Abstract
Background
Ginseng, which is widely used in functional foods and as an herbal medicine, has been reported to reduce the proliferation of prostate cancer cells by mechanisms that are not yet fully understood.
Methods
This study was designed to investigate the changes in ginsenoside content in ginseng after treatment with a microwave-irradiation thermal process and to verify the anticancer effects of the extracts. To confirm the anticancer effect of microwave-irradiated processed ginseng (MG), it was tested in three human prostate cancer cell lines (DU145, LNCaP, and PC-3 cells). Involvements of apoptosis and autophagy were assessed using Western blotting.
Results
After microwave treatment, the content of ginsenosides Rg1, Re, Rb1, Rc, Rb2, and Rd in the extracts decreased, whereas the content of ginsenosides 20(S)-Rg3, 20(R)-Rg3, Rk1, and Rg5 increased. Antiproliferation results for the human cancer cell lines treated with ginseng extracts indicate that PC-3 cells treated with MG showed the highest activity with an half maximal inhibitory concentration of 48 μg/mL. We also showed that MG suppresses the growth of human prostate cancer cell xenografts in athymic nude mice as an in vivo model. This growth suppression by MG is associated with the inductions of cell death and autophagy.
Conclusion
Therefore, heat processing by microwave irradiation is a useful method to enhance the anticancer effect of ginseng by increasing the content of ginsenosides Rg3, Rg5, and Rk1.
Keywords: anticancer, ginsenoside, microwave, Panax ginseng, prostate cancer
1. Introduction
Prostate cancer is a commonly diagnosed tumor in men that represents a broad spectrum of severity, ranging from indolent to highly lethal [1]. Because prostate cancer cannot grow or differentiate without androgens, hormone therapy has become the standard treatment for prostate cancer [2]. However, cancer recurrence normally develops in years when the patient with prostate cancer no longer responds to hormone therapy. Therefore, chemotherapy with cytotoxic agents has been suggested as an alternative growth inhibitor for hormone-refractory prostate cells [3]. However, the effectiveness of cytotoxic agents against prostate cancer cells is markedly diminished due to the slow proliferation of these cells [4].
Several anticancer agents inhibiting proliferation of cancer cells, inducing apoptosis, or modulating signal transductions are currently used for the treatment of cancers, and a combination of multiple chemopreventive agents with multiple targets is considered to be more effective [5]. Therefore, herbal therapy has been suggested, partly because herbal medicines consist of several constituents with multiple targets, and partly because there is a long history of using herbal medicines in Asian and European countries [6]. Natural products have appreciably contributed to the development of a large number of anticancer drugs. Approximately 50% of all anticancer drugs approved internationally are either natural products or natural product mimics and were developed based on the knowledge obtained from small molecules or macromolecules existing in nature [7], [8], [9].
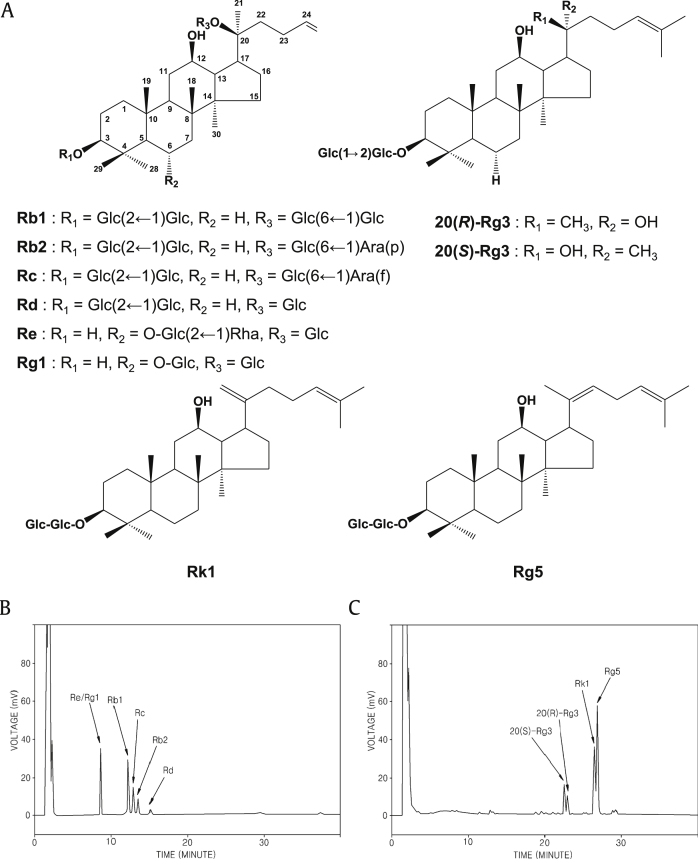
Ginseng, generally the root of Panax ginseng Meyer, has been used in Oriental medicine and is now used widely around the world. There are a variety of commercial ginseng products available, such as white, red, sun, and black ginsengs [10], [11]. More than 30 different ginsenosides have been identified and isolated, all with several pharmacological effects. Ginsenosides are divided into 20(S)-protopanaxadiols (ginsenoside Rb1, Rb2, Rc, Rd, and Rg3) and 20(S)-protopanaxatriols (ginsenoside Re and Rg1) groups on the basis of their aglycone moieties (Fig. 1) [11], [12].
