Abstract
Aspergillus fumigatus is an important opportunistic human pathogen known for its production of a large array of extrolites. Up to 63 species have been described in Aspergillus section Fumigati, some of which have also been reliably reported to be pathogenic, including A. felis, A. fischeri, A. fumigatiaffinis, A. fumisynnematus, A. hiratsukae, A. laciniosus, A. lentulus, A. novofumigatus, A. parafelis, A. pseudofelis, A. pseudoviridinutans, A. spinosus, A. thermomutatus, and A. udagawae. These species share the production of hydrophobins, melanins, and siderophores and ability to grow well at 37°C, but they only share some small molecule extrolites, that could be important factors in pathogenicity. According to the literature gliotoxin and other exometabolites can be contributing factors to pathogenicity, but these exometabolites are apparently not produced by all pathogenic species. It is our hypothesis that species unable to produce some of these metabolites can produce proxy-exometabolites that may serve the same function. We tabulate all exometabolites reported from species in Aspergillus section Fumigati and by comparing the profile of those extrolites, suggest that those producing many different kinds of exometabolites are potential opportunistic pathogens. The exometabolite data also suggest that the profile of exometabolites are highly specific and can be used for identification of these closely related species.
Keywords: Aspergillus, gliotoxin, fumagillin, extrolites, proxy-exometabolites
Introduction
The genus Aspergillus comprises 344 species (Samson et al., 2014), and some of these can cause human diseases. A. fumigatus is the most important species (Latgé, 1999), but several other species in Aspergillus section Fumigati have been shown to be pathogenic in humans and animals with an inefficient immune system, including A. lentulus (Balajee et al., 2005a; Alhambra et al., 2008; Alcazar-Fuoli et al., 2014; Howard, 2014), A. fumisynnematus (Alcazar-Fuoli et al., 2014), A. fumigatiaffinis (Alcazar-Fuoli et al., 2014), A. novofumigatus (Peláez et al., 2013), A. felis (Barrs et al., 2013), A. fischeri (Kano et al., 2015), A. viridinutans (Vinh et al., 2009a; Coelho et al., 2011; Alcazar-Fuoli et al., 2014), A. pseudofelis, A. pseudoviridinutans, and A. parafelis (Sugui et al., 2014), A. thermomutatus (Toskova et al., 2013; Alcazar-Fuoli et al., 2014; Howard, 2014; Khare et al., 2014), A. laciniosus (Malejczyk et al., 2013), A. hiratzukae (Guarro et al., 2002; Alcazar-Fuoli et al., 2014), A. spinosus (Sutton et al., 2002); and A. udagawae (Kano et al., 2008; Vinh et al., 2009b; Sugui et al., 2010; Posteraro et al., 2011; Gyotoku et al., 2012; Kano et al., 2013). The taxonomy and identification of the causing Aspergilli is not always clear-cut and some isolates have been misidentified (Balajee et al., 2005a,b, 2006; Álvarez-Pérez et al., 2014; Howard, 2014). For example pathogenic isolates identified as A. viridinutans (Varga et al., 2000; Vinh et al., 2009a; Kano et al., 2013) proved to be A. felis, A. pseudoviridinutans, A. parafelis, or A. pseudofelis (Barrs et al., 2013; Novaková et al., 2014; Sugui et al., 2014). Aspergillus species in subgenus Circumdati have also been reported as pathogenic including Aspergillus terreus in section Terrei, A. flavus in section Flavi and A. tubingensis in section Nigri, A. persii, and A. tanneri in section Circumdati, A. nidulans in section Nidulantes, (Sugui et al., 2012, 2015; Howard, 2014; Visagie et al., 2014) and Aspergillus section Phialosimplex [Ph. caninus = Aspergillus caninus and Ph. salinarum = Aspergillus salinarus (Sigler et al., 2010; Greiner et al., 2014)]. Small molecule extrolites (secondary metabolites) have been shown to be involved in the infection process (Kamei and Watanabe, 2005; Abad et al., 2010), so it might be expected that the pathogenic Aspergilli produce the same extrolites. In this review we examine whether the closely related pathogenic species in Aspergillus section Fumigati produce the same extrolites.
Aspergillus taxonomy
Since 2011, all ascomycetous species can only have one name (Hawksworth et al., 2011; Hawksworth, 2012; McNeill et al., 2012). All species formerly included in Dichotomomyces, Cristaspora, Phialosimplex, Polypaecilum, in addition to Penicillium inflatum, have been formally combined into Aspergillus (Houbraken et al., 2014; Samson et al., 2014). Furthermore, all species of Eurotium, Emericella, Chaetosartorya, Fennellia, Neocarpenteles, Neopetromyces, Neosartorya, Petromyces, Saitoa, and Stilbothamnium have also been transferred to Aspergillus (Samson et al., 2014). Ascoma producing species in section Fumigati were originally described under the name Neosartorya (Samson et al., 2006, 2007), but have now all been transferred to Aspergillus (Samson et al., 2014). Several of the species originally thought to produce only the asexual state have later been shown to be able to produce mature ascomata when crossed with the opposite mating type, for example A. fumigatus (O'Gorman et al., 2009) and A. lentulus (Swilaiman et al., 2013). Other opportunistically pathogenic species such as A. flavus (Horn et al., 2009), A. tubingensis (Horn et al., 2013), and A. terreus (Samson et al., 2011; Arabatsis and Velegraki, 2013) can also produce mature ascomata when crossed with the opposite mating type. All species in Aspergillus and Penicillium have now been placed in the family Aspergillaceae (Houbraken and Samson, 2011). Species in Aspergillus section Fumigati are both phenotypically and genotypically distinct (Raper and Fennell, 1965; Geiser et al., 1998; Hong et al., 2005, 2006, 2008; Katz et al., 2005; Geiser et al., 2007; Samson et al., 2007; Yaguchi et al., 2007). Aspergillus lentulus was originally claimed to be a sibling species of A. fumigatus, but was later shown to be phenotypically very different from A. fumigatus, especially concerning extrolite profiles (Larsen et al., 2007; Tamiya et al., 2015). The species A. pseudofelis, A. parafelis, and A. pseudoviridinutans have not been examined chemically, but they are very close phylogenetically and morphologically to A. felis and may be real sibling species with no phenotypic differences (Sugui et al., 2014). The 63 species listed in Table 1 are all those that have been described in Aspergillus section Fumigati and Neosartorya, but some of them are not yet available for the scientific community, so their identity and probably synonymy with other species is unknown. Samson et al. (2007) indicated that several species were synonyms of already known species in Aspergillus section Fumigati and Neosartorya. Thus the total number of species in Fumigati may be less than 63.
Table 1.
Species in Aspergillus section Fumigati and their extrolite production (species written in bold are known to be pathogenic to humans and/or other mammals).
| Aspergillus arcoverdensis: N.E. (Matsusawa et al., 2015) |
| Aspergillus assulatus: aszonapyrone A, indole alkaloids and apolar metabolites (Samson et al., 2007) |
| Aspergillus auratus: helvolic acid (Samson et al., 2007) |
| Aspergillus aureolus: fiscalins, fumagillin, fumiquinazolines, helvolic acid, pseurotin A, tryptoquivalines, tryptoquivalones, viriditoxin (Samson et al., 2007; Kaur et al., 2013) |
| Aspergillus australensis: aszonalenins, wortmannins (Samson et al., 2007) |
| Aspergillus beijingensis: N.E. (Li et al., 1998) |
| Aspergillus botucatensis (= A. spinosus) (Horie et al., 1995; Samson et al., 2007) |
| Aspergillus brevipes: roquefortine C, cf. meleagrin, viriditoxin (trace) (Lillehoj and Milburn, 1973; Samson et al., 2007) |
| Aspergillus brevistipitatus: N.E. (Novaková et al., 2014) |
| Aspergillus caatingaensis: N.E. (Matsusawa et al., 2014b) |
| Aspergillus conversis: N.E. (Novaková et al., 2014) |
| Aspergillus “coreanus” (Neosartorya coreana): aszonalenins (Samson et al., 2007) |
| Aspergillus delicatus (= A. tatenoi) (Samson et al., 2007) |
| Aspergillus denticulatus: gliotoxin, viriditoxin (Samson et al., 2007) |
| Aspergillus duricaulis: asperdurin, asperpentyn, cyclopaldic acid, duricaulic acid, fumagillin, 3-O-methylcyclopolic acid, furochromanols and phthalides, pseurotin A (Brillinger et al., 1978; Achenbach et al., 1982a,b, 1985a,b; Mühlenfeld and Achenbach, 1988a,b; Samson et al., 2007) |
| Aspergillus felis: fumagillin, fumigaclavine C, fumitremorgin A and C, helvolic acid, monomethylsulochrin, pyripyropen A, E, O, S, trypacidin (reported as “A. viridinutans”, Tamiya et al., 2015, but A. viridinutans has a very different profile of extrolites, and many isolates reported as A. viridinutans have been shown to be A. felis; Barrs et al., 2013) |
| Aspergillus fennelliae: asperfuran, aszonalenins, fumigaclavines, viridicatumtoxin (Samson et al., 2007) |
| Aspergillus ferenczii: asperfuran, aszonalenins, fumigaclavine, fumigatins, cf. gliotoxin, viridicatumtoxin (Samson et al., 2007) |
| Aspergillus fischeri: 5-N-acetylardeemin, 5-N-acetyl-15b-didehydroardeemin, 5-N-acetyl-16-hydroxyardeemin, acetylaszonalenin, ardeemin, aszonalenin, aszonapyrone A, B, cottoquinazolin E & F, cyclotryprostatin B, 12α,13α-dihydroxyfumitremorgin C, rel-(8S)-19,20-dihydroxy-8-methoxy-9,18-epifumitremorgin C, fiscalin A, B, C, fischerin, 1-formyl-5-hydroxyaszonalenin, fumitremorgin A, B, C, helvolic acid, 6-hydroxyaszonalenin, 15b-β-hydroxy-5-N-ardeemin, isoterrein, neofipiperazine A, B, C, neosartorin, nortryptoquivalone, 13-oxofumitremorgin B, pyripyropene A, pyripyrone S, sarcins, sartorypyrone B & D, sesterfischeric acid, sesterfischerol, terrein, TR-2, trypacidin, tryptoquivalines, verruculogen (Samson et al., 1990; Wong et al., 1993; Wakana et al., 2006; Samson et al., 2007; Yin et al., 2009; Eamvijarn et al., 2013a; Lee et al., 2013; Gomes et al., 2014; Shan et al., 2014; Sodngam et al., 2014) (as “Xylaria humosa”) (Zhang et al., 2014; Zheng et al., 2014; Kaifuchi et al., 2015; Shan et al., 2015; Ye et al., 2015). There are indications that A. fischeri can also produce fumagillin (Lin et al., 2013; Wiemann et al., 2013) |
| [fiscalin B, helvolic acid, helvolinic acid, 27-epi-nortryptoquivaline, setosusin, 2-(1-oxo-2-hydroxyethyl)furan, 27-epi-tryptoquivaline, was found in “Corynascus setosus,” which is probably an Aspergillus fischeri or alternatively the Corynascus culture was overgrown by A. fischeri; Fujimoto et al., 1996] |
| [cladoquinazoline, epi-cladoquinazoline, CS-C, deoxynortryptoquivaline, deoxytryptoquivaline, glyantrypine, 3-hydroxyglyantrypine, norquinadoline A, oxoglyantrypine, prelapatin B, quinadoline A, B, tryptoquivaline was reported from a Cladosporium sp., but the culture may have been overgrown with an Aspergillus fischeri; Peng et al., 2013] |
| Aspergillus fumigatiaffinis: auranthine, cycloechinuline, fumigaclavines, helvolic acid, neosartorin, palitantin, pyripyropen A, E, O, S, tryptoquivalins (Samson et al., 2007; Ola et al., 2014) |
