Abstract
Background
Pancreatitis is a highly prevalent medical condition associated with a spectrum of endocrine and exocrine pancreatic insufficiencies. While high alcohol consumption is an established risk factor for pancreatitis, its relationship with specific types of pancreatitis and a potential threshold have not been systematically examined.
Methods
We conducted a systematic literature search for studies on the association between alcohol consumption and pancreatitis based on PRISMA guidelines. Non-linear and linear random-effect dose–response meta-analyses using restricted cubic spline meta-regressions and categorical meta-analyses in relation to abstainers were conducted.
Findings
Seven studies with 157,026 participants and 3618 cases of pancreatitis were included into analyses. The dose–response relationship between average volume of alcohol consumption and risk of pancreatitis was monotonic with no evidence of non-linearity for chronic pancreatitis (CP) for both sexes (p = 0.091) and acute pancreatitis (AP) in men (p = 0.396); it was non-linear for AP in women (p = 0.008). Compared to abstention, there was a significant decrease in risk (RR = 0.76, 95%CI: 0.60–0.97) of AP in women below the threshold of 40 g/day. No such association was found in men (RR = 1.1, 95%CI: 0.69–1.74). The RR for CP at 100 g/day was 6.29 (95%CI: 3.04–13.02).
Interpretation
The dose–response relationships between alcohol consumption and risk of pancreatitis were monotonic for CP and AP in men, and non-linear for AP in women. Alcohol consumption below 40 g/day was associated with reduced risk of AP in women. Alcohol consumption beyond this level was increasingly detrimental for any type of pancreatitis.
Funding
The work was financially supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21AA023521) to the last author.
Keywords: Alcohol, Pancreatitis, Acute pancreatitis, Chronic pancreatitis, Meta-analysis
Highlights
-
•
The dose–response relationships between alcohol use and different types of pancreatitis in men and women are different.
-
•
The relationship was linear for chronic and acute pancreatitis in men, but non-linear for acute pancreatitis in women.
-
•
There is a threshold effect for acute pancreatitis in women at the level of up to 40 g/day.
-
•
The risk of pancreatitis was higher than previously thought beyond the level of 40 g of pure alcohol/day.
The article updates existing knowledge on the relationship between average alcohol consumption and the risk of pancreatitis. Specifically, there are differences between acute and chronic pancreatitis and different sexes. For women there is a threshold of 40 g of ethanol per day — below this level alcohol use is not increasing the risk of acute pancreatitis and might even be beneficial. Above this threshold alcohol use is detrimental. Beyond this threshold the risk of pancreatitis, acute and chronic, in both sexes is greater than previously thought, and increases with increases of average consumption.
1. Introduction
Pancreatitis is a prevalent inflammatory disorder of the pancreas that is associated with high mortality and is a source of significant global socioeconomic burden. (Lankisch et al., 2015, Yadav and Lowenfels, 2013) The annual incidence of acute pancreatitis (AP) ranges from 13 to 45 per 100,000 population (Lankisch et al., 2015), mainly based on studies from high-income countries. Among patients treated in US hospitals in 2009, AP was the most frequent discharge diagnosis in gastrointestinal disease and hepatology, the second highest cause of all hospital stays, the largest contributor to aggregate costs, and the fifth leading cause of in-hospital deaths (Lankisch et al., 2015, Peery et al., 2012). Chronic pancreatitis (CP) leads to progressive replacement of pancreatic parenchyma with fibrotic tissue (Braganza et al., 2011). CP is less prevalent than AP, with annual prevalence rates ranging between 5 and 12 per 100,000 (Yadav and Lowenfels, 2013, Braganza et al., 2011). CP is associated with a spectrum of chronic endocrine and exocrine pancreatic insufficiencies, manifesting in malnutrition, diabetes mellitus, disability, medical costs, and quality of life loss (Yadav and Lowenfels, 2013, Braganza et al., 2011).
Alcohol consumption is considered one of the major causative agents for pancreatitis (Lankisch et al., 2015, Irving et al., 2009); after gallstones, alcohol is the second major leading cause of AP and the most common cause of CP (Yadav and Lowenfels, 2013, Yadav and Lowenfels, 2006). In 2009, a systematic review and meta-analysis on the association between alcohol consumption and pancreatitis (Irving et al., 2009), based on data from six studies, found a monotonically increasing dose–response relationship between average alcohol consumption and pancreatitis. Since then, a number of original studies were published (Kume et al., 2015, Gonzalez-Perez et al., 2010, Yang et al., 2014, Lai et al., 2011, Lembke et al., 2011, Lin et al., 2014) which have led to recent summaries on etiological and pathobiological aspects of different subtypes of pancreatitis (Lankisch et al., 2015, Yadav and Lowenfels, 2013), which allow for more in-depth analyses of the risk relationships. In particular, these new studies allow for differentiation of dose–response relationships by type of pancreatitis (acute vs. chronic) and by sex. This differentiation is important, as the biological mechanisms underlying are different — gallstones are the primary cause of AP, along with factors associated with biliary disease and choledocholithiasis, such as obesity and hypertriglyceridemia, whereas they play a negligible role in CP, except in cases where AP progresses into CP (Yadav and Lowenfels, 2013). On the other hand, CP is associated primarily with the impact of chronic toxic influences that makes alcohol use the most common causative agent for CP (Yadav and Lowenfels, 2013). Consequently, while AP and CP are still seen on a continuum of clinical manifestations of one inflammatory disease (Whitcomb, 2004, Mitchell et al., 2003), the progression and outcomes of CP and AP significantly differ (Lankisch et al., 2015, Yadav and Lowenfels, 2013).