Fig. 1.
Structures and analysis of ginsenosides. (A) Structures of ginsenosides contained in Panax ginseng. (B) HPLC chromatogram analyzing ginsenosides (Re/Rg1, Rb1, Rc, Rb2 and Rd) in processed ginseng by microwave. (C) HPLC chromatogram analyzing ginsenosides (20(S)-Rg3, 20(R)-Rg3, Rk1, and Rg5) in processed ginseng by microwave. -Glc, D-glucopyranosyl; -Rha, L-rhamnopyranosyl; -Ara(f), L-arabinofuranosyl; -Ara(p), L-arabinopyranosyl.
Recently, our group developed a novel ginseng extract by microwave-assisted processing. This novel microwave-irradiated processed ginseng (MG) extract has increased content of ginsenosides Rg3, Rg5, and Rk1 [13]. In this study, we demonstrate that MG inhibits prostate cancer cell growth in three human prostate cancer cell lines (DU145, LNCaP, and PC-3 cells). Furthermore, we sought to investigate the anticancer efficacy of MG on the growth of prostate cancer cells in vivo (as xenografts in athymic nude mice).
2. Materials and methods
2.1. Chemicals and reagents
Dulbecco modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen Co. (Grand Island, NY, USA). EZ-Cytox enhanced cell viability assay kit was purchased from ITSBIO (Seoul, Korea). Ginsenoside standards Rb1, Rb2, Rc, Rd, Re, 20(S)-Rg3, 20(R)-Rg3, Rk1, and Rg5 were purchased from Ambo Institute (Seoul, Korea). Monoclonal antibodies against cleaved caspase-8 and β-actin and polyclonal antibodies against cleaved caspase-3, cleaved caspase-9, Bcl-2, Bax, and poly (ADP-ribose) polymerase (PARP) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The water and acetonitrile used were of HPLC grade from Fisher Scientific (Pittsburgh, PA, USA) and glacial acetic acid was of analytical grade from Sigma-Aldrich (Saint Louis, MO, USA).
2.2. Preparation of ginseng extract
Four-year-old fresh ginseng (Panax ginseng) was purchased from a local ginseng market in Seoul (Korea). White ginseng was prepared by drying 100 g of fresh ginseng at 50°C for 3 days. White ginseng was ground to pass an 80-mesh sieve and extracted under reflux with 50% ethanol three times at 70°C for 2 h, filtered through filter paper (Advantec, Tokyo, Japan), and the solvent was evaporated in vacuo to give a 50% ethanol extract with a yield of about 20%, by weight, of the original ginseng powder.
2.3. Preparation of processed ginseng by using microwave irradiation
The 50% ethanol extract of ginseng was processed by using microwave. Each of 200 mg of the ginseng dry extracts was added to 1 mL of water in a 10-mL container of a microwave irradiator (model no.: 908005) manufactured by CEM Company (Matthews, NC, USA). The ginseng dry extract was irradiated with microwaves in the sealed container at a temperature of 150°C and a power of 100 W (a frequency of 2,455 MHz) for 60 min. The microwave-irradiated ginseng dry extracts were freeze-dried to obtain microwave-irradiated process products. A pressure for the microwave irradiation was 20 atm.