| Aspergillus fumigatus: Wang compound 1,2, 3, Zhao compound 1, 2, 3, Zuck compound 1,2,3, N-acetyltyramine, asperfumigatin, asperfumin, asperfumoid, azaspirene, bisdechlorogeodin, chaetominine, bisdethio(bismethylthio)gliotoxin, brevianamide F, 4-carboxy-5,5′-dihydroxy-3,3′-dimethyldiphenylether, cephalimycin A, B, C, D, 2-chloro-1,3,8-trihydroxy-6-methyl-9-anthrone, cyclo-(Ala-Val), cyclotryprostatin A, B, C, D, cyclo(L-4-hydroxyproline-L-leucine), cyclo(L-4-hydroxyproline-L-phenylalanine), cyclo(L-Pro-L-Pro), cyclo(L-Pro-L-Gly), cyclo(L-Pro-L-Leu), cyclo(L-Pro-L-Pro), cyclo(L-Pro-L-Val), cyclo(L-Val-L-Leu), cyclotrypostatin C, 9-deacetoxyfumigaclavine C, 9-deacetylfumigaclavine C, 13-dehydroxycyclotryprostatin C, demethoxyfumitremorgin C, (4S,5S,6S,8S,9S,10R,13R,14S,16S,17Z)-6,16-diacetoxy-25-hydroxy-3,7-dioxy-29-nordammara-1,17(20)-dien-21-oic acid, didehydrobisdethiobis(methylthio)gliotoxin, difructosedianhydride, 1,2-dihydrohelvolic acid, 12,13-dihydroxyfumitremorgin C = TR-3, 2,3-dihydroxy-5-methyl-1,4-benzoquinone, 5,8-dihydroxy-9,12-octadecadienoic acid, 2,6-dihydroxyphenylacetic acid, dimethoxyfumitremorgin C, emodin, emodin 1,6-dimethylether, epoxysuccinic acid, ferrichrome C, festuclavine, FD-889, FK-463, fumagillin, fumagiringillin, fumifungin, fumigaclavine A, B, C,D, E, F, G, H (fumigaclavine A reported also from A. tamarii, but this was an A. fumigatus, Janardhanan et al., 1984), fumigatin, fumigatin chlorohydrin, fumigatin oxide, fumigatin quinol, fumigatonin (identity of producer not verified), fumigatoside B, C, and D, fumipyrrole, fumiquinazolin A, B, C, D, E, F, G, J, and K, fumiquinone A and B, fumitremorgin A, B, C, and D, (GERI-BP002-A), fusarinine C, glionitrin A and B, gliotoxin, gliotoxin E and G, helvolic acid, helvolinic acid, hexahydropolyprenol-18, 19, 20, 21, 22, 23, 24, 3-β-hydroxy-cyclo-L-tryptophyl-L-proline, 2-hydroxy-3-methoxy-5-methyl-1,4-benzoquinone, N-(2-(4-hydroxyphenyl)ethenyl)formamide, 14-hydroxyterezine D, 3-hydroxytoluquinone, 20-hydroxytryrpostatin B, isochaetominin, isorhodoptilometrin, LL-S490β, 6-methoxyspirotryprostatin B, 8′-O-methylasterric ac id, 11-O-methylpseurotin, monomethylsulochrin, orsellinic acid, 13-oxofumitremorgin B, 18-oxotryprostatin A, 13-oxo-verruculogen, 14-norpseurotin A, N-prenyl-cyclo-L-tryptophyl-L-proline, pseurotin A, A1, A2, D, F1, F2, pyripyropen A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P, Q, R, and S, questin, RK-95113, cis and trans-ruakuric acid (Cutler et al., 1996, identity of fungus could not be checked), Sch-528647, sphingofungin A, B, C, D, spinulosin, spinulosin hydrate, spinulosin quinol hydrate, spirotryprostatin A, B, C, D, and E, synerazol, terezine D, TR-2, 1,2,3,4-tetrahydroxy-5-methylbenzene, 4,8,10,14-tetramethyl-6-acetoxy-14-[16-acetoxy-19-(20,21-dimethyl)-18-ene]phenanthrene-1-ene-3,7-dione, 3-thiomethyl-cyclo(Ser, Phe), triacetylfusarinine, tryptostatin A, B, trypacidin, 1,2-seco-tryprostatin, tryptoquivaline F, G, H, I, J, L, M, and N, tryptoquivalin R?, S?, verruculogen (Samson et al., 1990; Land et al., 1993; Cui et al., 1996; Tepsic et al., 1997; Furtado et al., 2005; Han et al., 2007a,b; Samson et al., 2007; Wang et al., 2008; Zhang et al., 2008 (as “A. sydowi”); Frisvad et al., 2009; Zhao et al., 2010; Afiyatullov et al., 2012; Zhang et al., 2012; Cano et al., 2013; Ding et al., 2013; Zhou et al., 2013; Alcazar-Fuoli et al., 2014; Haas, 2014; Kim et al., 2014; Owens et al., 2014; Wiekmann et al., 2014; Xu et al., 2014; Liang et al., 2015; Liu et al., 2015; MacHeleidt et al., 2015; Tamiya et al., 2015; Xie et al., 2015) |
| [Aspergillus fumigatus (fungus misidentified): antafumicin A and B, cytochalasin E, expansolide A and B Macías et al., 2003, the strain used was misidentified and was an Aspergillus clavatus; isosclerone Li et al., 2014] |
| Aspergillus fumisynnematus: cyclopiazonic acid, fumimycin, neosartorin, pyripyropens (Kwon et al., 2007; Samson et al., 2007) |
| Aspergillus galapagensis: gregatins (Samson et al., 2007) |
| Aspergillus hiratsukae: avenaciolide (Samson et al., 2007) |
| Aspergillus huiyaniae: N.E. (Matsusawa et al., 2014a) |
| Aspergillus indohii: N.E. (Horie et al., 2003) |
| Aspergillus laciniosus: aszonalenins, aszonapyrone A and B, 3′-(4-oxoquinazolin-3-yl)spiro[1H-indol-3,5′-oxolane]2,2′-dione, 4(3H)-quinazoline, tryptoquivaline L & T (Samson et al., 2007; Eamvijarn et al., 2013a; Gomes et al., 2014) |
| Aspergillus lentulus: auranthine, cyclopiazonic acid, fumifungin, fumigaclavine A, B, C, fumiquinazoline F or G, monomethylsulochrin, neosartorin, pyripyropen A, E, O, S, sphingofungin A, B, C, D, terrein, trypacidin (Larsen et al., 2007; Samson et al., 2007,: Frisvad et al., 2009; Tamiya et al., 2015) |
| Aspergillus marvanovae: apolar indoloterpenes (Hubka et al., 2013) |
| Aspergillus multiplicatus: aszonapyrone A, helvolic acid (Samson et al., 2007) |
| Aspergillus neoglaber: asperpentyn, avenaciolide, glabramycin A, B, C, Mer-NF8054A, Mer-NF8054X, NK-372135A, B, C, sartoryglabrin A, B, C, wortmannins (Ellis et al., 1964; Morino et al., 1994; Samson et al., 2007; Jayasuriya et al., 2009; Kijjoa et al., 2011) |
| Aspergillus nishimurae: Anishidiol, 4-hydroxybenzaldehyde, 4-methylbenzylalcohol, monochaetin (Hosoe et al., 2011) |
| Aspergillus novofumigatus: Dihydroterrein, epi-aszonalenin A, B, C, ent-cycloechinulin, dihydroterrein, fiscalins, helvolic acid, neosartorin, novoamauromin, novobenzomalvin A, B, C, novofumigatamide, novofumigatonin, palitantin, terrein, territrem B (Rank et al., 2006; Samson et al., 2007; Rank et al., 2008; Hosoe et al., 2009; Ishikawa et al., 2010a,b, 2011) |
| Aspergillus otanii = A. fennelliae (Takeda et al., 2001; Samson et al., 2007) |
| Aspergillus papuensis: wortmannins (Samson et al., 2007) |
| Aspergillus parafelis: N.E. (Sugui et al., 2014) |
| Aspergillus paulistensis [= A. spinosus according to Samson et al. (2007)]: 3′-(4-oxoquinazolin-3-yl)spiro[1H-indol-3,5′-oxolane]2,2′-dione, 4(3H)-quinazoline, sartorypyrone C, tryptoquivaline L (Horie et al., 1995; Gomes et al., 2014) |
| Aspergillus pernambucoensis: N.E. (Matsusawa et al., 2014b) |
| Aspergillus primulinus = A. quadricinctus (Samson et al., 2007) |
| Aspergillus pseudofelis: N.E. (Sugui et al., 2014) |
| Aspergillus pseudoviridinutans: N.E. (Sugui et al., 2014) |
| Aspergillus quadricinctus: aszonalenins, PF1223, quinolactacin (Ozoe et al., 2004; Samson et al., 2007) |
| Aspergillus qizutongii: N.E. (Li et al., 1998) |
| Aspergillus shendawei: N.E. (Yaguchi et al., 2010) |
| Aspergillus siamensis: chevalone B, C, 4-dihydroxy-3-methylacetophenone, fiscalin A, C, epi-fiscalin A, C, neofiscalin A, epi-neofiscalin A, 3′-(4-oxoquinazolin-3-yl)spiro[1H-indole-3,5′-oxolane]-2,2′-dione, sartorymensin, tryptoquivaline, tryptoquivaline F, H, L, O (Buttachon et al., 2012; Eamvijarn et al., 2013b) |
| Aspergillus similanensis: chevalone, B, C, E, 6,8-dihydroxy-3,7-dimethylisocoumarin, 6,8-dihydroxy3-methylisocoumarin, p-hydroxybenzaldehyde, 5-hydroxy-8-methyl-2H,6H-pyrano[3,4-g]chromene-2,6-dione, pyripyropen E, S, and T, reticulol, S14-95, similanamide, similanpyrone C (Prompanya et al., 2014, 2015) |
| Aspergillus solicola: aszonalenins, chromanols, tryptoquivalines, tryptoquivalones, wortmannins (Samson et al., 2007, 2014) |
| Aspergillus spathulatus: aszonalenins, xanthocillins (Samson et al., 2007) |
| Aspergillus spinosus: aszonalenins, pseurotins, 2-pyrovoylaminobenzamide, fumigachlorin (Atsumi et al., 1970; Samson et al., 2007) |
| Aspergillus stramenius: avenaciolide, quinolactacin (Samson et al., 2007) |
| Aspergillus sublevisporus: N.E. (Someya et al., 1999) |
| Aspergillus takakii = A. spinosus (?) (Samson et al., 2007): acetylaszonalenin, aszonalenin, aszonapyrone A, chevalone B, 6-hydroxymellein, 3′-(4-oxoquinazolin-3-yl) spiro[1H-indole-3,5′-oxalone]-2-2′-dione, tryptoquivaline F, H, L, U (Zin et al., 2015) |
| Aspergillus tatenoi: aszonalenin, aszonapyrone A, B, tatenoic acid (Samson et al., 2007; Yim et al., 2014) |
| Aspergillus thermomutatus: 6-acetylbis(methylthio)gliotoxin, acetylgliotoxin, asperfuran, bisdethiobis(methylthio)gliotoxin, bis-N-norgliovictin, brasilianamide B, cadinene, CJ-12662?, 3,8-diacetyl-4-(3-methoxy-4,5-methylenedioxy)benzyl-7-phenyl-6-oxa-3,8-diazabicyclo[3.2.1]octane, didehydrobisdethiobis(methylthio)gliotoxin, euchevalierine, fiscalins (?), fischerindoline, gliotoxin, helvolic acid, 3-hydroxy-5-methylphenyl-2,4-dihydroxy-6-methylbenzoate, N-methyl-1H-indole-2-carboxamide, neosartorin A, B, C, pseudofischerine, pyripyropen A, E, O, S, 1,2,3,4-tetrahydro-2,3-dimethyl-1,4-dioxopyrazino[1,2-a]indole, 1,2,3,4-tetrahydro-2-methyl-3-methylene-1,4-dioxopyrazino[1,2-a]indole, 1,2,3,4-tetrahydro-2-methyl-1,3,4-trioxopyrazino[1,2-a]indole, (tryptoquivalin R, S ?) [maybe: misidentified as “Eurotium chevalieri”: cadinene, chevalone A, B, C, D, aszonapyrone A, B, euchevalerine, CJ-12662 Kanokmedhakul et al., 2011] (Samson et al., 2007; Eamvijarn et al., 2012; Masi et al., 2013; Xu et al., 2013; Liang et al., 2014) |
| Aspergillus tsunodae: helvolic acid, sartorypyrone A and B (Yaguchi et al., 2010; Eamvijarn et al., 2013a; Gomes et al., 2014) |
| Aspergillus tsurutae: N.E. (Horie et al., 2003) |
| Aspergillus turcosus: aszonalenins, gliotoxin, kotanins (Samson et al., 2007; Hubka et al., 2013) |
| Aspergillus udagawae: fumagillin, fumigaclavine A and C, fumigatins, fumiquinazolin F or G, helvolic acid, monomethylsulochrin, pyripyropene A, E, trypacidin, tryptoquivalines, tryptoquivalones (Samson et al., 2007; Tamiya et al., 2015) |
| Aspergillus unilateralis: aszonapyrones, mycophenolic acid (Samson et al., 2007; Hubka et al., 2013) |
| Aspergillus viridinutans: 4-acetyl-6,8-dihydroxy-5-methyl-2-benzopyran-1-1 A, 13-O-methylviriditin, phomaligin A, SC-28763, SC-30532, semiviriditoxin, viriditoxin, viritin, viriditin, wasabidienone B0 and B1 (Omolo et al., 2000; Samson et al., 2007) |
| Aspergillus waksmanii: apolar indoloterpenes (Hubka et al., 2013) |
| Aspergillus wangduanglii: N.E. (Li et al., 1998) |
| Aspergillus wyomingensis: N.E. (Novaková et al., 2014) |
N.E: Not Examined.
Chemotaxonomy of Aspergillus subgenus Fumigati
Species in subgenus Fumigati can produce many different extrolites (Frisvad and Samson, 1990; Samson et al., 2007; Stack et al., 2007; Varga et al., 2007; Frisvad et al., 2009; Sanchez et al., 2012; Kang et al., 2013; Frisvad and Larsen, 2015) of which some are specific to section Fumigati, while others are shared with the closely related section Clavati and the Dichotomomyces clade. Aspergillus cejpii in the Dichotomomyces clade produces gliotoxin, acetylgliotoxin, acetylgliotoxin G, bis(dethio)bis(methylthio)gliotoxin, fiscalin B, xanthocillin X monomethylether, tryptoquivalones, emindole SB, emindole SB β-mannoside, and 27-O-methylasporyzin (Varga et al., 2007; Harms et al., 2014; Rodrigues et al., 2015) possibly in addition to asporyzin A-C, emeniveol, JBIR-03, and asporyergosterol and other sterols (Qiao et al., 2010a,b). The producing strain of the latter exometabolites was probably misidentified as A. oryzae, since none of these exometabolites have ever been found in A. oryzae (Rank et al., 2012). Apart from some few other shared extrolites with Aspergillus species in other sections, most extrolites are unique to section Fumigati.
Aspergillus section Clavati contains species mostly associated to dung, and have not been reported to cause infections of vertebrate lungs (Varga et al., 2007). Species in Aspergillus section Clavati produce several bioactive extrolites, but few of these are found in Aspergillus section Fumigati. Examples of such Aspergillus section Clavati specific extrolites include patulin, cytochalasin E and K, antafumicins, expansolides, and clavatols, and these extrolites may be important for competition in a dung habitat, rather than in the compost habitats in which species of Aspergillus section Fumigati thrives. Some similar extrolites are in common between species in Aspergillus sections Fumigati and Clavati, however. Ribotoxins like the sarcins in Aspergillus section Clavati (Varga and Samson, 2008) are closely related to mitogillin and restrictocin in Aspergillus section Fumigati (Kao et al., 2001; Schwienbacher et al., 2005; Virágh et al., 2014). Furthermore, some tryptoquivalins are produced by species in both Aspergillus sections.
