Abstract
Background
It is not clear whether ginseng affects cyclosporine A (CsA)-induced desirable immunosuppressive action. In this study, we evaluated the immunological influence of combined treatment of ginseng with CsA.
Methods
Using CD4+ T cells from mouse spleens stimulated with the T cell receptor (TCR) or allogeneic antigen-presenting cells (APCs), we examined the differentiation of naïve T cells into T helper 1 (Th1), Th2, Th17, and regulatory T cells (Tregs), and their cytokine production during treatment by Korean Red Ginseng extract (KRGE) and/or CsA. The influence of KRGE on the allogeneic T cell response was evaluated by mixed lymphocyte reaction (MLR). We also evaluated whether signal transducer and activator of transcription 3 (STAT3) and STAT5 are implicated in this regulation.
Results
Under TCR stimulation, KRGE treatment did not affect the population of CD4+interferon gamma (IFNγ)+ and CD4+interleukin (IL)-4+ cells and their cytokine production compared with CsA alone. Under the Th17-polarizing condition, KRGE significantly reduced the number of CD4+IL-17+ cells and CD4+/phosphorylated STAT3 (p-STAT3)+ cells, but increased the number of CD4+CD25+forkhead box P3 (Foxp3)+ cells and CD4+/p-STAT5+ cells compared with CsA alone. In allogeneic APCs-stimulated CD4+ T cells, KRGE significantly decreased total allogeneic T cell proliferation. Consistent with the effects of TCR stimulation, KRGE reduced the number of CD4+IL-17+ cells and increased the number of CD4+CD25+Foxp3+ cells under the Th17-polarizing condition.
Conclusion
KRGE has immunological benefits through the reciprocal regulation of Th17 and Treg cells during CsA-induced immunosuppression.
Keywords: cyclosporine A, immune tolerance, Panax ginseng, regulatory T cell, T helper type 17
1. Introduction
Calcineurin inhibitors (CNIs) such as cyclosporine A (CsA) and tacrolimus are widely used immunosuppressants in solid organ transplantation. CNIs efficiently inhibit interleukin (IL)-2 transcription and therefore ultimately prevent T cell activation [1], [2]. In addition, CNIs reduce the frequency and function of CD4+ regulatory T cells (Tregs) and upregulates T helper type 17 (Th17) cell-associated pathways [3], [4], [5], [6]. It is well known that Th17 secretes proinflammatory cytokine IL-17 [7], [8] and is regulated by signal transducer and activator of transcription (STAT) 3. By contrast, the Treg population is associated with immune tolerance and is regulated by STAT5, which binds to the forkhead box P3 (Foxp3) promoter [9], [10], [11]. A recent paper suggested a competition model in which relative activation of STAT3 and STAT5 directly dictates the outcome of IL-17 production in CD4+ T cells [12].
Recently, ginseng has received attention as a potential therapy for preventing autoimmune and allogeneic immune disorders [13], [14], [15]. Interestingly, Jhun et al. [16] suggested that Korean Red Ginseng (KRG) could ameliorate arthritis in mice with collagen-induced arthritis, through suppression of Th17 differentiation by inhibiting phosphorylation of STAT3 and reciprocally increasing Treg population. Based on these observations of STAT3 inhibition by ginseng, it is expected that combined treatment of KRG would suppress the differentiation of pathogenic Th17 cells through the inhibition of STAT3 during CsA treatment.
Therefore, we designed this study to evaluate the influence of KRG on CsA-induced immunosuppression using isolated CD4+ splenocytes. First, we verified the frequency and expression of the Th17 and Treg cells as well as Th1 and Th2 during stimulation with the T cell receptor (TCR). Second, we further examined those affected by stimulation of alloantigen, which is mimicking rejection conditions. We also tested the expression of phosphorylation of STAT3 and STAT5 as the regulation mechanism of Th17 and Treg.
2. Materials and methods
2.1. Mice and drugs
C57BL/6 (H-2Kb) and BALB/c (H-2Kd) mice, 8–10-wk-old, were purchased from OrientBio (Sungnam, Korea). The mice were maintained under specific pathogen-free conditions in an animal facility with controlled humidity (55 ± 5%), light (12 h/12 h light/dark), and temperature (22 ± 1°C). The air in the clean bench was passed through a high efficiency particulate air filter system designed to eliminate bacteria and viruses. Mice were fed animal chow and tap water ad libitum. The animal protocol was approved by the Animal Care and Use Committee of the Catholic University of Korea. CsA (Sigma-Aldrich, St. Louis, MO, USA) was diluted in sterile phosphate-buffered saline. KRG extract (KRGE) was obtained from Korea Ginseng Corporation (Seoul, Korea) and was diluted in sterile phosphate-buffered saline. According to the manufacturer's data, the main components of the KRGE were Rg1 (2.01%), Rb1 (8.27%), Rg3 (1.04%), Re (2.58%), Rc (3.90%), Rb2 (3.22%), Rd (1.09%), Rf (1.61%), Rh1 (0.95%), and Rg2 (s) (1.35%).
2.2. Cytotoxicity assay
Cell viability was assessed using propidium iodide (PI) staining solution (BD Biosciences, San Jose, CA, USA). Isolated CD4+ T cells were cultured with various concentrations of CsA (3 ng/mL, 10 ng/mL, 30 ng/mL, 60 ng/mL) or KRGE (3 μg/mL, 10 μg/mL, 30 μg/mL) for 72 h. Viable and dead cells were distinguished using flow cytometric analysis on a fluorescence-activated cell sorting LSRII Fortessa (BD Biosciences).
