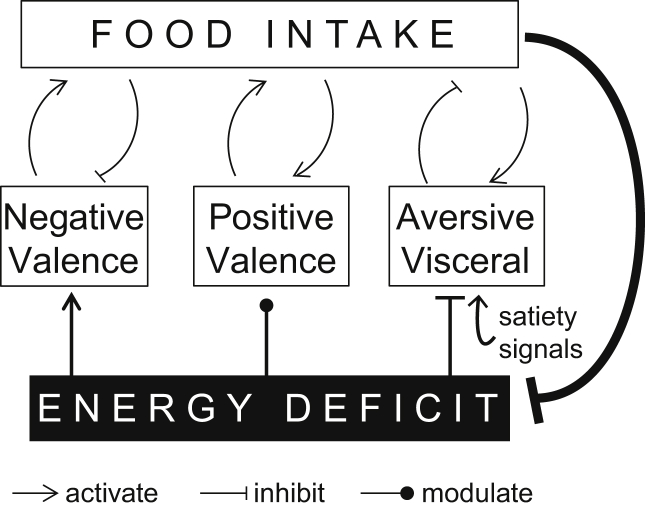
The distinction between feeding driven by homeostatic energy deficit versus non-homeostatic reward is a frequently heard dichotomy in the neurobiology of appetite. Although oversimplified, the underlying idea is that there are multiple appetitive systems. One important homeostatic system is mediated by Agouti related protein (Agrp)-expressing neurons, a hypothalamic cell type that is activated by conditions of homeostatic energy deficit. Optogenetic [1] or chemogenetic [2] activation of AGRP neurons rapidly elicits intense food intake, and transient inhibition of these neurons reduces appetite [2], [3]. Recently, this population has also been found to transmit a signal with negative valence, and this may serve as a motive for mice to consume food, which consequently reduces AGRP neuron activity [3]. In fact, 96% of AGRP neurons have been shown by in vivo calcium imaging to rapidly reduce activity upon just the sight of food, such that the neurons have low activity during food consumption [3]. This indicates that AGRP neurons are involved in food-seeking but not food consumption. However, sustained reduction of AGRP neuron activity requires food consumption, which is consistent with a homeostatic role [3]. Conversely, a population of inhibitory neurons in the lateral hypothalamus increases feeding, is active during food consumption, and shows rewarding characteristics [4]. These neurons preferentially elicit palatable food consumption and interact with the ventral tegmental area (VTA), a brain region associated with incentive (reward)-based learning [5]. Notably, lateral hypothalamic neurons and VTA dopamine neurons have also been demonstrated to have receptors for key homeostatic hormones such as ghrelin [6] or leptin, indicating that positive valence (i.e., ‘hedonic’) systems are also homeostatically sensitive. Taken together, these systems mediate appetite regulation by both the ‘carrot and the stick’, where the positive valence of lateral hypothalamic/dopamine circuits associated with food consumption or the expectation of consumption pulls an animal towards incentives, and the negative valence AGRP neurons push an animal to attend to physiological needs. Both positive and negative valence systems are homeostatically sensitive, but the positive valence system also operates in the absence of homeostatic need if the reward value of the food is high enough (Figure 1).
Figure 1.
Regulation of food intake by negative valence and positive valence appetitive systems, visceral aversive pathways, and the relationship with homeostatic deficit. Negative valence appetitive systems (e.g., AGRP neurons) are under homeostatic control, but AGRP neurons are rapidly inhibited by cues that predict food, thus consumption is associated with the positive valence system. Positive valence-driven eating (e.g., via the lateral hypothalamus) is modulated by energy deficit but also maintains appetite irrespective of a homeostatic need state. Aversive visceral signals, with different motivational properties than negative valence AGRP neurons, are activated by visceral and hormonal satiety signals and lead to cessation of food consumption. Interactions between these systems exist but are not shown.
