction, and failure of different organs, especially the eyes, kidneys, nerves, heart, and blood vessels (American Diabetes Association, 2014, Abdullah et al., 2014, Kahn et al., 2014). It is a major global health problem that affected 387 million people worldwide in 2014 and cost 612 billion US dollars (Abdullah et al., 2014). In the United States, estimated 24.4 million people (9.2% population) had diabetes in 2013, costing 306 billion dollars (Abdullah et al., 2014).
Etiologically, various factors have been postulated to be involved in the development of T2DM, such as autoimmunity, the metabolic syndrome, diets, obesity, infection, ethnicity, genetic polymorphism and predisposition, drugs, stress, sedentary lifestyle, pregnancy, etc. (Velloso et al., 2013, Kahn et al., 2014, Stumvoll et al., 2005, Masters et al., 2011). Recently, a low-grade systemic inflammation, induced by persistently increased levels of endotoxin lipopolysaccharides (LPS) in blood (metabolic endotoxemia), has been implicated as an etiological factor for T2DM (Cani et al., 2007). In our previous work, we have shown that mice deficient in the brush-border enzyme intestinal alkaline phosphatase (IAP) (Akp3 knockout, Akp3−/−) develop T2DM (Kaliannan et al., 2013). We have also shown that IAP detoxifies LPS and reduces metabolic endotoxemia, and oral supplementation with IAP not only prevents but also cures high-fat diet-induced T2DM in wild-type mice (Kaliannan et al., 2013). Because IAP deficiency results in T2DM in mice, we hypothesized that a high level of IAP might play a protective role against T2DM in humans. Therefore, using a case-control design we recruited T2DM and non-T2DM subjects from a community in the suburb of Dhaka, Bangladesh, and determined the levels of IAP in the stools of these participants. Here, we report that people with diabetes have less amounts of IAP in their stool compared to their healthy counterparts. Also, we found that obese people with high IAP do not develop T2DM. Further, results of this study suggest that ‘temporal IAP profiling’ might be a valuable tool for identifying ‘incipient’ T2DM and other metabolic disorders.
2. Materials and Methods
2.1. Study Design and Participants
A case-control study was used to assess the difference in the concentrations of IAP in stools of diabetic and control healthy populations. Participants, aged 30–70 yr., were recruited from a suburban community of Dhaka, Bangladesh by advertisement through local dignitaries, hospitals, clinics and physicians' offices. Based on preliminary data (unpublished) the sample size of each group was calculated to achieve statistical power of 80% or more (continuous endpoint, α = 0.05). The study included 202 diabetic cases (63 males and 139 females) and 445 healthy control subjects (114 males and 331 females) of the same ethnicity. The study included more females than males just because of more accessibility to female participants. People with T2DM were diagnosed by a physician for at least 6 months prior to their recruitment, and were on oral antihyperglycemic agents and/or insulin medication. Newly diagnosed persons with hyperglycemia (FPG > 7.0 mmol/l) were also included in the diabetic group (see below). All participants were on unrestricted diets. Any person suffering from an acute disease was excluded from the study. Pregnant women, patients with Type 1 diabetes and history of cancer were excluded. All participants were tested for renal, hepatic and cardiovascular diseases, and participants with clinically significant renal, hepatic and cardiovascular diseases were excluded. The participants did not have any history of chronic alcohol consumption. Underweight persons (body mass index, BMI < 18.5 kg/m2) were also excluded.
The Institutional Review Board (IRB) of Harvard Biotech BD Ltd. (Dhaka, Bangladesh) reviewed and approved the study. An informed consent form to participate in the study was signed by each participant.
2.2. Laboratory Tests, Physical Examination and Socio-Medical History
Laboratory tests were performed using biochemical assay kits from Linear Chemicals S.L. (Barcelona, Spain) and an automatic biochemistry analyzer from Sinnowa Medical Science & Technology Co., Ltd. (Nanjing, Jiangsu, China; Model: Sinnolab MT 5000, Version 5.00). The diabetes status of each participant (diabetes patients and healthy controls) was confirmed by measuring fasting (at least 10 h) plasma glucose (FPG). An FPG level > 7.0 mmol/l (126 mg/dl), was considered diagnostic for diabetes (American Diabetes Association, 2014). Any newly diagnosed diabetes patient (FPG > 7.0 mmol/l) was included in the diabetic group. All participants were also subjected to serum biochemical tests for cholesterol, low-density lipoproteins (LDL), high-density lipoprotein (HDL), triglycerides, creatinine and alanine aminotransferase (ALT). Height and weight of each participant were measured to calculate the BMI defined as weight in kg divided by the square of height in meter (kg/m2). Participants were asked for the history of alcohol consumption. Medical history was used to discover participants with kidney, liver and heart diseases. The status of cardiovascular disease was also evaluated by measuring blood pressure.
2.3. Homogenization of Stool
The supernatant of a homogenized stool suspension was used for alkaline phosphatase (AP) assay. A small amount of fresh stool (mgs) was measured and then the ‘stool dilution buffer’ (10 mM Tris–HCl, pH 8.0, 1 mM magnesium chloride, 10 μM zinc chloride) at a defined ratio was added. Usually, 50 μl of stool dilution buffer was added to 1 mg of stool. The sample was vigorously vortexed to prepare a homogenized stool suspension, which was then centrifuged at 10,000 × g for 20 min, and the supernatant containing AP was collected and assayed for AP concentration. It is to point out that stool suspended in water shows a bit lower AP activity compared to stool suspended in stool dilution buffer.
2.4. Alkaline Phosphatase Assay
The stool supernatant was assayed for alkaline phosphatase (AP) following an established protocol using the automatic biochemistry analyzer mentioned above (Nanjing, Jiangsu, China). In brief, 20 μl of supernatant was added to 1 ml of enzyme assay buffer (1.25 M diethanolamine (DEA) buffer, pH 10.2, 0.6 mM magnesium chloride) containing 10 mM p-nitrophenyl phosphate (pNPP), and the reaction mixture was incubated for one min at 37 °C followed by measuring the AP concentration by the analyzer pre-calibrated with AP standards. To determine the major isoform among stool APs, prior to assaying for AP activity an aliquot of stool sample was treated for 10 min with l-phenylalanine (l-Phe, 10 mM final conc.), a specific inhibitor of IAP, and another aliquot with L-homoarginine (L-Arg, 10 mM final conc.), a specific inhibitor of tissue-nonspecific alkaline phosphatase (TNAP) (Kaliannan et al., 2013, Sergienko et al., 2009). Each aliquot treated with an inhibitor was then mixed with the reaction buffer containing an equal concentration (10 mM) of the respective inhibitor, and assayed for AP activity using the analyzer. Because most of the AP activity in stool is due to IAP (see Results) the stool AP values are expressed as units of IAP/g stool. All AP assays were performed by a single laboratory technologist who was blinded to the diagnoses of participants.