2.3. CD4+ T cell isolation and differentiation
Spleens were removed from C57BL/6 mice and minced. Splenic red blood cells were removed with ammonium-chloride-potassium lysis buffer (0.15M NH4Cl, 1mM KHCO3, 0.1mM Na2 EDTA, pH 7.2∼7.4). The cells were resuspended in complete media containing RPMI 1640 supplemented with 5% fetal bovine serum and 1% antibiotics (all from Gibco, Grand Island, NY, USA). CD4+ T cells were isolated using a CD4+ T cell isolation kit (Miltenyi Biotec, San Diego, CA, USA), according to the manufacturer's protocol. The purity of isolated CD4+ T cells was assessed as > 95%. Negatively selected non-CD4+ cells were regarded as antigen-presenting cells (APCs) and irradiated at 3,000 rad before coculture. Isolated CD4+ T cells were stimulated with plate-bound anti-CD3 (0.5 mg/mL) and soluble anti-CD28 (1 mg/mL) (both from BD Biosciences) in the presence or absence of CsA (30 ng/mL) and KRGE (3 μg/mL or 10 μg/mL) for 72 h. For Th17 cell-polarizing condition, isolated CD4+ T cells were stimulated with plate-bound anti-CD3 mAb (0.5 mg/mL), soluble anti-CD28 mAb (1 mg/mL), anti-interferon (IFN) γ (2 mg/mL), anti-IL-4 (2 mg/mL), anti-IL-2 (2 mg/mL), IL-6 (20 ng/mL) (all from R&D Systems, Minneapolis, MN, USA), and transforming growth factor-beta (2 ng/mL, PeproTech, London, UK) for 72 h [16], [17].
2.4. Flow cytometry
Expression of cytokines and transcription factors was assessed by intracellular staining. The following antibodies were used for intracellular staining of mouse cells: anti-CD4- peridinin chlorophyll or -fluorescein isothiocyanate, anti-CD25-eFluor 450, anti-IL-17-phycoerythrin (PE), anti-Foxp3-allophycocyanin (APC), anti-IFNγ-peridinin chlorophyll-cyanine 5.5, and anti-IL-4-PE-cyanine 7 (all from eBioscience, San Diego, CA, USA). Cells were stimulated for 4 h with phorbol 12-myristate 13-acetate (Sigma) and ionomycin (Sigma) with the addition of GolgiStop (BD Bioscience). Intracellular staining was performed using an intracellular staining kit (eBioscience) according to the manufacturer's protocol. To examine the expression of phosphorylated STAT (p-STAT)3 and p-STAT5, cultured cells were stimulated with IL-6 for 30 min before harvesting and were stained with anti-p-STAT3-PE and anti-p-STAT5-Alexa Fluor 488 (both from BD Bioscience). Appropriate isotype controls were used for gate setting. Cells were analyzed on fluorescence-activated cell sorting LSRII Fortessa, and the data were analyzed with FlowJo software version 7.6 (Tree star, Ashland, OR, USA). The experiments were conducted at least three times. The experiments were performed with individual samples from separate experiments and not using different wells from the same culture plate.
2.5. Quantitation of subset of CD4+T cell by flow cytometry
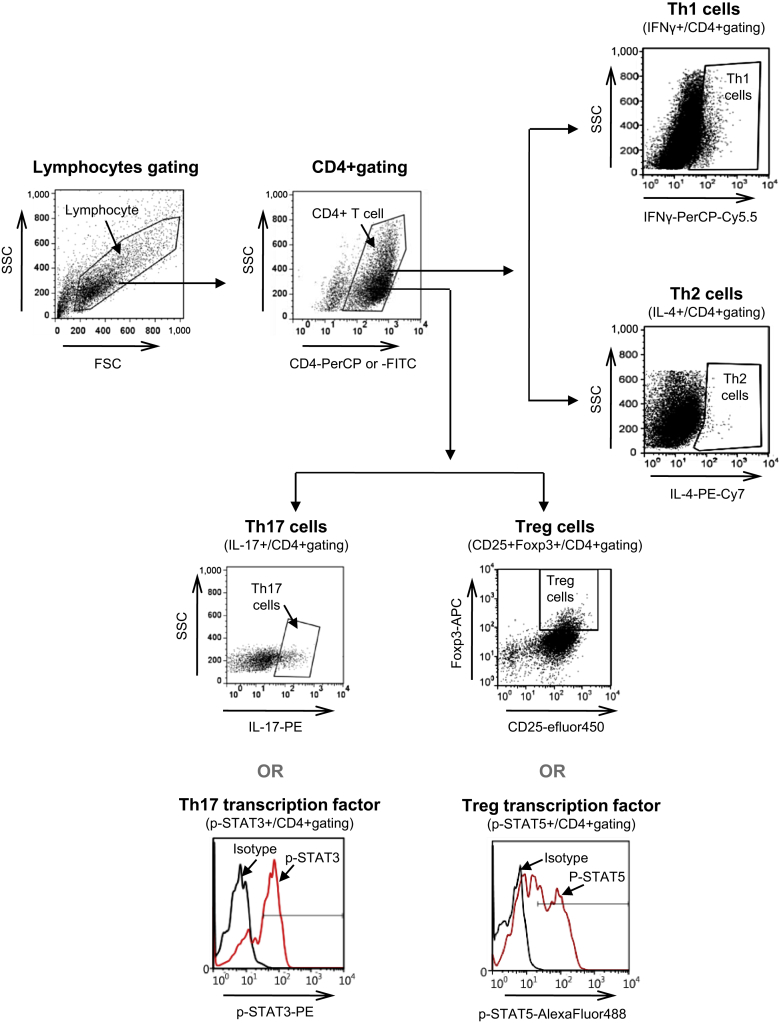
In the analysis of the subsets of CD4+ T cells, the lymphocyte population was divided by the forward-scattered light/side-scattered light. From this primary gate, CD4+ T cells are identified by their expression of CD4 and their characteristic light scatter properties. From this CD4+ T cell gating, relative percentages of interested cell populations (e.g., IFNγ+, IL-4+, IL-17+, CD25+Foxp3+, p-STAT3+, p-STAT5+) were identified by drawing around the enlarged cluster and by subtracting isotype control versus side scatter analysis. The results are expressed as the mean of triplicate samples ± the standard deviation (SD).
2.6. Enzyme-linked immunosorbent assay
Antibodies to IFNγ, IL-4, and IL-17 were obtained from R&D Systems. The concentrations of IFNγ, IL-4, and IL-17 in the culture supernatants were measured by sandwich enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instruction. A standard curve was drawn by plotting the optical density versus the log of the concentration of IFNγ, IL-4, or IL-17.