Like other filamentous fungi, A. fumigatus isolates produce extrolites in a species specific manner (Larsen et al., 2005; Frisvad et al., 2008), but some strains do not produce all the extrolites expected. This weaker exometabolic vigor is most pronounced in isolates directly isolated from patients (Frisvad and Samson, 1990; Tamiya et al., 2015). These isolates are often floccose and less strongly sporulating. However, isolates from natural habitats, such as compost, always sporulate heavily and produce most of the expected species specific extrolites (Frisvad and Samson, 1990; Tepsic et al., 1997; Hong et al., 2010a,b). Production of small molecule extrolites is depending on the growth conditions and the growth media (Nielsen et al., 2011; Frisvad, 2012; Brakhage, 2013), and some of these extrolites may need biological / chemical stimulants of the producing fungus to be expressed (Brakhage and Schroeckh, 2011; Zuck et al., 2011; Netzker et al., 2015).
Being species specific, the difference between the extrolites profiles of different species of Aspergillus section Fumigati can be used in identification of the species in Aspergillus section Fumigati as an alternative to sequence—based or MALDI-TOF based identification (Panda et al., 2015), or used together with morphology and physiology in a polyphasic identification approach (Samson et al., 2007). For example A. fumigatus can be distinguished from A. lentulus by exometabolite profiling (Larsen et al., 2007), MALDI-TOF (Verwer et al., 2014), and sequencing (Balajee et al., 2005a; Samson et al., 2007), but only partially by morphology and Raman spectroscopy (Verwer et al., 2014).
Extrolites produced by Aspergillus fumigatus and other pathogenic species in Fumigati
A. fumigatus has been reported to produce many different extrolites that are bioactive and may contribute to infection in humans and other animals (Amitani et al., 1995; Tomee and Kauffman, 2000; Reeves et al., 2006; Cramer et al., 2009; Abad et al., 2010; Coleman et al., 2011). Melanins are polyketide derived conidium pigments that may have an influence on the infection process (Tsai et al., 1998; Jahn et al., 2000; Tsai et al., 2001; Langfelder et al., 2003). Since all species in Aspergillus section Fumigati produce green conidia, it is expected that they all contain melanin (Perrin et al., 2007). Another more general small molecule pathogenicity factor is siderophores, of which A. fumigatus produces fusarinine C and triacetylfusarinine C extracellularly (Haas, 2014; Petrik et al., 2014). Furthermore hydrophobins are also present in all species of Aspergillus section Fumigati (Geiser et al., 1998; Pedersen et al., 2011). These proteins will protect conidia from being recognized by the immune system in mammals (Aimanianda et al., 2009). Other proteins, especially proteases also play a role in the infection process and may be expected to be produced by many pathogenic species (Tomee and Kauffman, 2000; Abad et al., 2010; Dhingra et al., 2012). Small molecule siderophores are also considered to be important pathogenicity factors, and given the general importance for fungi they can be expected to be produced by all pathogenic species of Aspergillus (Fedorova et al., 2008; Abad et al., 2010; Haas, 2014), but probably also by non-pathogenic species.
However, other extrolites are not produced by all species in Aspergillus section Fumigati. Gliotoxin has long been known to be important for the infection process by inhibiting the immune response, phagocytosis and angiogenesis (Watanabe et al., 2003, 2004; Tsunawaki et al., 2004; Bok et al., 2005; Lewis et al., 2005; Stanzani et al., 2005; Coméra et al., 2007; Sugui et al., 2007; Ben-Ami et al., 2009; Abad et al., 2010). Gliotoxin has been reported from the pathogenic species A. fumigatus and A. thermomutatus, but also from A. denticulatus, A. ferenczii and A. turcosus (Table 1) the latter three not yet known to be pathogenic. Annotation of the genomes of A. fumigatus and A. fischeri indicates that the latter species can also produce gliotoxin given the right conditions (Inglis et al., 2013). However, many other Aspergillus section Fumigati extrolites appear to be involved in pathogenesis. Verruculogen, produced by A. fumigatus and A. fischeri, modifies electrophysical properties of the human nasal epithelial cells (Khoufache et al., 2007) but is also a potent tremorgen (Land et al., 1993; Kelman et al., 2004). Verruculogen and fumitremorgin C (Rabindran et al., 2000) are produced by A. fumigatus and A. fischeri (Table 1) in section Fumigati. Fumagillin suppresses the immune response, neutrophil function and angiogenesis (Fallon et al., 2010, 2011) and is produced by the pathogenic species A. felis, A. fumigatus, and A. udagawae, but also by species in Aspergillus section Fumigati, such as A. aureolus and A. viridinutans that have not been reported as yet to be pathogenic (Table 1). Pseurotin A is an inhibitor of immunoglobulin E and is responding to hypoxia (Schmeda-Hirschmann et al., 2008; Ishikawa et al., 2009; Vödisch et al., 2011). Pseurotins are produced by the pathogenic A. fumigatus and A. spinosus, but are also produced by A. duricalis and A. aureolus (Table 1). Sulochrin inhibits eosinophil activation (Ohashi et al., 1997, 1998) and is produced by four pathogenic species in section Fumigati: A. felis, A. fumigatus, A. lentulus, and A. udagawae (Table 1). The related asterric acid is produced by the same species and this extrolite inhibits vascular endothelial growth factor induced tube formation (Lee et al., 2013). Another related extrolite is trypacidin, which is cytotoxic (Gauthier et al., 2012), but was originally isolated as an antiprotozoan metabolite (Balan et al., 1963). The fumiquinazolins are also cytotoxic (Lim et al., 2014), and are produced consistently by A. fumigatus (Frisvad et al., 2009). The fumiquinazolines (Takahashi et al., 1995) are produced by the pathogenic A. fumigatus and A. lentulus, but are also produced by A. aureolus (Table 1). The chemically similar fiscalins and cottoquinazolins (norfumiquinazolins; Ames and Walsh, 2010; Shan et al., 2015) are produced by A. fischeri, indicating that these metabolites are of importance for the competitiveness of these fungi. The pyripyropenes have antiangiogenic activity (Hayashi et al., 2009) and are produced by nearly all the known pathogenic species in section Fumigati: A. fumigatus, A. fumigatiaffinis, A. fumisynnematus, A. lentulus, A. thermomutatus, and A. udagawae (Table 1). In addition pyripyropens are produced by A. similanensis, a species that has not yet been tested for pathogenicity or isolated from any animal tissues.
Helvolic acid has been reported as an antibiotic and antifungal extrolite (Rementeria et al., 2005), but it also has been reported to affect human respiratory epithelium (Amitani et al., 1995) and the metabolism of macrophages (Shinohara et al., 1992). Helvolic acid has been reported from Aspergillus auratus, A. aureolus, A. felis, A. fischeri, A. fumigatiaffinis, A. fumigatus, A. multiplicatus, A. novofumigatus, A. thermomutatus, A. tsunodae, and A. udagawae. It is upregulated with gliotoxin in A. fumigatus (O'Keeffe et al., 2014). Thus helvolic acid may also be a pathogenicity factor, but of the species listed above A. auratus, A. aureolus, A. multiplicatus, and A. tsunodae have not been reported as pathogenic. Among bioactive proteins it seems that mitogillin is playing a role in the infection process (Schwienbacher et al., 2005; Abad et al., 2010), but these ribotoxins have not been screened in the other 62 species in Aspergillus section Fumigati.
Several small molecule extrolites have not yet been claimed to be involved in pathogenesis. The fumigaclavines are produced by the pathogenic species A. felis, A. fumigatus, A. fumigatiaffinis, and A. lentulus, but are also produced by A. fennelliae and A. ferenczii (Table 1). Even though these ergot alkaloids are associated with conidiation in A. fumigatus (Coyle et al., 1981), their role in animal pathogenesis is unknown. The fumigatins have mostly been found in soil-borne strains of A. fumigatus (Frisvad and Samson, 1990), and may rather have a role in competitiveness in compost and soil, than in animal pathogenesis.
Prediction of other potential opportunistic pathogenic species in Aspergillus section Fumigati based on extrolites
Among the 63 species described in Aspergillus section Fumigati, 17 have until now been reported to be opportunistic pathogens of vertebrate animals (in bold, Table 1). Several extrolites have been shown to have a certain role in the infection process, but these extrolites may have a different role in the natural habitats of these fungi, of which plant compost may be the primary habitat (Latgé, 1999; Abad et al., 2010). It appears that when growing on plant compost these fungi need a certain profile of extrolites (small molecule extrolites and exoproteins), while as vertebrate opportunistic pathogens they may need quite a different profile of extrolites (Abad et al., 2010). For example cellulases would be important in the compost situation (Srivastava et al., 2014; Miao et al., 2015), while hemolysins are probably only important for the vertebrate infection process (Abad et al., 2010). The same would be the case for antifungals and antibiotics, especially anti-streptomycete metabolites, as A. fumigatus and other members of Aspergillus section Fumigati are thermotolerant / thermophilic species competing with other thermotolerant and thermophilic species of fungi and bacteria (Langarica-Fuentes et al., 2014). Several species, such as A. assulatus, A. australensis, A. brevipes, A. “coreanus,” A. duricaulis, A. fennelliae, A. galapagensis, A. neoglaber, A. marvanovae, A. nishimurae, A. papuensis, A. quadricinctus, A. solicola, A. spathulatus, A. tatenoi, A. unilateralis, A. viridinutans, and A. waksmanii produce few if any of the extrolites suspected to play a role in the infection process, and so would not be predicted to be potential opportunistic pathogens of vertebrates. Some species, such as A. auratus, A. denticulatus, A. similanensis, A. tsunodae, and A. turcosus only produce one of the extrolites believed to play a role in pathogenesis, and may or may not be prospective vertebrate pathogens. Finally A. aureolus, A. ferenczii, and A. siamensis produce several of the extrolites potentially involved in pathogenesis, and thus may be predicted to be potential opportunistic vertebrate pathogens.
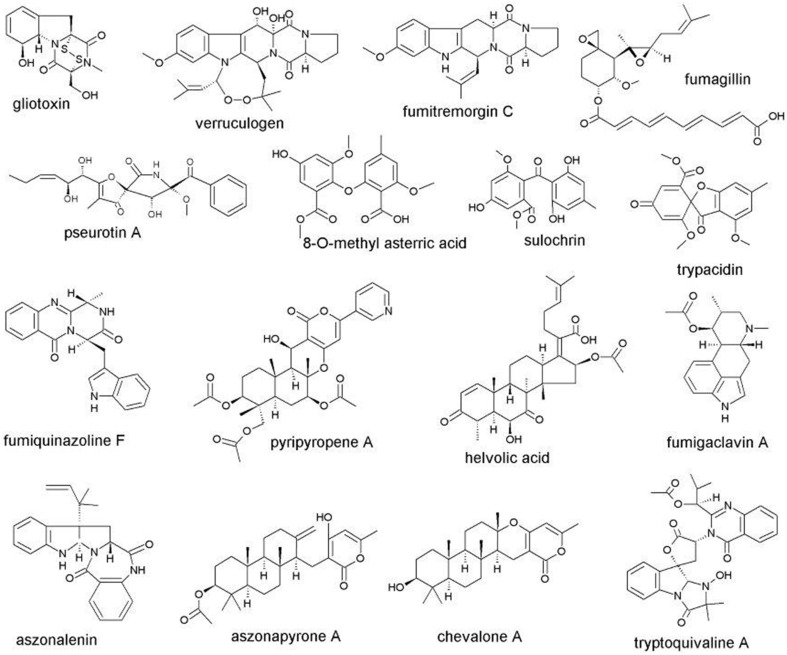
The many extrolites that have been suspected to be pathogenicity factors and are produced by species in Aspergillus section Fumigati are biosynthetically derived from polyketides, amino acids, terpenes, shikimic acid or are of mixed biosynthetic origin. The formula of some of the most important extrolites common in Aspergillus section Fumigati are shown in Figure 1. Some of the extrolites are not produced in the same patterns in different species in Aspergillus section Fumigati. While A. fumigatus produces fumiquinazolins A–G, J, and K, A. fischeri produces the related norfumiquinazolins (Shan et al., 2015). These extrolites may have the same function, even though they are chemically somewhat different. Whether such proxy-extrolites have the same function for pathogenicity in vertebrates is unknown. It is known, however, that other opportunistic pathogenic aspergilli in other sections of Aspergillus produce secondary metabolites that are biosynthetically and functionally closely related. While A. fumigatus, A. thermomutatus, and other species in section Fumigati produce gliotoxin, A. flavus in Aspergillus section Flavi can produce aspirochlorine and A. terreus in Aspergillus section Terrei can produce acetylaranotin (Frisvad and Larsen, 2015). While not identical to gliotoxin, these epidithiodioxopiperazines could be predicted to play a role in pathogenicity of A. flavus and A. terreus. The reports that A. niger, A. flavus, and A. terreus could produce gliotoxin (Lewis et al., 2005; Kupfahl et al., 2008) have not been confirmed (Samson et al., 2011; Varga et al., 2011a,b).
Figure 1.
Structures of the most important extrolites from Aspergillus section Fumigati potentially involved in pathogenesis.
Close phylogenetic relationships seem to be less suited pathogenicity predictors. For example A. viridinutans seems to be non-pathogenic, while the closely related A. felis is pathogenic (Barrs et al., 2013; Novaková et al., 2014; Sugui et al., 2014). Good growth at 37°C also seems to be a contributing factor to pathogenicity, and for example A. brevipes, A. duricaulis and A. viridinutans grow relatively poorly at 37°C, and in addition are not considered potentially pathogenic Aspergillus species in section Fumigati, based on extrolite evidence and absence of reports of pathogenicity. However, while there a many data on the involvement of exometabolites for A. fumigatus (Abad et al., 2010), there are few data on production of exoproteins for other opportunistic pathogenic species such as A. thermomutatus.
