Abstract
Objective
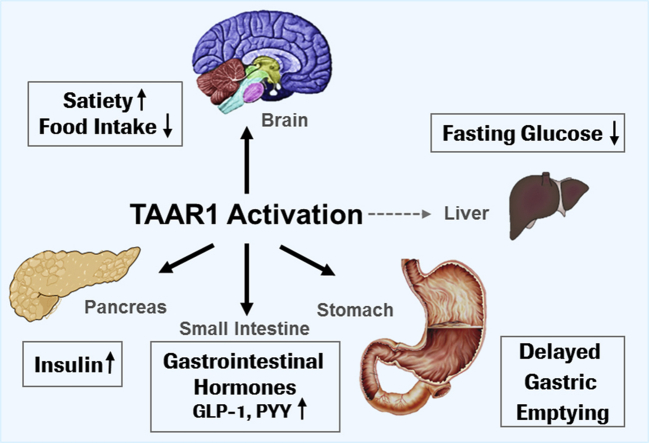
Type 2 diabetes and obesity are emerging pandemics in the 21st century creating worldwide urgency for the development of novel and safe therapies. We investigated trace amine-associated receptor 1 (TAAR1) as a novel target contributing to the control of glucose homeostasis and body weight.
Methods
We investigated the peripheral human tissue distribution of TAAR1 by immunohistochemistry and tested the effect of a small molecule TAAR1 agonist on insulin secretion in vitro using INS1E cells and human islets and on glucose tolerance in C57Bl6, and db/db mice. Body weight effects were investigated in obese DIO mice.
Results
TAAR1 activation by a selective small molecule agonist increased glucose-dependent insulin secretion in INS1E cells and human islets and elevated plasma PYY and GLP-1 levels in mice. In diabetic db/db mice, the TAAR1 agonist normalized glucose excursion during an oral glucose tolerance test. Sub-chronic treatment of diet-induced obese (DIO) mice with the TAAR1 agonist resulted in reduced food intake and body weight. Furthermore insulin sensitivity was improved and plasma triglyceride levels and liver triglyceride content were lower than in controls.
Conclusions
We have identified TAAR1 as a novel integrator of metabolic control, which acts on gastrointestinal and pancreatic islet hormone secretion. Thus TAAR1 qualifies as a novel and promising target for the treatment of type 2 diabetes and obesity.
Keywords: Pancreatic β-cell, Insulin secretion, Type 2 diabetes, Incretin hormones, Obesity
Graphical abstract
Highlights
-
•
TAAR1 is a novel key player in metabolic control.
-
•
TAAR1 is expressed in β-cells and intestinal enteroendocrine cells in mice and humans.
-
•
TAAR1 agonist improved glucose tolerance and reduced body weight in mouse disease models.
Abbreviations
- β gal
beta galactosidase
- DIO
diet induced obesity
- GI
gastrointestinal
- GLP1
glucagon like peptide 1
- GPCR
G-protein coupled receptor
- ivGTT
intravenous glucose tolerance test
- NASH
nonalcoholic steatohepatitis
- oGTT
oral glucose tolerance test
- PYY
peptide YY
- TAAR1
trace amine associated receptor 1
1. Introduction
TAAR1 is a G protein-coupled receptor (GPCR) belonging to the trace amine-associated receptor family [1], [2], [3]. It was identified in 2001 as a receptor for endogenous trace amines, i.e. p-tyramine, β-phenylethylamine, octopamine and tryptamine, which are metabolites of amino acids with structural similarity to biogenic amines [4], [5]. Upon activation, TAAR1 signals via Gαs proteins leading to increased intracellular cAMP levels [1], [4], [5], [6]. Taar1 knockout mice expressing the LacZ gene under control of the Taar1 promoter (Taar1−/−/LacZ) demonstrated TAAR1 expression in restricted areas of the brain, where it modulates monoaminergic neurotransmission [2], [7]. Therefore, TAAR1 recently emerged as a novel target for the treatment of psychiatric disorders [7], [8], [9]. In the periphery, TAAR1 is expressed in the stomach, the duodenum and pancreatic β-cells in mice [7], [10], [11]. In particular, in mouse islets, TAAR1 has been shown to be among the most highly expressed and enriched GPCRs as revealed by quantitative real time PCR analysis of 373 GPCRs [10]. However, little is known about the physiological effects of TAAR1 modulation in these tissues, which is attributed to the polypharmacology of trace amines and the hitherto lack of selective ligands for TAAR1. Recently, selective TAAR1 ligands have been described [8], [9], allowing the exploration of the effects of specific TAAR1 activation in metabolic disease. We describe here for the first time that TAAR1 activation has beneficial effects on glucose control and body weight in animal models of type 2 diabetes and obesity.
2. Methods
2.1. Generation of anti-human TAAR1-specific mouse monoclonal antibodies
An expression construct encoding Glutathione-S-Transferase (GST) fused in-frame to the N-terminal segment and all extracellular domains of human TAAR1 coupled with GSSG linkers was expressed in Escherichia coli [12]. Five mice were immunized by i.p. injection with recombinant protein. Animals were tail bled after two boosts, and sera were tested by ELISA on the immunogen to select the best candidate for hybridoma production. From the animals showing a specific immune response to human TAAR1, the spleens were removed and the cells were fused to Ag8 cells according to [13]. Positive hybridomas were selected by immunofluorescence and Western blotting using a recombinant human TAAR1 N-terminus/extracellular domain construct fused to His tag and expressed in HEK293 cells. Clonal purity was achieved in limited dilution conditions. Final selection of the anti-human TAAR1 (anti-hTAAR1) mAb was done by immunofluorescence on SF9 cells expressing full length human TAAR1 from baculovirus vector.
2.2. Gene expression analysis
mRNA purification from INS1E cells or human islets was performed with RNeasy Mini or Micro Kit (both Qiagen, Hombrechtikon, Switzerland) including an RNAse free DNAse I treatment according to the manufacturer's instructions. cDNA was synthesized using cDNA Synthesis System (Roche Applied Science, Rotkreuz, Switzerland). Quantitative real-time PCR assays (qRT-PCR) were performed using the QuantiFast SYBR Green PCR Kit and the Rotor-Gene 6000 (Qiagen, Hombrechtikon, Switzerland) with specific DNA primers. Analysis was done by the ΔCt threshold method to determine expression relative to GAPDH mRNA. Each analysis reaction was performed in duplicate, with two samples per condition.
2.3. Immunohistochemistry
Paraffin sections (4–5 μm) from adult normal human tissues (Asterand, Herts, UK; Cureline, San Francisco, USA), male C57BL/6J, or Taar1−/−/LacZ mice tissues were incubated with following primary and secondary antibodies: mouse anti-hTAAR1 mAb (Roche clone 6/6); rabbit anti-chromogranin A and anti-peptide YY (Abcam, Cambridge, UK); guinea pig anti-swine insulin (Dako, Glostrup, Denmark); rabbit anti-GLP-1 (7–36) (Peninsula, San Carlos, USA), rabbit anti beta-galactosidase (MP Biomedicals Santa Ana, California, USA) and Alexa Fluor® 488- or 555-conjugated or peroxidase conjugated secondary antibodies (Invitrogen, Basel, Switzerland).