2.7. Mixed lymphocyte reactions culture in vitro
CD4+ T cells derived from C57BL/6 mice were used as the responder cells and irradiated non-CD4+ cells derived from BALB/c or C57BL/6 mice were used as APC for allogeneic or syngeneic stimulator cells, respectively. The cells were resuspended in complete media containing RPMI 1640 supplemented with 5% fetal bovine serum and 1% antibiotics. Aliquots of 2 × 105 CD4+ T cells were cultured with 2 × 105 irradiated (3,000 cGy) APCs in 96-well plates containing 200 μL of complete medium and were incubated for 72 h. Cocultured cells were pulsed with 1 μCi of (3H)-TdR (thymidine) (NEN Life Science, Boston, MA, USA) for 18 h before harvesting and were counted using an automated harvester (PHD Cell Harvester, Cambridge Technology, Cambridge, MA, USA). The results are expressed as the mean count/min of triplicate samples ± the SD.
2.8. Statistical analysis
One-way analysis of variance and the paired-samples t test were applied using SPSS software (version 19.0; IBM, Armonk, NY, USA). Data are shown as mean ± SD. A p value < 0.05 was taken to be significant.
3. Results
3.1. Determination of the doses of KRGE and CsA
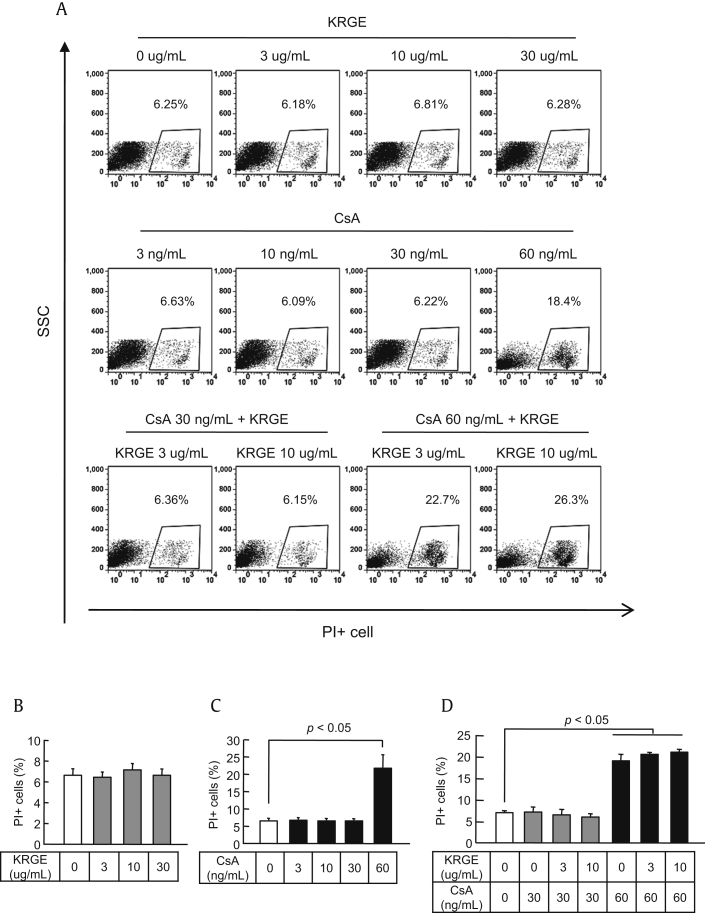
First, we used PI staining to determinate the noncytotoxic concentrations of KRGE and CsA. CD4+ T cells isolated from splenocytes were exposed to various concentrations of CsA (3 ng/mL, 10 ng/mL, 30 ng/mL, and 60 ng/mL) and KRGE (3 μg/mL, 10 μg/mL, and 30 μg/mL) when stimulated with anti-CD3 and anti-CD28 antibodies. In this assay, the viable cells are negative for PI uptake and dead cells are positive for PI uptake (PI+), as shown in Fig. 1A. The percentages of dead cells did not differ between control and KRGE-treated cells at 3–30 ng/mL of CsA (Figs. 1B–D). However, the percentage of dead cells was significantly higher at 60 ng/mL of CsA compared with the control condition (p < 0.05). Based on these results, we used the concentrations of 3 μg/mL and 10 μg/mL for KRGE and 30 μg/mL for CsA for further studies.
Fig. 1.
Determination of the dose of Korean Red Ginseng extract (KRGE) and cyclosporine A (CsA) for the in vitro test. CD4+ T cells from the spleens of normal C57BL/6 mice were cultured with antibodies to CD3 and CD28 with CsA and/or KRGE. After 72 h of culture, the cells were stained with propidium iodide (PI) to detect dead cells using flow cytometry. (A) Representative flow cytometric dot plots showing the side-scattered light (SSC) (cell granularity)/PI gate. The percentage of PI+ cells at the various concentrations of KRGE (B), CsA (C), and the KRGE + CsA combination (D). Note that there were no significant changes in the number of PI+ cells except at 60 ng/mL of CsA. The data are presented as means ± standard deviation (SD) of three independent experiments. The p value was < 0.05 vs. the other treatment conditions.