Genome sequencing and systematic comparison of the genomes and transcriptomes of other members of Aspergillus section Fumigati may help in predicting which pathogenicity factors are especially important (Galaghan et al., 2005; Nierman et al., 2005; Wortman et al., 2006; Fedorova et al., 2008; McDonagh et al., 2008; Chooi et al., 2013; Inglis et al., 2013; Cerqueira et al., 2014; Kusuya et al., 2015; Lind et al., 2015). These data should be compared to phenotypic data such as profiles of large and small molecule extrolites, growth temperatures, carbon dioxide tolerance etc.
Altogether, approximately one third of the species in Aspergillus section Fumigati are common pathogenic species, one third are rare species of unknown pathogenicity and one third are predicted to be non-pathogenic, based on their production of relatively few exometabolites. Exometabolite pathogenicity factors found in the successful opportunistic pathogenic fungus A. fumigatus may have proxy-exometabolites with the same function in other species in that section, but also in less closely related pathogenic Aspergilli, especially species from sections Nigri, Terrei, Circumdati, and Flavi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Agilent technologies for Agilent Thought Leader Award # 2871 and the Novo Nordisk Foundation for grant NNF 13OC0005201.
References
- Abad A., Fernández-Molina J. V., Bikandi J., Ramírez A., Margareto J., Sendino J., et al. (2010). What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 27, 155–182. 10.1016/j.riam.2010.10.003 [DOI] [PubMed] [Google Scholar]
- Achenbach H., Mühlenfeld A., Brillinger G. U. (1985a). Stoffwechselprodukte von mikroorganismen. XXX. phthalide und chromanole aus Aspergillus duricaulis. Liebigs Annalen der Chemie 1985, 1596–1628. [Google Scholar]
- Achenbach H., Mühlenfeld A., Kohl W., Brillinger G. U. (1985b). Stoffwechselprodukte von mikroorganismen, XXXI [1]. Duricaulinsäure, ein neuer Naturstoff vom phthalimidin-Typ aus Aspergillus duricaulis. Z. Naturfors. 40b, 1219–1225. [Google Scholar]
- Achenbach H., Mühlenfeld A., Weber B., Brillinger G. U. (1982b). Highly substituted chromanols of Aspergillus duricaulis. Tetrahedron Lett. 23, 4659–4660. [Google Scholar]
- Achenbach H., Mühlenfeld A., Weber B., Kohl W., Brillinger G. U. (1982a). Stoffwechselprodukte von mikroorganismen. XXVII [1]. Cyclopaldsäure und 3-O-methylcyclopolsäure, zwei antibiotisch worksame Substanzen aus Aspergillus duricaulis. Z. Naturfors. 37b, 1091–1097. [Google Scholar]
- Afiyatullov S. S., Zhuravleva O. I., Antonov A. S., Kalinovsky A. I., Pivkin M. V., Mentinskaya E. S., et al. (2012). New metabolites from the marine-derived fungus Aspergillus fumigatus. Nat. Prod. Commun. 7, 497–500. [PubMed] [Google Scholar]
- Aimanianda V., Bayry J., Bozza S., Kniemeyer O., Perrucio K., Elluru S. R., et al. (2009). Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460, 1117–1121. 10.1038/nature08264 [DOI] [PubMed] [Google Scholar]
- Alcazar-Fuoli L., Cairns T., Lopen J. F., Zonja B., Pérez J., Barceló D., et al. (2014). A modified recombineering protocol for the genetic manipulation of gene clusters in Aspergillus fumigatus. PLoS ONE 9:e111875. 10.1371/journal.pone.0111875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhambra A., Catalán M., Moragues M. D., Brena S., Pontón J., Montejo J. C., et al. (2008). Isolation of Aspergillus lentulus in Spain from a critically ill patient with chronic obstructive pulmonary disease. Rev. Iberoam. Micol. 25, 246–249. 10.1016/S1130-1406(08)70058-5 [DOI] [PubMed] [Google Scholar]
- Álvarez-Pérez S., Mellado E., Serrano P., Blanco J. L., Garcia M. E., Kwon M., et al. (2014). Polyphasic characterization of fungal isolates from a published case of invasive aspergillosis reveals misidentification of Aspergillus felis as Aspergillus viridinutans. J. Med. Microbiol. 63, 617–619. 10.1099/jmm.0.068502-0 [DOI] [PubMed] [Google Scholar]
- Ames B. D., Walsh C. T. (2010). Anthranilate-activating modules from fungal non-ribosomal peptide assembly lines. Biochemistry 49, 3351–3365. 10.1021/bi100198y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitani R., Taylor G., Elezis E. N., Llwellyn-Jones C., Mitchell J., Kuza F., et al. (1995). Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect. Immun. 63, 3266–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabatsis M., Velegraki A. (2013). Sexual reproduction cycle in the opportunistic human pathogen Aspergillus terreus. Mycologia 105, 71–79. 10.3852/11-426 [DOI] [PubMed] [Google Scholar]
- Atsumi K., Takada M., Mizuno K., Ando T. (1970). Fumigachlorin, a new antifungal antibiotic. J. Antibiot. 23, 223–224. 10.7164/antibiotics.23.223 [DOI] [PubMed] [Google Scholar]
- Balajee S. A., Gribskov J., Brandt M., Ito J., Fothergill A., Marr K. A. (2005b). Mistaken identity: Neosartorya pseudofischeri and its anamorph masquerading as Aspergillus fumigatus. J. Clin. Microbiol. 43, 5996–5999. 10.1128/JCM.43.12.5996-5999.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balajee S. A., Gibskov J. L., Hanley E., Nickle C., Marr K. A. (2005a). Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryotic Cell 4, 625–632. 10.1128/EC.4.3.625-632.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balajee S. A., Nickle D., Varga J., Marr K. A. (2006). Molecular studies reveal frequent misidentification of Aspergillus fumigatus by morphotyping. Eukaryotic Cell 5, 1705–1712. 10.1128/EC.00162-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan J., Ebringer L., Nemec P., Kovac S., Dobias J. (1963). Antiprotozoal antibiotics. II. Isolation and characterization of trypacidin, a new antibiotic, active against Trypanosoma cruzi and Toxoplasma gondii. J. Antibiot. 16, 157–160. [PubMed] [Google Scholar]
- Barrs V. R., van Doorn T. M., Houbraken J., Kidd S. E., Martin P., Pinheiro M. D., et al. (2013). Aspergillus felis sp. nov., an emerging agent of aspergillosis in humans, cats, and dogs. PLOS ONE 8:e64871. 10.1371/journal.pone.0064871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R., Lewis R. E., Leventakos K., Kontoyiannis D. P. (2009). Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 114, 5393–5399. 10.1182/blood-2009-07-231209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J. W., Chung D., Balajee S. A., Marr K. A., Andes D., Nielsen K. F., et al. (2005). GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 74, 6761–6768. 10.1128/IAI.00780-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage A. A. (2013). Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 11, 21–32. 10.1038/nrmicro2916 [DOI] [PubMed] [Google Scholar]
- Brakhage A. A., Schroeckh V. (2011). Fungal secondary metabolites – strategies to activate silent gene clusters. Fungal Genet. Biol. 48, 15–22. 10.1016/j.fgb.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Brillinger G. U., Heberle W., Weber B., Achenbach H. (1978). Metabolic products of microorganisms. 167. Cyclopaldic acid from Aspergillus duricaulis. 1. Production, isolation and biological properties. Arch. Microbiol. 116, 245–252. 10.1007/BF00417847 [DOI] [PubMed] [Google Scholar]
- Buttachon S., Chandrapatya A., Manoch L., Silva A., Gales L., Bruyère C., et al. (2012). Sartorymensin, a new indole alkaloid, and new analogues of tryptoquivaline and fiscalins produced by Neosartorya siamensis (KUFC 6349). Tetrahedron 68, 3253–3262. 10.1016/j.tet.2012.02-024 [DOI] [Google Scholar]
- Cano P. M., Jamin E. L., Tadrist S., Bourdau'hui P., Pean M., Debrauwer L., et al. (2013). New untargeted metabolite profiling combining mass spectrometry and isotopic labelling: application to Aspergillus fumigatus grown on wheat. Anal. Chem. 85, 8412–8420. 10.1021/ac401872f [DOI] [PubMed] [Google Scholar]
- Cerqueira G. C., Arnaud M. B., Inglis D. O., Skrzypek M. S., Binkley G., Simison M., et al. (2014). The Aspergillus genome database: multispecies curation and incorporation of RNA-Seq data to improve structural gene annotations. Nucleic Acids Res. 42, D705–D710. 10.1093/nar/gkt1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chooi Y.-H., Fang J., Liu H., Filler S. G., Wang P., Tang Y. (2013). Genome mining of a prenylated and immunosuppressive polyketide from pathogenic fungi. Org. Lett. 15, 780–783. 10.1021/ol303435y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho D., Silva S., Vale-Silva L., Gomes H., Pinto E., Sarmento A., et al. (2011). Aspergillus viridinutans: an agent of adult chronic invasive aspergillosis. Med. Mycol. 49, 755–759. 10.3109/13693786.2011.556672 [DOI] [PubMed] [Google Scholar]
- Coleman J. J., Ghosh S., Okoli I., Mylonakis E. (2011). Antifungal activity of microbial secondary metabolites. PLoS ONE 6:e25321. 10.1371/journal.pone.0025321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coméra C., André K., Lafitte J., Collet X., Galtier P., Maridonneau-Parini I. (2007). Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signaling pathways in human neutrophils. Microb. Infect. 9, 47–54. 10.1016/j.micinf.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Coyle C. M., Kenaley S. C., Rittenour W. R., Panaccione D. G. (1981). Association of ergot alkaloids with conidiation in Aspergillus fumigatus. Mycologia 99, 804–811. 10.3852/mycologia.99.6.804 [DOI] [PubMed] [Google Scholar]
- Cramer R. A., Keats Shwab E., Jr., Keller N. P. (2009). Genetic regulation of Aspergillus secondary metabolites and their role in pathogenesis, in Aspergillus fumigatus and Aspergillosis eds Latgé J. P., Steinbach W. J. (Washington, DC: ASM Press; ), 185–199. ISBN 978-1-55581-438-0 [Google Scholar]
- Cui C. B., Kakeya H., Okada G., Onose R., Osada H. (1996). Novel mammalian cell cycle inhibitor, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. II. Physico-chemical properties and structures. J. Antibiot. 49, 527–533. 10.7164/antibiotics.49.527 [DOI] [PubMed] [Google Scholar]
- Cutler H. G., Lauren D. R., Wilkins A. L., Holland P. T., Hill R. A., Dugan F. M. (1996). Ruakuric acid: a natural product from Aspergillus fumigatus. Phytochemistry 43, 209–214. 10.1016/0031-9422(96)00224-5 [DOI] [PubMed] [Google Scholar]
- Dhingra S., Andes D., Calvo A. M. (2012). VeA regulated conidiation, Gliotoxin production, and protease activity in the opportunistic human pathogen Aspergillus fumigatus. Eukaryotic Cell 11, 1531–1543. 10.1128/EC.00222-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G.-Z., Liu J., Wang J.-M., Faing L., Yi S.-S. (2013). Secondary metabolites from the endophytic fungi Penicillium polonicum and Aspergillus fumigatus. J. Asian Nat. Prod. Res. 15, 446–452. 10.1080/10286020.2013.780349 [DOI] [PubMed] [Google Scholar]
- Eamvijarn A., Gomes N. M., Dethoup T., Buaruang J., Manoch L., Manoch L., et al. (2013a). Bioactive meroditerpenes and indole alkaloids from the soil fungus Neosartorya fischeri (KUFC 6344), and the marine-derived fungi Neosartorya laciniosa (KUFC 7896) and Neosartorya tsunodae (KUFC 9213). Tetrahedron 69, 8583–8591. 10.1016/j.tet.2013.07.078 [DOI] [Google Scholar]
- Eamvijarn A., Kijjoa A., Bruyère C., Mathieu V., Manoch L., Lefranc F., et al. (2012). Secondary metabolites from a culture of the fungus Neosartorya pseudofischeri and their in vitro cytostatic activity in human cancer cells. Planta Med. 78, 1767–1776. 10.1055/s-0032-1315301 [DOI] [PubMed] [Google Scholar]
- Eamvijarn A., Manoch L., Chamswarng C., Piasai O., Visarathanonth N., Langsa-ard J. J., et al. (2013b). Aspergillus siamensis sp. nov. from soil in Thailand. Mycoscience 54, 401–405. 10.1016/j.myc.2013.01.005 [DOI] [Google Scholar]
- Ellis J. J., Stodola F. H., Vesonder R. F., Glass C. A. (1964). C15H22O4 compound produced by the fungus Aspergillus fischeri var. glaber. Nature 203:1382 10.1038/2031382a0 [DOI] [Google Scholar]
- Fallon J. P., Reeves E. P., Kavanagh K. (2010). Inhibition of neutrophil function following exposure to the Aspergillus fumigatus toxin fumagillin. J. Med. Microbiol. 59, 625–633. 10.1099/jmm.0.018192-0 [DOI] [PubMed] [Google Scholar]
- Fallon J. P., Reeves E. P., Kavanagh K. (2011). The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology 157, 1481–1488. 10.1099/mic.0.043786-0 [DOI] [PubMed] [Google Scholar]