2.5. Statistical Analysis
The SAS System (SAS Institute, Cary, North Carolina) was used for statistical analysis. Mean and standard errors were calculated for T2DM cases and non-T2DM controls stratified by sex. The correlation between IAP levels and various risk factors for T2DM was assessed via Pearson correlation coefficient stratified by sex and T2DM status (T2DM patients or non-T2DM controls). Mean differences in IAP levels between T2DM cases and non-T2DM controls were assessed via linear regression models controlling for the effects of age, sex, FPG and BMI on IAP levels and T2DM status. The statistical significance of the variance associated with independent variables were assessed from sum of square III using GLM procedure in SAS. Multiple logistic regression using Proc Logist procedure in SAS assessed association between T2DM cases with independent risk factors including IAP. Regression coefficients and odds ratios were used to express the independent risk contribution of IAP to T2DM status. The statistical significance of the difference between two groups was determined using unpaired two-tailed Student's t-Test. The difference between two groups was considered significant when the p value was < 0.05. Student's t-Test was performed using Microsoft Excel program. Post-hoc statistical power analysis of two independent groups was performed using an online program (http://clincalc.com/Stats/Power.aspx).
2.6. Role of the Funding Source
The funding source (Harvard Biotech BD Ltd., Dhaka, Bangladesh) played no role in the development of theory and study design. Harvard Biotech BD Ltd. was also not involved in the collection, analyses, and interpretation of data, or writing and submission of the manuscript. The corresponding author had full access to all the data in the study, and took the decision for publication of the data.
3. Results
3.1. Stool Alkaline Phosphatase Activity Is Mostly Due to Intestinal Alkaline Phosphatase
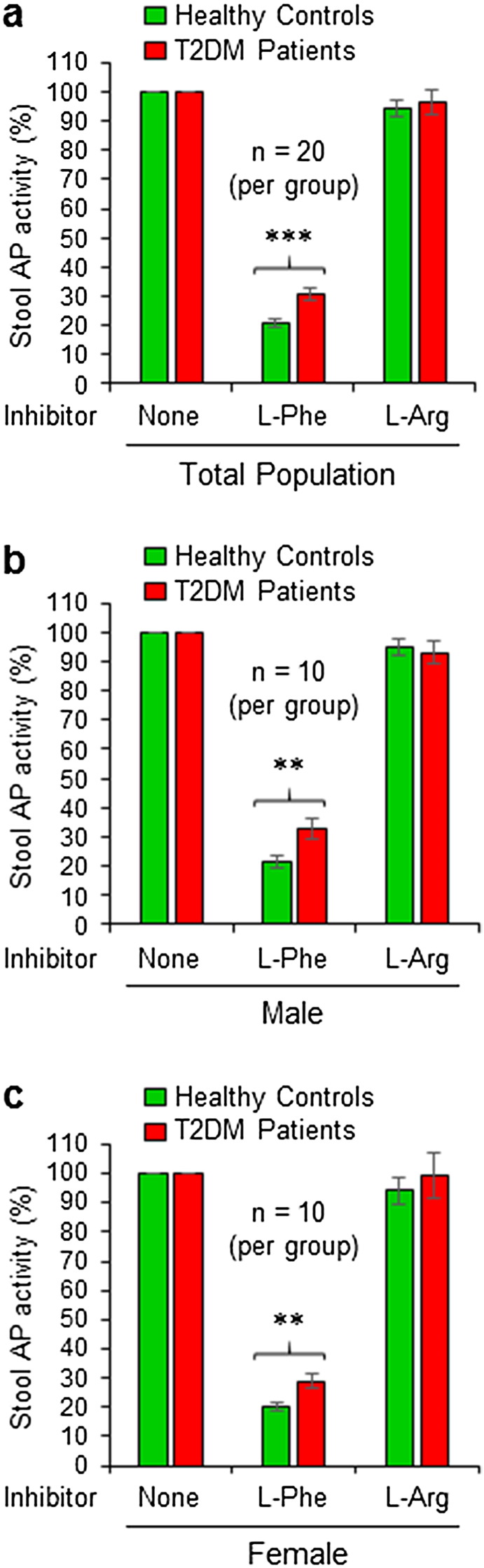
Intestinal alkaline phosphatase (IAP) knockout (Akp3−/−) mice develop T2DM (Kaliannan et al., 2013), and based on this observation we hypothesized that IAP might play a protective role against T2DM in humans. Accordingly, we decided to measure IAP concentrations in the stools of diabetic and control non-diabetic healthy people. However, taking in consideration of possible association of different isoforms of alkaline phosphatases (APs) with T2DM in humans, we first decided to determine the nature of major AP isoform in the stools of both diabetic and control healthy populations. We performed AP assays on individual aliquots of a stool sample in presence of l-phenylalanine (l-Phe, a specific inhibitor of IAP) as well as L-homoarginine (L-Arg, a specific inhibitor of TNAP). l-Phe inhibited approx. 80% of the AP activity in the stool of total healthy population, and the inhibition was approx. 70% in total diabetic population, however, the difference was statistically significant (p = 0.00014) (Fig. 1a). On the other hand, L-Arg had no inhibitory effect on the stool AP activity of either group. Gender-matched distributions also showed similar AP inhibitory effect of l-Phe in males (Fig. 1b) and females (Fig. 1c) of both control and diabetic groups. This result indicates that most of the stool AP activity is due to IAP, and accordingly, we decided to refer stool AP as IAP in the following sections as it is applicable.
Fig. 1.
Stool alkaline phosphatase activity is mostly due to intestinal alkaline phosphatase (IAP). Stool samples of non-diabetic healthy controls and T2DM patients were homogenized in a stool dilution buffer followed by centrifuging and collection of supernatant. The supernatant was assayed for alkaline phosphatase (AP) concentration without any inhibitors (total activity) or in presence of l-phenylalanine (l-Phe), a specific inhibitor of IAP as well as in presence of L-homoarginine (L-Arg), a specific inhibitor of tissue nonspecific alkaline phosphatase (TNAP), using an automatic biochemistry analyzer. (a) Effects of AP inhibitors on the AP activity in the stools of total control and diabetic populations. (b) Effects of AP inhibitors on the AP activity in the stools of male control and diabetic populations. (c) Effects of AP inhibitors on the AP activity in the stools of female control and diabetic populations. Statistics: Values are expressed as mean +/− SEM. Statistical significance of the difference between two respective groups was tested using the unpaired two-tailed Student's t-Test. p < 0.05 is considered significant. **, p < 0.01; ***, p < 0.001.
3.2. Patients with Type 2 Diabetes Mellitus (T2DM) Have Low Levels of IAP in Their Stool
We determined the levels of IAP in the stools of 202 T2DM patients (63 males, 139 females) and 445 non-diabetic healthy controls (114 males, 331 females). We recruited participants from all age groups (see below), and there was no significant difference in age between respective control and diabetic groups. Table 1 shows a few important physical and biochemical characteristics of participants, such as age, weight, height, body mass index (BMI), systolic and diastolic blood pressures, and levels of serum creatinine, cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, alanine aminotransferase (ALT) and fasting plasma glucose (FPG).
Table 1.