3.2. Influence of KRGE treatment on differentiation of Th1 and Th2 cells during CsA treatment
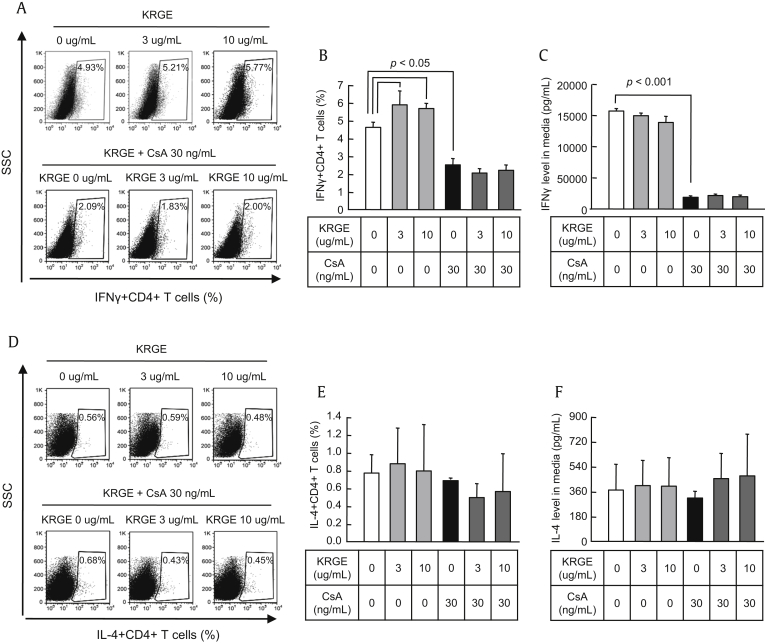
To determine the impact of CsA and KRGE on the differentiation of Th1 cells from naïve CD4+ T cells under distinct stimulating conditions, CD4+ T cells were treated with or without CsA and KRGE under stimulation with anti-CD3 and anti-CD28 antibodies to activate naïve CD4+ T cells. Th1 cells were identified as the IFNγ+ population of CD4+ cells (CD4+IFNγ+) by flow cytometry (Fig. 2), and the IFNγ concentration in the CD4+ T cell cultured supernatant was measured by ELISA. The resulting fluorescence profiles showed that CsA reduced the differentiation of Th1 cells (p < 0.05), but that KRGE did not affect the differentiation of Th1 cells (Fig. 3A, B). The IFNγ concentration in the culture supernatant was also reduced by CsA (p < 0.001), but not by KRGE (Fig. 3C). We assessed the influence of KRGE on the differentiation of Th2 cells in the CsA-treated condition. Differentiated Th2 cells were identified as IL-4+ cells in the CD4+ T cell population (CD4+IL-4+) using flow cytometry (Figs. 3D, E), and IL-4 concentration was measured by ELISA in the CD4+ T cell cultured supernatant (Fig. 3F). The resulting fluorescence profile showed that neither CsA nor KRGE affected the differentiation of Th2 cells or the production of IL-4.
Fig. 2.
Flow cytometric analysis of CD4+T cell subsets. CD4+T cells were isolated using CD4+ T cell isolation kit. Isolated CD4+T cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 with or without cyclosporine A (CsA) and Korean Red Ginseng extract (KRGE) for 72 h. Anti-interferon gamma (IFNγ), anti-interleukin (IL)-4, anti-IL-2, IL-6, and transforming growth factor-beta (TGF-β) also additionally applied into the isolated CD4+T cells for Th17 cell-polarizing condition. The lymphocyte gating was divided by the forward-scattered light (FSC) and side-scattered light (SSC). From this primary gate, CD4+T cells were identified by their expression of CD4 and their characteristic light scatter properties. Then, relative frequency (%) of interested cell populations [IFNγ+, IL-4+, IL-17+, CD25+forkhead box P3 (Foxp3)+, phosphorylated signal transducer and activator of transcription 3 (p-STAT3)+, p-STAT5+] in CD4+ T cells were identified by drawing around the enlarged cluster and by subtracting isotype control versus side scatter analysis. Efluor, efluorophore; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll.
Fig. 3.
Effect of cotreatment with Korean Red Ginseng extract (KRGE) on the population of Th1 and Th2 cells during cyclosporine A (CsA) treatment. CD4+ T cells from the spleens of normal C57BL/6 mice were cultured with antibodies to CD3 and CD28 with CsA and/or KRGE. After 72 h of culture, the cells were stained with antibodies to CD4 and either interferon gamma (IFNγ) (A and B) or interleukin (IL)-4 (D and E) and analyzed by intracellular flow cytometry. The concentrations of IFNγ (C) and IL-4 (F) in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA). Note that cotreatment with KRGE did not affect the CD4+ and either IFNγ or IL-4 cells compared with the CsA-only group. The data are presented as means ± standard deviation (SD) of three independent experiments. The p value was < 0.05 vs. the other treatment conditions. SSC, side-scattered light.
3.3. Influence of KRGE treatment on differentiation of Th17 cells and STAT3 expression under CsA treatment
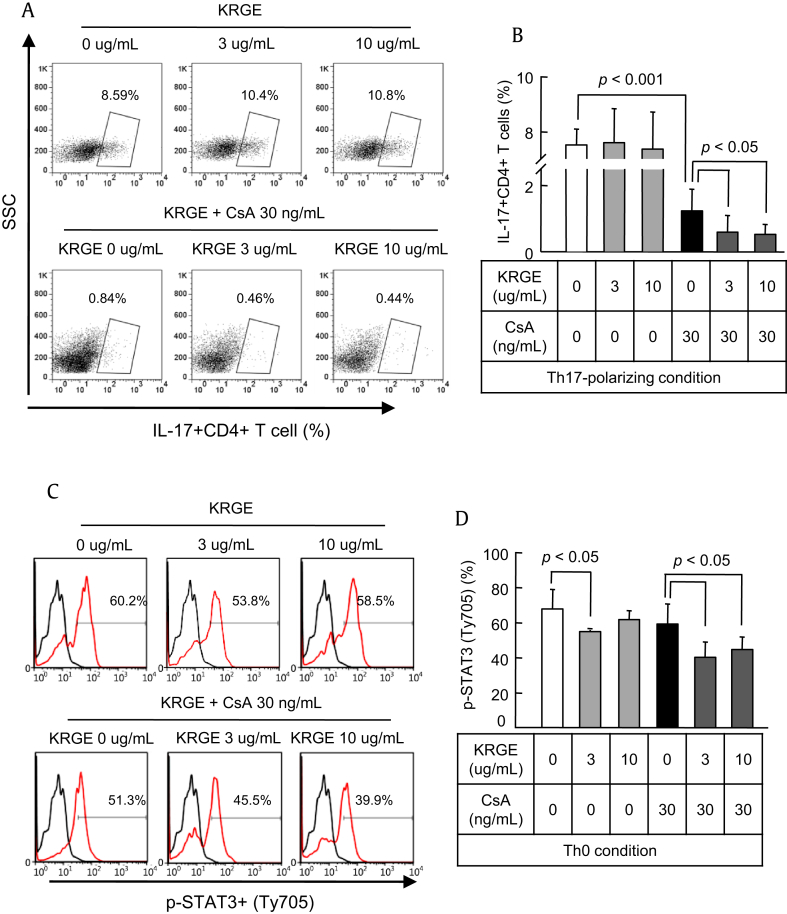
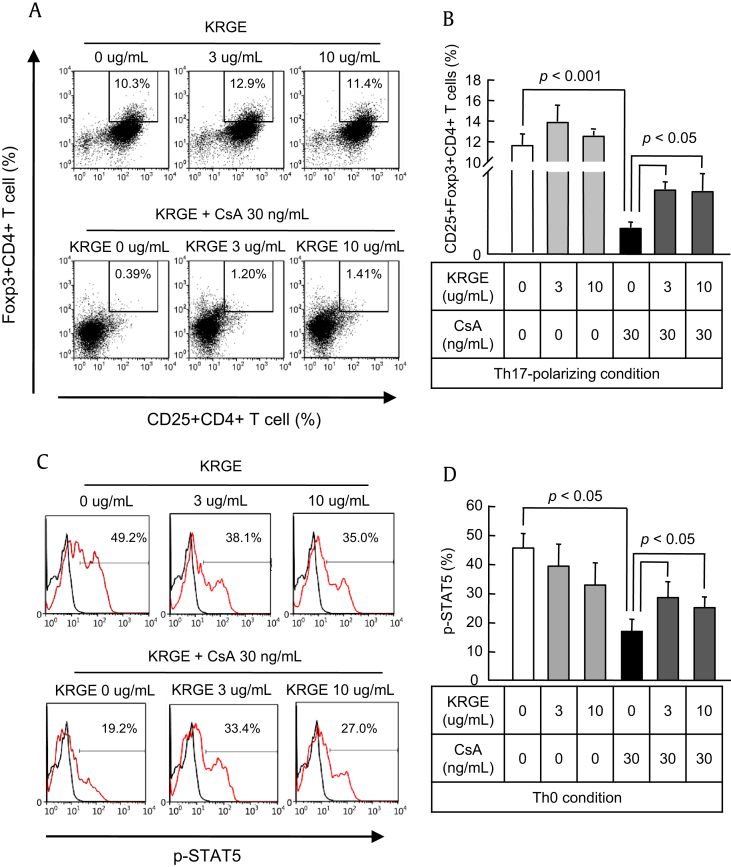