- Fedorova N. D., Khaldi N., Joarder V. S., Maiti R., Amedeo P., Anderson M. J., et al. (2008). Genomic islands in in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 4:e1000046. 10.1371/journal.pgen.1000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad J. C. (2012). Media and growth conditions for induction of secondary metabolites, in Fungal Secondary Metabolism: Methods and Protocols eds Keller N. P., Turner G. (New York, NY: Humana Press; ), 47–58. [DOI] [PubMed] [Google Scholar]
- Frisvad J. C., Andersen B., Thrane U. (2008). The use of secondary metabolite profiling in fungal taxonomy. Mycol. Res. 112, 231–240. 10.1016/j.mycres.2007.08.018 [DOI] [PubMed] [Google Scholar]
- Frisvad J. C., Larsen T. O. (2015). Chemodiversity in the genus Aspergillus. Appl. Microbiol. Biotechnol. 99, 7859–7877. 10.1007/s00253-015-6839-z [DOI] [PubMed] [Google Scholar]
- Frisvad J. C., Rank C., Nielsen K. F., Larsen T. O. (2009). Metabolomics of Aspergillus fumigatus. Med. Mycol. 47, S53–S71. 10.1080/13693780802307720 [DOI] [PubMed] [Google Scholar]
- Frisvad J. C., Samson R. A. (1990). Chemotaxonomy and morphology of Aspergillus fumigatus and related species, in Modern Concepts in Penicillium and Aspergillus Classification eds Samson R. A., Pitt J. I. (New York, NY: Plenum Press; ), 201-208. [Google Scholar]
- Fujimoto H., Negishi E., Yamaguchi K., Nishi N., Yamazaki M. (1996). Isolation of new tremorgenic metabolites from an Ascomycete, Corynascus setosus. Chem. Pharm. Bull. 44, 1843–1848. 10.1248/cpb.44.1843 [DOI] [Google Scholar]
- Furtado N. A. J. C., Pupo M. T., Carvalho I., Campo V. L., Duarte M. C. T., Bastos J. K. (2005). Diketopiperazines produced by an Aspergillus fumigatus Brazilian strain. J. Braz. Chem. Soc. 16, 1448–1453. 10.1590/S0103-50532005000800026 [DOI] [Google Scholar]
- Galaghan J. E., Calvo S. E., Cuomo C., Ma L. J., Wortman J. R., Batzoglou S., et al. (2005). Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438, 1105–1115. 10.1038/nature04341 [DOI] [PubMed] [Google Scholar]
- Gauthier T., Wang X., Dos Santos J. S., Fysikopoulos A., Tadrist S., Canlet C., et al. (2012). Trypacidin, a spore-borne toxin from Aspergillus fumigatus, is cytotoxic to lung cells. PLoS ONE 7:e29906. 10.1371/journal.pone.0029906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser D. M., Frisvad J. C., Taylor J. W. (1998). Evolutionary relationships in Aspergillus section Fumigati inferred from partial beta-tubulin and hydrophobin DNA sequences. Mycologia 90, 832–846. 10.2307/3761325 [DOI] [Google Scholar]
- Geiser D. M., Klich M. A., Frisvad J. C., Peterson S. W., Varga J., Samson R. A. (2007). The current status of species recognition and identification in Aspergillus. Stud. Mycol. 59, 1–10. 10.3114/sim.2007.59.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes N. M., Bessa L. J., Buttachon S., Costa P. M., Buaruang J., Dethoup T., et al. (2014). Antibacterial and antibiofilm activities of tryptoquivalines and meroditerpenes isolated from the marine-derived fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the soil fungi N. fischeri and N. siamensis. Mar. Drugs 12, 822–839. 10.3390/md12020822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner K., Peršoh D., Weig A., Rambold G. (2014). Phialosimplex salinarum, a new species of Eurotiomycetes from a hypersaline habitat. IMA Fungus 5, 161–172. 10.5598/imafungus.2014.05.02.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarro J., Kallas E. G., Godoy P., Karenina A., Gene J., Stchigel A., et al. (2002). Cerebral aspergillosis caused by Neosartorya hiratsukae infection. Emerging Infect. Dis. 8, 989–991. 10.3201/eid0809.020073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyotoku H., Izumikawa K., Ikeda H., Takazono T., Morinaga Y., Nakamura S., et al. (2012). A case of bronchial aspergillosis caused by Aspergillus udagawae and its mycological features. Med. Mycol. 50, 631–636. 10.3109/13693786.2011.639036 [DOI] [PubMed] [Google Scholar]
- Haas H. (2014). Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 31, 1266–1276. 10.1039/C4NP00071D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Xu X., Cui C., Gu Q. (2007a). Alkaloidal compounds produced by a marine-derived fungus, Aspergillus fumigatus H1-04, and their antitumor activities. Chin. J. Med. Chem. 17, 232–237. [Google Scholar]
- Han X., Xu X., Cui C., Gu Q. (2007b). Diketopiperazines produced by a marine-derived fungus, Aspergillus fumigatus H1-04, and their antitumor activities. Chin. J. Med. Chem. 17, 155–159. [Google Scholar]
- Harms H., Rempel V., Kehraus S., Kaiser M., Hufendick P., Müller C. E., et al. (2014). Indoloterpenes from a marine-derived fungal strain of Dichotomomyces cejpii with an antagonistic activity at GPR18 and cannabinoid receptors. J. Nat. Prod. 77, 673–677. 10.1021/np400850g [DOI] [PubMed] [Google Scholar]
- Hawksworth D. L. (2012). Managing and coping with names of pleomorphic fungi in a period of transition. IMA Fungus 3, 15–24. 10.5598/imafungus.2012.03.01.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth D. L., Crous P. W., Redhead S. A., Reynolds D. R., Samson R. A., Seifert K. A., et al. (2011). The Amsterdam declaration on fungal nomenclature. IMA Fungus 2, 105–112. 10.5598/imafungus.2011.02.01.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Artai M., Fujita M., Kobayashi M. (2009). Pyripyropens, fungal sesquiterpenes conjugated with alpha-pyrone and pyridine moieties, exhibits anti-angiogenicitivity against human umbilical vein endothelial cells. Biol. Pharm. Bull. 32, 1261–1265. 10.1248/bpb.32.1261 [DOI] [PubMed] [Google Scholar]
- Hong S. B., Cho H. S., Shin H. D., Frisvad J. C., Samson R. A. (2006). New Neosartorya species isolated from soil in Korea. Int. J. Syst. Evol. Microbiol. 56, 439–442. 10.1099/ijs.0.63980-0 [DOI] [PubMed] [Google Scholar]
- Hong S. B., Go S. J., Shin H. D., Frisvad J. C., Samson R. A. (2005). Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97, 1316–1329. 10.3852/mycologia.97.6.1316 [DOI] [PubMed] [Google Scholar]
- Hong S. B., Kim D. H., Park I. C., Choi Y. J., Shin H. D., Samson R. A. (2010a). Re-identification of Aspergillus fumigatus sensu lato based on a new concept of species delimitation. J. Microbiol. 48, 607–615. 10.1007/s12275-010-0084-z [DOI] [PubMed] [Google Scholar]
- Hong S. B., Kim D.-H., Park I. C., Samson R. A., Shin H. D. (2010b). Isolation and identification of Aspergillus section Fumigati strains from arable soil in Korea. Mycobiology 38, 1–6. 10.4489/MYCO.2010.38.1.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. B., Shin H. D., Hong J., Frisvad J. C., Nielsen P. V., et al. (2008). New taxa of Neosartorya and Aspergillus in Aspergillus section Fumigati. Antonie van Leeuwenhoek 93, 87–98. 10.1007/s10482-007-9183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie Y., Abliz P., Fukushima K., Okada K., Takaki G. M. C. (2003). Two new species of Neosartorya from Amazonian soil, Brazil. Mycoscience 44, 397–402. 10.1007/S10267-003-0132-1 [DOI] [Google Scholar]
- Horie Y., Miyaji M., Nishimura K., Franco M. F., Colho K. I. R. (1995). Two new species of Neosartorya from Brazilian soil. Mycoscience 36, 159–165. 10.1007/BF02268552 [DOI] [Google Scholar]
- Horn B. W., Moore G. G., Carbone I. (2009). Sexual reproduction in Aspergillus flavus. Mycologia 103, 174–183. 10.3852/10-115 [DOI] [PubMed] [Google Scholar]
- Horn B. W., Olarte R. A., Peterson S. W., Carbone I. (2013). Sexual reproduction in Aspergillus tubingensis from section Nigri. Mycologia 105, 1153–1163. 10.3852/13-101 [DOI] [PubMed] [Google Scholar]
- Hosoe T., Mori N., Kamano K., Itabashi T., Yaguchi T., Kawai K. (2011). A new antifungal yellow pigment from Aspergillus nishimurae. J. Antibiot. 64, 211–212. 10.1038/ja.2010.132 [DOI] [PubMed] [Google Scholar]
- Hosoe T., Moriyama H., Wakana D., Itabashi T., Kawai K., Yaguchi T., et al. (2009). Inhibitory effects of dihydroterrein and terrain isolated from Aspergillus novofumigatus on platelet aggregation. Mycotoxins 59, 75–82. 10.2520/myco.59.75 [DOI] [Google Scholar]
- Houbraken J., de Vries R. P., Samson R. A. (2014). Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Adv. Appl. Microbiol. 86, 199–249. 10.1016/B978-0-12-800262-9.00004-4 [DOI] [PubMed] [Google Scholar]
- Houbraken J., Samson R. A. (2011). Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud. Mycol. 70, 1–51. 10.3114/sim.2011.70.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. J. (2014). Multiresistant aspergillosis due to cryptic species. Mycopathologia 178, 427–433. 10.1007/s11046-014-9774-0 [DOI] [PubMed] [Google Scholar]
- Hubka V., Peterson S. W., Frisvad J. C., Yaguchi T., Kubátová A., Kolaøik M., et al. (2013). Aspergillus waksmanii sp. nov. and Aspergillus marvanovae sp. nov., two new closely related species in section Fumigati. Int. J. Syst. Evol. Microbiol. 63, 763–789. 10.1099/ijs.0.047076-0 [DOI] [PubMed] [Google Scholar]
- Inglis D. O., Binkley J., Skrzypek M. S., Arnaud M. B., Cerqueira G. C., Shah P., et al. (2013). Comprehensive annotation of secondary biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol. 13:91. 10.1186/1471-2180-13-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Hosoe T., Itabashi T., Sato F., Wachi H., Nagase H., et al. (2011). Quinazolinobenzodiazepine derivatives, novobenzomalvins A-C: Fibronectin expression regulators from Aspergillus novofumigatus. Sci. Pharm. 79, 937–950. 10.3797/scipharm.1106-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Hosoe T., Itabashi T., Takizawa K., Yaguchi T., Kawai K. (2010b). Novofumigatamide, new cyclic tripeptide from Aspergillus novofumigatus. Heterocycles 81, 2143–2148. 10.3987/COM-10-12005 [DOI] [Google Scholar]
- Ishikawa K., Hosoe T., Itabashi T., Wakana D., Takizawa K., Yuguchi T., et al. (2010a). Novoamauromine and ent-cycloechinulin: two new diketopiperazine derivatives from Aspergillus novofumigatus. Chem. Pharm. Bull. 58, 717–719. 10.1248/cpb.58.717 [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Nionomiya T., Akabane H., Kushida N., Tsujiuchi G., Ohyama M., et al. (2009). Pseurotin A and its analogues as inhibitors of immunoglobulin E production. Bioorg. Med. Chem. Lett. 19, 1457–1460. 10.1016/j.bmcl.2009.01.029 [DOI] [PubMed] [Google Scholar]
- Jahn B., Boukhallouk F., Lotz J., Langfelder K., Wanner G., Brakhage A. A. (2000). Interaction of human phagocytes with pigmentless Aspergillus conidia. Infect. Immun. 68, 3736–3739. 10.1128/IAI.68.6.3736-3739.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhanan K. K., Satta A., Husain A. (1984). Production of fumigaclavine by Aspergillus tamarii Kita. Can. J. Microbiol. 30, 247–250. 10.1139/m84-036 [DOI] [PubMed] [Google Scholar]
- Jayasuriya H., Zink D., Basilio A., Vicente F., Collado J., Bills G., et al. (2009). Discovery and antibacterial activity of glabramycin A-C from Neosartorya glabra by an antisense strategy. J. Antibiot. 62, 265–269. 10.1038/ja.2009.26 [DOI] [PubMed] [Google Scholar]
- Kaifuchi S., Mori M., Nonaka K., Musauma R., Omura S., Shiomi K., et al. (2015). Sartorypyrone D: a new NADH-fumarate reductase inhibitor produced by Neosartorya fischeri FO-5897. J. Antibiot. 68, 403–405. 10.1038/ja.2014.167 [DOI] [PubMed] [Google Scholar]
- Kamei K., Watanabe A. (2005). Aspergillus mycotoxins and their effects on the host. Med. Mycol. 43, S95–S99. 10.1080/13693780500051547 [DOI] [PubMed] [Google Scholar]
- Kang D., Son G. H., Park H. M., Kim J., Choi J. N., Kim H. Y., et al. (2013). Culture condition-dependant metabolite profiling of Aspergillus fumigatus with antifungal activity. Fungal Biol. 117, 211–219. 10.1016/j.funbio.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Kano R., Itamoto K., Okuda M., Inokuma H., Hasegawa A., Balajee S. A. (2008). Isolation of Aspergillus udagawae from a fatal case of feline orbital aspergillosis. Mycoses 51, 360–361. 10.1111/j.1439-0507.2008.01493.x [DOI] [PubMed] [Google Scholar]
- Kano R., Shinahashi A., Fujino Y., Sakai H., Mori T., Tsujimoto H., et al. (2013). Two cases of feline orbital aspergillosis due to Aspergillus udagawae and A. viridinutans. J. Vet. Med. Sci. 75, 7–10. 10.1292/jvms.12-0119 [DOI] [PubMed] [Google Scholar]