2.4. Insulin secretion
Experiments with INS1E cells were performed as described [14]. Experiments with transplantation-grade human islets (∼80% purity, male donors 59–61 year old, BMI < 28) were performed using handpicked islets (10 islets/condition). Islets were starved for 2 h at 2.8 mM glucose, before insulin secretion was assessed by 1 h incubation with indicated glucose and compound concentrations.
Insulin secretion was presented as % secreted insulin of total insulin content. Human islet experiments were approved by the University of Geneva ethics committee and were conducted in adherence to all relevant laws and ethical guidelines regulating the collection, transfer and use of human tissue.
2.5. Animals
All procedures were conducted in strict adherence to the Swiss federal ordinance on animal protection and welfare, according to the rules of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), and with the explicit approval of the local veterinary authority. Experiments using Glp1r−/− mice were performed following the approval and guidelines of the institutional animal care and use committees of the University of Cincinnati.
Male C57BL/6J and db/db mice (BKS. Cg-m+/+ Leprdb/J) were purchased from Charles River Laboratories (Lyon, France). Taar1−/−/LacZ mice are described elsewhere [2].
DIO mice were generated by placing C57BL/6J mice on SSNIFF diet (EF M D12492: 60% energy from fat, 21% from sugar) starting at 9 weeks of age at Charles River Laboratories (Lyon, France). The DIO mice were 39 weeks of age at the time of the experiment. Glp1r−/− mice were generated as previously described [15]. Animals were fed a HFD (Research Diets D12331: 58% energy from fat plus sucrose) starting at 8 weeks of age and maintained on a 12-light, 12-h dark cycle (lights on 6 am, lights off 6 pm).
2.6. Glucose tolerance tests
Animals were fasted for 10 h prior to glucose tolerance tests. For the oGTT, mice were treated per oral gavage with indicated doses of RO5166017 or vehicle (0.3% Tween 80 in water) 45 min prior to oral glucose challenge (2 g glucose/kg for C57BL/6J, Taar1−/−/LacZ, DIO and Glp1r−/− mice and 1 g glucose/kg for db/db mice). For the ivGTT, mice were treated with RO5166017 (3 mg/kg s.c.), or vehicle (saline) 30 min prior to an i.v. glucose challenge (1 g glucose/kg). Blood was collected at indicated time points after glucose load.
Blood glucose levels were measured by tail vein sampling using a handheld glucometer (Accu-Chek Aviva, Roche) at indicated time points. Plasma insulin was determined by ELISA (Mercodia®, Uppsala Sweden), total GLP-1 by MSD mouse/rat total GLP-1 Assay kit (Mesoscale Discovery, USA) and total PYY with a rat/mouse radioimmunoassay kit (Millipore, MA. USA).
2.7. Gastric emptying
Gastric emptying was assessed using a carbohydrate-and protein-rich semisolid [16] and a liquid meal protocol. Overnight fasted animals were dosed p.o. with vehicle (0.3% Tween 80 in H2O) or 0.3 mg/kg RO5166017 45 min prior to meal administration, or dosed s.c. with vehicle (saline) or propantheline at 30 min prior to meal. Gastric emptying rates were determined according to [16]. Liquid phase gastric emptying was determined as described [17] in overnight fasted animals treated with 0.3 mg/kg RO5166017 or vehicle (0.3% Tween 80 in H2O) 45 min prior to oral glucose load containing 1% acetaminophen (Sigma Aldrich, Switzerland). Quantification of plasma acetaminophen levels was performed by LC-MS/MS.
2.8. Food intake and body weight studies
Taar1−/−/LacZ mice or wild-type (wt) littermates were placed into an automated food monitoring system (TSE system©: TSE drinking and feeding monitor, TSE Systems GmbH, Bad Homburg, Germany). 10 h fasted animals were treated orally with 0.3 mg/kg RO5166017 or vehicle (0.3% Tween 80 in H2O) 45 min prior to ad libitum food access. For the sub-chronic study, 10 h fasted DIO mice were provided unlimited access either to food containing vehicle or RO5166017 as admix at 0.06 mg/g food. Food intake was recorded automatically (TSE system©). The cumulative daily dose of RO5166017 was calculated by animal weight and amount of food consumed. Liver TG content was determined at the end of the study by 1H-magnetic resonance relaxometry (MRR) [18].
2.9. Statistics
Statistical analysis was performed using unpaired T-test, unless otherwise stated. Data are expressed as mean ± SEM unless otherwise stated and p values < 0.05 were considered statistically significant.