The dose–response relationships between alcohol and chronic disease typically vary by sex, with women experiencing higher risks at comparatively lower levels of intake (Shield et al., 2013) due to different absorption and metabolism of alcohol (Mumenthaler et al., 1999). New studies will also allow for determining a threshold for alcohol consumption associated with the risk of pancreatitis.
Thus, the goal of the present systematic review and series of meta-analyses was to examine the association between alcohol consumption and risk of different types of pancreatitis (acute and chronic) by sex, including but not limited to analyses of potential threshold effects.
2. Methods
2.1. Literature Search Strategy
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009), we performed a search of OVID Medline, Embase, PsycINFO, PubMed, Scopus and Web of Science databases to identify epidemiological studies that contained information on the association between alcohol consumption and pancreatitis. The search was conducted using a combination of alcohol consumption related terms (“ethanol*”, “alcohol*”, “drink*”) and the term “pancreat*” as subject terms (descriptors). As our previous search ended in early 2009, the timeframe of this search was set between January 2009 and May 2015. In addition, we manually searched the content pages of the major epidemiological journals in the field, reference lists of relevant articles and recent reviews. No language restrictions were applied.
2.2. Study Eligibility Criteria
To be included into the meta-analyses, primary studies had to: 1) be of cohort or case–control study design; 2) have a control group of abstainers; 3) report relative risks (RR), odds ratios (OR), hazard ratios (HR), or contain data sufficient for their calculation; 4) have acute or chronic pancreatitis as an endpoint; and 5) include two or more categories of level of alcohol consumption in comparison to abstainers. We excluded studies if they: 1) were of cross-sectional design; 2) did not have enough information to calculate a risk estimate; 3) reported only on alcoholic pancreatitis (alcohol-induced acute or chronic pancreatitis, K85.2 or K86.0); and 4) were not published as full reports (e.g. conference abstracts) or contained partial or incomplete data.
2.3. Pancreatitis Ascertainment
Diagnoses of acute (AP) or chronic pancreatitis (CP) were defined using the International Classification of Disease (ICD) codes reported in the studies. Based on the study timeframe, we used the ICD-10 codes (K85.x for acute pancreatitis of various etiologies, K86.x for chronic pancreatitis). For earlier studies, the corresponding ICD-8 and ICD-9 codes were used (577.0 and 577.1 for AP and CP).
2.4. Data Extraction
Data extracted from each study included name of the first author, date of publication, timeframe of the study, design of the study, duration of follow-up for cohort studies, exposure measures, number of observed pancreatitis cases among participants by drinking group, number of total participants by drinking group, study endpoints, measures of association (RR, HR, OR) and corresponding 95% confidence intervals for each category of alcohol consumption, while adjusting for potential confounders. We converted alcohol intake into average grams of pure alcohol per day (g/day) using the midpoints (mean) of reported drinking group categories. The midpoint for open-ended categories was calculated by adding 75% of the preceding category's range to the lower bound of the open-ended category. We used reported conversion factors when standard drinks were the unit of measurement. Depending on the country, one standard drink is approximately 8–14 g of pure alcohol (World Health Organization, 2000). Assessments of full-text articles with uncertain eligibility and data abstraction were conducted independently by AVS and MR. Two authors discussed differences until consensus was reached.
2.5. Data Analysis
We gave priority to estimates where 1) lifetime abstainers were the risk reference group; 2) data were adjusted for potential confounders; and 3) data were sex-specific. If necessary, risk estimates within studies were re-calculated based on the method described by Hamling et al. (2008) or combined using fixed-effect models to derive one estimate for each analysis per study. Each case of pancreatitis was used only once in each of the analyses conducted.
Using studies that reported data for two or more drinking groups with current alcohol intake (midpoints of categories) in relation to abstainers, we conducted the two-stage restricted cubic spline regression (three knots) in multivariable meta-regression models, taking into account the variance–covariance matrix for multiple risk estimates derived from one reference group to calculate non-linear dose–response curves for average alcohol consumption (g/day) using the two-step approach described by Orsini and colleagues (Orsini and Greenland, 2006, Orsini et al., 2012). Evidence for non-linearity was determined by the significance level of the second meta-regression coefficient. When no evidence for non-linearity was found, we conducted linear two-stage meta-regressions (Hamling et al., 2008, Orsini and Greenland, 2006).
To determine potential threshold effects, we conducted categorical meta-analyses on the relationship between alcohol consumption (cut-points defined by 20 g/day intervals). Risk estimates were pooled with inverse-variance weighting using DerSimonian–Laird random-effect models to allow for between-study heterogeneity (DerSimonian and Laird, 1986). Variation in the effect size because of heterogeneity between studies was quantified using the Q- and I-statistics (Yadav and Lowenfels, 2013; Higgins and Thompson, 2002). Publication bias were examined using Egger's regression-based test (Egger et al., 1997). All meta-analytical procedures were conducted on the log scale in Stata statistical software, version 13.1; p < 0.05 (two-sided) was considered statistically significant.