- Kano R., Takahashi T., Hayawaka T., Yamayaka Y., Hasegawa A., Kamata H. (2015). The first case of feline sinonasal aspergillosis due to Aspergillus fischeri in Japan. J. Vet. Med. Sci. 77, 1183–1185. 10.1292/jvms.14-0454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanokmedhakul K., Kanokmedhakul S., Suwannatrai R., Soytong K., Prabpai S., Kongsaeree P. (2011). Bioactive meroterpenoids and alkaloids from the fungus Eurotium chevalieri. Tetrahedron 67, 5461–5468. 10.1016/j.tet.2011.05.066 [DOI] [Google Scholar]
- Kao R., Martinez-Ruiz A., del Poozo A. M., Crameri R., Davies J. (2001). Mitogillin and related fungal ribotoxins. Meth. Enzymol. 34, 324–335. 10.1016/S0076-6879(01)41161-X [DOI] [PubMed] [Google Scholar]
- Katz M. E., Dougall A. M., Weeks K., Cheetham B. F. (2005). Multiple genetically distinct groups revealed among clinical isolates identified as atypical Aspergillus fumigatus. J. Clin. Microbiol. 43, 551–555. 10.1128/JCM.43.2.551-555.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A., Swenson D. C., Wicklow D. T., Gloer J. B. (2013). New fiscalin, tryptoquivaline, and fumiquinazoline analogues from an endophytic isolate of Neosartorya aureola. Planta Med. 79, 852 10.1055/s-0033-1348666 [DOI] [Google Scholar]
- Kelman B. J., Robbins C. A., Swenson L. J., Hardin B. D. (2004). Risk from inhaled mycotoxins in indoor office and residential environments. Int. J. Toxicol. 23, 3–10. 10.1080/10915810490265423 [DOI] [PubMed] [Google Scholar]
- Khare R., Gupta S., Arif S., Jentoft M. E., Denziel P. H., Roden A. C., et al. (2014). Misidentification of Neosartorya pseudofischeri as Aspergillus fumigatus in a lung transplant patient. J. Clin. Microbiol. 52, 2722–2725. 10.1128/JCM.00216-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoufache K., Puel O., Loiseau N., Delaforge M., Rivollet D., Coste A., et al. (2007). Verruculogen associated with Aspergillus fumigatus hyphae and conidia modifies the electrophysiological properties of human nasal epithelial cells. BMC Microbiol. 25:5 10.1186/1471-2180-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijjoa A., Santos S., Dethoup T., Manoch L., Almeida A. P., Vasconselos M. H., et al. (2011). Sartoryglabrins, analogs of ardeemins, from Neosartorya glabra. Nat. Prod. Commun. 6, 807–812. [PubMed] [Google Scholar]
- Kim Y.-J., Park H. B., Yoo J.-H., Kwon H. C., Kim J., Yang H. O. (2014). Glionitrin A, a new diketopiperazine disulphide, asctivates ATM-ATR-Chk1/2 via 53BP1 phosphorylation in DU145 cells and shows antitumor effect in xenograft model. Biol. Pharm. Bull. 37, 378–386. 10.1248/bpb.b13-00719 [DOI] [PubMed] [Google Scholar]
- Kupfahl C., Michalka A., Lass-Flörl C., Fischer G., Haase G., Ruppert T., et al. (2008). Gliotoxin production by clinical and environmental Aspergillus fumigatus strains. Int. J. Med. Microbiol. 298, 319–327. 10.1016/j.ijmm.2007.04.006 [DOI] [PubMed] [Google Scholar]
- Kusuya Y., Takahashi-Nakaguchi A., Takahashi H., Yaguchi T. (2015). Draft genome sequence of the pathogenic filamentous fungus Aspergillus udagawae strain IFM 46973T. Genome Announc. 3, e00834–e00815. 10.1128/genomeA.00834-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y.-J., Sohn M.-J., Zheng C.-J., Kim W.-G. (2007). Fumimycin: a peptide deformylase inhibitor with an unusual skeleton produced by Aspergillus fumisynnematus. Org. Lett. 9, 2449–2451. 10.1021/ol0703231 [DOI] [PubMed] [Google Scholar]
- Land C. J., Lundström H., Werner S. (1993). Production of tremorgenic mycotoxins by isolates of Aspergillus fumigatus from sawmills in Sweden. Mycopathologia 124, 87–93. 10.1007/BF01103107 [DOI] [PubMed] [Google Scholar]
- Langarica-Fuentes A., Handley P. S., Houlden A., Fox G., Robson G. D. (2014). An investigation of the biodiversity of thermophilic and thermotolerant fungal species in composts using culture-based and molecular techniques. Fungal Ecol. 11, 132–144. 10.1016/j.funeco.2014.05.007 [DOI] [Google Scholar]
- Langfelder K., Streibel M., Jahn B., Haase G., Brakhaage A. A. (2003). Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38, 143–158. 10.1016/S1087-1845(02)00526-1 [DOI] [PubMed] [Google Scholar]
- Larsen T. O., Smedsgaard J., Nielsen K. F., Hansen M. E., Frisvad J. C. (2005). Phenotypic taxonomy and metabolite profiling in microbial drug discovery. Nat. Prod. Rep. 22, 672–695. 10.1039/b404943h [DOI] [PubMed] [Google Scholar]
- Larsen T. O., Smedsgaard J., Nielsen K. F., Hansen M. E., Samson R. A., Frisvad J. C. (2007). Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section Fumigati. Med. Mycol. 45, 225–232. 10.1080/13693780601185939 [DOI] [PubMed] [Google Scholar]
- Latgé J. P. (1999). Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12, 310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. Y., Park H. M., Son G. H., Lee C. H. (2013). Liquid chromatography-mass spectrometry based chemotaxonomic classification of Aspergillus species and evaluation of the biological activity of its unique metabolite neosartorin. J. Microbiol. Biotechnol. 23, 932–941. 10.4014/jmb.1212.12068 [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Wiederhold N. P., Lionakis M. S., Prince R. A., Kontoyiannis D. P. (2005). Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J. Clin. Microbiol. 43, 6120–6122. 10.1128/JCM.43.12.6120-6122.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. M., Horie Y., Wang Y., Li R. (1998). Three new Aspergillus species isolated from clinical sources as a causal agent of human aspergillosis. Mycoscience 39, 299–305. 10.1007/BF02464012 [DOI] [Google Scholar]
- Li Y.-X., Himaya S. W. A., Dewapriya P., Kim H. J., Kim S. K. (2014). Anti-proliferative effects of isosclerone from marine fungus Aspergillus fumigatus in MCF-7 human breast cancer cells. Process Biochem. 49, 2292–2298. 10.1016/j.procbio.2014.08.016 [DOI] [Google Scholar]
- Liang W.-L., Le X., Li H.-J., Yang X.-L., Chen J.-X., Xu J., et al. (2014). Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 12, 5657–5676. 10.3390/md12115657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Zhang T., Zhang X., Zhang J., Zhao C. (2015). An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules 20, 1424–1433. 10.3390/molecules20011424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj E. B., Milburn M. S. (1973). Viriditoxin production by Aspergillus viridi-nutans and related species. Appl. Microbiol. 26, 202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F. Y., Ames B., Walsch C. T., Keller N. P. (2014). Co-ordination between BrlA regulation and secretion of the oxidoreductase FmqD directs selective accumulation of fumiquinazoline C to conidial tissues in Aspergillus fumigatus. Cell. Microbiol. 16, 1267–1283. 10.1111/cmi.12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.-C., Chooi Y.-H., Dhingra S., Xu W., Calvo A. M., Tang Y. (2013). The fumagillin biosynthetic gene cluster in Aspergillus fumigatus encodes a cryptic terpene cyclase involved in the formation of β-trans-bergamotene. J. Am. Chem. Soc. 135, 4616–4619. 10.1021/ja312503y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind A. L., Wisecaver J. H., Smith T. D., Feng X., Calvo A. M., Rokas A. (2015). Examining the evolution of the regulatory circuit controlling secondary metabolism and development in the fungal genus Aspergillus. PLoS Genet. 11:e1005096. 10.1371/journal.pgen.1005096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wei X., Kim E. L., Lin X., Yang X.-W., Zhou X., et al. (2015). New glucosidated pyrazinoquinazoline indole alkaloids from the fungus Aspergillus fumigatus derived from a jellyfish. Tetrahedron 71, 271–275. 10.1016/j.tet.2014.11.063 [DOI] [Google Scholar]
- MacHeleidt J., Scherlach K., Neuwirth T., Schmidt-Heck W., Strassburger M., Spraker J., et al. (2015). Transcriptome analysis of cyclic AMP-dependent protein kinase A-regulated genes reveals the production of the novel natural compound fumipyrrole by Aspergillus fumigatus. Mol. Microbiol. 96, 148–162. 10.1111/mmi.12926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macías F. A., Varela R. M., Simonet A. M., Cutler H. G., Cutler S. J., Hill R. A. (2003). Absolute configuration of bioactive expansolides A and B from Aspergillus fumigatus Fresenius. Tetrahedron Lett. 44, 941–943. 10.1016/S0040-4039(02)02778-8 [DOI] [Google Scholar]
- Malejczyk K., Sigler L., Gibas G. F. C., Smith S. W. (2013). Invasive sino-orbital mycosis in an aplastic anaemia patient caused by Neosartorya laciniosa. J. Clin. Microbiol. 51, 1316–1319. 10.1128/JCM.02919-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi M., Andolfi A., Mathieu V., Boari A., Cimmino A. Y., Banuls L. M., et al. (2013). Fischerindoline, a pyrrolindole sesquiterpenoid isolated from Neosartorya pseudofischeri, with in vitro growth inhibitory activity in human cancer cell lines. Tetrahedron 69, 7466–7470. 10.1016/j.tet.2013.06.031 [DOI] [Google Scholar]
- Matsusawa T., Horie Y., Abliz P., Gonoi T., Yaguchi T. (2014a). Aspergillus huiyaniae sp. nov., a teleomorphic species in sect. Fumigati isolated from desert soil in China. Mycoscience 55, 213–220. 10.1016/j.myc.2013.08.007 [DOI] [Google Scholar]
- Matsusawa T., Takaki G. M. C., Yaguchi T., Okada K., Abliz P., Gonoi T., et al. (2015). Aspergillus arcoverdensis, a new species of Aspergillus section Fumigati isolated from caatinga soil in the state of Pernambuco, Brazil. Mycoscience 56, 123–131. 10.1016/j.myc.2014.04.006 [DOI] [Google Scholar]
- Matsusawa T., Takaki G. M. C., Yaguchi T., Okada K., Gonoi T., Horie Y. (2014b). Two new species of Aspergillus section Fumigati isolated from caatinga soil in the state of Pernambuco, Brazil. Mycoscience 55, 79–88. 10.1016/j.myc.2013.04.001 [DOI] [Google Scholar]
- McNeill J., Barrie F. R., Buck W. R., Demoulin V., Greuter W., Gams W., et al. (2012). International Code of Nomenclature for Algae, Fungi and Plants (Melbourne Code) Adopted by the 18th International Botanical Congress, Melbourne, Australia, Jul 2011. Regnum Vegetabile 154. Koenigstein: Koeltz Scientific Books. [Google Scholar]
- McDonagh A., Fedorova N. D., Crabtree J., Yu Y., Kim S., Chen D., et al. (2008). Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 4:e1000154. 10.1371/journal.ppat.1000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y.-Z., Liu D.-Y., Li G.-Q., Li P., Xu Y.-C., Shen Q.-R., et al. (2015). Genome-wide transcriptomic analysis of a superior biomass-degrading strain of Aspergillus fumigatus releated active lignocellulose-degrading genes. BMC Genomics 16:459. 10.1186/s12864-015-1658-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino T., Nishimoto M., Itou N., Nishikiori T. (1994). NK372135s, novel antifungal agents produced by Neosartorya fischeri. J. Antibiot. 47, 1546–1548. 10.7164/antibiotics.47.1546 [DOI] [PubMed] [Google Scholar]
- Mühlenfeld A., Achenbach H. (1988a). Asperpentyn, a novel acetylenic cyclohexene epoxide from Aspergillus duricaulis. Phytochemistry 27, 3853–3855. [Google Scholar]
- Mühlenfeld A., Achenbach H. (1988b). Stofwechselprodukte von mikroorganismen. 35 mitt. Asperdurin, ein neues antimykotisches phthalide aus Aspergillus duricaulis. Archiv der Pharmazie (Weinheim) 321, 803–805. [DOI] [PubMed] [Google Scholar]
- Netzker T., Fischer J., Weber J., Mattem D. J., Konig C. C., Valiante V., et al. (2015). Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 6:299. 10.3389/fmicb.2015.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M. L., Nielsen J. B., Rank C., Klejnstrup M. L., Holm D. M. K., Brogaard K. H., et al. (2011). A genome-wide polyketide synthase deletion library uncovers novel genetic links to polyketides and meroterpenoids in Aspergillus nidulans. FEMS Microbiol. Lett. 321, 157–166. 10.1111/j.1574-6968.2011.02327.x [DOI] [PubMed] [Google Scholar]
- Nierman W. C., Pain A., Anderson M. J., Wortman J. R., Kim H. S., Arroyo J., et al. (2005). Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438, 1151–1156. 10.1038/nature04332 [DOI] [PubMed] [Google Scholar]