2.4. Analysis of ginsenosides
Ginsenosides were analyzed by the comparison of retention times and molecular weights with those of standard samples [14], [15]. Analytical reversed-phase HPLC system was composed of a solvent degasser (Agilent, G1322A, Santa Clara, CA, USA), binary pump (Agilent, G1312C), an autosampler (Agilent, G1329B) and model 380 Evaporative Light Scattering Detector (ELSD) (Agilent). ELSD conditions were optimized in order to achieve maximum sensitivity: temperature of the nebulizer was set for 50°C, and N2 was used as the nebulizing gas at a pressure of 2.0 bar. The Phenomenex Luna C18 column (150 × 4.6 mm, 5 μm, Torrance, CA, USA) was used, and the mobile phase consisted of a binary gradient of solvent A (acetonitrile: water: 5% acetic acid in water = 15:80:5) and solvent B (acetonitrile: water = 80:20) at a flow rate of 1.0 mL/min. The gradient flow program was as follows: initial; 0% B, 6 min; 30% B, 18 min; 50% B, 30 min; 100% B, 37 min; 100% B, 42 min; 0% B. Low-resolution electrospray ionisation (ESI)-MS data were measured with an Agilent Technologies VS/Agilent 1100 system (Santa Clara, CA, USA). The amounts of ginsenosides in samples were quantified as reported previously [12]. The standard solutions containing 1–50 μg of each ginsenoside were injected into the HPLC and all calibration curves showed good linearity (R2 > 0.995). The analysis was repeated two times for the verification of repeatability.
2.5. Antiproliferative effect on three prostate cancer cell lines
Androgen-responsive LNCaP, androgen-insensitive DU145, and PC3 prostate cancer cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were grown in DMEM or RPMI1640 medium (Cellgro, Manassas, VA, USA) supplemented with 10% fetal bovine serum (Gibco BRL, Carlsbad, MD, USA), 100 units/mL penicillin and 100 μg/mL streptomycin and incubated at 37°C in a humidified atmosphere with 5% CO2. Ez-cytox Cell viability assay kit (Itsbio, Seoul, Korea) was used to determine cell viability. Briefly, cells were seeded in 96-well plates at a density of 1 × 104 cells/well and incubated for 24 h at 37°C. The cells were treated with different concentrations of compounds. After incubation for 24 h, 10 μL of the kit reagent was added to each well, and the cells were incubated for an additional hour. Cell proliferation was measured by scanning with a microplate reader at 450 nm. Control cells were exposed to culture media containing 0.5% v/v dimethyl sulfoxide (DMSO).
2.6. Growth of cancer cell xenografts in athymic nude mice
All procedures involving the use of live animals as described in this study were approved in May 2013 by the Institutional Animal Care and Use Committee of the Korea Institute of Science and Technology, Gangneung, Taiwan and strictly followed the National Institutes of Health guidelines for humane treatment of animals. Male athymic nu/nu mice, 4–5 wk of age, were obtained from Orient Bio Co., Ltd. (Seongnam, Korea). They were exposed to a 12-h light/12-h dark cycle, and had free access to water and normal diet (38057, Agribrands Purina Korea, Seongnam, Korea) containing 10 kcal% fat for a period of 1 wk after arrival. Mice were housed under aseptic conditions (positive air pressure in a designated mouse room, with microisolator tops) and all mouse handling procedures were carried out under a laminar flow hood. DU145 cells (5 × 106 cells/100 μL PBS) were injected subcutaneously into the right and left flank of the mice. Two wks after inoculation, mice were randomly grouped according to body weight and tumor size and started feeding on the experimental diets. They were fed on either normal diet (vehicle) or diet containing 0.1% MG (MG group) throughout the experimental period (approximately equivalent to the oral dose of 200 mg/kg). The maximum and minimum diameters of the tumor were measured once/wk using a slide caliper. Tumor volume was calculated using the formula [π/6 × d3], where d is the mean diameter. Body weight of each mouse was measured twice/wk. At the end of the experiment, the mice were killed with CO2 overdose followed by cervical dislocation.