CD4+ T cells were cultured in the Th17-polarizing condition with or without KRGE and CsA for 72 h. Differentiation of Th17 cells from naive CD4+ T cells was assessed by counting the number of IL-17+ cells using flow cytometry, and IL-17 concentration was measured by ELISA in the CD4+ T cell cultured supernatant in the Th17-polarizing condition. Compared with CsA-only treatment, differentiation of Th17 cells was significantly inhibited by CsA treatment (p < 0.001) and was inhibited further by KRGE (p < 0.05; Figs. 4A, B). Expression of STAT3 induces activation and differentiation of Th17 cells. Expression of STAT3 was not influenced by CsA treatment, whereas KRGE inhibited the expression of STAT3. KRGE also significantly inhibited the expression of STAT3 when cotreated with CsA (p < 0.05; Figs. 4C, D).
Fig. 4.
Effect of cotreatment with Korean Red Ginseng extract (KRGE) on the population of Th17 cells and the expression of signal transducer and activator of transcription 3 (STAT3) during cyclosporine A (CsA) treatment. CD4+ T cells from the spleens of normal C57BL/6 mice were cultured with CsA and/or KRGE in the Th0- or Th17-polarizing condition. After 72 h of culture, the cells were stained with antibodies to CD4 and either interleukin (IL)-17 in the Th17-polarized condition (A and B) or phosphorylated STAT3 (p-STAT3) in the Th0 condition (C and D) and analyzed by intracellular flow cytometry. Note that cotreatment with KRGE significantly reduced both CD4+IL-17+ cells and p-STAT3 cells compared with the CsA group. The data are presented as means ± standard deviation (SD) of three independent experiments. The p value was < 0.05 vs. the other treatment conditions. SSC, side-scattered light.
3.4. Influence of KRGE treatment on the differentiation of Tregs and STAT5 expression under CsA treatment
Next, we assessed the effect of KRGE on Tregs under CsA treatment. Foxp3 is a unique transcription factor in CD4+CD25+ Tregs and has been demonstrated to be critical to the development of Tregs in the mouse and humans. To determine the effect of KRGE on Foxp3 protein in CD4+CD25+ T cells, we performed intracellular staining for Foxp3 protein using anti-Foxp3 mAb with gating on the CD4+CD25+ population in flow cytometry. As shown in Figs. 5A and B, most CD4+CD25+ T cells expressed Foxp3 with or without treatment with KRGE and CsA. KRGE-only treatment had no effect on the differentiation of Tregs, whereas CsA significantly inhibited Treg differentiation (p < 0.001). The inhibition of Treg differentiation by CsA was increased by the addition of KRGE (p < 0.05; Figs. 5A, B). STAT3 activation induces the activation and differentiation of Th17 cells. STAT3 regulates the generation of Th17 cells, whereas activation of STAT5 is required for the expression of Foxp3 and the differentiation of Tregs. Addition of KRGE augmented the CsA-induced decrease in the expression of STAT5 (p < 0.05; Figs. 5A, B).
Fig. 5.
Effect of cotreatment with Korean Red Ginseng extract (KRGE) on the regulatory T cell (Treg) population and signal transducer and activator of transcription 5 (STAT5) expression during cyclosporine A (CsA) treatment. CD4+ T cells from the spleens of normal C57BL/6 mice were cultured with CsA and/or KRGE in the Th0- or Th17-polarizing condition. After 72 h of culture, the cells were stained with antibodies to CD4, CD25, and forkhead box P3 (Foxp3) in the Th17-polarizing condition (A and B) and phosphorylated STAT5 (p-STAT5) in the Th0 condition (C and D) and analyzed by intracellular flow cytometry. Note that cotreatment with KRGE significantly recovered both CD4+CD25+Foxp3+ cells and p-STAT5 cells compared with the CsA-only condition. The data are presented as means ± standard deviation (SD) of three independent experiments. The p value was < 0.05 vs. the other treatment conditions.