- Novaková A., Hubka V., Dudova Z., Matsuzawa T., Kubatová A., Yaguchi T., et al. (2014). New species in Aspergillus section Fumigati from reclamation sites in Wyoming (USA) and revision of A. viridinutans complex. Fungal Divers. 64, 253–274. 10.1007/s13225-013-0262-5 [DOI] [Google Scholar]
- O'Gorman C. M., Fuller H. T., Dyer P. S. (2009). Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457, 471–474. 10.1038/nature07528 [DOI] [PubMed] [Google Scholar]
- Ohashi H., Ishikawa M., Ito J., Ueno A., Gleich G. J., Kita H., et al. (1997). Sulochrin inhibits eosinophil degranulation. J. Antibiot. 50, 972–974. 10.7164/antibiotics.50.972 [DOI] [PubMed] [Google Scholar]
- Ohashi H., Motegi Y., Kitas H., Gleich G. J., Miura T., Ishikawa M., et al. (1998). Sulochrin inhibits eosinophil activation and chemotaxis. Inflamm. Res. 47, 409–415. 10.1007/s000110050352 [DOI] [PubMed] [Google Scholar]
- O'Keeffe G., Hammel S., Owens R. A., Keane T. M., FitzPatrick O. A., Jones G. W., et al. (2014). RNA-sequencing reveals the pan-transcriptomic impact of attenuating the gliotoxin self-protection mechanism in Aspergillus fumigatus. BMC Genomics 15:894. 10.1186/1471-2164-15-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola A. R. B., Debbab A., Aly A. H., Mandi A., Zerfass I., Hamacher A., et al. (2014). Absolute configuration and antibiotic activity of neosartorin from the endophytic fungus Aspergillus fumigatiaffinis. Tetrahedron Lett. 55, 1020–1023. 10.1016/j.tetlet.2013.12.070 [DOI] [Google Scholar]
- Omolo J. O., Anke H., Chhabara S., Sterner O. (2000). New variotin analogues from Aspergillus viridi-nutans. J. Nat. Prod. 63, 975–977. 10.1021/np990509b [DOI] [PubMed] [Google Scholar]
- Owens K. A., Hammel S., Sheridan K. J., Jones G. W., Doyle S. (2014). A proteomic approach to investigating gene cluster expression and secondary metabolite functionality in Aspergillus fumigatus. PLoS ONE 9:e106942. 10.1371/journal.pone.0106942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozoe Y., Kuriyama T., Tachibana Y., Hariyama K., Takahashi N., Yuguchi T., et al. (2004). Isocoumarin derivative as a novel GABA receptor ligand from Neosartorya quadricincta. J. Pestic. Sci. 29, 328–333. 10.1584/jpestics.29.328 [DOI] [Google Scholar]
- Panda A., Ghosh A. K., Mirdha B. R., Xess I., Paul S., Samanaray J. C., et al. (2015). MALDI-TOF mass spectrometry for rapid identification of clinical fungal isolates based on ribosomal protein markers. J. Microbiol. Methods 109, 93–105. 10.1016/j.mimet.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Pedersen M. H., Borodina I., Moresco J. L., Svendsen W. E., Frisvad J. C., Søndergaard I. (2011). High-yield production of hydrophobins RodA and RodB from Aspergillus fumigatus in Pichia pastoris. Appl. Microbiol. Biotechnol. 90, 1923–1932. 10.1007/s00253-011-3235-1 [DOI] [PubMed] [Google Scholar]
- Peláez T., Álvarez-Perez S., Mellado E., Serrano D., Valerio M., Blanco J. L., et al. (2013). Invasive aspergillosis caused by cryptic Aspergillus species: a report of two consecutive episodes in a patient with leukemia. J. Med. Mycol. 62, 474–478. 10.1099/jmm.0.044867-0 [DOI] [PubMed] [Google Scholar]
- Peng J., Lin T., Wang W., Xin Z., Zhu T., Gu Q., et al. (2013). Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. J. Nat. Prod. 76, 1133–1140. 10.1021/np400200k [DOI] [PubMed] [Google Scholar]
- Perrin R. M., Fedorova N. D., Bok J. W., Cramer R. A., Jr., Wortman J. R., Kim H. S., et al. (2007). Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 3:e50. 10.1371/journal.ppat.0030050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik M., Haas J. H., Laverman P., Schrettl M., Franssen G. M., Blatzer M., et al. (2014). 68Ga-triacetylfusarinine C and 68Ga-ferrioxamine E for Aspergillus infection imaging: Uptake specificity in various microorganisms. Mol. Imag. Biol. 16, 102–108. 10.1007/s11307-013-0654-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posteraro B., Mattei R., Trivella F., Maffei A., Torre A., De Carolis E., et al. (2011). Uncommon Neosartorya udagawae fungus as a causative agent of severe corneal infection. J. Clin. Microbiol. 49, 2357–2360. 10.1128/JCM.00134-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompanya C., Dethoup T., Bessa L. J., Pinto M. M. M., Gales L., Costa P. M., et al. (2014). New isocoumarin derivatives and meroterpenoids from the marine sponge-associated fungus Aspergillus similanensis. Mar. Drugs 12, 5160–5173. 10.3390/md12105160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompanya C., Fernandes C., Cravo S., Pinto M. M. M., Dethoup T., Silva A. M. S., et al. (2015). A new cyclic hexapeptide and a new isocoumarin derivative from the marine sponge-associated fungus Aspergillus similanensis KUFA 0013. Mar. Drugs 13, 1432–1450. 10.3390/md13031432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M.-F., Ji N.-Y., Liu X.-H., Li F., Xue Q.-Z. (2010b). Asporyergosterol, a new steroid from an algiocolous isolate of Aspergillus oryzae. Nat. Prod. Commun. 5, 1575–1578. [PubMed] [Google Scholar]
- Qiao M.-F., Ji N.-Y., Liu X.-H., Li K., Zhu Q.-M., Xue Q.-Z., et al. (2010a). Indoloterpenes from an algicolous isolate of Aspergillus oryzae. Biorg. Med. Chem. Lett. 20, 5677–5680. 10.1016/j.bmcl.2010.08.024 [DOI] [PubMed] [Google Scholar]
- Rabindran S. K., Ross D. D., Doyle L. A., Yang W., Greenberger L. M. (2000). Fumitremorgin C reverses multidrug resistance in cells transfected with the cancer resistance protein. Cancer Res. 60, 47–50. [PubMed] [Google Scholar]
- Rank C., Phipps R. K., Harris P., Frisvad J. C., Gotfredsen C. H., Larsen T. O. (2006). Epi-aszonalenin A, B, and C from Aspergillus novofumigatus. Tetrahedron Lett. 47, 6099–6102. 10.1016/j.tetlet.2006.06.086 [DOI] [Google Scholar]
- Rank C., Phipps R. K., Harris P., Fristrup P., Larsen T. O., Gotfredsen C. H. (2008). Novofumigatonin, a new orthoester meroterpenoid from Aspergillus novofumigatus. Org. Lett. 10, 401–404. 10.1021/ol7026834 [DOI] [PubMed] [Google Scholar]
- Rank C., Klejnstrup M. L., Petersen L. M., Kildegaard S., Frisvad J. C., Gotfredsen C. H., et al. (2012). Comparative chemistry of Aspergillus oryzae (RIB40) and A. flavus (NRRL 3357). Metabolites 2, 39–56. 10.3390/metabo2010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper K. B., Fennell D. I. (1965). The Genus Aspergillus. Baltimore, MD: Williams and Wilkins. [Google Scholar]
- Reeves E. P., Reiber K., Neville C., Schreibner O., Kavanagh K., Doyle S. (2006). A nonribosomal peptide synthetase (Pes1) confers protection against oxidative stress in Aspergillus fumigatus. FEBS J. 273, 3038–3053. 10.1111/j.1742-4658.2006.05315.x [DOI] [PubMed] [Google Scholar]
- Rementeria A., Lopez-Molina N., Ludwig A., Vivanco A. B., Belen A., Bikandi J., et al. (2005). Genes and molecules involved in Aspergillus fumigatus virulence. Rev. Iberoam. Micol. 22, 1–23. 10.1016/S1130-1406(05)70001-2 [DOI] [PubMed] [Google Scholar]
- Rodrigues B. S. F., Sahm B. D. B., Jimenez P. C., Pinto F. C. L., Mafezoli J., Mattos M. C., et al. (2015). Bioprospection of cytotoxic compounds in fungal strains recovered from sediments of the Brazilian coast. Chem. Biodivers. 12, 432–442. 10.1002/cbdv.201400193 [DOI] [PubMed] [Google Scholar]
- Samson R. A., Hong S.-B., Frisvad J. C. (2006). Old and new concepts of species differentiation in Aspergillus. Med. Mycol. 44, S133–S144. 10.1080/13693780600913224 [DOI] [PubMed] [Google Scholar]
- Samson R. A., Hong S.-B., Peterson S. W., Frisvad J. C., Varga J. (2007). Polyphasic taxonomy of Aspergillus section Fumigati and its teleomorph Neosartorya. Stud. Mycol. 59, 147–203. 10.3114/sim.2007.59.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R. A., Nielsen P. V., Frisvad J. C. (1990). The genus Neosartorya: differentiation by scanning electron microscopy and mycotoxin profiles, in Modern Concepts in Penicillium and Aspergillus Classification, R. A. Samson and J. I. Pitt (New York, NY: Plenum Press; ), 455-467. 10.1007/978-1-4899-3579-3_40 [DOI] [Google Scholar]
- Samson R. A., Peterson S. W., Frisvad J. C., Varga J. (2011). New species in Aspergillus section Terrei. Stud. Mycol. 69, 39–55. 10.3114/sim.2011.69.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R. A., Visagie C. M., Houbraken J., Hong S.-B., Hubka V., Klaasen C. H. W., et al. (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 78, 141–173. 10.1016/j.simyco.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J. F., Somoza A. D., Keller N. P., Wang C. C. (2012). Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 29, 351–371. 10.1039/c2np00084a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeda-Hirschmann G., Hormazabal E., Rodriguez J. A., Theoduloz C. (2008). Cycloaspeptide A and pseurotin A from the endophytic fungus Penicillium janczewskii. Z. Naturforsch. C 63, 383–388. 10.1515/znc-2008-5-612 [DOI] [PubMed] [Google Scholar]
- Schwienbacher M., Weig M., Thies S., Regula J. T., Heeseman J., Ebel F. (2005). Analysis of the major proteins secreted by the human opportunistic pathogen Aspergillus fumigatus under in vitro conditions. Med. Mycol. 43, 623–630. 10.1080/13693780500089216 [DOI] [PubMed] [Google Scholar]
- Shan W., Wang S., Ying Y., Ma L., Zhan Z. (2014). Indole-benzodiazepine-2,5-dione derivatives from Neosartorya fischeri. J. Chem. Res. 38, 692–694. 10.3184/174751914X14140034695581 [DOI] [Google Scholar]
- Shan W.-G., Wang S.-L., Lang H. Y., Chen S. M., Ying Y.-M., Zhan Z.-J. (2015). Cottoquinazolins E and F from Neosartorya fischeri NRRL 181. Helv. Chim. Acta 98, 552–556. 10.1002/hlca.201400270 [DOI] [Google Scholar]
- Shinohara C., Hasumi K., Endo A. (1992). Inhibition of oxidized low-density lipoprotein metabolism in macrophage J774 by helvolic acid. Biochim. Biophys. Acta 1167, 303–306. 10.1016/0005-2760(93)90233-Y [DOI] [PubMed] [Google Scholar]
- Sigler L., Sutton D. A., Gibas C. F. C., Summerbell R. C., Noel R. K., Iwen P. C. (2010). Phialosimplex, a new anamorphic genus associated with infections in dogs and having phylogenetic affinity to the Trichomaceae. Med. Mycol. 48, 335–345. 10.3109/13693780903225805 [DOI] [PubMed] [Google Scholar]
- Sodngam S., Sawadsitang S., Suwannasai N., Mongkoltharanuk W. (2014). Chemical constituents, and their cytotoxicity, of the rare wood decaying fungus Xylaria humosa. Nat. Prod. Commun. 9, 157–158. [PubMed] [Google Scholar]
- Someya A., Yaguchi T., Udagawa S. (1999). Neosartorya sublevispora, a new species of soil-borne Eurotiales. Mycoscience 40, 405–409. 10.1007/BF02464395 [DOI] [Google Scholar]
- Srivastava N., Rawal R., Sharma R., Oberoi H. S., Srivastava M., Singh J. (2014). Effect of nickel-cobaltite nanoparticles on production and thermostability of cellulases from newly isolated thermotolerant Aspergillus fumigatus NS (Class: Eurotiomycetes). Appl. Biochem. Biotechnol. 174, 1092–1103. 10.1007/s12010-014-0940-0 [DOI] [PubMed] [Google Scholar]
- Stack D., Neville C., Doyle S. (2007). Nonribosomal peptide synthesis in Aspergillus fumigatus and other fungi. Microbiology 153, 1297–1306. 10.1099/mic.0.2006/006908-0 [DOI] [PubMed] [Google Scholar]
- Stanzani M., Orciuolo E., Lewis R., Kontoyiannis D. P., Martins S. L. R., St. John L. S., et al. (2005). Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 105, 2258–2265. 10.1182/blood-2004-09-3421 [DOI] [PubMed] [Google Scholar]
- Sugui J. A., Pardo J., Chang Y. C., Zarember K. A., Nardone G., Galvez E. M., et al. (2007). Gliotoxin is a virulence factor of Aspergillus fumigatus; gliP deletion attenuates virulence in mice immunocompromised with hydrocortisone. Eukaryotic Cell 6, 1562–1569. 10.1128/EC.00141-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugui J. A., Kwon-Chung K. J., Juvvadi P. R., Latg,é J. P., Steinbach W. J. (2015). Aspergillus fumigatus and related species. Cold Spring Harb. Perspect. Med. 5:a019786. 10.1101/cshperspect.a019786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugui J. A., Peterson S. W., Clark L. P., Nardone G., Folio L., Riedlinger G., et al. (2012). Aspergillus tanneri sp. nov., a new pathogen that causes invasive disease refractory to antifungal therapy. J. Clin. Microbiol. 50, 3309–3317. 10.1128/JCM.01509-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugui J. A., Peterson S. W., Figat A. B., Hansen B., Samson R. A., Mellado E., et al. (2014). Genetic relatedness versus biological compatibility between Aspergillus fumigatus and related species. J. Clin. Microbiol. 52, 3707–3721. 10.1128/JCM.01704-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugui J. A., Vinh D. C., Nardone G., Sheat Y. R., Chang Y. C., Zelazny A. M., et al. (2010). Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J. Clin. Microbiol. 48, 220–228. 10.1128/JCM.01556-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D. A., Summerbell R. C., Samson R., Rinalde M. F. (2002). First report of Neosartorya spinosa (Raper & Fennell) Kozakiewicz inciting human disease, in Abstracts of the General Meeting of ASM, Vol. 102 (Salt Lake City, UT: ), 212. [Google Scholar]