2.7. Western blotting analysis
Cells (8 × 105 cells) grown in 60-mm dishes were treated with the indicated concentration of samples for 24 h. Whole-cell extracts were then prepared according to the manufacturer's instructions using RIPA buffer (Cell Signaling) supplemented with 1 × protease inhibitor cocktail and 1mM phenylmethylsulfonyl fluoride (PMSF). Proteins (whole-cell extracts, 30 μg/lane) were separated by electrophoresis in a precast 4–15% Mini-PROTEAN TGX gel (Bio-Rad, Hercules, CA, USA) blotted onto PVDF transfer membranes and analyzed with epitope-specific primary and secondary antibodies. Bound antibodies were visualized using ECL Advance Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK) and a LAS 4000 imaging system (Fujifilm, Tokyo, Japan).
2.8. Statistical analysis
Statistical significance was determined through analysis of variance followed by a multiple comparison test with a Bonferroni adjustment. A p value < 0.05 was considered statistically significant. The analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
Methods for improving the anticancer effect of ginseng by conversion of ginsenosides, the major active components of ginseng, by microwave-assisted high-temperature and high-pressure processing has been developed. To quantify the ginsenosides, the HPLC condition was optimized by changing the elution gradient. The chromatograms for each of the prepared ginseng extracts are shown in Figs. 1B and C. The processing conditions for microwave treatment of ginseng extracts were optimized [13] and the conditions resulting in the highest generation of ginsenosides Rg3, Rg5, and Rk1 were selected. As shown in Figs. 1B and C, in the ginseng extract that was microwave-assisted processed at 150°C for 60 min, the contents of ginsenosides Re, Rb1, Rc, and Rb2 gradually decreased whilst the contents of ginsenoside Rg3, Rk1, and Rg5 increased.
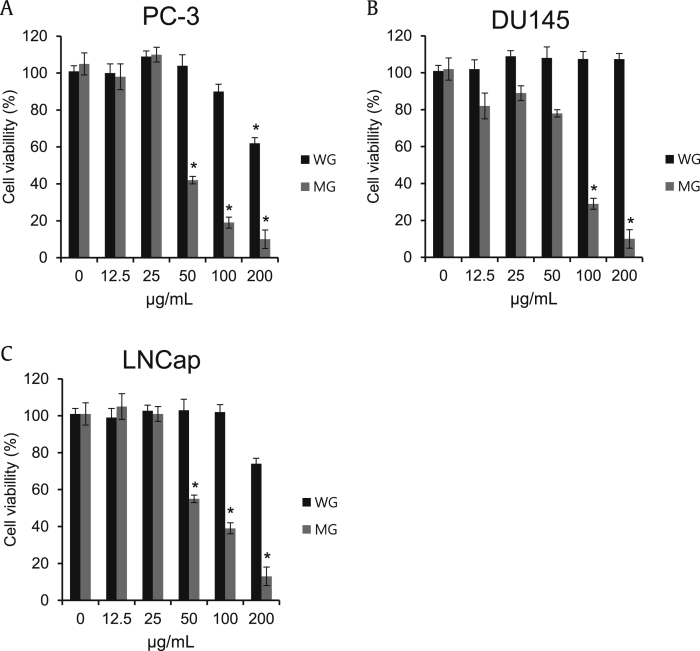
To confirm the anticancer effect of MG, it was tested in three human prostate cancer cell lines (DU145, LNCaP, and PC-3 cells). MG inhibited proliferation of DU145, LNCaP, and PC-3 cells more strongly than WG in a dose-dependent manner as shown in Fig. 2. The half-maximal inhibitory concentration (IC50) values were determined by interpolation from the dose–response curves. Antiproliferation results for the three prostate cancer cell lines treated with ginseng extracts showed that MG had IC50 values of 48 μg/mL, 74 μg/mL, and 62 μg/mL in PC-3, DU145, and LNCap cells, respectively.
Fig. 2.
Comparison in the effect of ginseng extracts on the proliferation of human prostate cancer cells. (A) PC-3 prostate cancer cells were exposed to complete medium containing indicated concentrations of white ginseng (WG) and microwave-irradiated processed ginseng (MG) (12.5–200 μg/mL) for 24 h. (B) DU145 prostate cancer cells were exposed to complete medium containing indicated concentrations of WG and MG (12.5–200 μg/mL) for 24 h. (C) LNCap prostate cancer cells were exposed to complete medium containing indicated concentrations of WG and MG (12.5–200 μg/mL) for 24 h. Ez-cytox Cell viability assay kit was used to determine cell viability. The data are presented as mean ± standard deviation (*p < 0.05, n = 3).