3.5. Influence of KRGE on the alloreactive T cell response under CsA treatment
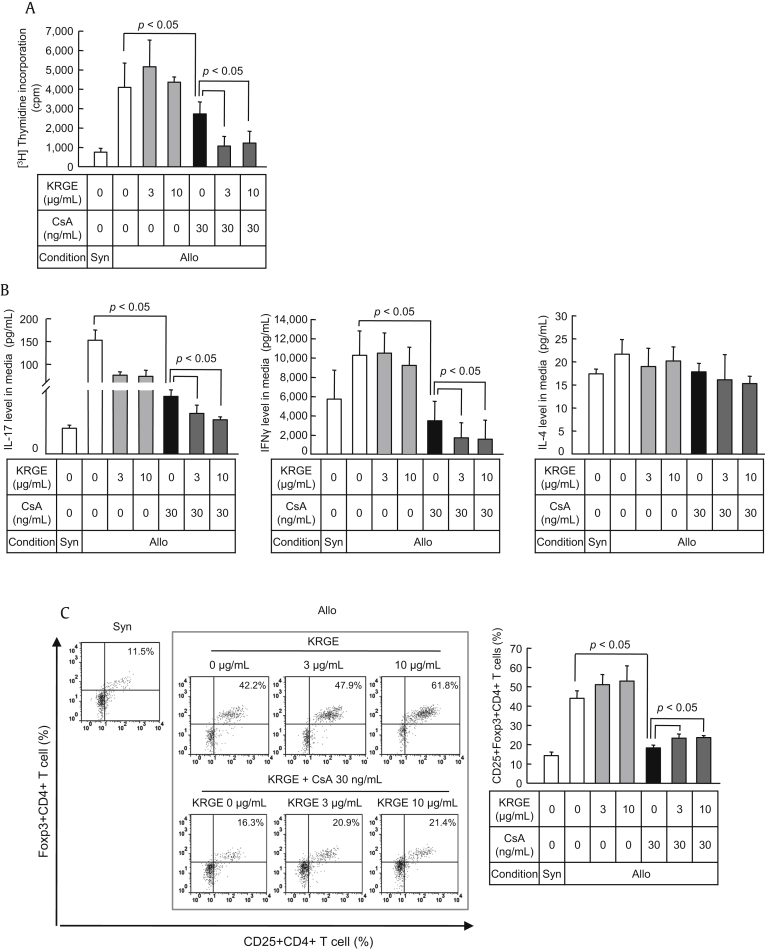
To assess the effects of KRGE on the proliferative capacity of donor CD4+ T cells in response to alloantigens following transplantation, the alloreactivity of T cells was measured by (3H)-thymidine incorporation to assess T cell proliferation in the mixed lymphocyte reaction (MLR). A proliferative response to BALB/c cells (allogeneic stimulator) was observed in C57BL/6 CD4+ T cells (responder cells). KRGE had no significant effect on this alloreactive T cell response, whereas CsA inhibited the alloreactivity-induced proliferation of T cells by inhibiting T cell activation (p < 0.001). The alloreactivity-induced proliferation of T cells was inhibited further by KRGE compared with CsA-only treatment (p < 0.05; Fig. 6A). Cytokine concentrations were measured in the supernatant from the coculture of allogeneic stimulator and responder cells. Both IL-17 production by Th17 cells and IFNγ production by Th1 cells were significantly reduced by KRGE in cells cotreated with RGE and CsA compared with CsA-only treatment (p < 0.05). By contrast, IL-4 production by Th2 cells was not influenced by KRGE and CsA (Fig. 6B).To investigate the effects of KRGE on immune tolerance during the allogeneic T cell response, we assessed the differentiation of Tregs in response to alloantigens following transplantation. The resulting fluorescence profile showed that the increase in the number of Foxp3+CD25+ Tregs in the allogeneic CD4+ T cell population was reduced by CsA treatment (p < 0.05). By contrast, KRGE recovered the differentiation of Tregs under stimulation with CsA (p < 0.05; Fig. 6C).
Fig. 6.
Effect of cotreatment with Korean Red Ginseng extract (KRGE) on the alloreactive T cell response during cyclosporine A (CsA) treatment. CD4+ T cells from the spleens of normal C57BL/6 mice were cultured with irradiated non-CD4+ T cells from BALB/c mice in a mixed lymphocyte reaction (MLR) during treatment with CsA and/or KRGE for 72 h. (A) Alloreactive T cell proliferation detected by (3H)-thymidine incorporation. The concentrations of interleukin (IL)-17, interferon gamma (IFNγ), and IL-4 in the culture supernatants (B) and intracellular flow cytometry analysis of cells stained with CD4, CD25, and forkhead box P3 (Foxp3) (C). Note that the cotreatment with KRGE significantly increased regulatory T cells (Tregs) and decreased IL-17 concentration and alloreactive T cell proliferation compared with the CsA-only condition. The data are presented as means ± standard deviation (SD) of three independent experiments. The p value was < 0.05 vs. the other treatment conditions. Allo, allogeneic stimulator; Syn, syngeneic stimulator.
4. Discussion
The present study was undertaken to investigate the effect of ginseng on the subpopulation of CD4+ T cells and the ability of these cells to produce cytokines during CsA treatment after stimulation with the TCR or alloantigen. We found that the combined treatment with KRGE and CsA did not affect the differentiation of naïve T cells into Th1 or Th2 cells or their ability to produce their related cytokines. Interestingly, the Th17 cell population and its transcription factor STAT3 were suppressed significantly by KRGE, and the CsA-induced suppression of Treg differentiation was markedly recovered by KRGE treatment. Overall, our results provide evidence that supplementation with ginseng has beneficial effects against CsA-based immunosuppression.
First, we investigated the effect of KRGE on the differentiation of Th1/Th2 cells under CsA treatment, because published data have indicated that CD4+ Th1 cells are involved in allograft rejection [18]. Our results showed that CsA inhibited the differentiation of CD4+IFNγ+ and production of IFNγ but did not alter CD4+IL-4+ cells and production of IL-4. CsA is known to downregulate IL-2 synthesis, thereby inhibiting Th1, CD8, and natural killer cells functions and IFNγ synthesis. In a study of clinical transplantation, CD4+ T cell clones isolated from human kidney allografts during acute rejection produced a high concentration of IFNγ, but not of IL-4 or IL-5, after stimulation with phytohemagglutinin, indicating that alloreactive Th1 cells, but not Th2 cells, are involved in the acute allograft rejection [19]. Similarly, it is known that ginseng extract or its components (e.g., Re or Rg1) suppress IFNγ secretion in response to immune activation, which increases cell viability [20], [21]. In this study, when used together in the nontoxic dose range, KRGE did not add to the CsA-induced suppression of CD4+IFNγ+ cells or to the reduced IFNγ concentration in the cell medium. KRGE treatment had no effect on the differentiation of CD4+IL-4+ Th2 cells or the secretion of the cytokine IL-4. These findings suggest that KRGE treatment does not influence CsA-based suppression of Th1 cells.