- Swilaiman S. S., O'Gorman C. M., Balajee S. A., Dyer P. S. (2013). Discovery of a sexual cycle in Aspergillus lentulus, a close relative of A. fumigatus. Eukaryotic Cell 12, 962–969. 10.1128/EC.00040-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi C., Matsushida T., Doi M., Minoura K., Shingu T., Komeda Y., et al. (1995). Fumiquinazolions A-G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J. Chem. Soc. Perkin Trans. I 1995, 2345–2353. 10.1039/p19950002345 [DOI] [Google Scholar]
- Takeda M., Horie Y., Abliz P. (2001). Two new heterothallic Neosartorya from African soil. Mycoscience 42, 361–367. 10.1007/BF02461219 [DOI] [Google Scholar]
- Tamiya H., Ochiai E., Kikuchi K., Yahiro M., Toyotome T., Watanabe A., et al. (2015). Secondary metabolite profiles and antifungal drug susceptibility of Aspergillus fumigatus and closely related species, A. lentulus, A. udagawae, and A. viridinutans. Journal of Infection and Chemotherapy. 21, 385–391. 10.1016/j.jiac.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Tepsic K., Gunde-Cimerman N., Frisvad J. C. (1997). Growth and mycotoxin production by Aspergillus fumigatus strains isolated from a saltern. FEMS Microbiol. Lett. 157, 9–12. 10.1111/j.1574-6968.1997.tb12745.x [DOI] [Google Scholar]
- Tomee J. F., Kauffman H. F. (2000). Putative virulence factors of Aspergillus fumigatus. Clin. Exp. Allergy 30, 476–484. 10.1046/j.1365-2222.2000.00796.x [DOI] [PubMed] [Google Scholar]
- Toskova M., Palousova D., Kocmanova I., Pavlovsky Z., Timilsinas S., Langerova M., et al. (2013). Invasive mould disease involving the gastro-intestinal tract caused by Neosartorya pseudofischeri in a haematological patient. Mycoses 56, 385–388. 10.1111/myc.12038 [DOI] [PubMed] [Google Scholar]
- Tsai H.-F., Chang Y. C., Washburn R. G., Wheeler M. H., Kwon-Chung K. J. (1998). The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180, 3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.-F., Fujii I., Watanabe A., Wheeler M. H., Chang Y. C., Yasuaka Y., et al. (2001). Pentaketide melanin biosynthesis in Aspergillus fumigatus requires chain-length shortening of a heptaketide precursor. J. Biol. Chem. 276, 29292–29298. 10.1074/jbc.M101998200 [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Yoshida L. S., Nishida S., Kobayashi T., Shimoyama T. (2004). Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 72, 3373–3382. 10.1128/IAI.72.6.3373-3382.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J., Due M., Frisvad J. C., Samson R. A. (2007). Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Stud. Mycol. 59, 89–106. 10.3114/sim.2007.59.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J., Frisvad J. C., Kocsubé S., Brancovics B., Szigeti G., Samson R. A. (2011a). New and revisited species in Aspergillus section Nigri. Stud. Mycol. 69, 1–17. 10.3114/sim.2011.69.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J., Frisvad J. C., Samson R. A. (2011b). Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud. Mycol. 69, 57–80. 10.3114/sim.2011.69.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J., Samson R. A. (2008). Ribotoxin genes in Aspergillus section Clavati. Antonie van Leeuwenhoek 94, 481–485. 10.1007/s10482-008-9266-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J., Tóth B., Rigó K., Debets F., Kozakiewicz Z. (2000). Genetic variability within the Aspergillus viridinutans species. Folia Microbiol. 45, 423–428. 10.1007/BF02817615 [DOI] [PubMed] [Google Scholar]
- Verwer P. E. B., van Leuwen W. B., Girard V., van Belkum A., Staab J. F., Verbrugh H. A., et al. (2014). Diuscrimination of Aspergillus lentulus from Aspergillus fumigatus by Raman spectroscopy and MALDI-TOF MS. Eur. J. Clin. Microbiol. 33, 245–251. 10.1007/s10096-013-1951-4 [DOI] [PubMed] [Google Scholar]
- Vinh D. C., Shea Y. R., Jones P. A., Freeman A. F., Zelazny A., Holland S. M. (2009a). Chronic invasive aspergillosis caused by Aspergillus viridinutans. Emerging Infect. Dis. 15, 1292–1294. 10.3201/eid1508.090251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh D. C., Shea Y. R., Sugui J. A., Parrilla-Castellar E. R., Freeman A. F., Campbell J. W., et al. (2009b). Invasive aspergillosis due to Neosartorya udagawae. Clin. Infect. Dis. 49, 102–111. 10.1086/599345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virágh M., Vörös D., Kele Z., Kovács L., Fizilo Á., Lakatos G., et al. (2014). Production of a defensin-like antifungal protein NFAP from Neosartorya fischeri in Pichia pastoris and its antifungal activity against filamentous fungal isolates from human infections. Protein Expr. Purif. 94, 78–84. 10.1016/j.pep.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Visagie C., Varga J., Houbraken J., Meijer M., Yilmaz N., Fotedar R., et al. (2014). Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati). Stud. Mycol. 78, 1–61. 10.1016/j.simyco.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vödisch M., Scherlach K., Winkler R., Hertweck C., Braun H. P., Roth M., et al. (2011). Analysis of the Aspergillus fumigatus proteome reveals metabolic changes and the activation of the pseurotin A biosynthesis gene cluster in response to hypoxia. J. Proteome Res. 10, 2508–2524. 10.1021/pr1012812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana D., Hosoe T., Itabashi T., Nozawa K., Okada K., Campos Takaki G. M., et al. (2006). Isolation of isoterrein from Neosartorya fischeri. Mycotoxins 56, 3–6. 10.2520/myco.56.3 [DOI] [Google Scholar]
- Wang F., Fang Y., Zhu T., Zhang M., Lin A., Gu Q., et al. (2008). Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron 64, 7986–7991. 10.1016/j.tet.2008.06.013 [DOI] [Google Scholar]
- Watanabe A., Kamei K., Sekine T., Hiraguchi H., Ochiai E., Hashimoto Y., et al. (2004). Cytotoxic substances from Aspergillus fumigatus in oxygenated or poorly oxygenated environment. Mycopathologia 158, 1–7. 10.1023/B:MYCO.0000038439.56108.3c [DOI] [PubMed] [Google Scholar]
- Watanabe A., Kamei K., Sekine T., Waku M., Nishimura K., Miyaji M., et al. (2003). Immunosuppressive substances in Aspergillus fumigatus culture filtrate. J. Infect. Chemother. 9, 114–121. 10.1007/s10156-002-0227-1 [DOI] [PubMed] [Google Scholar]
- Wiekmann I., Lechner B. E., Baccile J. A., Velk T. A., Yin W.-B., Bok J. B., et al. (2014). Pertubations in small molecule synthesis uncovers an iron-responsive secondary metabolite network in Aspergillus fumigatus. Front. Microbiol. 5:530. 10.3389/fmicb.2014.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann P., Guo C. J., Palmer J. M., Sekonyela R. R., Wang C. C. C., Keller N. P. (2013). Prototype of an intertwined secondary-metabolite supercluster. Proc. Natl. Acad. Sci. U.S.A. 110, 17065–17070. 10.1073/pnas.1313258110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. M., Musza L. L., Kydd G. C., Kullnig R., Gillum A. M., Cooper R. (1993). Fiscalins: new substance P inhibitors produced by the fungus Neosartorya fischeri. Taxonomy, fermentation, structures, and biological properties. J. Antibiot. 46, 545–553. 10.7164/antibiotics.46.545 [DOI] [PubMed] [Google Scholar]
- Wortman J. R., Fedorova N., Crabtree J., Joardar V., Maiti R., Haas B. J., et al. (2006). Whole genome comparison of the Aspergillus fumigatus family. Med. Mycol. 44, S3–S7. 10.1080/13693780600835799 [DOI] [PubMed] [Google Scholar]
- Xie F., Li X.-B., Zhou J.-C., Xu Q.-Q., Wang X.-N., Yuan H.-Q., et al. (2015). Secondary metabolites from Aspergillus fumigatus, an endophytic fungus from the liverwort Heteroscyphus tener (STEPH.) SCHIFFN. Chem. Biodivers. 12, 1313–1321. 10.1002/cbdv.201400317 [DOI] [PubMed] [Google Scholar]
- Xu N., Cao Y., Wang L., Chen G., Pei Y.-H. (2013). New alkaloids from a marine-derived fungus Neosartorya sp. HN-M-3. J. Asian Nat. Prod. Res. 15, 731–736. 10.1080/10286020.2013.797895 [DOI] [PubMed] [Google Scholar]
- Xu J., Song Y. C., Guo Y., Mei Y. N., Tan R. X. (2014). Fumigaclavine D-H, new ergot alkaloids from endophytic Aspergillus fumigatus. Planta Med. 80, 1131–1137. 10.1055/s-0034-1382958 [DOI] [PubMed] [Google Scholar]
- Yaguchi T., Horie Y., Tanaka R., Matsuzawa T., Ito J., Nishimura K. (2007). Molecular phylogenetics of multiple genes on Aspergillus section Fumigati isolated from clinical specimens in Japan. Jpn. J. Med. Mycol. 48, 37–46. 10.3314/jjmm.48.37 [DOI] [PubMed] [Google Scholar]
- Yaguchi T., Matsuzawa T., Tanaka R., Abliz P., Hui Y., Horie Y. (2010). Two new species of Neosartorya isolated from soil in Xinjiang, China. Mycoscience 51, 253–262. 10.1007/S10267-010-0037-8 [DOI] [Google Scholar]
- Ye Y., Minami A., Mandi A., Liu C., Taniguchi T., Kuzuyama T., et al. (2015). Genome mining for sesterpenes using bifunctional terpene synthases reveals a unified intermediate of di/sesterpenes. J. Am. Chem. Soc. 137, 11846–11853. 10.1021/jacs.5b08319 [DOI] [PubMed] [Google Scholar]
- Yim T., Kanokmedhakul K., Kanokmedhakul S., Sanmanoch W., Boonlue S. (2014). A new meroterpenoid tatenoic acid from the fungus Neosartorya tatenoi. Nat. Prod. Res. 28, 1847–1852. 10.1080/14786419.2014.951353 [DOI] [PubMed] [Google Scholar]
- Yin W.-B., Grundmann A., Cheng Y., Li S.-M. (2009). Acetylaszonalenin biosynthesis in Neosartorya fischeri. Identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical characterization. J. Biol. Chem. 284, 100–109. 10.1074/jbc.M807606200 [DOI] [PubMed] [Google Scholar]
- Zhang H.-W., Ying C., Tang Y.-F. (2014). Four ardeemin analogs from endophytic Aspergillus fumigatus SPS-02 and their reversal effects on multi-drug resistant tumor cells. Chem. Biodivers. 11, 85–91. 10.1002/cbdv.201300220 [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang W.-L., Fang Y.-C., Zhu T.-J., Gu Q.-Q., Zhu W.-M. (2008). Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 71, 985–989. 10.1021/np700737g [DOI] [PubMed] [Google Scholar]
- Zhang H.-C., Ma Y.-M., Liu R., Zhou F. (2012). Endophytic fungus Aspergillus tamarii from Ficus carica L., a new source of indolyl diketopiperazines. Biochem. Syst. Ecol. 45, 31–33. 10.1016/j.bse.2012.07.020 [DOI] [Google Scholar]
- Zhao W. Y., Zhu T. J., Fan G. T., Liu H. B., Fang Y. C., Gu Q. Q., et al. (2010). Three new dioxopiperazine metabolites from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 24, 953–957. 10.1080/14786410902726134 [DOI] [PubMed] [Google Scholar]
- Zheng Z. Z., Shan W. C., Wang S.-L., Ying Y.-M., Ma L.-F., Zhan Z.-J. (2014). Three new prenylated diketopiperazines from Neosartorya fischeri. Helv. Chim. Acta 97, 1020–1026. 10.1002/hlca.201300416 [DOI] [Google Scholar]
- Zhou F., Zhang H., Liu R., Zhang D. (2013). Isolation and biological evaluation of secondary metabolites from the endophytic fungus Aspergillus fumigatus from Astralagus membranaceus. Chem. Nat. Comp. 49, 568–570. 10.1007/s10600-013-0675-0 [DOI] [Google Scholar]
- Zin W. W. M., Buttachon S., Buaruang J., Gales L., Pereira J. A., Pinto M. M. M., et al. (2015). A new meroditerpene and a new tryptoquivaline analog from the algicolous fungus Neosartorya takakii KUFC 7898. Mar. Drugs 13, 3776–3790. 10.3390/md13063776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuck K. M., Shipley S., Newman D. J. (2011). Induced production of N-formyl alkaloids from Aspergillus fumigatus by co-culture with Streptomyces peucetius. J. Nat. Prod. 74, 1653–1657. 10.1021/np200255f [DOI] [PubMed] [Google Scholar]