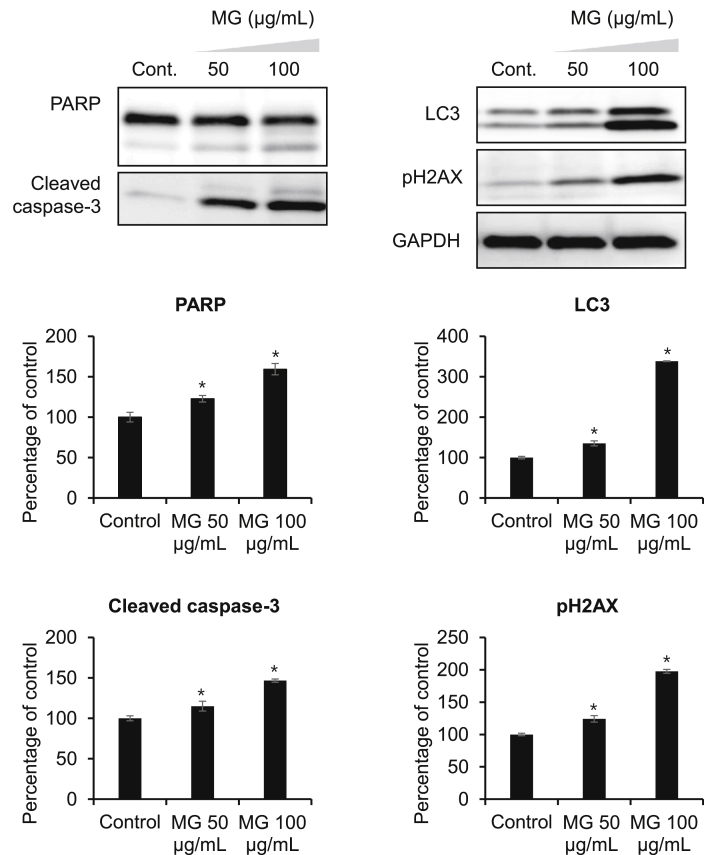
The roles of DNA damage, autophagy, and apoptosis have highly researched and reviewed for cancer prevention in ginseng research [15], [16], [17], [18], [19]. PARP inhibitors are currently undergoing clinical evaluation in advanced sporadic prostate cancer both as a single agent and in combination with multiple chemopreventive agents. PARP inhibitors may be the first of a generation of novel therapeutic strategies that improve or possibly avoid the use of hormone therapy with prostate cancer [20]. After treatment with 100 μg/mL MG, the expression of apoptosis-related proteins PARP (cleaved PARP) increased slightly (Fig. 3).
Fig. 3.
MG decreases the proliferative and antiapoptotic proteins expression. PC-3 cells were exposed to vehicle (0.1% dimethyl sulfoxide) or indicated concentrations of MG (50 μg/mL and 100 μg/mL) for 24 h. Cell lysates from each sample were used for Western blot analyses performed with antibodies directed against poly (ADP-ribose) polymerase (PARP), cleaved caspase-3, LC3, and pH2AX. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. The data are presented as mean ± standard deviation (*p < 0.05, n = 3).
Superoxides, hydroxyl radicals, and peroxides are reactive oxygen species (ROS) that are generated during normal metabolic processes in cells. However, ROS that generated either endogenously or from external sources [21] play a critical role in regulating several biological phenomena. The increased ROS generation not only has traditionally been associated with tissue injury or DNA damage, but also plays an essential role in several cellular processes associated with neoplastic transformation and aberrant proliferation and growth [22], [23]. Ginsenosides have reported to inhibit cancer cell proliferation by inducing gene or protein expression of the cell cycle regulatory protein p21 and arresting cell cycle progression by inducing apoptosis through activation of caspases via a bcl-2-insensitive pathway and/or by inhibiting the mitogen-activated protein kinase signaling pathway in cancer cells [24], [25], [26], [27]. Co-treatment of cells with MG resulted in the activation of the inactive 32-kDa caspase-3 precursor to the cleaved p17 subunit, indicating that caspase-3 was activated during drug-induced apoptosis (Fig. 3).