Next, we investigated the influence of KRGE on the differentiation of Th17 cells during CsA treatment based on accumulating evidence that Th17 cells are implicated in allograft rejection. The Th17 cell is a third subset of effector T cells and is characterized by the secretion of the proinflammatory cytokine IL-17 [7], [8]. Th17 cells act through the secretion of IL-17, which recruits monocytes and neutrophils, and acts in synergy with other local inflammatory cytokines [22]. In this study, we found that CsA treatment significantly inhibited the population of CD4+IL-17+ cells and the IL-17 concentration in the supernatant and that cotreatment with KRGE further reduced both parameters. Interestingly, p-STAT3, a main transcription factor in Th17 cells [23], [24], was suppressed significantly by KRGE treatment. This finding confirms that KRGE can inhibit the differentiation of Th17 cells by inhibiting the transcription of Th17.
There is emerging evidence that allograft function correlates with the number of Tregs [25]. However, CsA inhibits the generation of Tregs by inhibiting IL-2 transcription in the thymus and periphery, and inhibition of calcineurin by CsA restricts the access of the nuclear factor for Foxp3 [26]. In this study, we tested whether KRGE treatment would be effective in inducing Tregs, whose induction is inhibited by CsA-based immunosuppression. We found that CsA inhibition of the CD4+CD25+Foxp3+ Treg population was recovered by KRGE treatment. p-STAT5, a transcription factor in Tregs, was also consistently increased by KRGE treatment. This finding confirms the effect of ginseng on the generation of Tregs in immune disorders such as autoimmune encephalomyelitis and atopic dermatitis [13], [27].
Reciprocal regulation of Th17 cells and Tregs has been proposed recently in a competition model, in which the relative activation of STAT3 and STAT5 directly dictates the outcome of IL-17 production in CD4+ T cells [12]. It was demonstrated that STAT3 and STAT5 bind to multiple common sites across the locus encoding IL-17 and that the induction of STAT5 binding by IL-2 is associated with less binding of STAT3 at these sites. Therefore, therapeutic agents that inhibit STAT3 may simultaneously upregulate STAT5. Consistent with these findings, our results showed that KRGE upregulated Foxp3 expression in CD4+ T cells even in conditions fostering Th17 cell differentiation. In addition, KRGE increased the number of Tregs that expressed Foxp3 or STAT5, both of which are critical transcription factors in Treg cells (Fig. 5, Fig. 6). These findings suggest that ginseng treatment is beneficial by reciprocally regulating Th17 and Treg cells during CsA treatment.
In this study to extend our previous findings, we confirmed the in vitro alloreactive CD4+ T cell response to KRGE when administered with CsA in an MLR. Wang et al. reported that the ginsenoside Rd by itself has immunosuppressive effects on skin allograft rejection in rats [28], but they did not compare this effect when combined with CsA. In our study, the combined treatment of KRGE and CsA suppressed alloreactive T cell proliferation (Fig. 6). This result suggests that KRGE treatment may play an important role in regulating transplant rejection, although this requires confirmation by in vivo studies.
Our study has some limitations. First, we determined the noncytotoxic concentration of CsA and KRGE for lymphocytes in an in vitro study, as shown in Fig. 1. We did not measure the absorption rate and pharmacokinetics in an in vitro study, and further experiments are needed to identify the optimum doses of CsA and KRGE in vivo. Second, in this study, we selected two doses of KRGE (3 μg/mL and 10 μg/mL) based on the results of cellular toxicity (Fig. 1) and a preliminary test of subset of CD4+T cells. Much lower or high doses of KRGE did not influence CsA effects. We expected dose-dependent effects between 3 μg/mL and 10 μg/mL of KRGE but could not get statistical significance, although there was tendency to dose-dependency. Third, we applied commercial KRGE rather than specific ginsenoside compounds. It would be valuable to study the functions of specific constituents of ginseng, because ginseng extracts contain multiple functional constituents.
In this study, combined treatment of KRGE minimally changed the population of Th17 cells or Treg cells against CsA-based immunosuppression. Therefore, we suggest several possibilities. First, because CsA itself is too strong an immunosuppressant, changes of subset of CD4+T cells are not easy to detect, unlike immune activation-related diseases (e.g., autoimmune arthritis, autoimmune encephalomyelitis, and atopic dermatitis). Otherwise, indeed, the immune-modulatory effect of KRGE may be too weak to recover during CsA treatment. However, cotreatment with KRGE did not show side effects on CsA-induced immune suppression at least. Therefore, we suggest that supplements of KRGE should be taken as subside materials in transplant patients receiving CsA.
In conclusion, combined treatment with ginseng showed immunologic safety in CsA-based immunosuppression. It also acts as an immune regulator through the reciprocal regulation of Th17 and Treg cells. These findings provide a rationale for the use of ginseng in transplant and immune-disorder patients receiving with CsA.