Most quality scores are tailored for meta-analyses of randomized trials of interventions (Moher et al., 1998, Chalmers et al., 1981, Detsky et al., 1992, Greenland and O'Rourke, 2001) and many criteria do not apply to epidemiological studies examined in this study. Additionally, quality score used in meta-analyses remains controversial (Greenland and O'Rourke, 2001, Herbison et al., 2006). As a result, quality assessment was incorporated by including quality components, such as measurement of alcohol consumption and ascertainment of pancreatitis, in the inclusion and exclusion criteria, and further by investigating potential heterogeneity in several subgroup analyses outlined above.
2.6. Ethics
Data were derived from published articles with anonymized data. No ethics board review and approval were required.
3. Results
3.1. Search Results
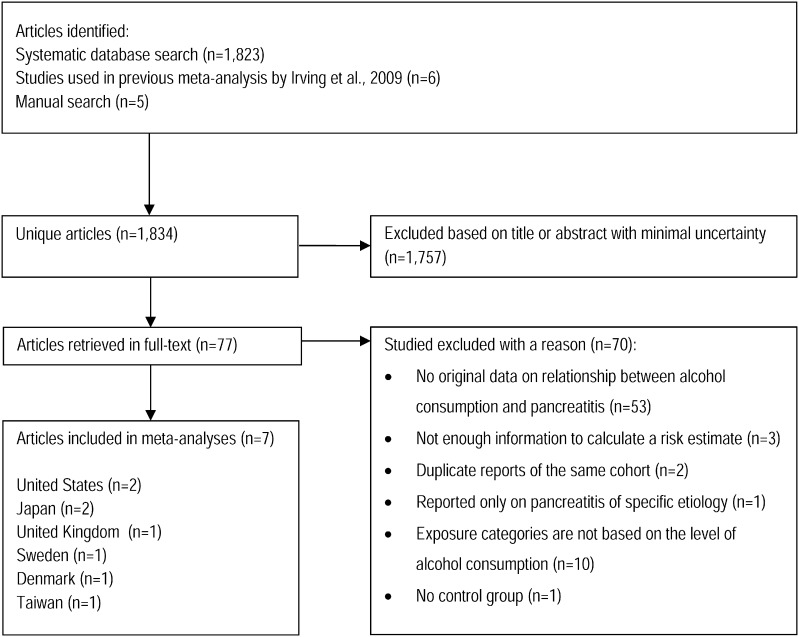
The search identified 1823 unique articles (excluding duplicates), six additional articles were taken from the previous meta-analysis, and five more were identified manually. Of these, 1757 were excluded based on title or abstract. 77 articles were retrieved in full text for further review. Out of these, 55 did not meet our inclusion criteria. In total, seven studies met the inclusion criteria: two each in the United States and Japan; and one each from the UK, Sweden, and Denmark. Detailed descriptions of the studies included are presented in Table 1. These studies included 157,026 participants with 3618 cases of pancreatitis — 2490 cases of first episode or recurrent AP and 1128 cases of CP. Five studies had datasets specific to AP, four studies had datasets specific for CP. Five studies had a case–control design and two were cohort studies (Fig. 1).
Table 1.
Characteristics of the 7 articles included in the meta-analysis.
| Reference | Design, sex, place, timeframe | Setting, follow-up, participants | Eligibility criteria | Alcohol consumption categories | Endpoint | Adjustment |
|---|---|---|---|---|---|---|
| Blomgren et al. (2002) | Case–control, M/F, Sweden, 1995–1998 | Population-based. 462 cases; 1781 controls | Age 20–95, both sexes; excluded those who didn't have access to telephone or were not able to speak Swedish, had history of malignancies, previous AP/CP, or those hospitalized for ≥ 30 days | Non-drinkers; > 0–<20 g/week; 20–<120 g/week; 120–220 g/week; 220–<320 g/week; 320–<420 g/week; 420 g/week and more. | AP | Unadjusted data used for analyses |
| Lin et al. (2001) | Case–control, M, Japan, 1997–1998 | Hospital-based. 91 cases, 175 controls | Excluded those who failed to return the study questionnaire. | Ex-Drinkers; Non-drinkers; < 50 g/day; 50–99 g/day; 100 + g/day. | CP | BMI, education level, cigarette smoking. |
| Morton et al. (2004) | Cohort, M/F, US, 1978–1998 | Population-based. 439 cases; 128,495 controls | Prepaid health plan members able to complete research questionnaire. Data must be sufficient to diagnose pancreatitis. | Ex-drinkers; never drinkers; < 1 drink/month; 1 drink/month–<1 drink/day; 1–2 drinks per day; 3 + drinks per day. | Any pancreatitis | Age, sex, race, education, marital status, BMI, smoking. |