The pH2AX foci mark sites of double-strand DNA breaks and subsequently recruit multiple proteins involved in DNA strand break repair and damage signaling [28], [29]. The pH2AX is typically deactivated at the completion of proper DNA repair. In genomically unstable cells with dysfunctional DNA damage, pH2AX remains activated and cells continue to replicate without proper DNA repair [30]. Therefore, the expression of pH2AX in PC-3 cells after MG treatment was investigated. As shown in Fig. 3, protein expression of pH2AX in PC-3 cells was markedly increased after MG treatment in a dose-dependent manner.
Autophagy, a cellular process responsible for the degradation of cytoplasmic components through an autophagosomal–lysosomal pathway, has been implicated to play a key role in cancer initiation and progression. Microtubule-associated protein light chain 3 (LC3) is widely used to monitor autophagosome formation. The conversion of LC3-I (18 kDa) to LC3-II (16 kDa) and its translocalization from the cytosol to autophagosomes is a reliable marker of autophagy [31]. Western blot analysis for LC3 showed a remarkable increase in LC3-II in response to MG treatment in a concentration-dependent manner (Fig. 3). Thus, these results suggest that MG induces autophagic cell death as well as apoptosis in PC-3 cells.
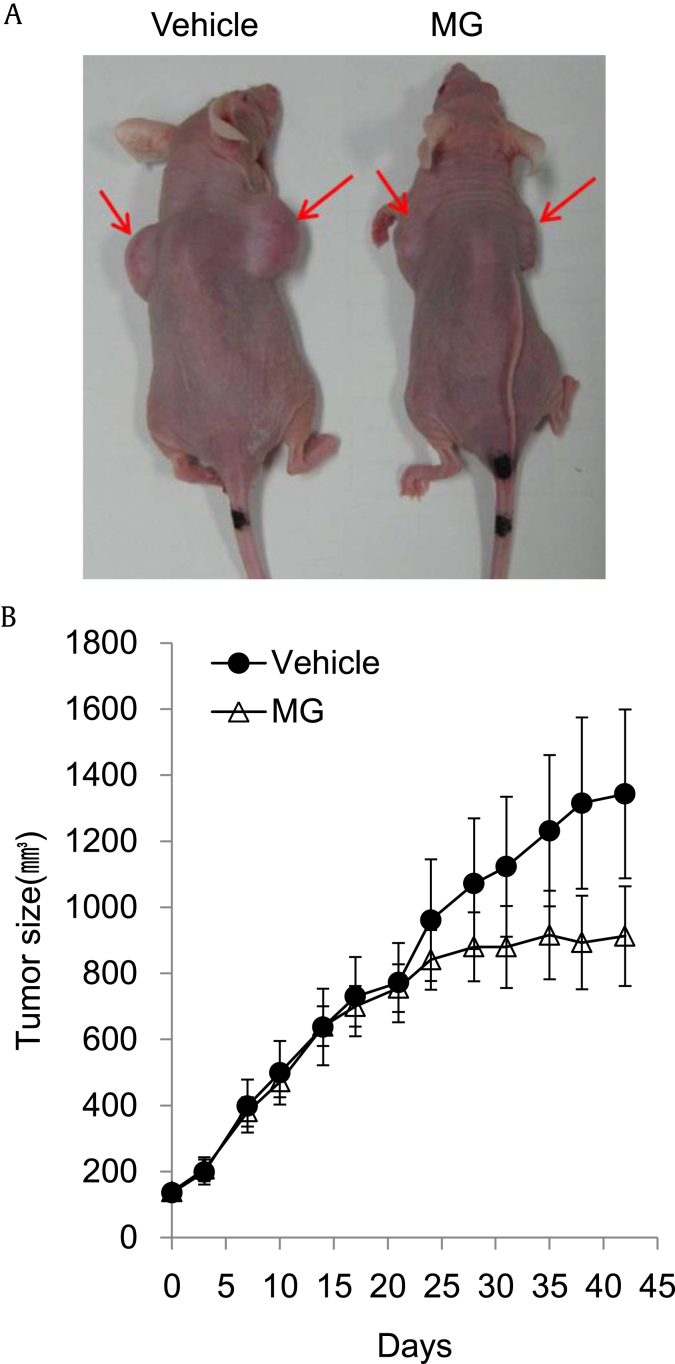
To evaluate the anticancer effect of MG in vivo, we used the growth of human cervical cancer cell xenografts in athymic nude mice as a model. Fig. 4 shows the effect of MG on the growth of human cervical cancer HeLa cells in athymic nude mice. A significant increase in tumor growth volume was observed during the course of and at the end of experimentation in vehicle-treated mice (Fig. 4). However, treatment with MG significantly suppressed tumor growth over the 5-wk experimental period (Fig. 4B). These data indicate that MG suppresses prostate cancer growth in vivo by promoting cancer cell death, likely resulting from increased induction of apoptosis and autophagy.