A new report in Cell Metabolism by Denis et al. examines the food consumption characteristics of mice that lack AGRP neurons [7]. This work builds on prior studies showing that permanent ablation of AGRP neurons in adult mice leads to short-term anorexia [8] due to visceral malaise [9]; however, when AGRP neurons are ablated in neonatal mice, they grow up mostly normal as a result of compensatory circuits [8], [9]. Denis et al. found that mice with neonatal AGRP neuron ablation, after developing to adulthood, displayed an enhanced reliance on incentive-driven appetite. After food deprivation, AGRP neuron-ablated mice showed a blunted re-feeding response with grain-based chow, which is a moderately rewarding food, but they ate as much of a palatable high fat high sugar (HFHS) diet as intact mice. Similarly, the homeostatic hormone ghrelin did not elicit feeding with grain-based food in AGRP neuron-ablated mice (as it does in intact mice), but ghrelin was effective at increasing HFHS diet consumption. Furthermore, elevated dopamine signaling resulted in increased grain food consumption only in AGRP neuron-ablated mice, presumably due to greater reliance on dopamine release for appetite regulation. Thus, in the absence of AGRP neurons, appetite is faulty with a moderately palatable grain-based diet, but this is normalized by excess dopamine or by feeding a HFHS diet, which can even lead to overeating in AGRP neuron-ablated mice [7].
These experiments give some insight as to why we have an AGRP neuron system. Mice and people have, for most of their history, often lacked easy access to highly palatable foods. Therefore, many animals have to make due with whatever can be found, which depends on the important AGRP neuron system to keep them alive. This AGRP neuron system is a stern nurse, seemingly eliciting discomfort to make sure that we take care of our body's nutritional needs. Its negative valence signal is activated by energy deficit and suppressed by nutritive food consumption, so mice learn quickly to eat when they get hungry in order to minimize this unpleasant state [3]. Once consumption has commenced, it is sustained by the positive valence system, which has a positive feedback relationship with food taste and ingested nutrients and is a major contributor to learning food-seeking behaviors. Consumption is subsequently halted by an additional aversive pathway that is activated by visceral and hormonal satiety signals [9], [10] (Figure 1). Therefore, elevated AGRP neuron activity effectively imposes a cost on not consuming food, so eating becomes prioritized when the mouse is hungry. However, in the absence of AGRP neurons, mice are left with a fair-weather friend instead of the old AGRP taskmaster. These are the homeostatically regulated, positive valence neurons in the lateral hypothalamus, VTA, and elsewhere. In this case, incentives related to food are potentiated by homeostatic energy deficit signals such as ghrelin, but the emergency AGRP neuron system is absent. The lower intake of moderately palatable food in these mice after deprivation indicates that homeostatic control of the positive valence system is not sufficient to compensate for the loss of the AGRP neuron system. However, with a highly palatable diet, the positive valence system is able to fully accommodate the energetic requirements of the animal.
It is unclear to what degree well-fed humans rely on their AGRP neurons, due to near constant access to high calorie foods. The main instance is likely on a weight-loss diet, where the negative emotional attributes of the AGRP neuron ‘stick’ become all too noticeable, leading many diets to fail. Would inhibiting AGRP neurons help people lose weight? Denis and colleagues suggest that AGRP neuron suppression may actually lead to overeating and obesity because of reliance on the hedonic system. An alternative view is that, with the behavioral changes that some people implement to avoid high calorie foods during weight loss diets, they could use just a little help by blunting the countervailing influence of AGRP neurons. Uncertainty about these issues highlights the need to consider contributions from multiple interacting systems when devising strategies for controlling appetite and body weight.
Acknowledgements
S.M.S. is funded by the Howard Hughes Medical Institute.
Footnotes
This commentary refers to “Palatability can drive feeding independent of AgRP neurons” by Denis et al., Cell Metabolism Volume 22, Issue 4, 6 October 2015, Pages 646–657.
References
- 1.Aponte Y., Atasoy D., Sternson S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neuroscience. 2011;14(3):351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krashes M.J., Koda S., Ye C., Rogan S.C., Adams A.C., Cusher D.S. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of Clinical Investigation. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betley J.N., Xu S., Huang Cao Z.F., Gong R., Magnus C.J., Yu Y. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015 doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennings J.H., Ung R.L., Resendez S.L., Stamatakis A.M., Taylor J.G., Huang J. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160(3):516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieh E.H., Matthews G.A., Allsop S.A., Presbrey K.N., Leppla C.A., Wichmann R. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160(3):528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zigman J.M., Jones J.E., Lee C.E., Saper C.B., Elmquist J.K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. Journal of Comparative Neurology. 2006;494(3):528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis R.G., Joly-Amado A., Webber E., Langlet F., Schaeffer M., Padilla S.L. Palatability can drive feeding independent of AgRP neurons. Cell Metabolism. 2015 doi: 10.1016/j.cmet.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q., Boyle M., Palmiter R. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter M.E., Soden M.E., Zweifel L.S., Palmiter R.D. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]