| Kristiansen et al. (2008) | Cohort, M/F, Denmark, 1976–2007 | Population-based. 268 cases; 17,670 controls | Participants of the Copenhagen City Heart Study. Excluded people with previously diagnosed pancreatitis. | Non-drinkers; 1–6 drinks/week; 7–13 drinks/week; 14–20 drinks/week; 21–34 drinks/week; 35–48 drinks/week; 48 + drinks/week | Both AP and CP | Age, sex, smoking, education, BMI |
| Yadav et al. (2009) | Case–control, M/F, US, 2000–2006 | Hospital-based, 985 cases (CP, n = 536 and recurrent AP, n = 449), 663 controls. | North American Pancreatitis Study 2 (NAPS 2). Exclusion criteria not reported. | < 20 drinks in a lifetime; ≤ 0.5 drinks per day or ≤ 3 drinks per week; 0.5–1 drinks per day, or 4–7 drinks per week for women, 0.5–2 drinks per day or 4–14 drinks per week for men; 1–5 drinks per day or 8–34 drinks per week for women and 2–5 drinks per day or 15–34 drinks per week for men; 5 or more drinks per day or 35 or more drinks per week. | Recurrent AP and CP | Unadjusted data used for analyses |
| Gonzalez-Perez et al. (2010) | Case–control, M/F, UK, 1996–2006 | Population-based. 419 cases and 5000 controls | 20–79 years of age, both sexes, who were enrolled at least 2 years with their GP, first prescription > 1 year before entering the study and at least 1 health contact in the last 2 years. Excluded if history cancer other than non-melanoma skin cancer or pancreatic disease; people who were 70 + years of age with a FU over 1 year and less than 2 health contacts. | Non-drinkers; 1–7 drinks/week; 8–29 drinks per week; 30 + drinks per week | AP | Age, sex, calendar year, BMI, Townsend deprivation index, smoking status, alcohol intake, general comorbidities, and several medication groups (antidiabetic drugs, antibiotics, antidepressants, corticosteroids, acid-suppressing drugs, NSAIDs, antihypertensives, lipid-lowering drugs and HRT). |
| Kume et al. (2015) | Case–control, M/F, Japan, 2005–2010 | Hospital-based. 982 cases, (574 cases of AP and 574 cases of CP), 1015 controls. | Non-drinkers; < 20 g/day; 20–40 g/day; 40–60 g/day; 60–80 g/day; 80–100 g/day; 100 + g/day |
Both AP and CP | Age, sex, hospital, time to first hospital visit. |
Fig. 1.
PRISMA flowchart.
3.2. Dose–Response Relationship
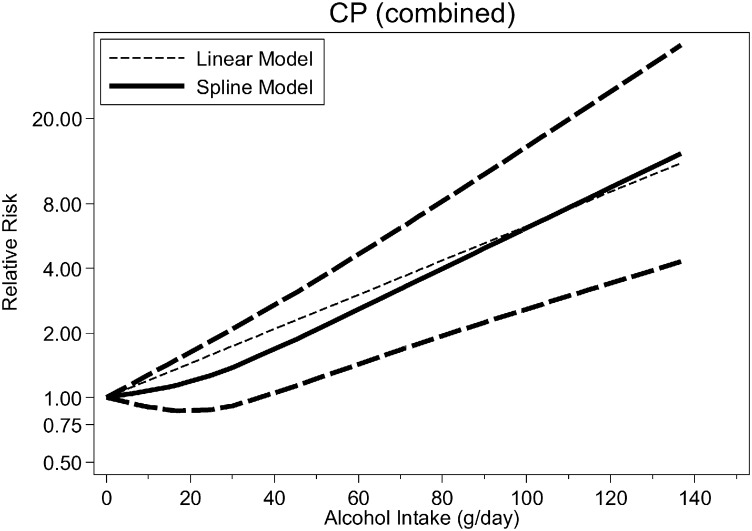
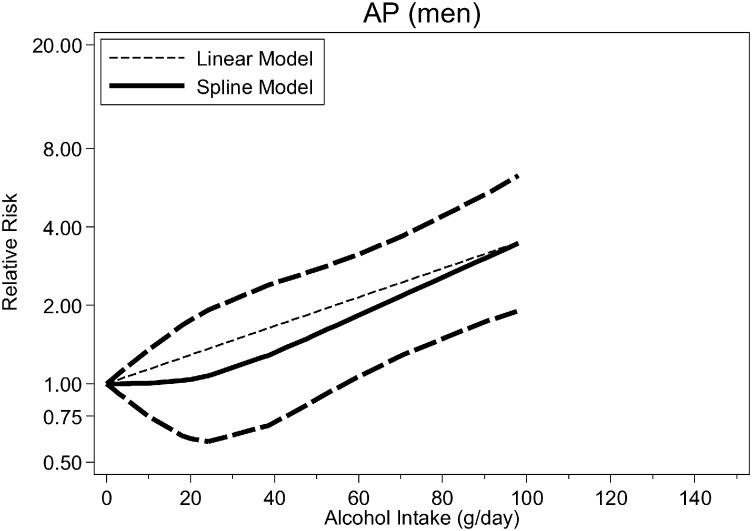
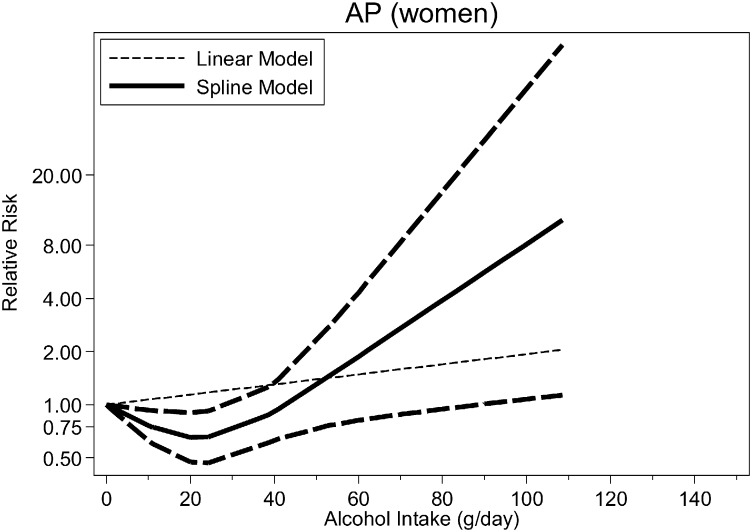
Fig. 2, Fig. 3, Fig. 4 (see also Table 2) show the dose–response relationship between average alcohol consumption and CP (n = 4 studies, Fig. 2), AP among men (n = 3 studies, Fig. 3) and women (n = 3 studies, Fig. 4). For CP, the relationship was monotonically increasing with no evidence for non-linearity (p = 0.091) (RRs at 25 g/day = 1.58, 95%CI: 1.32–1.90; 50 g/day = 2.51, 95%CI: 1.74–3.61; 75 g/day = 3.97, 95%CI: 2.30–6.85; 100 g/day = 6.29; 95%CI: 3.04–13.02) (Fig. 3, linear model). There were not enough data to systematically