3. Results
3.1. TAAR1 has restricted peripheral tissue distribution and is co-localized with insulin in pancreatic β-cells, where it contributes to glucose-dependent insulin secretion
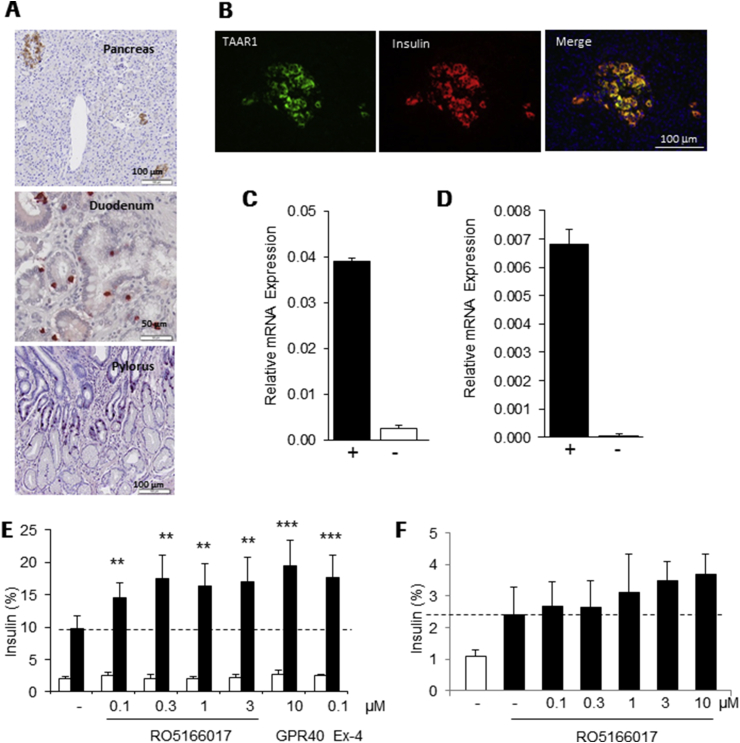
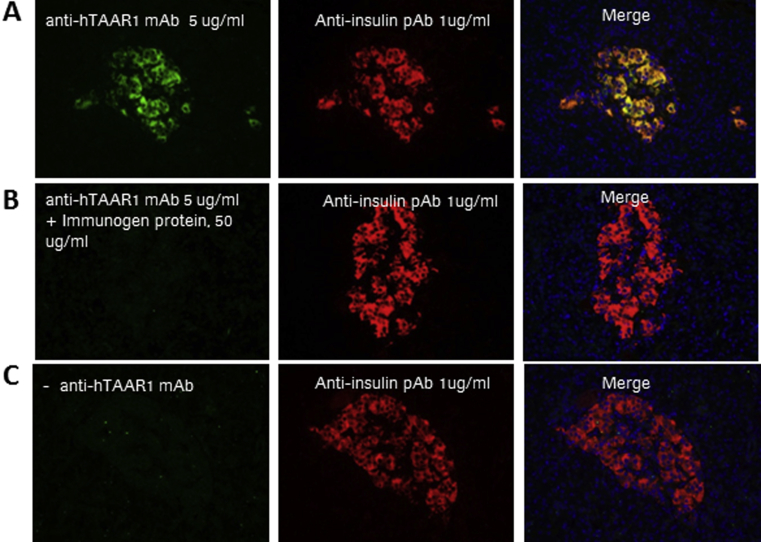
We isolated a mouse monoclonal antibody, which showed specific affinity towards human TAAR1 as investigated in SF9 cells overexpressing human TAAR1 protein versus an unrelated GPCR (not shown). Immunohistochemical staining using this antibody revealed a similar peripheral distribution of TAAR1 in human tissues as previously described in mice [7], [10], [11] with restricted expression in pancreatic islets, duodenum and jejunum and pylorus of the stomach (Figure 1A). In control experiments with secondary antibody only or anti-hTAAR1 mAb preincubated with the immunogen, no immunostaining was obtained (Supplement Figure 1). High TAAR1 immunoreactivity was co-localized with insulin in pancreatic β-cells (Figure 1B), while no co-staining was seen with glucagon (not shown). TAAR1 expression was not detected in other human or mouse tissues investigated, such as heart, kidney and liver (Supplement Figure 2).
Figure 1.
TAAR1 is expressed in pancreatic β-cells and increases insulin secretion (A) Specific staining with anti-hTAAR1 Ab in human pancreas, duodenum and pylorus and (B) co-localization (yellow) of TAAR1 (green) with insulin (red) in human islets. Relative expression of TAAR1 in (C) human islets and (D) INS1E cells was detected by qRT-PCR, expression levels were normalized to GAPDH mRNA (+). In negative PCR controls (−) reverse transcription (RT) was omitted. (E) Increased insulin secretion in INS1E cells at elevated glucose (16 mM) concentration (white bars 2 mM, black bars 16 mM glucose) by RO5166017, a GPR40 agonist (GPR40) or Exendin-4 (Ex-4); n = 6. (F) Glucose-stimulated (11.2 mM glucose, black bars; 2 mM glucose, white bar) insulin secretion is increased by RO5166017 in human islets; n = 3. Dotted lines reflect glucose-induced insulin secretion without compound treatment. Bars in (E) and (F) represent mean ± standard deviation. Statistical analysis: One way Anova followed by Dunnett. **p < 0.01, ***p < 0.001.
We investigated the functional role of TAAR1 in β-cells using the potent and selective small molecule TAAR1 agonist RO5166017, which has been described in details [8]. In cAMP assays using TAAR1-expressing HEK293 cells, RO5166017 activates the human, rat and mouse TAAR1 with EC50s of 55, 14 and 3 nM, respectively [8]. Insulin secretion experiments were performed in rat INS1E cells and in isolated human islets, where TAAR1 mRNA was shown to be expressed (Figure 1C,D). In the presence of low glucose concentration (2 mM), RO5166017 had no effect on insulin secretion in INS1E cells. At 16 mM glucose, RO5166017 significantly potentiated glucose-induced insulin secretion to a similar extent as Exendin-4 or a GPR40 agonist (cpd. B in [19]), both well-characterized insulin secretagogues (Figure 1E). In isolated human islets, RO5166017 also showed a trend to increasing glucose-dependent insulin secretion, although this effect was not significant (Figure 1F).
3.2. TAAR1 agonist improved glucose tolerance
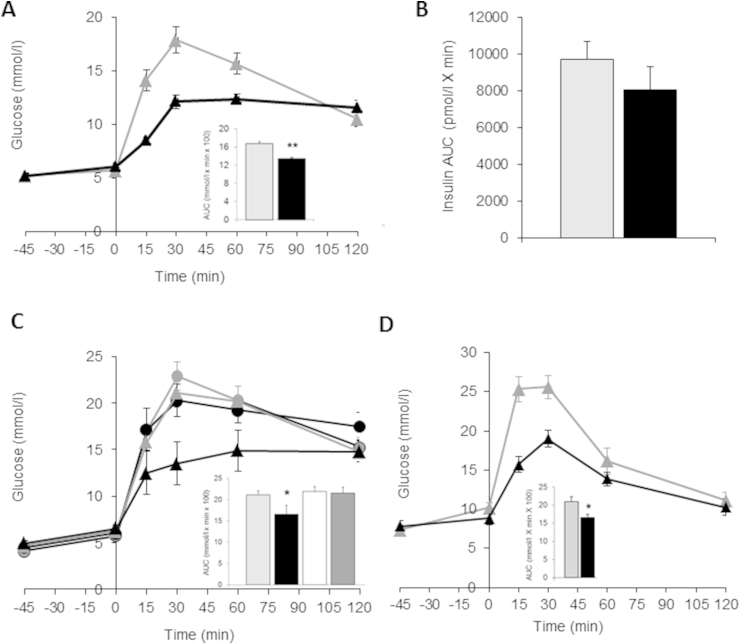
During an oral glucose tolerance test (oGTT) in C57BL/6 mice, a single dose of RO5166017 (0.3 mg/kg, p.o.) significantly reduced glucose area under the curve (AUC0–120) by 20% (Figure 2A) with no effect on fasting glucose. Insulin AUC0–120 was not changed (Figure 2B). To see if this effect is specifically driven by TAAR1 activation, we conducted an oGTT in Taar1−/−/LacZ mice vs. their wt littermates. As shown in Figure 2C, in the absence of TAAR1, no effect of RO5166017 on glucose excursion was seen, demonstrating that the glucose lowering effect is specifically mediated by TAAR1.
Figure 2.