Characteristics of healthy control participants and T2DM patients.
| Type of participants | Total participants |
Males |
Females |
|||
|---|---|---|---|---|---|---|
| Healthy | T2DM | Healthy | T2DM | Healthy | T2DM | |
| No. of participants | 445 | 202 | 114 | 63 | 331 | 139 |
| Age of participants (yr.) | 47.1 +/− 0.4 | 47.6 +/− 0.7 | 48.7 +/− 0.8 | 50.9 +/− 1.2 | 46.7 +/− 0.5 | 46.0 +/− 0.8 |
| Weight (kg) | 59.3 +/− 0.5 | 59.6 +/− 0.7 | 65.0 +/− 0.9 | 65.7 +/− 1.3 | 57.3 +/− 0.6 | 56.9 +/− 0.8 |
| Height (m) | 1.52 +/− 0.00 | 1.52 +/− 0.01 | 1.62 +/− 0.01 | 1.61 +/− 0.01 | 1.48 +/− 0.00 | 1.48 +/− 0.01 |
| BMI (kg/m2) | 25.7 +/− 0.2 | 26.0 +/− 0.3 | 24.7 +/− 0.3 | 25.5 +/− 0.5 | 26.1 +/− 0.2 | 26.2 +/− 0.4 |
| Systolic blood pressure (mm Hg) | 134.2 +/− 1.1 | 137.3 +/− 1.5 | 134.4 +/− 1.7 | 133.3 +/− 2.5 | 134.2 +/− 1.4 | 139.0 +/− 1.8 |
| Diastolic blood pressure (mm Hg) | 78.9 +/− 0.6 | 80.1 +/− 0.8 | 80.0 +/− 1.1 | 80.8 +/− 1.4 | 78.5 +/− 0.7 | 79.8 +/− 1.0 |
| Creatinine (mg/dl) | 0.98 +/− 0.15 | 0.9 1 +/− 0.02 | 0.89 +/− 0.03 | 0.95 +/− 0.03 | 1.01 +/− 0.20 | 0.89 +/− 0.03 |
| Cholesterol (mg/dl) | 162.7 +/− 1.3 | 172.5 +/− 2.2*** | 160.3 +/− 2.6 | 164.4 +/− 4.1 | 162.7 +/− 1.3 | 176.2 +/− 2.5*** |
| HDL (mg/dl) | 38.1 +/− 0.4 | 38.8 +/− 0.5 | 36.7 +/− 0.7 | 38.6 +/− 0.9 | 38.6 +/− 0.4 | 38.8 +/− 0.6 |
| LDL (mg/dl) | 94.6 +/− 1.0 | 102.2 +/− 2.0*** | 93.2 +/− 2.5 | 94.5 +/− 4.0 | 95.0 +/− 1.1 | 105.7 +/− 2.2*** |
| Triglycerides (mg/dl) | 152.2 +/− 2.3 | 159.4 +/− 3.8 | 153.5 +/− 5.9 | 162.3 +/− 7.6 | 151.7 +/− 2.4 | 158.1 +/− 4.3 |
| ALT (U/l) | 44.8 +/− 0.9 | 47.7 +/− 1.7 | 46.9 +/− 2.4 | 49.0 +/− 2.8 | 44.1 +/− 0.8 | 47.1 +/− 2.1 |
| FPG (mmol/l) | 4.4 +/− 0.0 | 8.2 +/− 0.2*** | 4.4 +/− 0.1 | 8.3 +/− 0.4*** | 4.4 +/− 0.0 | 8.2 +/− 0.3*** |
The participants were recruited from a suburb of Dhaka, Bangladesh. All participants were on overnight (10 h) fasting and investigated for all the physical and biochemical tests described above. Statistics: Values are expressed as mean +/− SEM. Statistical significance of the difference between two respective groups was tested using the unpaired two-tailed Student's t-Test. p < 0.05 is considered significant. ***, p < 0.001.
IAP concentrations in the stools of entire non-diabetic healthy control and diabetic populations are shown in Fig. 2a. Compared to healthy controls, T2DM patients have approximately 47.6% less IAP in their stools and the difference is highly significant (67.4 +/− 3.2 vs 35.3 +/− 2.5 U/g stool, respectively; p = 5.6E-10). Also, sex-dependent distributions show similar reduction in IAP levels in male and female diabetes patients (Fig. 2a). Compared to healthy males, diabetic males have 51.4% less IAP (57.1 +/− 4.5 vs 27.7 +/− 3.2 U/g stool, respectively; p = 0.000011). Similarly, in comparison to healthy females, diabetic females have 45.4% less IAP (71.0 +/− 4.0 vs 38.8 +/− 3.3 U/g stool, respectively; p = 0.0000016). Healthy control males have the IAP levels 19.6% less than the healthy females, however, the difference is not significant (p = 0.061). On the other hand, the diabetic males have 28.6% lower IAP levels compared to diabetic females, and the difference is statistically significant (p = 0.041). The above data suggest a protective role of high IAP against T2DM.
Fig. 2.
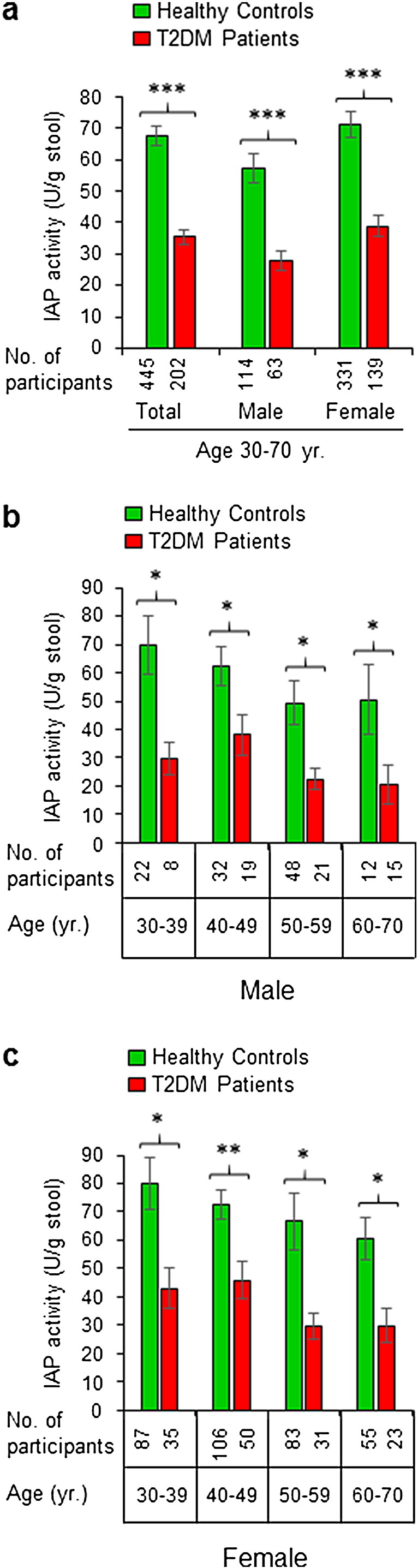
Patients with type 2 diabetes mellitus (T2DM) have low levels of intestinal alkaline phosphatase (IAP) in their stool. Stool samples of healthy participants and T2DM patients were assayed for IAP concentration using an automatic biochemistry analyzer (see Fig. 1). (a) IAP concentrations in the stools of total non-diabetic healthy control and diabetic populations. (b) Age-dependent distribution of IAP concentrations in the stools of male control and diabetic populations. (c) Age-dependent distribution of IAP concentrations in the stools of female control and diabetic populations. Statistics: Values are expressed as mean +/− SEM. Statistical significance of the difference between two groups was tested using the unpaired two-tailed Student's t-Test. p < 0.05 is considered significant. *, p < 0.05; **, p < 0.01; ***, p < 0.001. The post-hoc statistical power analyses revealed the powers for respective total, male and female groups to be 100%, 100% and 100%, respectively, validating the adequacy of power (conventionally, > 80% power at α = 0.05) for respective sample sizes. Percentage loss of IAP in T2DM patients compared to healthy controls: Total, 47.6%; male, 51.4%; female, 45.4%. The average IAP level is 19.6% less in healthy control males compared to healthy females, however, the difference is not significant (p = 0.061). T2DM males have 28.6% less IAP compared to T2DM females, and the difference is significant (p = 0.041). The difference in IAP values between younger and older groups of the same population (diabetic or non-diabetic) is not statistically significant.