Fig. 4.
Effect of microwave-assisted processed ginseng on the growth of human prostate cancer PC-3 cells in athymic nude mice. (A) Representative photographs of tumors in athymic nude mice. (B) The changes in tumor growth. DU145 cells (5 × 106 cells/100 μL PBS) were injected subcutaneously into the right and left flank of the mice. Two wks after inoculation, mice were randomly grouped according to body weight and tumor size and started to feed the experimental diets.
In summary, the microwave-irradiated processed ginseng has a higher content of ginsenosides Rg3, Rg5, and Rk1, and thus has an increased medicinal effect from them. It was shown that MG suppressed the growth of human prostate cancer cells both in vitro and in vivo. This growth suppression by MG is associated with the inductions of apoptotic cell death and autophagy. Therefore, heat processing by microwave-assisted irradiation is a useful method to enhance the apoptotic effect of ginseng due to the increased content of ginsenosides Rg3, Rg5, and Rk1.
Conflicts of interest
The authors declare no conflicts of interest in this work.
Acknowledgments
This work was also supported by the Korea Institute of Science and Technology institutional program (2Z04390). This research was also conducted under the industrial infrastructure program for fundamental technologies (N0000885), which is funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Ki Sung Kang, Email: kkang@gachon.ac.kr.
Jungyeob Ham, Email: ham0606@kist.re.kr.
References
- 1.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 2.Bidoli E., Talamini R., Bosetti C., Negri E., Maruzzi D., Montella M., Franceschi S., La Vecchia C. Macronutrients, fatty acids, cholesterol and prostate cancer risk. Ann Oncol. 2005;16:152–157. doi: 10.1093/annonc/mdi010. [DOI] [PubMed] [Google Scholar]
- 3.Garnick M.B., Fair W.R. Combating prostate cancer. Sci Am. 1998;279:74–83. doi: 10.1038/scientificamerican1298-74. [DOI] [PubMed] [Google Scholar]
- 4.Kyle E., Neckers L., Takimoto C., Curt G., Bergan R. Genistein-induced apoptosis of prostate cancer cells is preceded by a specific decrease in focal adhesion kinase activity. Mol Pharmacol. 1997;51:193–200. doi: 10.1124/mol.51.2.193. [DOI] [PubMed] [Google Scholar]
- 5.Hong W.K., Sporn M.B. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 6.Belaiche P., Lievoux O. Clinical studies on the palliative treatment of prostatic adenoma with extract of Urtica root. Phytother Res. 1991;5:267–269. [Google Scholar]
- 7.Bhanot A., Sharma R., Noolvi M.N. Natural sources as potential anti-cancer agents: a review. Int J Phytomedicine. 2011;3:9–26. [Google Scholar]
- 8.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 9.Lee H.W., Jang K.S., Choi H.J., Jo A., Cheong J.H., Chun K.H. Celastrol inhibits gastric cancer growth by induction of apoptosis and autophagy. BMB Rep. 2014;47:697–702. doi: 10.5483/BMBRep.2014.47.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang K.S., Ham J., Kim Y.J., Park J.H., Cho E.J., Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: active components and action mechanism. J Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang K.S., Yokozawa T., Yamabe N., Kim H.Y., Park J.H. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolated from Panax ginseng C A Meyer. Biol Pharm Bull. 2007;30:917–921. doi: 10.1248/bpb.30.917. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y.J., Yamabe N., Choi P., Lee J.W., Ham J., Kang K.S. Efficient thermal deglycosylation of ginsenoside Rd and its contribution to the improved anticancer activity of ginseng. J Agric Food Chem. 2013;61:9185–9191. doi: 10.1021/jf402774d. [DOI] [PubMed] [Google Scholar]