Acute TAAR1 activation improves glucose tolerance (A) Improvement of glucose tolerance and reduction in glucose AUC0–120min (inset) by single oral dose of 0.3 mg/kg RO5166017 in C57BL/6J mice after oral glucose bolus (2 g/kg at t = 0, n = 8/group). (B) No change in insulin AUC0–120min seen after 0.3 mg/kg RO5166017 p.o. during the oGTT. (C) Glucose tolerance is not changed by RO5166017 (0.3 mg/kg, p.o.) in Taar1−/−/LacZ mice (n = 7/group), in contrast to wt littermates (n = 5/group). Inset shows glucose AUC0–120min reduction for vehicle or RO5166017 treated wt and Taar1−/−/LacZ mice (D) RO5166017 (0.3 mg/kg, p.o., n = 8/group) improved glucose tolerance in diabetic db/db mice during an oGTT and reduced glucose AUC0–120min (inset). (A–D): wt mice: vehicle – grey triangles and bars; RO5166017 – black triangles and bars; Taar1−/−/LacZ mice: vehicle – grey circles and white bar; RO5166017 – black circle and dark grey bar. Data are mean ± SEM. *p < 0.05, **p < 0.01.
We next investigated, if RO5166017 also shows efficacy in the condition of type 2 diabetes. In diabetic db/db mice TAAR1 activation by RO5166017 (0.3 mg/kg, p.o.) resulted in a significant reduction of glucose AUC0–120 by 21% during the oGTT (Figure 2D). Elevated fasting blood glucose levels were slightly but not significantly reduced (−13%). To avoid stress during the study, blood withdrawal was minimized, and insulin levels were not assessed.
3.3. TAAR1 activation delayed gastric emptying and increased insulin secretion during an ivGTT
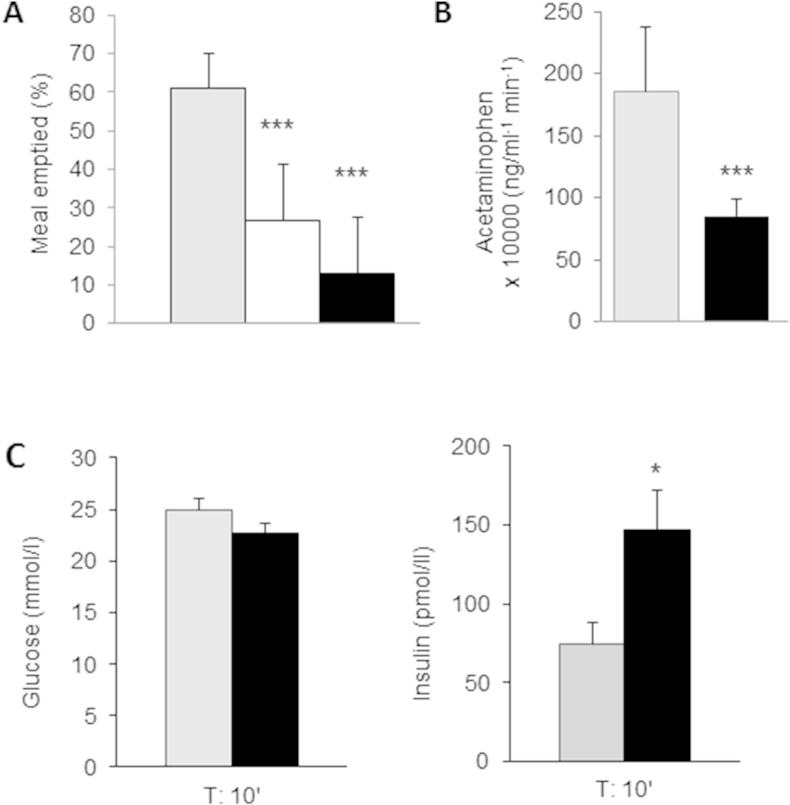
The effect of RO5166017 on gastric emptying in wt mice was determined after a semi-solid meal by calculation of the mass of stomach content relative to the amount of ingested food. Gastric emptying was reduced by 123% in RO5166017 (0.3 mg/kg, p.o.) treated mice (Figure 3A). This effect was greater than that observed for propantheline used as a positive control [16]. Next the effect of TAAR1 activation on acetaminophen absorption, a clinically established assay for the assessment of gastric emptying [20], was investigated. Plasma acetaminophen AUC0–120 was significantly reduced by RO5166017 treatment (0.3 mg/kg, p.o.) following a liquid meal (Figure 3B), indicating delayed gastric emptying.
Figure 3.
Acute TAAR1 activation impairs gastric emptying in mice and elicits insulin secretion during an ivGTT (A) 30 min after a semi-solid meal a lower amount of meal was emptied from the stomachs of propantheline (10 mg/kg s.c., white bar) and RO5166017 (0.3 mg/kg p.o.) treated C57BL/6J mice (n = 10/group). Statistical analysis: Anova with Tukey–Kramer multiple comparison post test. (B) Plasma acetaminophen excursion (AUC0–120) after a liquid meal was reduced by RO5166017 (0.3 mg/kg p.o., n = 8/group) in C57BL/6J mice. Statistical analysis: Anova followed by Dunnett. (C) plasma glucose levels (left) and plasma insulin levels (right) 10 min post i.v. glucose bolus (1 g/kg at t = 0) after acute RO5166017 (3 mg/kg s.c.) treatment in C57BL/6J mice (n = 8/group). Vehicle – grey bars, RO5166017 – black bars. Data are mean ± SEM. *p < 0.05, ***p < 0.001.
To test the direct insulin secretagogue effect of the TAAR1 agonist, we determined the acute insulin response after an ivGTT in C57BL/6J mice. When RO5166017 was given at a dose of 3 mg/kg subcutaneously (s.c.), to assure sufficient drug exposure at the pancreas consistent with the pharmacokinetic properties of the compound, a 2-fold increase in plasma insulin levels in comparison to the vehicle group was observed (Figure 3C).