We determined age group- and gender-matched distributions of IAP. We observed a decreasing trend of enzyme levels with age both in males (Fig. 2b) and females (Fig. 2c) of non-diabetic and diabetic populations. It is apparent that compared to non-diabetic group, T2DM patients have approx. 35–60% less mean stool IAP, and the difference between two respective groups is statistically significant. The least reduction (36.8%) in IAP level is observed in 40–49 yr. old diabetic females, whereas the highest reduction (59.0%) is evidenced in 60–70 yr. old male diabetes patients. Although it appears that IAP levels decrease with age, however, we observed no statistically significant difference in IAP levels between the younger and older groups of the same population.
3.3. IAP Levels Are Low in T2DM Patients at All Percentile Points
Taking in consideration of any influence of ‘outliers’ (a few extremely high or low values compared to the most other values) on the average (mean) values of IAP levels as shown above (see Fig. 2a), we further verified the difference in IAP levels of diabetic and non-diabetic groups at different percentile points. We evaluated the percentile distribution of IAP values in 202 T2DM and 445 healthy controls. We organized individual IAP values from each group (diabetic or non-diabetic) from the lowest to the highest, and then calculated the average IAP value within each 10 percentile divisions. It is apparent that at all percentile points IAP values are less in T2DM patients compared to the healthy population, and the difference is highly significant (Fig. 3). The percentile distribution of IAP levels confirms that T2DM patients, indeed, have lower levels of IAP compared to their counterparts. This distribution further confirms that IAP plays a protective role against diabetes.
Fig. 3.
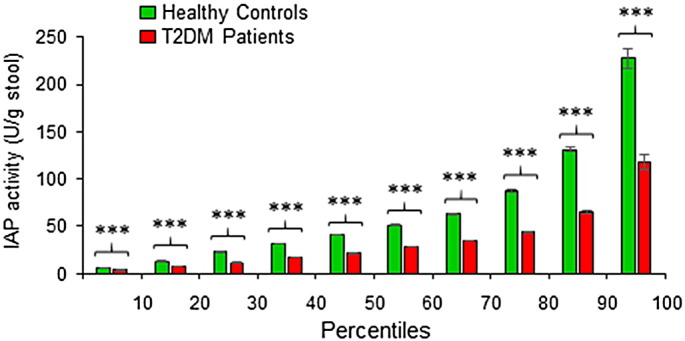
IAP levels are low in T2DM patients at all percentile points. Individual IAP values from each group (Healthy Controls or T2DM Patients) were arranged from the lowest to the highest, and then the average IAP value within each 10th percentile was calculated (n = 20 within each 10th percentile for T2DM Patients, and n = 44 within each 10th percentile for Healthy Controls). Average values for corresponding percentiles are plotted. Statistics: Values are expressed as mean +/− SEM. Statistical significance of the difference between two respective groups was tested using the unpaired two-tailed Student's t-Test. p < 0.05 is considered significant. ***, p < 0.001. Note: Only the values in first and last 10 percentile divisions will be greatly affected if an ‘outlier’ (a few extremely high or low values, compared to the most other values, affecting the mean value) is present. The values within 10th and 90th percentiles are real, not affected by outliers.
3.4. IAP Deficiency Is Associated with T2DM
Pearson correlation coefficient analysis showed no correlation between the IAP level and age, gender, FPG, BMI, ALT, serum creatinine, blood pressure and lipid parameters (Table 2). A generalized linear regression model predicted a strong association of IAP with T2DM (Table 3). It also predicted mild association of IAP with age validating the observation of decreasing trend of IAP with age (see Fig. 2b & c). We performed multiple logistic regression analyses controlling for age, FPG, BMI, ALT, serum creatinine, blood pressure and lipid parameters. The data showed an independent inverse relationship between the IAP level and diabetes status (Table 4). With each 25 U/g decrease in stool IAP, there is a 35% increased risk of diabetes. We conclude that IAP deficiency is associated with diabetes independent of other known diabetes risk factors, and a high IAP level is protective against T2DM.
Table 2.
Pearson correlation coefficients showing no correlation of IAP levels with different risk factors of T2DM.
| Type of participants |
Male |
Female |
||
|---|---|---|---|---|
| Healthy |
T2DM |
Healthy |
T2DM |
|
| Risk factors | IAP correlation | IAP correlation | IAP correlation | IAP correlation |
| Age | − 0.192 | − 0.221 | − 0.08 | − 0.131 |
| BMI | − 0.024 | 0.088 | 0.085 | 0.008 |
| Creatinine | − 0.013 | − 0.069 | 0.07 | 0.055 |
| Total cholesterol | 0.267 | − 0.132 | 0.004 | 0.127 |
| HDL-cholesterol | 0.101 | − 0.007 | 0.005 | 0.206 |
| LDL-cholesterol | 0.176 | − 0.130 | − 0.014 | 0.080 |
| Triglycerides | 0.138 | 0.014 | − 0.007 | 0.074 |
| ALT | 0.156 | 0.107 | − 0.065 | − 0.013 |
| FPG | − 0.076 | 0.039 | 0.05 | − 0.014 |
| Systolic blood pressure | 0.169 | − 0.257 | − 0.031 | − 0.153 |
| Diastolic blood pressure | − 0.040 | − 0.059 | 0.039 | − 0.060 |
A Pearson correlation coefficient close to + 1 or − 1 indicates that the two variables are highly correlated (positively or negatively, respectively). A correlation coefficient between 0 and + 0.30 or between 0 and − 0.30 was considered of having no correlation between the two variables.
Table 3.