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Acknowledgments
This work was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1A1A3A04000946) and the Korean Health Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (HI09C1555).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Grinyó J.M., Cruzado J.M., Millán O., Caldés A., Sabaté I., Gil-Vernet S., Serón D., Brunet M., Campistol J.M., Torras J. Low-dose cyclosporine with mycophenolate mofetil induces similar calcineurin activity and cytokine inhibition as does standard-dose cyclosporine in stable renal allografts. Transplantation. 2004;78:1400–1403. doi: 10.1097/01.tp.0000141227.63639.63. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T., Momoi Y., Iwasaki T. Cyclosporine A inhibits the mRNA expressions of IL-2, IL-4 and IFN-gamma, but not TNF-alpha, in canine mononuclear cells. J Vet Med Sci. 2007;69:887–892. doi: 10.1292/jvms.69.887. [DOI] [PubMed] [Google Scholar]
- 3.Chung B.H., Kim K.W., Kim B.M., Piao S.G., Lim S.W., Choi B.S., Park C.W., Kim Y.S., Cho M.L., Yang C.W. Dysregulation of Th17 cells during the early post-transplant period in patients under calcineurin inhibitor based immunosuppression. PLoS One. 2012;7:e42011. doi: 10.1371/journal.pone.0042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deteix C., Attuil-Audenis V., Duthey A., Patey N., McGregor B., Dubois V., Caligiuri G., Graff-Dubois S., Morelon E., Thaunat O. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5344–5351. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 5.Kim K.W., Chung B.H., Kim B.M., Cho M.L., Yang C.W. The effect of mammalian target of rapamycin inhibition on T helper type 17 and regulatory T cell differentiation in vitro and in vivo in kidney transplant recipients. Immunology. 2015;144:68–78. doi: 10.1111/imm.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mas V.R., Archer K.J., Scian M., Maluf D.G. Molecular pathways involved in loss of graft function in kidney transplant recipients. Expert Rev Mol Diagn. 2010;10:269–284. doi: 10.1586/erm.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 8.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 9.Burchill M.A., Yang J., Vogtenhuber C., Blazar B.R., Farrar M.A. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 10.Passerini L., Allan S.E., Battaglia M., Di Nunzio S., Alstad A.N., Levings M.K., Roncarolo M.G., Bacchetta R. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25- effector T cells. Int Immunol. 2008;20:421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 11.Yao Z., Kanno Y., Kerenyi M., Stephens G., Durant L., Watford W.T., Laurence A., Robinson G.W., Shevach E.M., Moriggl R. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X.P., Ghoreschi K., Steward-Tharp S.M., Rodriguez-Canales J., Zhu J., Grainger J.R., Hirahara K., Sun H.W., Wei L., Vahedi G. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang I., Ahn G., Park E., Ha D., Song J.-Y., Jee Y. An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunology Letters. 2011;138:169–178. doi: 10.1016/j.imlet.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Oh G., Son S. Efficacy of Korean Red Ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391–395. doi: 10.5142/jgr.2012.36.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu D., Liu M., Yang Y., Ma L., Jiang Y., Zhou L., Huang Q., Pi R., Chen X. Ginsenoside Rd ameliorates experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neurosci Res. 2014;92:1217–1226. doi: 10.1002/jnr.23397. [DOI] [PubMed] [Google Scholar]
- 16.Jhun J., Lee J., Byun J.K., Kim E.K., Woo J.W., Lee J.H., Kwok S.K., Ju J.H., Park K.S., Kim H.Y. Red ginseng extract ameliorates autoimmune arthritis via regulation of STAT3 pathway, Th17/Treg balance, and osteoclastogenesis in mice and human. Mediators Inflamm. 2014;2014:351856. doi: 10.1155/2014/351856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son H.J., Lee J., Lee S.Y., Kim E.K., Park M.J., Kim K.W., Park S.H., Cho M.L. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014;2014:973986. doi: 10.1155/2014/973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang S., Herrera O., Lechler R.I. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Curr Opin Immunol. 2004;16:550–557. doi: 10.1016/j.coi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 19.D'Elios M.M., Josien R., Manghetti M., Amedei A., de Carli M., Cuturi M.C., Blancho G., Buzelin F., del Prete G., Soulillou J.P. Predominant Th1 cell infiltration in acute rejection episodes of human kidney grafts. Kidney Int. 1997;51:1876–1884. doi: 10.1038/ki.1997.256. [DOI] [PubMed] [Google Scholar]
- 20.Lee E.J., Ko E., Lee J., Rho S., Ko S., Shin M.K., Min B.I., Hong M.C., Kim S.Y., Bae H. Ginsenoside Rg1 enhances CD4(+) T-cell activities and modulates Th1/Th2 differentiation. Int Immunopharmacol. 2004;4:235–244. doi: 10.1016/j.intimp.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Son Y.M., Kwak C.W., Lee Y.J., Yang D.C., Park B.C., Lee W.K., Han S.H., Yun C.H. Ginsenoside Re enhances survival of human CD4+ T cells through regulation of autophagy. Int Immunopharmacol. 2010;10:626–631. doi: 10.1016/j.intimp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell P., Afzali B., Lombardi G., Lechler R.I. The T helper 17-regulatory T cell axis in transplant rejection and tolerance. Curr Opin Organ Transplant. 2009;14:326–331. doi: 10.1097/MOT.0b013e32832ce88e. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.M., Tato C., Yoshimura A., Hennighausen L., O'Shea J.J. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durant L., Watford W.T., Ramos H.L., Laurence A., Vahedi G., Wei L., Takahashi H., Sun H.W., Kanno Y., Powrie F. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis S., Braudeau C., Giral M., Dupont A., Moizant F., Robillard N., Moreau A., Soulillou J.P., Brouard S. Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation. 2006;81:398–407. doi: 10.1097/01.tp.0000203166.44968.86. [DOI] [PubMed] [Google Scholar]
- 26.Bocian K., Borysowski J., Wierzbicki P., Wyzgal J., Klosowska D., Bialoszewska A., Paczek L., Gorski A., Korczak-Kowalska G. Rapamycin, unlike cyclosporine A, enhances suppressive functions of in vitro-induced CD4+CD25+ Tregs. Nephrol Dial Transplant. 2010;25:710–717. doi: 10.1093/ndt/gfp586. [DOI] [PubMed] [Google Scholar]
- 27.Sohn E.H., Jang S.A., Lee C.H., Jang K.H., Kang S.C., Park H.J., Pyo S. Effects of Korean Red Ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice. J Ginseng Res. 2011;35:479–486. doi: 10.5142/jgr.2011.35.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L., Zhang Y., Chen J., Li S., Wang Y., Hu L., Wang L., Wu Y. Immunosuppressive effects of ginsenoside-Rd on skin allograft rejection in rats. J Surg Res. 2012;176:267–274. doi: 10.1016/j.jss.2011.06.038. [DOI] [PubMed] [Google Scholar]