3.4. TAAR1 activation increased plasma GLP-1 and PYY concentrations
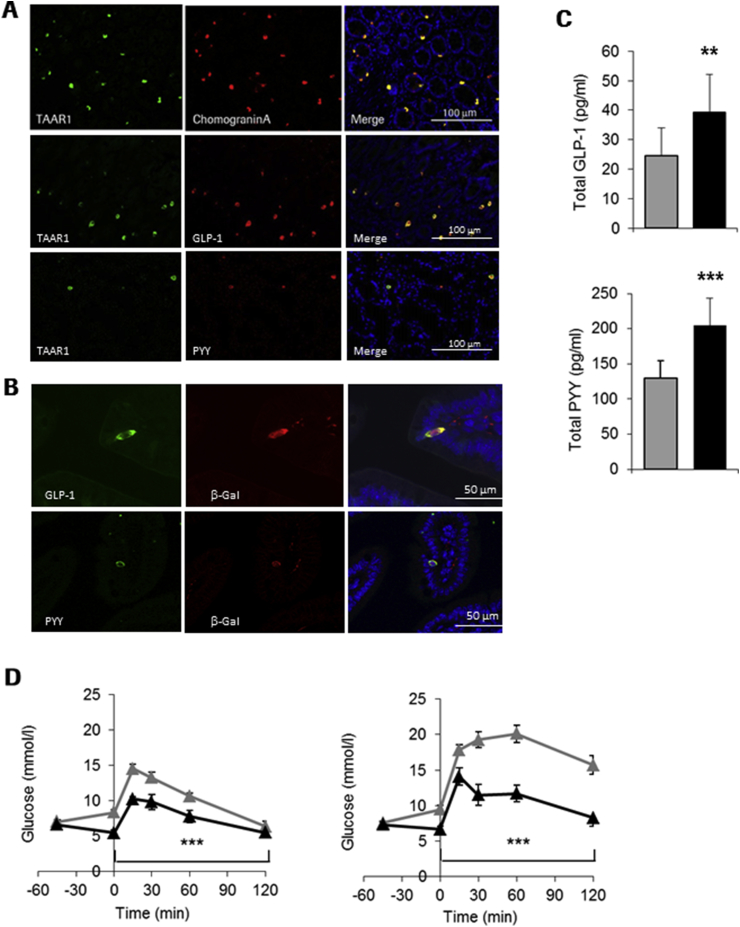
We next investigated the localization of TAAR1 in intestinal tissues by co-staining experiments with the anti-hTAAR1 mAb and enteroendocrine cell markers. TAAR1 co-localized with Chromogranin A (Figure 4A upper panel) and with GLP-1 (Figure 4A middle panel) in human duodenal sections; we also observed some cells co-expressing TAAR1 and peptide YY (PYY) (Figure 4A, lower panel). Subsequently we determined co-localization of TAAR1 with GI hormones in intestinal sections in mice by using Taar1−/−/LacZ mice. We observed co-staining of β−Gal with PYY and GLP-1 (Figure 4B) in the duodenum similar to what was seen in human tissue.
Figure 4.
TAAR1 is co-expressed with GI hormones and activation results in increased GI hormone plasma levels (A) Co-staining (merge, yellow) of TAAR1 (green) with chromogranin A (red, upper panel) GLP-1 (red, middle panel) and PYY (red, lower panel) in human duodenal tissue sections. (B) Co-staining of TAAR1 (beta-galactosidase activity, red), GLP-1 (green, upper panel) and PYY (green, lower panel) in duodenal sections of Taar1−/−/LacZ mice. (C) Increase in total GLP-1 and PYY plasma levels in RO5166017 (0.3 mg/kg, p.o.) treated C57BL/6J mice 30 min after an oral glucose bolus (2 g/kg). (D) Reduced glucose excursion during oGTT by RO5166017 (10 mg/kg, p.o.) treatment in DIO mice (left) and in diet-induced obese Glp1r−/− mice (right). Vehicle – grey triangles and bars, RO5166017 – black triangles and bars. (n = 8/group). Data are mean ± SEM. Statistical analysis in (D): 2 Way Anova followed by LSMeans Contrast. **p < 0.01, ***p < 0.001.
In order to determine if this is of physiological relevance, we determined plasma PYY and GLP-1 levels after single dose of RO5166017 (0.3 mg/kg, p.o.) followed by an oral glucose load in C57BL/6J mice. Plasma levels of both total GLP-1 and PYY were significantly increased 30 min after glucose challenge in RO5166017-treated mice (Figure 4C), linking co-localization of TAAR1 with these hormones with physiological secretion. To test whether GLP-1 secretion in response to the RO5166017 is the main component of the glucose lowering efficacy, we performed an oGTT in GLP-1 receptor knockout (Glp1r−/−, [15]) mice fed a high fat diet compared to diet induced obese (DIO) wt mice. A single dose of RO5166017 resulted in reduction of both fasting glucose levels and glucose excursion in wt DIO mice (Figure 4D, left). In DIO Glp1r−/− mice RO5166017 was still efficacious in both reducing fasting glucose and improving glucose tolerance (Figure 4D, right), indicating that the metabolic effects of a TAAR1 agonist involve other mechanisms in addition to intestinal GLP-1 secretion.
3.5. TAAR1 activation reduced food intake and body weight in DIO mice resulting in improved insulin sensitivity
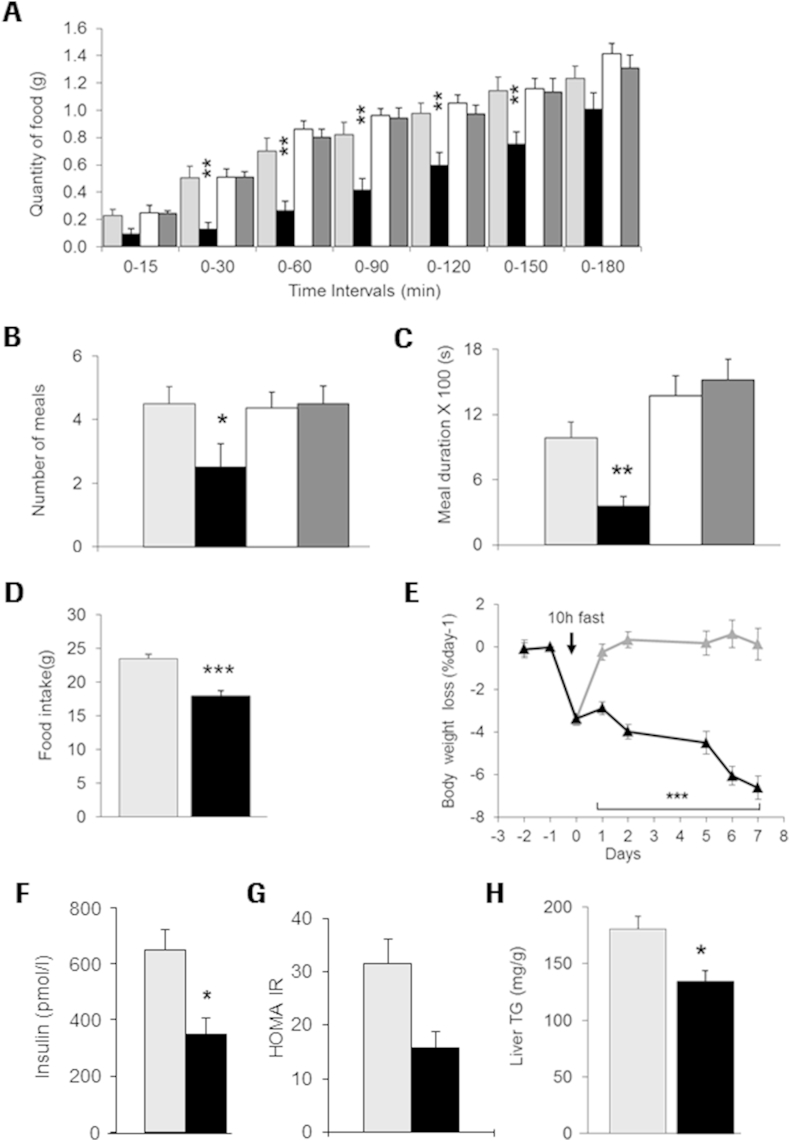
We next investigated the effect of TAAR1 activation with RO5166017 on food intake in wt vs. Taar1−/−/LacZ mice. Acute treatment with RO5166017 in over-night fasted C57Bl6 mice resulted in significant reduction in food consumption for up to 150 min after food presentation (Figure 5A), which is consistent with the T1/2 of the compound [8]. In contrast, Taar1−/−/LacZ mice were completely refractory to the food consumption-reducing effects of RO5166017. Despite resumption of food intake, total food consumption over 24 h remained lower in treated vs. control animals, demonstrating absence of compensatory response (data not shown). TAAR1 agonist-treated wt mice showed a reduction in number of meal events and shorter meal duration (Figure 5B,C) during the first hour after compound application, while no effect on meal pattern was observed in Taar1−/−/LacZ mice.