A generalized linear regression model predicts an association of IAP with T2DM.
| Source | DF | Type III SS | F Value | Pr > F |
|---|---|---|---|---|
| Diabetes | 1 | 81665.00 | 23.30 | < 0.0001 |
| Sex | 1 | 12309.33 | 3.51 | 0.0614 |
| FPG (mmol/l) | 1 | 180.46 | 0.05 | 0.8206 |
| BMI (kg/m2) | 1 | 5335.29 | 1.52 | 0.2177 |
| Age | 1 | 23707.51 | 6.76 | 0.0095 |
The model predicts a strong association of IAP with T2DM. It also predicts a mild association of IAP with age (IAP level gradually decreases with age; see Fig. 2b & c).
Table 4.
Multiple logistic regression analyses predict an association of IAP deficiency with T2DM.
| Explanatory variables | Logistic coefficients per unit change | Odds ratio (95% CI) |
|---|---|---|
| Age (yr.) | 0.0226 | 1.023 (0.993–1.054) |
| Body mass index (BMI, kg/m2) | 0.0466 | 1.048 (0.986–1.114) |
| Creatinine (mg/dl) | − 0.0166 | 0.983 (0.847–1.142) |
| Total cholesterol (mg/dl) | 0.00183 | 1.002 (0.984–1.020) |
| HDL-cholesterol (mg/dl) | − 0.0599 | 0.942 (0.900–0.985) |
| LDL-cholesterol (mg/dl) | 0.0206 | 1.021 (1.001–1.041) |
| Triglycerides (mg/dl) | 0.00473 | 1.005 (0.998–1.011) |
| ALT (U/l) | 0.00944 | 1.009 (0.998–1.021) |
| Systolic blood pressure (mm Hg) | − 0.00012 | 1.000 (0.984–1.016) |
| Diastolic blood pressure (mm Hg) | − 0.0139 | 0.986 (0.958–1.015) |
| FPG (mmol/l) | 1.3563 | 3.882 (2.972–5.070) |
| IAP (U/g stool) | − 0.0145 | 0.986 (0.978–0.993) |
With each U/g decrease in stool IAP level there is a 1.4% increase in the odds of diabetes diagnosis. For example, if there is 25 U/g decrease in IAP level there will be a 35% increased risk of diabetes. Statistics: Proc Logist procedure (SAS) was used for multiple logistic regression analyses determining association between T2DM with independent risk factors including IAP.
3.5. Hyper- and Normo-Glycemic T2DM Patients Have Similar Levels of Stool IAP
T2DM patients on rigorous antidiabetic medications and life-style changes very often achieve normoglycemic status (FPG < 7.0 mmol/l). We were interested to know whether the glycemic status of diabetes patients has any effect on the stool IAP level. We observed that both hyperglycemic (FPG > 7.0 mmol/l) and normoglycemic diabetes patients have similar levels of IAP in their stools, and the difference in values of respective groups is not statistically significant (Total population: 37.7 +/− 3.4 vs 32.0 +/− 3.8 U/g stool, respectively, p = 0.26; Male: 29.6 +/− 4.4 vs 25.1 +/− 4.6 U/g stool, respectively, p = 0.50; Female: 41.3 +/− 4.4 vs 35.2 +/− 5.2 U/g stool, respectively, p = 0.38). These data suggest that glycemic status and/or antidiabetic medications do not affect IAP concentrations.
3.6. Obese People with High IAP Do Not Develop T2DM
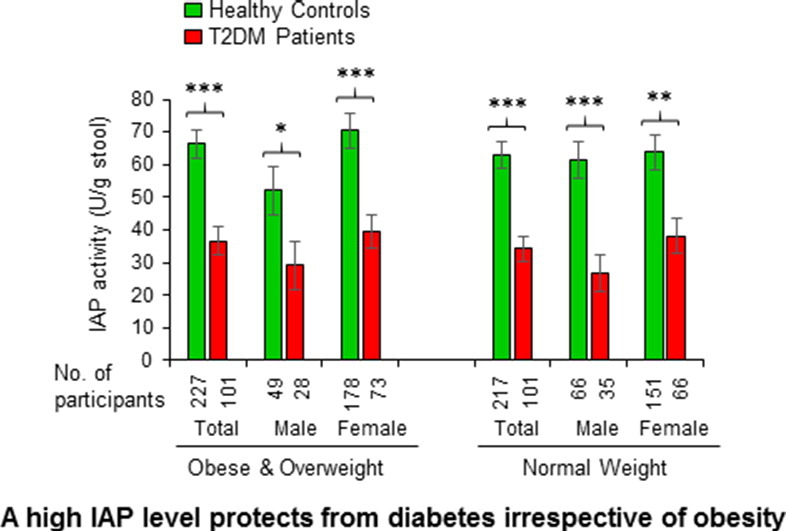
It is well recognized that overweight (BMI > 25.0 kg/m2) and obese (BMI > 30.0 kg/m2) populations are more vulnerable for developing T2DM than the population with normal weights (BMI < 25.0–18.5 kg/m2). We categorized the T2DM patients as well as healthy controls of this study in two groups, one with BMI > 25.0 kg/m2 and the other having BMI < 25.0 kg/m2. Fig. 4 is a graphical presentation of IAP levels in high- and low-BMI groups. It is evident that both groups of diabetic participants have significantly lower amounts of IAP compared to their healthy counterparts. It is also obvious that an obese or overweight person with high IAP belongs to the non-diabetic control group, but not to the diabetic group. These data suggest that IAP plays a protective role against the development of T2DM irrespective of BMI.
Fig. 4.
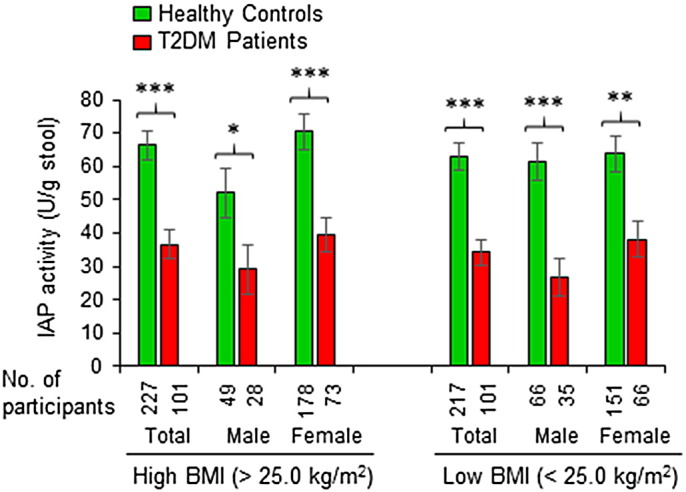
A high level of IAP is protective against T2DM irrespective of body mass index (BMI). Stool samples of healthy participants and T2DM patients were assayed for IAP concentration using an automatic biochemistry analyzer (see Fig. 1). The healthy controls as well as T2DM patients were categorized in two groups, one with high BMI (> 25.0 kg/m2) and the other having low BMI (< 25.0 kg/m2). Statistics: Values are expressed as mean +/− SEM. Statistical significance of the difference between two groups was tested using the unpaired two-tailed Student's t-Test. p < 0.05 is considered significant. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Note: There was no significant difference in IAP levels between high- and low-BMI groups of healthy controls as well as between high- and low-BMI groups of diabetes patients.