investigate sex-specific relationships for CP. There was no evidence for non-linearity for AP among men (p = 0.396, Table 2) (RRs [linear model] at 25 g/day = 1.38, 95%CI: 1.12–1.69; 50 g/day = 1.89, 95%CI 1.25–2.86; 75 g/day = 2.60, 95%CI: 1.40–4.83; 100 g/day = 3.58, 95%CI: 1.57–8.16). Among women, there was strong evidence for a non-linear association both for any type of pancreatitis (p < 0.001) and AP (p = 0.008), with a potential beneficial effect of up to 40 g/day and a steep increase in risk beyond this threshold (Fig. 4), higher than among men.
Fig. 2.
Pooled dose–response risk relationships between average alcohol consumption and chronic pancreatitis, both sexes.
Fig. 3.
Pooled dose–response risk relationships between average alcohol consumption and acute pancreatitis in men.
Fig. 4.
Pooled dose–response risk relationships between average alcohol consumption and acute pancreatitis in women.
Table 2.
Pooled restricted cubic spline and linear regression coefficients for dose–response relationships.
| Diagnosis/sex | Model | Number of studies | Number of cases | Coefficient 1 |
Coefficient 2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p | Beta | SE | p | ||||
| Any pancreatitis | |||||||||
| Men | Spline | 3 | 1451 | − 0.003 | 0.017 | 0.863 | 0.028 | 0.021 | 0.186 |
| Men | Linear | 3 | 1451 | 0.017 | 0.003 | < 0.001 | NA | NA | NA |
| Women | Spline | 3 | 978 | − 0.033 | 0.011 | 0.002 | 0.077 | 0.016 | < 0.001 |
| CP | |||||||||
| Both sexes | Spline | 4 | 1128 | 0.007 | 0.009 | 0.427 | 0.014 | 0.008 | 0.091 |
| Both sexes | Linear | 4 | 1128 | 0.018 | 0.004 | < 0.001 | NA | NA | NA |
| AP | |||||||||
| Both sexes | Spline | 6 | 2490 | 0.003 | 0.009 | 0.771 | 0.017 | 0.013 | 0.201 |
| Both sexes | Linear | 6 | 2490 | 0.011 | 0.002 | < 0.001 | NA | NA | NA |
| Men | Spline | 3 | 847 | 0.0002 | 0.015 | 0.988 | 0.015 | 0.018 | 0.396 |
| Men | Linear | 3 | 847 | 0.013 | 0.004 | 0.002 | NA | NA | NA |
| Women | Spline | 3 | 638 | − 0.028 | 0.010 | 0.007 | 0.059 | 0.022 | 0.008 |
SE, standard error.
Analyses of the dose–response relationship between alcohol consumption and the risk of CP conducted separately using the data from different geographical areas – Asia and Europe or US – showed that the risk of CP in Asia (n = 2) is increasing in a linear fashion with a higher slope than in Europe or US (n = 2) (Suppl. Figs. S1–S2). Only one study from Asia reported the risk for AP (Kume et al., 2015) showing a similar relationship compared to all other studies.
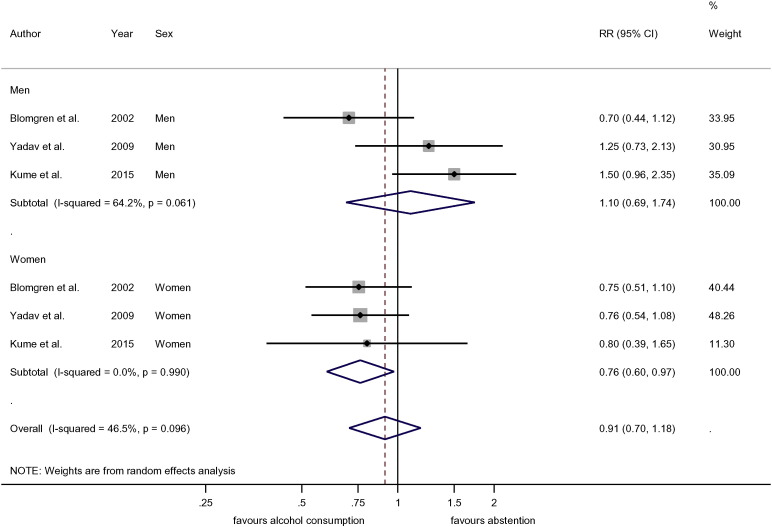
Categorical meta-analyses supported these findings (Suppl. Figs. S3–S5). The difference between men and women is highlighted by a comparison of < 40 g/day alcohol intake compared to abstention (Fig. 5). While there was no difference among men (RR = 1.10, 95%CI: 0.69–1.74), women showed a significantly lower risk (RR = 0.76, 95%CI: 0.60–0.97). Analyses for any pancreatitis (Table 2, Figs. S6–S7) confirmed evidence for a non-linear relationship among women and a linear relationship among men.