Figure 5.
TAAR1 activation results in reduced food intake and body weight Single dose of RO5166017 (0.3 mg/kg, p.o.) in C57BL/6J mice resulted in (A) reduction in food intake for 3 h after treatment and reduction in the total number of meals (B) and cumulative meal duration (C) during the first hour after treatment. No effect was observed in Taar1−/−/LacZ mice. 7 days treatment of DIO mice with RO5166017 (cumulative dose 3.5 mg/kg/day, food admix) resulted in reduction in (D) cumulative food intake, (E) body weight from baseline (day −1; the drop in body weight at day 0 (treatment start) reflects that mice were fasted for 10 h) and (F) postprandial insulin levels, (G) HOMA IR, and (H) liver triglycerides. wt mice: vehicle – grey triangles and bars; RO5166017 – black triangles and bars; Taar1−/−/LacZ mice: vehicle – white bar; RO5166017 – dark grey bar. n = 8/group (A–C) and n = 6/group (D–H). Statistical analysis in (A and E): 2 Way Anova followed by LSMeans Contrast. *p < 0.05, **p < 0.01, ***p < 0.001.
To determine TAAR1-mediated effects on food intake and body weight, we treated weight stable DIO mice with RO5166017 for 7 days. To compensate for the short T1/2, RO5166017 was provided as an admixture in the diet to deliver an approximate daily cumulative dose of 3.5 mg/kg (0.06 mg/g food). Cumulative food intake over a 7-day treatment period was significantly reduced by 25% by RO5166017 (Figure 5D), resulting in a body weight loss of 6.6% in the RO5166017-treated mice vs. controls after 7 days at the end of the study (Figure 5E). No difference was observed in postprandial glucose levels (not shown), but mice treated with RO5166017 had significantly reduced plasma insulin levels (Figure 5F) resulting in improved insulin sensitivity as reflected by a reduction of the homeostatic model assessment (HOMA IR) index (Figure 5G). Furthermore, the TAAR1 agonist reduced postprandial plasma triglycerides by 13% (not shown) and hepatic triglyceride content by 27% as compared to the vehicle-treated animals (Figure 5H).
4. Discussion
We report for the first time a role of TAAR1 in type 2 diabetes and obesity. We demonstrated limited expression of TAAR1 in peripheral human tissues using a novel monoclonal anti-hTAAR1 antibody. We detected TAAR1 in key endocrine organs that secrete a variety of hormones crucial for the regulation of energy homeostasis and metabolism, such as the pancreas, pylorus and small intestine. TAAR1 is expressed in enteroendocrine cells and co-localized with insulin in the pancreas, and with GLP-1 and PYY in the small intestine. Notably, we did not see TAAR1 protein expression in any other peripheral mouse [7] or human tissues, which is in contrast to reports showing broader TAAR1 mRNA tissue distribution with expression e.g. in the heart [21], adipose tissue [22], kidney and lung [5] using reverse transcription PCR.
Trace amines, which have been described as endogenous ligands for TAAR1, are not selective for TAAR1, since they have been shown to modulate other targets, such as monoaminergic receptors and transporters [23], [24]. Due to the lack of selective ligands for the TAAR1 receptor, little was known about the role of TAAR1 in the peripheral tissues and the effect of specific modulation of TAAR1 on metabolic parameters. We addressed this question using a synthetic selective TAAR1 agonist. When RO5166017 was tested in cellular and animal models we observed an effect on glucose-dependent insulin secretion in vitro and an improvement in glucose tolerance and body weight in vivo. The evidence that the observed effects are triggered solely by TAAR1 activation was demonstrated by lack of efficacy on glucose control and food consumption of the compound in mice lacking TAAR1.
In contrast to our in vivo pharmacological findings, 3- iodothyramine (T1AM), a molecule that is structurally related to trace amines and is reported to activate TAAR1 [25], has been shown to increase blood glucose levels and decrease insulin levels after acute treatment in mice. This effect is unlikely to be TAAR1-mediated and in fact it has been shown that T1AM exhibits polypharmacology and also activates α2A adrenergic receptor (Adra2a), which is coupling to Gαi protein upon activation. Thus, in isolated mouse islets, where Adra2a is much higher expressed than TAAR1 [26], T1AM led to a net reduction in insulin secretion [10], indicating that under physiological conditions Adra2a activation is masking the TAAR1 secretagogue effect. With the discovery of a selective small molecule TAAR1 agonist, we demonstrate that indeed TAAR1 activation in a rat β-cell line and human islets increases glucose-stimulated insulin secretion.
Metabolic control by TAAR1 activation in vivo is glucose-dependent as there were no effects on lowering fasting glucose levels by the TAAR1 agonist in normoglycemic mice, whereas in db/db and DIO mice elevated fasting glucose levels were decreased by RO5166017 treatment, suggesting that there is no risk of inducing hypoglycemia by activating this receptor.
We did not see any increase in plasma insulin levels during an oGTT in TAAR1 agonist-treated C57Bl6 mice compared to the vehicle group. A similar observation has been described for GLP-1 and its analogs in humans [27], [28] and in rodent models [17], where a delay in gastric emptying induced by these peptides was triggering the reduction in glucose excursion without inducing insulin secretion after oral nutrient stimulation. Similarly, we demonstrate that TAAR1 activation induced a delay in gastric emptying in mice after both a liquid and solid oral nutrient challenge, which is the main mechanism for reduced glucose excursion in this experimental setting. When the stomach is bypassed by an i.v. glucose challenge, an increase in insulin secretion was seen after TAAR1 agonist treatment, which is in line with the TAAR1 mediated insulin secretagogue effect observed in vitro.