3.7. The Majority of Population Has the Incipient Metabolic Syndrome
The ‘overt’ metabolic syndrome is defined as co-existence of 3 out of 5 criteria encompassing obesity (BMI > 30.0 kg/m2), hyperglycemia (FPG > 5.5 mmol/l), hypertension (systolic blood pressure > 130 mm Hg or diastolic blood pressure > 85 mm Hg), hypertriglyceridemia (> 150 mg/dl) and low high-density lipoprotein (HDL) (< 40 mg/dl for males or < 50 mg/dl for females) (Huang, 2009). It is to point out that contributions of total cholesterol and LDL-cholesterol are not considered in defining the metabolic syndrome. We have previously shown that IAP deficiency causes the metabolic syndrome in mice (Kaliannan et al., 2013). Therefore, we predict that the association of IAP deficiency with T2DM in humans might be useful to isolate the people having ‘the incipient metabolic syndrome’ who are healthy persons vulnerable to develop the metabolic syndrome due to IAP deficiency. People with ‘the incipient metabolic syndrome’ would include the persons having ‘incipient’ diabetes, heart disease, nonalcoholic fatty liver disease, hypertension, and other metabolic disorders. We observed that the average IAP level in healthy population is approx. 67.4 +/− 3.2 U/g stool (see Fig. 2), and hence we define that, under the conditions of AP assay described here, a healthy person having IAP level less than 65.0 U/g stool should be considered as having ‘the incipient metabolic syndrome’ that would include ‘incipient diabetes’. We found that out of 445 healthy subjects 299 people have IAP level < 65.0 U/g stool, which means that approx. 67.2% of the healthy population have ‘the incipient metabolic syndrome’. We also noticed that 39.8% of control participants have IAP levels that are less than the average IAP level in diabetes patients (35.0 U/g stool), and we consider these persons have ‘the severe incipient metabolic syndrome’ and are extremely vulnerable to develop the metabolic syndrome within the near future.
4. Discussion
Type 2 diabetes mellitus (T2DM) is a major global health problem. Based on our previous observation that IAP deficiency leads to T2DM in mice (Kaliannan et al., 2013), we hypothesized that humans with high IAP levels might be protected from T2DM, and indeed, the present study validated our hypothesis. We found that diabetes patients of all age groups have less amounts of IAP in their stool compared to their healthy counterparts. The study established that IAP deficiency is associated with T2DM, and a loss of 25 U/g IAP increases the risk of developing T2DM by 35% (see Fig. 2, Fig. 3, Table 3, Table 4). We discovered a protective role of high IAP against diabetes irrespective of obesity (see Fig. 4).
In the context of confounding factors affecting the AP levels in stool, we presumed that the stool AP activity could be due to different human AP isoforms as well as bacterial AP. However, we found that most of the human stool AP activity is due to IAP (see Fig. 1). We believe that the difference between total AP and IAP might represent microbial AP because the activity of TNAP isoform is very low.
It is worthy to note that IAP excreted in stool reflects the balance among production, digestion and degradation of IAP in the intestine, and these processes could be modulated by different factors, especially diets (Lallès, 2010, Lalles, 2014). This study was performed on participants on unrestricted diets. We believe, it is unlikely that diets had any significant effect on these data because the sample sizes were relatively large (achieved 100% statistical power) and even 40% of the control healthy participants with ‘the severe incipient metabolic syndrome’ (see above) had IAP values less than the average IAP value in T2DM patients. However, we believe, it will be interesting to include dietary questionnaires in future studies for evaluating any possible diet-induced IAP deficiency.
We observed a distinctly visible decreasing trend of IAP with age (Fig. 2b & c), however, the difference between the younger and older groups of the same population (T2DM patients or healthy controls) was not statistically significant that, we believe, could probably be due to small number of sample size for each age-group. We anticipate that it will require larger sample size for each age group to establish any probable inverse relationship with IAP levels and age. It is noteworthy that the generalized linear regression model predicted an inverse relationship of age with IAP levels (see Table 3).
We found that healthy males have approximately 19.6% less stool IAP compared to healthy females, however, the difference is not statistically significant. On the other hand, T2DM males have 28.6% less IAP compared to T2DM females, and the difference is significant (p = 0.041). This observation indicates that, in the context of developing T2DM, males can accept more loss of IAP compared to females. We speculate that a relatively sedentary life or other risk factors may make females more vulnerable to T2DM even when they have higher levels of IAP.
Obesity or high BMI has long been considered a contributing factor to T2DM, however, this study makes a striking revelation that if a person's IAP level is high then the person is resistant to develop T2DM even if the person is obese (see Fig. 4). In the context of childhood obesity and associated metabolic syndrome (Steinberger, 2003), it will be interesting to investigate the levels of IAP in these children. We anticipate that IAP deficiency might be an early event of life.
Antidiabetic therapy aims to achieve normoglycemic status, and we therefore, evaluated the effects of glycemic status on stool IAP levels (see Results). We observed that IAP levels are not significantly different in hyperglycemic and normoglycemic diabetes patients, which indicates that glucose levels and/or antidiabetic drugs do not play any significant role in modulating IAP levels.
We observed that the majority (approx. 65%) of the healthy population has ‘the incipient metabolic syndrome’ (see Results). People with ‘the incipient metabolic syndrome’ would also include people with ‘the pre-metabolic syndrome’ exhibiting at least one criterion of the metabolic syndrome (Kaliannan et al., 2013, Huang, 2009). We believe, the observed 65% prevalence rate of ‘the incipient metabolic syndrome’ in this study is in concordance with the 71% prevalence rate of the pre-metabolic syndrome in Saudi soldiers (Al-Qahtani and Imtiaz, 2005). Also, it was reported that 68–81% of Pakistani people have low levels of HDL-cholesterol, one criterion of the metabolic syndrome (Basit and Shera, 2008). We calculated that approx. 40% of healthy subjects have amounts of IAP that are less than the average IAP value of the diabetic group (35.0 U/g stool). We predict that these people have ‘the severe incipient metabolic syndrome’ and are extremely vulnerable to develop T2DM or other metabolic disorders within a few years (see Results). Interestingly, the prevalence rate of ‘overt’ metabolic syndrome is also 40% (Kaliannan et al., 2013). We also observed that a few T2DM patients (approx. 15%) have stool IAP level > 67.0 U/g (the average value of healthy controls), and this observation prompted us to speculate that either IAP is not associated with the pathogenesis of T2DM in these specific patients or a persistent loss of IAP from a previously high level might also lead to T2DM. A loss of approx. 50% IAP activity might be significant to precipitate T2DM as diabetes patients on average have approx. 50% less IAP compared to their healthy counterparts (see Fig. 2).