- 13.Choi P., Park J.Y., Kim T., Park S.H., Kim H., Kang K.S., Ham J. Improved anticancer effect of ginseng extract by microwave-assisted processing through the generation of ginsenosides Rg3, Rg5 and Rk1. J Funct Foods. 2015;14:613–622. [Google Scholar]
- 14.Yamabe N., Kim Y.J., Lee S., Cho E.J., Park S.H., Ham J., Kim H.Y., Kang K.S. Increase in antioxidant and anticancer effects of ginsenoside Re-lysine mixture by Maillard reaction. Food Chem. 2013;138:876–883. doi: 10.1016/j.foodchem.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Jang H.J., Han I.H., Kim Y.J., Yamabe N., Lee D., Hwang G.S., Oh M., Choi K.C., Kim S.N., Ham J. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J Agric Food Chem. 2014;62:2830–2836. doi: 10.1021/jf5000776. [DOI] [PubMed] [Google Scholar]
- 16.Kim A.D., Kang K.A., Kim H.S., Kim D.H., Choi Y.H., Lee S.J., Kim H.S., Hyun J.W. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D.G., Jung K.H., Lee D.G., Yoon J.H., Choi K.S., Kwon S.W., Shen H.M., Morgan M.J., Hong S.S., Kim Y.S. 20(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget. 2014;5:4438–4451. doi: 10.18632/oncotarget.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang L.D., He T., Du T.W., Fan Y.G., Chen D.S., Wang Y. Ginsenoside Rg5 induces apoptosis and DNA damage in human cervical cancer cells. Mol Med Rep. 2015;11:940–946. doi: 10.3892/mmr.2014.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z.J., Chee C.E., Huang S., Sinicrope F.A. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner J.C., Ateeq B., Li Y., Yocum A.K., Cao Q., Asangani I.A., Patel S., Wang X., Liang H., Yu J. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664–678. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barzilai A., Rotman G., Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair(Amst) 2002;22:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 22.Lambeth J.D. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naka K., Muraguchi T., Hoshii T., Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 24.Jang J.H., Cho Y.C., Kim K.H., Lee K.S., Lee J., Kim D.E., Park J.S., Jang B.C., Kim S., Kwon T.K. BAI, a novel Cdk inhibitor, enhances farnesyltransferase inhibitor LB42708-mediated apoptosis in renal carcinoma cells through the downregulation of Bcl-2 and c-FLIP (L) Int J Oncol. 2014;45:1680–1690. doi: 10.3892/ijo.2014.2534. [DOI] [PubMed] [Google Scholar]
- 25.Murphy L.L., Rice J.A., Zong W. Ginsenosides Rc and Rh2 inhibit MCF-7 cell proliferation through distinctly different mechanisms. Mol Biol Cell. 2001;12(Suppl):764. [Google Scholar]
- 26.Oh M., Choi Y.H., Choi S., Chung H., Kim K., Kim S.I., Kim D.K., Kim N.D. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 1999;14:869–875. doi: 10.3892/ijo.14.5.869. [DOI] [PubMed] [Google Scholar]
- 27.Park J.A., Lee K.Y., Oh Y.J., Kim K.W., Lee S.K. Activation of caspase-3-protease via a Bcl-2-insensitive pathway during the process of ginsenoside Rh2-induced apoptosis. Cancer Lett. 1997;121:73–81. doi: 10.1016/s0304-3835(97)00333-9. [DOI] [PubMed] [Google Scholar]
- 28.Clingen P.H., Wu J.Y., Miller J., Mistry N., Chin F., Wynne P., Prise K.M., Hartley J.A. Histone H2AX phosphorylation as a molecular pharmacological marker for DNA interstrand crosslink cancer chemotherapy. Biochem Pharmacol. 2008;76:19–27. doi: 10.1016/j.bcp.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Mah L.J., El-Osta A., Karagiannis T.C. GammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 30.Cook P.J., Ju B.G., Telese F., Wang X., Glass C.K., Rosenfeld M.G. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458:591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janku F., McConkey D.J., Hong D.S., Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]