Fig. 5.
Pooled relative risk of acute pancreatitis for 0·1–40 g/day drinking category compared to abstainers.
3.3. Heterogeneity and Publication Bias
Between-study heterogeneity was low to moderate in analyses on AP among women, and moderate to high for CP and AP among men. Using all studies available, there was no evidence for publication bias (Egger's test, Figs. S8–S9) or single influential studies (Figs. S10–S11) for estimates up to 40 g/day.
4. Discussion
There were differential dose–response relationships between men and women with respect to alcohol consumption, as well as type of pancreatitis. The dose–response relationship between the level of alcohol consumption and CP was linear, and monotonically increasing with no identifiable threshold. On the contrary, the relation between average alcohol consumption and AP was non-linear (J-shaped) among women, and monotonically increasing among men. High alcohol consumption was consistently associated with an increased risk for pancreatitis at the alcohol consumption level above 40 g/day (higher than previously reported) (Irving et al., 2009).
There are two plausible explanations for a J-shaped relationship between alcohol consumption and AP as it appeared in women. First, the beneficial effect may be explained by contamination of the reference group that included ex-drinkers who quit drinking due to health problems and were at increased risk of developing pancreatitis. Two studies reported on the risk of pancreatitis in former drinkers (Lin et al., 2001, Morton et al., 2004). In comparison to lifetime abstainers, former drinkers showed an elevated risk for any pancreatitis (RR = 2.20, 95%CI: 1.45–3.34), and there was no beneficial association (RR = 1.01, 95%CI: 0.82–1.24) of moderate alcohol consumption (< 40 g/day). There were not enough data to stratify this relationship by pancreatitis diagnosis or sex.
The second explanation is related to the distribution of etiological factors for different types of pancreatitis in men and women, and accordingly, the pathogenesis and natural history of the disease. Specifically, alcohol consumption is the predominant cause of CP in Western countries, with the proportion of cases of alcoholic pancreatitis reaching 80% and sometimes even 95% (Braganza et al., 2011, Pezzilli, 2009). The pathophysiology of CP is based on damage to acinar cells of the pancreas (mediated by sustained elevation of the cytosolic Ca2 + levels) (Gerasimenko et al., 2014), which start releasing synthesized pancreatic enzymes into interstitium (pancreastasis), thus triggering inflammation (Braganza et al., 2011). While a number of agents and factors can trigger pancreastasis, it was indicated that alcohol metabolism plays a significant role in it as it is associated with the production of reactive oxygen species (ROS) via acetaldehyde pathway (Gonzalez, 2005) and fatty acid ethyl esters (FAEE) via non-oxidative route (Pandol and Raraty, 2007, Witt et al., 2007) which in turn cause injury to acinar cells and activate stellate cells (Braganza et al., 2011). Since the quantities of alcohol metabolites and their impact directly correlate with the amounts of alcohol consumed, the relationship between alcohol consumption level and the risk of CP is monotonic.
The interaction between alcohol and other etiological factors in AP is quite similar to CP from pathobiological standpoint — a cascade of intra- and extracellular reactions leads to FAEE-induced increase of the Ca2 + release that in turn leads to necrosis of pancreatic acinar cells (Gerasimenko et al., 2014). At the same time a more complex interplay between variety of etiological factors is observed — alcohol is the second leading cause of AP after gallstones and biliary problems leading to them (Lankisch et al., 2015). It was shown that low alcohol consumption is reducing the risk of symptomatic choledocholithiasis, and thus lowering the risk of biliary AP (Leitzmann et al., 1999).
This hypothesis also provides some insight into more pronounced beneficial effects of low alcohol consumption on the risk of AP in women compared to men. Specifically, women are more likely to develop biliary problems and gallstones (Shen et al., 2013, Russell et al., 1998) and therefore constitute a larger proportion of the cases of biliary AP, whereas men are more likely to consume larger amounts of alcohol on daily basis and to binge drink, and therefore represent the majority of cases of alcoholic AP. Thus, the proportion of women consuming alcohol at the levels of less than 40 g per day, which reduces their risk of biliary AP, is higher compared to men, and the beneficial effect of low levels of alcohol consumption is therefore more pronounced. This can be illustrated by data from the study by Morton et al. (2004). The subset of cases of alcoholic pancreatitis demonstrated an exponential increase of the risk of pancreatitis with the increase of the daily alcohol consumption. At the same time, in the subset of gallstone pancreatitis cases, the risk of pancreatitis was lower in subjects consuming low amounts of alcohol, and increased with higher alcohol consumption.
While both sick-quitter and biliary effect hypotheses appear to be plausible explanations, there are no data available to test and to determine their relative contribution. Further research is required here, with specific focus on comparisons between ex-drinkers and lifetime abstainers, and the distribution of different types of pancreatitis depending on the alcohol consumption levels, preferably separated by sex.