T2DM is diagnosed by hyperglycemia (FPG > 7.0 mmol/l), which is associated with the established T2DM or with the terminal stage of development of T2DM. Therefore, it is obvious that measuring FPG cannot be used to predict years in advance whether the person have incipient diabetes. Currently, there is no protocol for predicting incipient diabetes. We anticipate that diagnosis and treatment of incipient diabetes will be the critical steps to control the devastating global pandemic of T2DM. This study establishes a protocol for diagnosing incipient diabetes, the metabolic syndrome and other metabolic disorders based on regular monitoring of stool IAP, defined here as ‘temporal IAP profiling’.
This study points towards a possibility of using oral IAP supplementation as a therapeutic approach for preventing/treating incipient diabetes and/or other overt or incipient metabolic diseases. We believe that any therapeutic aim should be at least to preserve/restore the normal level of IAP in stool (approx. 65.0 U/g stool). Another therapeutic approach might involve up-regulation of IAP by small molecules, such as short-chain fatty acids (as sodium butyrate and propionate), thyroid hormone, curcumin, omega-3 fatty acid, etc. (Meng et al., 1999, Malo et al., 2004, Ghosh et al., 2014, Kaliannan et al., 2015). Mahmood et al. (2003) have shown that corn oil feeding increases IAP secretion in rats, which we believe, might be a physiological response to prevent the high-fat diet-associated endotoxemia (Kaliannan et al., 2013). We anticipate that it will be critically important to decipher the mechanisms of IAP deficiency for understanding the pathophysiology of T2DM. Dysbiosis has been implicated in the pathogenesis of the metabolic syndrome and diabetes (Kaliannan et al., 2013, D'Aversa et al., 2013, Tilg and Moschen, 2014, Parekh et al., 2015), and based on this observation we postulate that dysbiosis might be responsible for IAP deficiency that ultimately leads to the metabolic syndrome and diabetes. We believe, future projects should focus on finding out whether any genetic, environmental and/or nutritional factor is involved in IAP deficiency.
We would like to point out that a previous study by Kim et al. (2013) reported 6.5% higher serum alkaline phosphatase activity in patients with the metabolic syndrome compared to healthy counterparts. We believe, the difference is within experimental error (conventionally, +/− 10% variation from the mean value), and hence total serum alkaline phosphatase levels cannot be used to predict an individual vulnerability to the metabolic syndrome. Also, total serum alkaline phosphatase activity serves as a non-specific marker of inflammation (Lalles, 2014), and the intestinal contribution to total serum AP activity is less than 5% (Matsushita et al., 2002).
Intestinal alkaline phosphatase (IAP) is a membrane-bound glycoprotein that is exclusively expressed in villus-associated enterocytes of proximal small intestine and hence recognized as an enterocyte differentiation marker (Lalles, 2014, Malo et al., 2006). Its regulation and function have been extensively reviewed (Lallès, 2010, Lalles, 2014, Estaki et al., 2014, Sharma et al., 2014, Buchet et al., 2013, Vaishnava and Hooper, 2007). From the enterocytes the enzyme is bidirectionally secreted into the intestinal lumen as well as the systemic circulation (Eliakim et al., 1991). IAP in the intestinal lumen travels downwards from the proximal small intestine to the distal large intestine and then excreted with stool (Malo et al., 2010).
Physiologically, IAP exerts two very important functions with respect to its existence in the luminal bacterial environment; firstly, it maintains the normal homeostasis of intestinal microbiota, and secondly, it detoxifies bacterial toxins. We have shown that IAP knockout mice harbor fewer bacteria compared to its wild-type littermates (Malo et al., 2010), and IAP promotes the gut bacterial growth by reducing the concentrations of intestinal luminal nucleotide triphosphates that have toxic effect on bacterial growth (Malo et al., 2014). We and others have shown that IAP detoxifies various bacterial toxins, such as lipopolysaccharides (LPS), CpG DNA, flagellin and uridine diphosphate (UDP), and IAP probably destroys these targets by dephosphorylation (phosphohydrolysis) (Bentala et al., 2002, Chen et al., 2010, Moss et al., 2013). Further, IAP limits fat absorption (Narisawa et al., 2003) and maintains the gut mucosal integrity (Hamarneh et al., 2015).
Pharmacologically, oral supplementation of IAP prevents antibiotic-induced susceptibility to enteric pathogens such as Salmonella Typhimurium, and Clostridium difficile (Malo et al., 2010, Alam et al., 2014). We have shown that oral IAP supplementation not only prevents but also cures the high fat diet-induced metabolic syndrome in mice (Kaliannan et al., 2013). IAP supplementation have been shown to have efficacious values in treating colitis in humans and mice (Lukas et al., 2010, Ramasamy et al., 2011, Whitehouse et al., 2010), and peritonitis in animal models (van Veen et al., 2005, Ebrahimi et al., 2011).
We have previously shown that IAP deficiency causes T2DM in mice (Kaliannan et al., 2013), and here we demonstrate that IAP deficiency is associated with T2DM in humans. We strongly believe that IAP deficiency also causes T2DM in humans, and to confirm this hypothesis, we anticipate, it will require a long-term cohort study monitoring ‘temporal IAP profiles’ and incidence rates of T2DM among the people with ‘incipient diabetes’ and other healthy populations.
5. Conclusions
In conclusion, this study links IAP deficiency in stool with T2DM, and shows that a high level of IAP is protective against diabetes irrespective of obesity. The study also provides a tool for diagnosing ‘the incipient metabolic syndrome’ including ‘incipient diabetes’ based on ‘temporal profiling of IAP’.
Author Contribution
The theory, study concept and research design were developed by M.S.M. Data analysis and manuscript preparation were also performed by M.S.M.
Conflict of Interests
M.S.M. has a patent on ‘Diagnosis and Treatment of Incipient Diabetes’ pending, and he also has equity interests on Atoxin Biotech, LLC and Harvard Biotech BD Ltd.
Acknowledgments
The study was supported by a grant (HB.1401) from Harvard Biotech BD Ltd., Dhaka, Bangladesh (to M.S.M). We are thankful to Jagannath Malo and Gopal Chandra for their technical assistance including data collection and biochemical analyses. We are grateful to Syed S. Islam, MD, PhD (Epidemiology) for his critical review of the statistical analysis as well as the manuscript.
Footnotes
Funding: Harvard Biotech BD Ltd., Dhaka, Bangladesh.