TAAR1 co-localized with GLP-1 and PYY in the small intestine and we could demonstrate that plasma levels of both total GLP-1 and PYY were elevated during an oGTT. Given the nature of the receptor with coupling to cAMP increases [1], [4], [5], [6], its activation can trigger a hormone secretion pathway as seen in pancreatic β-cells [29], [30], [31]. Unfortunately, we were not able to investigate direct secretagogue mechanisms in enteroendocrine cells in vitro, since all available cellular models like GLUTag or NCI-H716 lines exhibited too little or no TAAR1 expression. Further work is needed using isolated primary intestinal enteroendocrine cells to investigate the direct effects of TAAR1 activation on GI hormone secretion in vitro. Accordingly, it has been demonstrated very recently that RO5166017 exhibited a direct effect on somatostatin secretion from primary D-cells isolated from mouse stomach [32], underlining a physiological role of TAAR1 in the gastrointestinal system.
When tested in Glp1r−/− mice, RO5166017 still demonstrated efficacy during an oGTT, indicating that the secretion of GLP-1 is not a main trigger for the improvement in glucose tolerance. There is more than increased GLP-1 secretion induced by TAAR1 activation that contributes to the glucose lowering effect, which may involve other hormones like PYY or oxyntomodulin, which are co-secreted with GLP-1 from intestinal enteroendocrine cells [33], [34].
Our data also show that TAAR1 activation results in improvement of insulin sensitivity after subchronic treatment. There was a significant reduction in body weight in weight stable DIO mice, likely by inhibiting food intake and slowing gastric emptying, as it is seen for metabolically active hormones such as GLP-1 and PYY [35]. Besides inducing direct peripheral, paracrine or exocrine effects in the GI system, TAAR1 activation may also affect key neuronal pathways that regulate energy balance either directly or indirectly, similar to GLP-1 [36]; both receptors are expressed in the periphery and the brain [2], [37]. Potentially as a consequence of the body weight reduction, both plasma and liver triglyceride levels were improved, opening the possibility to have beneficial effects on diabetes co-morbidities such as nonalcoholic steatohepatitis (NASH).
Finally, our data suggest TAAR1 as a novel nutrient sensor in endocrine cells. Trace amines, the endogenous ligands, are widely distributed and found in food such as chocolate and fermented products like cheese and wine. They are also generated in the vertebrate GI tract by cleavage of amino acids by bacterial aromatic amino acid decarboxylases after a meal [38], [39]. Thus, with restricted peripheral expression of TAAR1 in the GI system, the receptor is found where trace amine concentrations seem to be highest due to ingested food or bacterial metabolism.
In conclusion, by using a selective small molecule agonist we demonstrate beneficial metabolic consequences of specific TAAR1 activation. Our data support multiple effects of a selective TAAR1 agonist likely mediated by β-cells in the pancreas and enteroendocrine cells in the intestine. These findings suggest TAAR1 as a new target for the treatment of type 2 diabetes and obesity with an incretin-like mechanism of action amenable to orally delivered small molecule drugs.
Author contributions
SR, KCK, MH, MHT and SS planned and discussed the experiments. RN, MB, HM provided reagents and discussed data; SR, NC HW, SU, RAS BK, NOP, DPT and CU conducted experiments and analyzed the data. SS wrote the manuscript.
Acknowledgments
We thank, M. Brecheisen, M. Osborne, C. Karrer, V. Griesser, U. Sprecher and E. Zirwes (Roche Innovation Center Basel, Switzerland) for their excellent technical support. We thank Daniel Drucker and his laboratory (University of Toronto, Canada) for enabling use of the Glp1r−/− mice. We are grateful to Dr. D. Bosco and the Cell Isolation and Transplantation Centre, Geneva University Hospital, Geneva, Switzerland for the provision of human islets. We thank A. Konkar, E. Sebokova, S. Pomposiello and M. Wright (Roche Innovation Center Basel, Switzerland) for fruitful discussions and for critical reading of the manuscript.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2015.09.015.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplement Figure 1.
Negative control experiments for anti-hTAAR1 mAb. (A) Co staining (yellow, right) of TAAR1 (green, left) and insulin (red, middle) in human islets. (B) after pre-incubation of the anti-hTAAR1 mAb with 50 μg/ml recombinant TAAR1 antigen or (C) omitting the anti-hTAAR1 mAb from the staining procedure, no TAAR1 immunostaining was observed (left) while insulin staining remained (middle).
Immunohistochemistry experiments on human tissue sections revealed that TAAR1 is not broadly expressed. For each assessment, tissue sections from 3 independent healthy donors were stained for TAAR1 expression using anti-hTaar1 mAb. Staining was performed using AEC-red as substrate for peroxidase conjugated secondary antibody. No TAAR1 expression was obtained in human liver, lung, kidney, spleen, aorta, heart (left ventricle), coronary artery, gall bladder, muscle and testis, while a clear TAAR1 signal was seen in the jejunum (black arrows).