The data on the risk of pancreatitis in different populations are scarce. While the data on AP were not sufficient to draw any conclusions regarding the impact of ethnicity on the risk of pancreatitis we were able to conduct separate analyses for CP based on geographical area of the study and there are indications that the risk of CP in predominantly Asian populations is linear and higher than in non-Asians or mixed populations of Europe and US. These variations may be related to genetic factors (e.g. Brooks et al., 2009), different drinking patterns as well as dietary variations.
4.1. Strengths and Limitations
A major strength of our study was the testing of alcohol consumption as a non-linear risk factor for pancreatitis as a continuum of inflammatory diseases of the pancreas, as well as subanalyses for specific types (acute and chronic) of pancreatitis and for men and women. At the same time, it must be noted that certain subgroup analyses were subject to low power due to a limited number of studies included, and we were not able to conduct separate analyses for men and women having CP as well as for the studies conducted in ethnically different populations or geographical areas. Also, there were not enough data to separate recurrent AP from other forms of AP, but from studies which were able to do this separation, it seems to be justified to combine them. Also, further research is needed to explore the impact of alcohol on the risk of pancreatitis in specific populations such as former drinkers; the interplay of alcohol, diet and biliary problems in men and women; as well as the role of alcohol in development of pancreatitis of non-alcoholic etiology.
Another potential limitation is related to the fact that alcohol consumption was self-reported in all of the studies, which adds variability to alcohol consumption estimates in that they are subject to recall bias and other biases. Finally, our analyses are based on the data from published studies, and thus are a subject to potential publication bias. Though we did not find evidence for such bias, we cannot completely exclude such a possibility.
4.2. Implications
We have collected and systematized all the data available on the relationship between alcohol consumption and risk of pancreatitis. Our analyses allowed us to describe this relationship differentially for the major types of pancreatitis in men and women. These differences might be due to several factors, but primarily, they highlight the high risk of heavy alcohol consumption, as well as the potentially beneficial effects of moderate intake (< 40 g/day), especially among women. These findings can be used for development of recommendations for pancreatitis prevention. Also, we have identified several gaps in knowledge that can guide further research.
5. Conclusions
There are differential dose–response relationships between average volume of alcohol consumption and risk of different types of pancreatitis in men and women. The relationship was linear for CP and AP in men, but non-linear for AP in women. There was strong evidence supporting a threshold effect for AP in women at the level of alcohol consumption of up to 40 g/day. Beyond 40 g of pure alcohol/day, the risk of pancreatitis, both acute and chronic, regardless of sex, was higher than previously thought.
Role of the Funding Source
The sponsor of the study (NIAAA) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors collected the data, and had full access to all of the data in the study. The authors also had final responsibility for the decision to submit the study results for publication.
Contributors
AVS, MR and JR shared responsibility to design the study. AVS was responsible for the literature review, and data extraction. MR conducted the statistical analyses. AVS and MR were responsible for data interpretation, and article preparation. JR contributed to design and data interpretation, article preparation, and article review. All authors contributed to the article and approved the final version.
Funding
The work was financially supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21AA023521) to MR.
Declaration of Conflict of Interest
MR and JR report grants from the National Institutes of Health (NIH), National Institute on Alcohol Abuse and Alcoholism (NIAAA, R21AA023521), during the conduct of the study. AVS has no conflict of interest.
Acknowledgments
We would like to thank Mr. Kyle Runeckles, MSc for proofreading.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.11.023.
Appendix A. Supplementary Data
Supplementary figures
Supplementary material
References
- Blomgren K., Sundström A., Steineck G., Genell S., Sjöstedt S., Wiholm B. A Swedish case-control network for studies of drug-induced morbidity – acute pancreatitis. Pharmacoepidemiology and Prescription. 2002;58(4):275–283. doi: 10.1007/s00228-002-0471-4. [DOI] [PubMed] [Google Scholar]
- Braganza J., Lee S., McCloy R., McMahon M. Chronic pancreatitis. Lancet. 2011;377(9772):1184–1197. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- Brooks P.J., Enoch M.A., Goldman D., Li T.K., Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6(3) doi: 10.1371/journal.pmed.1000050. (e50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers T., Smith H., Blackburn B. A method for assessing the quality of a randomized control trial. Control. Clin. Trials. 1981;2(1):31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Detsky A., Naylor C., O'Rourke K., McGreer A., L'Abbé K. Incorporating variations in the quality of individual randomized trials into meta-analysis. Journal of Clinical Epidemiology. 1992;45(3):255–265. doi: 10.1016/0895-4356(92)90085-2. [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko J.V., Gerasimenko O.V., Petersen O.H. The role of Ca(2 +) in the pathophysiology of pancreatitis. J. Physiol. 2014;592(Pt 2):269–280. doi: 10.1113/jphysiol.2013.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat. Res. 2005;569(1–2):101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez A., Schlienger R.G., Rodriguez L.A. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care. 2010;33(12):2580–2585. doi: 10.2337/dc10-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S., O'Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2(4):463–471. doi: 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- Hamling J., Lee P., Weitkunat R., Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat. Med. 2008;27(7):254–270. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- Herbison P., Hay-Smith J., Gillespie W. Adjustment of meta-analyses on the basis of quality scores should be abandoned. J. Clin. Epidemiol. 2006;59(12):1249–1256. doi: 10.1016/j.jclinepi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Higgins J., Thompson S. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Irving H., Samokhvalov A.V., Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. J. Pancreas. 2009;10(4):387–392. [PMC free article] [PubMed] [Google Scholar]
- Kristiansen L., Grønbæk M., Becker U., Tolstrup J. Risk of Pancreatitis According to Alcohol Drinking Habits: A Population-based Cohort Study. Am. J. Epidemiol. 2008;168(8):932–937. doi: 10.1093/aje/kwn222. [DOI] [PubMed] [Google Scholar]
- Kume K., Masamune A., Ariga H., Shimosegawa T. Alcohol consumption and the risk for developing pancreatitis: a case–control study in Japan. Pancreas. 2015;44(1):53–58. doi: 10.1097/MPA.0000000000000256. [DOI] [PubMed] [Google Scholar]
- Lai S.W., Muo C.H., Liao K.F., Sung F.C., Chen P.C. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am. J. Gastroenterol. 2011;106(9):1697–1704. doi: 10.1038/ajg.2011.155. [DOI] [PubMed] [Google Scholar]
- Lankisch P., Apte M., Banks P. Acute pancreatitis. Lancet. 2015;386(9988):85–96. doi: 10.1016/S0140-6736(14)60649-8. [DOI] [PubMed] [Google Scholar]
- Leitzmann M.F., Giovannucci E.L., Stampfer M.J. Prospective study of alcohol consumption patterns in relation to symptomatic gallstone disease in men. Alcohol. Clin. Exp. Res. 1999;23(5):835–841. [PubMed] [Google Scholar]
- Lembke A., Bradley K.A., Henderson P., Moos R., Harris A.H. Alcohol screening scores and the risk of new-onset gastrointestinal illness or related hospitalization. J. Gen. Intern. Med. 2011;26(7):777–782. doi: 10.1007/s11606-011-1688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.H., Chang H.Y., Chiang Y.T., Wu M.S., Lin J.T., Liao W.C. Smoking, drinking, and pancreatitis: a population-based cohort study in Taiwan. Pancreas. 2014;43(7):1117–1122. doi: 10.1097/MPA.0000000000000209. [DOI] [PubMed] [Google Scholar]
- Lin Y., Tamakoshi A., Hayakawa T., Ogawa M., Ohno Y. Associations of alcohol drinking and nutrient intake with chronic pancreatitis: findings from a case-control study in Japan. Am. J. Gastroenterol. 2001;96(9):2622–2627. doi: 10.1111/j.1572-0241.2001.04121.x. [DOI] [PubMed] [Google Scholar]
- Mitchell R., Byrne M., Baillie J. Pancreatitis. Lancet. 2003;361(9367):1447–1455. doi: 10.1016/s0140-6736(03)13139-x. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Pham B., Jones A. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- Morton C., Klatsky A., Udaltsova N., Morton C1., Klatsky A.L., Udaltsova N. Am. J. Gastroenterol. 2004;99(4):731–738. doi: 10.1111/j.1572-0241.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- Mumenthaler M.S., Taylor J.L., O'Hara R., Yesavage J.A. Gender differences in moderate drinking effects. Alcohol Res. Health. 1999;23(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- Orsini N., Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6:40–57. [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol S.J., Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology. 2007;7(2–3):105–114. doi: 10.1159/000104235. [DOI] [PubMed] [Google Scholar]
- Peery A.F., Dellon E.S., Lund J. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187. doi: 10.1053/j.gastro.2012.08.002. (e3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzilli R. Etiology of chronic pancreatitis: has it changed in the last decade? World J. Gastroenterol. 2009;15(38):4737–4740. doi: 10.3748/wjg.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.C., Walsh S.J., Reed-Fourquet L., Mattie A., Lynch J. Symptomatic cholelithiasis: a different disease in men? Connecticut Laparoscopic Cholecystectomy Registry. Ann. Surg. 1998;227(2):195–200. doi: 10.1097/00000658-199802000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H.-N., Wang W.-C., Lu C.-L., Li C.-Y. Effects of gender on severity, management and outcome in acute biliary pancreatitis. PLoS One. 2013;8(2):e57504. doi: 10.1371/journal.pone.0057504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield K.D., Parry C., Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res.: Curr. Rev. 2013;35(2):155–173. doi: 10.35946/arcr.v35.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb D. Mechanisms of disease: advances in understanding the mechanisms leading to chronic pancreatitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2004;1(1):46–52. doi: 10.1038/ncpgasthep0025. [DOI] [PubMed] [Google Scholar]
- Witt H., Apte M.V., Keim V., Wilson J.S. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132(4):1557–1573. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva, Switzerland: 2000. International Guide for Monitoring Alcohol Consumption and Related Harm. [Google Scholar]
- Yadav D., Lowenfels A. Trends in the epidemiology of the first attack of acute pancreatitis. A systematic review. Pancreas. 2006;33(4):323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- Yadav D., Lowenfels A.B. the epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D., Hawes R.H., Brand R.E. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch. Intern. Med. 2009;169(11):1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang L., Shi Y.H. Risk factors of acute pancreatitis in the elderly Chinese population: a population-based cross-sectional study. J. Dig. Dis. 2014;15(9):501–507. doi: 10.1111/1751-2980.12168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures
Supplementary material