References
- Abdullah N., Attia J., Oldmeadow C., Scott R.J., Holliday E.G. The architecture of risk for type 2 diabetes: understanding Asia in the context of global findings. Int. J. Endocrinol. 2014;2014:593982. doi: 10.1155/2014/593982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S.N., Yammine H., Moaven O. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann. Surg. 2014;259:715–722. doi: 10.1097/SLA.0b013e31828fae14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qahtani D.A., Imtiaz M.L. Prevalence of metabolic syndrome in Saudi adult soldiers. Saudi Med. J. 2005;26:1360–1366. [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl. 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Basit A., Shera A.S. Prevalence of metabolic syndrome in Pakistan. Metab. Syndr. Relat. Disord. 2008;6:171–175. doi: 10.1089/met.2008.0005. [DOI] [PubMed] [Google Scholar]
- Bentala H., Verweij W.R., Huizinga-Van der Vlag A., van Loenen-Weemaes A.M., Meijer D.K., Poelstra K. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock. 2002;18:561–566. doi: 10.1097/00024382-200212000-00013. [DOI] [PubMed] [Google Scholar]
- Buchet R., Millán J.L., Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol. Biol. 2013;1053:27–51. doi: 10.1007/978-1-62703-562-0_3. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Amar J., Iglesias M.A., Poggi M. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Chen K.T., Malo M.S., Moss A.K. Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G467–G475. doi: 10.1152/ajpgi.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aversa Gut microbiota and metabolic syndrome. Intern. Emerg. Med. 2013;8(Suppl. 1):S11–S15. doi: 10.1007/s11739-013-0916-z. [DOI] [PubMed] [Google Scholar]
- Ebrahimi F., Malo M.S., Alam S.N. Local peritoneal irrigation with intestinal alkaline phosphatase is protective against peritonitis in mice. J. Gastrointest. Surg. 2011;15:860–869. doi: 10.1007/s11605-010-1405-6. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Mahmood A., Alpers D.H. Rat intestinal alkaline phosphatase secretion into lumen and serum is coordinately regulated. Biochim. Biophys. Acta. 1991;1091:1–8. doi: 10.1016/0167-4889(91)90213-h. [DOI] [PubMed] [Google Scholar]
- Estaki M., DeCoffe D., Gibson D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 2014;20:15650–15656. doi: 10.3748/wjg.v20.i42.15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.S., Gehr T.W., Ghosh S. Curcumin and chronic kidney disease (CKD): major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules. 2014;19:20139–20156. doi: 10.3390/molecules191220139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamarneh S.R., Mohamed M.M., Economopoulos K.P. A novel approach to maintain gut mucosal integrity using an oral enzyme supplement. Ann. Surg. 2015;260:706–714. doi: 10.1097/SLA.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S.E., Cooper M.E., Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliannan K., Hamarneh S.R., Economopoulos K.P. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7003–7008. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliannan K., Wang B., Li X.Y. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci. Report. 2015;5:11276. doi: 10.1038/srep11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.K., Baek K.H., Kang M.I. Serum alkaline phosphatase, body composition, and risk of metabolic syndrome in middle-aged Korean. Endocr. J. 2013;60:321–328. doi: 10.1507/endocrj.ej12-0331. [DOI] [PubMed] [Google Scholar]
- Lallès J.P. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 2010;68:323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- Lalles J.P. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr. Rev. 2014;72:82–94. doi: 10.1111/nure.12082. [DOI] [PubMed] [Google Scholar]
- Lukas M., Drastich P., Konecny M. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm. Bowel Dis. 2010;16:1180–1186. doi: 10.1002/ibd.21161. [DOI] [PubMed] [Google Scholar]
- Mahmood A., Shao J.S., Alpers D.H. Rat enterocytes secrete SLPs containing alkaline phosphatase and cubilin in response to corn oil feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G433-G341. doi: 10.1152/ajpgi.00466.2002. [DOI] [PubMed] [Google Scholar]
- Malo M.S., Alam S.N., Mostafa G. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010;59:1476–1484. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- Malo M.S., Moaven O., Muhammad N. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G826–G838. doi: 10.1152/ajpgi.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo M.S., Mozumder M., Zhang X.B. Intestinal alkaline phosphatase gene expression is activated by ZBP-89. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G737–G746. doi: 10.1152/ajpgi.00394.2005. [DOI] [PubMed] [Google Scholar]
- Malo M.S., Zhang W., Alkhoury F. Thyroid hormone positively regulates the enterocyte differentiation marker intestinal alkaline phosphatase gene via an atypical response element. Mol. Endocrinol. 2004;18:1941–1962. doi: 10.1210/me.2003-0351. [DOI] [PubMed] [Google Scholar]
- Masters S.L., Latz E., O'Neill L.A. The inflammasome in atherosclerosis and type 2 diabetes. Sci. Transl. Med. 2011;3:81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Irino T., Kawaguchi T., Komoda T. The effect of different buffers and amounts of intestinal alkaline phosphatase isoforms on total alkaline phosphatase activity. Clin. Chim. Acta. 2002;319:49–55. doi: 10.1016/s0009-8981(02)00008-6. [DOI] [PubMed] [Google Scholar]
- Meng S., Wu J.T., Archer S.Y., Hodin R.A. Short-chain fatty acids and thyroid hormone interact in regulating enterocyte gene transcription. Surgery. 1999;126:293–298. [PubMed] [Google Scholar]
- Moss A.K., Hamarneh S.R., Mohamed M.M. Intestinal alkaline phosphatase inhibits the proinflammatory nucleotide uridine diphosphate. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G597–G604. doi: 10.1152/ajpgi.00455.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa S., Huang L., Iwasaki A., Hasegawa H., Alpers D.H., Millan J.L. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol. Cell. Biol. 2003;23:7525–7530. doi: 10.1128/MCB.23.21.7525-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh P.J., Balart L.A., Johnson D.A. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin. Transl. Gastroenterol. 2015;18:6. doi: 10.1038/ctg.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S., Nguyen D.D., Eston M.A. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm. Bowel Dis. 2011;17:532–542. doi: 10.1002/ibd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergienko E., Su Y., Chan X., Brown B. Identification and characterization of novel tissue-nonspecific alkaline phosphatase inhibitors with diverse modes of action. J. Biomol. Screen. 2009;14:824–837. doi: 10.1177/1087057109338517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U., Pal D., Prasad R. Alkaline phosphatase: an overview. Indian J. Clin. Biochem. 2014;29:269–278. doi: 10.1007/s12291-013-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger J. Diagnosis of the metabolic syndrome in children. Curr. Opin. Lipidol. 2003;14:555–559. doi: 10.1097/00041433-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Stumvoll M., Goldstein B.J., van Haeften T.W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- Tilg H., Moschen A.R. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- Vaishnava S., Hooper L.V. Alkaline phosphatase: keeping the peace at the gut epithelial surface. Cell Host Microbe. 2007;2:365–367. doi: 10.1016/j.chom.2007.11.004. [DOI] [PubMed] [Google Scholar]
- van Veen S.Q., van Vliet A.K., Wulferink M., Brands R., Boermeester M.A., van Gulik T.M. Bovine intestinal alkaline phosphatase attenuates the inflammatory response in secondary peritonitis in mice. Infect. Immun. 2005;73:4309–4314. doi: 10.1128/IAI.73.7.4309-4314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velloso L.A., Eizirik D.L., Cnop M. Type 2 diabetes mellitus–an autoimmune disease? Nat. Rev. Endocrinol. 2013;9:750–755. doi: 10.1038/nrendo.2013.131. [DOI] [PubMed] [Google Scholar]
- Whitehouse J.S., Riggle K.M., Purpi D.P. The protective role of intestinal alkaline phosphatase in necrotizing enterocolitis. J. Surg. Res. 2010;163:79–85. doi: 10.1016/j.jss.2010.04.048. [DOI] [PubMed] [Google Scholar]