References
- 1.Lindemann L., Hoener M.C. A renaissance in trace amines inspired by a novel GPCR family. Trends in Pharmacological Sciences. 2005;26:274–281. doi: 10.1016/j.tips.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Lindemann L., Meyer C.A., Jeanneau K., Bradaia A., Ozmen L., Bluethmann H. Trace amine-associated receptor 1 modulates dopaminergic activity. Journal of Pharmacology and Experimental Therapeutics. 2008;324:948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- 3.Liberles S.D. Trace amine-associated receptors: ligands, neural circuits, and behaviors. Current Opinion in Neurobiology. 2015;34C:1–7. doi: 10.1016/j.conb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunzow J.R., Sonders M.S., Arttamangkul S., Harrison L.M., Zhang G., Quigley D.I. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Molecular Pharmacology. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 5.Borowsky B., Adham N., Jones K.A., Raddatz R., Artymyshyn R., Ogozalek K.L. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proceedings of the National Academy of Sciences U S A. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindemann L., Ebeling M., Kratochwil N.A., Bunzow J.R., Grandy D.K., Hoener M.C. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Revel F.G., Moreau J.L., Pouzet B., Mory R., Bradaia A., Buchy D. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Molecular Psychiatry. 2013;18:543–556. doi: 10.1038/mp.2012.57. [DOI] [PubMed] [Google Scholar]
- 8.Revel F.G., Moreau J.L., Gainetdinov R.R., Bradaia A., Sotnikova T.D., Mory R. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proceedings of the National Academy of Sciences U S A. 2011;108:8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revel F.G., Moreau J.L., Gainetdinov R.R., Ferragud A., Velázquez-Sánchez C., Sotnikova T.D. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biological Psychiatry. 2012;72:934–942. doi: 10.1016/j.biopsych.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Regard J.B., Kataoka H., Cano D.A., Camerer E., Yin L., Zheng Y.W. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. Journal of Clinical Investigation. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito J., Ito M., Nambu H., Fujikawa T., Tanaka K., Iwaasa H. Anatomical and histological profiling of orphan G-protein-coupled receptor expression in gastrointestinal tract of C57BL/6J mice. Cell and Tissue Research. 2009;338:257–269. doi: 10.1007/s00441-009-0859-x. [DOI] [PubMed] [Google Scholar]
- 12.Larsson K., Hofstrom C., Lindskog C., Hansson M., Angelidou P., Hökfelt T. Novel antigen design for the generation of antibodies to G-protein-coupled receptors. Journal of Immunological Methods. 2011;370:14–23. doi: 10.1016/j.jim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Kohler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 14.Sebokova E., Christ A.D., Wang H., Sewing S., Dong J.Z., Taylor J. Taspoglutide, an analog of human glucagon-like peptide-1 with enhanced stability and in vivo potency. Endocrinology. 2010;151:2474–2482. doi: 10.1210/en.2009-1459. [DOI] [PubMed] [Google Scholar]
- 15.Scrocchi L.A., Brown T.J., MaClusky N., Brubaker P.L., Auerbach A.B., Joyner A.L. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nature Medicine. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 16.Osinski M.A., Seifert T.R., Cox B.F., Gintant G.A. An improved method of evaluation of drug-evoked changes in gastric emptying in mice. Journal of Pharmacological and Toxicological Methods. 2002;47:115–120. doi: 10.1016/s1056-8719(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 17.Flock G., Holland D., Seino Y., Drucker D.J. GPR119 regulates murine glucose homeostasis through incretin receptor-dependent and independent mechanisms. Endocrinology. 2011;152:374–383. doi: 10.1210/en.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunnecke B., Verry P., Benardeau A., von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obesity Research. 2004;12:1604–1615. doi: 10.1038/oby.2004.200. [DOI] [PubMed] [Google Scholar]
- 19.Tan C.P., Feng Y., Zhou Y.P., Eiermann G.J., Petrov A., Zhou C. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes. 2008;57:2211–2219. doi: 10.2337/db08-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blase E., Taylor K., Gao H.Y., Wintle M., Fineman M. Pharmacokinetics of an oral drug (acetaminophen) administered at various times in relation to subcutaneous injection of exenatide (exendin-4) in healthy subjects. Journal of Clinical Pharmacology. 2005;45:570–577. doi: 10.1177/0091270004274432. [DOI] [PubMed] [Google Scholar]
- 21.Chiellini G., Frascarelli S., Ghelardoni S., Carnicelli V., Tobias S.C., DeBarber A. Cardiac effects of 3-iodothyronamine: a new aminergic system modulating cardiac function. Faseb Journal. 2007;21:1597–1608. doi: 10.1096/fj.06-7474com. [DOI] [PubMed] [Google Scholar]
- 22.Regard J.B., Sato I.T., Coughlin S.R. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry M.D. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. Journal of Neurochemistry. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- 24.Burchett S.A., Hicks T.P. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Progress in Neurobiology. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Scanlan T.S., Suchland K.L., Hart M.E., Chiellini G., Huang Y., Kruzich P.J. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nature Medicine. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 26.Dinter J., Mühlhaus J., Jacobi S.F., Wienchol C.L., Cöster M., Meister J. 3-Iodothyronamine differentially modulates alpha-2A-adrenergic receptor-mediated signaling. Journal of Molecular Endocrinology. 2015;54:205–216. doi: 10.1530/JME-15-0003. [DOI] [PubMed] [Google Scholar]
- 27.Nauck M.A., Niedereichholz U., Ettler R., Holst J.J., Orskov C., Ritzel R. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. American Journal of Physiology. 1997;273:E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 28.Little T.J., Pilichiewicz A.N., Russo A., Phillips L., Jones K.L., Nauck M.A. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: relationships with postprandial glycemic and insulinemic responses. Journal of Clinical Endocrinology & Metabolism. 2006;91:1916–1923. doi: 10.1210/jc.2005-2220. [DOI] [PubMed] [Google Scholar]
- 29.Friedlander R.S., Moss C.E., Mace J., Parker H.E., Tolhurst G., Habib A.M. Role of phosphodiesterase and adenylate cyclase isozymes in murine colonic glucagon-like peptide 1 secreting cells. British Journal of Pharmacology. 2011;163:261–271. doi: 10.1111/j.1476-5381.2010.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker H.E., Wallis K., le Roux C.W., Wong K.Y., Reimann F., Gribble F.M. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. British Journal of Pharmacology. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furman B., Ong W.K., Pyne N.J. Cyclic AMP signaling in pancreatic islets. Advances in Experimental Medicine and Biology. 2010;654:281–304. doi: 10.1007/978-90-481-3271-3_13. [DOI] [PubMed] [Google Scholar]
- 32.Adriaenssens A., Yee Hong Lam B., Billing L., Skeffington K., Sewing S., Reimann F. A transcriptome-led exploration of molecular mechanisms regulating somatostatin-producing D-cells in the gastric epithelium. Endocrinology. 2015 doi: 10.1210/en.2015-1301. en20151301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Small C.J., Bloom S.R. Gut hormones as peripheral anti obesity targets. CNS & Neurological Disorders-Drug Targets. 2004;3:379–388. doi: 10.2174/1568007043336950. [DOI] [PubMed] [Google Scholar]
- 34.Wynne K., Bloom S.R. The role of oxyntomodulin and peptide tyrosine-tyrosine (PYY) in appetite control. Nature Clinical Practice Endocrinology & Metabolism. 2006;2:612–620. doi: 10.1038/ncpendmet0318. [DOI] [PubMed] [Google Scholar]
- 35.Talsania T., Anini Y., Siu S., Drucker D.J., Brubaker P.L. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 36.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metabolism. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Dunphy J.L., Taylor R.G., Fuller P.J. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Molecular and Cellular Endocrinology. 1998;141:179–186. doi: 10.1016/s0303-7207(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 38.Spano G., Russo P., Lonvaud-Funel A., Lucas P., Alexandre H., Grandvalet C. Biogenic amines in fermented foods. European Journal of Clinical Nutrition. 2010;3(64 Suppl.):S95–S100. doi: 10.1038/ejcn.2010.218. [DOI] [PubMed] [Google Scholar]
- 39.Pessione E. Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Frontiers in Cellular and Infection Microbiology. 2012;2:86. doi: 10.3389/fcimb.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemistry experiments on human tissue sections revealed that TAAR1 is not broadly expressed. For each assessment, tissue sections from 3 independent healthy donors were stained for TAAR1 expression using anti-hTaar1 mAb. Staining was performed using AEC-red as substrate for peroxidase conjugated secondary antibody. No TAAR1 expression was obtained in human liver, lung, kidney, spleen, aorta, heart (left ventricle), coronary artery, gall bladder, muscle and testis, while a clear TAAR1 signal was seen in the jejunum (black arrows).