Abstract
Aneurysmal bone cysts are benign bone tumors that usually present in childhood and early adulthood. They usually manifest as expansile osteolytic lesions with a varying potential to be locally aggressive. Since their first description in 1942, a variety of treatment methods has been proposed. Traditionally, these tumors were treated with open surgery. Either intralesional surgical procedures or en bloc excisions have been described. Furthermore, a variety of chemical or physical adjuvants has been utilized in order to reduce the risk for local recurrence after excision. Currently, there is a shift to more minimally invasive procedures in order to avoid the complications of open surgical excision. Good results have been reported during percutaneous surgery, or the use of embolization. Recently, sclerotherapy has emerged as a promising treatment, showing effective consolidation of the lesions and functional results that appear to be superior to the ones of open surgery. Lastly, non-invasive treatment, such as pharmaceutical intervention with denosumab or bisphosphonates has been reported to be effective in the management of the disease. Radiotherapy has also been shown to confer good local control, either alone or in conjunction to other treatment modalities, but is associated with serious adverse effects. Here, we review the current literature on the methods of treatment of aneurysmal bone cysts. The indication for each type of treatment along reported outcome of the intervention, as well as potential complications are systematically presented. Our review aims to increase awareness of the different treatment modalities and facilitate decision-making regarding each individual patient.
Key words: Aneurysmal bone cyst, surgery, sclerotherapy
Introduction
Aneurysmal bone cysts (ABC) are benign bone lesions which usually arise in childhood or early adulthood. Patients usually complain for pain in the affected skeletal region, and rarely a pathological fracture is evident. A plain radiogram typically reveals an expansile osteolytic lesion; whereas magnetic resonance tomography showing characteristic fluid-fluid levels due to blood sedimentation can give valuable information that assists in diagnosis. Biopsy is obligatory, as the telangiectatic variant of osteogenic sarcoma needs to be taken into account in the differential diagnosis of the lesion.1 The most widely accepted theory explaining the pathophysiology of the lesion suggests that there is a locally increased vascular pressure in the venous network of the bone tumor that results in the dilation of the small vessels. This in turn causes erosion and resorption of the bone matrix. Recently, cytogenetic analysis has revealed a specific genetic translocation of the USP6 oncogene in ABC, resulting in the upregulation of USP6 transcription. This aberration is though to arrest the normal maturation of osteoblasts and is implied in matrix metalloproteinase production.2 However, approximately one third of ABCs are considered to be secondary to another primary bone tumor, such as giant cell tumor of the bone or chondroblastoma. These tumors typically lack the aforementioned genetic aberration. The histological appearance is of blood-filled spaces and septa that are not lined by endothelial cells, whereas fibroblasts and multinucleate giant cells are evident in the stroma of the tumor. There is always absence of nuclear atypia or other signs of malignancy in ABCs.
The natural evolution of the lesion remains unclear, and there have been reports of involution and self-healing.3,4 However, as the tumor is generally symptomatic and occasionally locally aggressive, watchful waiting is seldom the case, and treatment is recommended. A variety of methods has been described in the medical literature, and optimal treatment is still a matter of debate. Open surgery has long been considered the gold standard, resulting in good local control, especially in the case of wide excision or when local adjuvants are used. However, the complication rate is not negligible, and some authorities prefer non-invasive methods such as embolization. Radiotherapy has also been used, but a major concern is the risk for secondary malignancies. More recently, the use of sclerotherapy has proved an easy and safe method which is associated with good local control and few side-effects. The introduction of novel medical treatments that block the osteolytic pathway, such as denosumab, has given encouraging preliminary results.
A literature review using Pubmed/Medline database was performed in order to identify scientific publications relevant to the treatment of ABC. The MESH terms aneurysmal bone cyst, operative treatment, sclerotherapy, radiotherapy and embolization were used in our search in order to retrieve the relevant publications. A total of 1198 articles in the English literature were identified, from the inception of the database to July 2015 and references were also retrieved from the publication list of the papers. Articles in written in languages other than English were excluded. Of these, 53 articles were found that met the criteria of this review article, where we aim to systematically present the outcome of therapeutic interventions, as well as their indications and limitations, so that the treating physician can choose the appropriate modality for each individual patient.
En bloc surgical excision
Complete (en bloc) surgical excision of ABC is associated with excellent local control rate. Early reports of en block excisions of ABC of the hand (and reconstruction with bone grafting) reported no recurrences.5,6 Recently, Jafari et al. observed a case of local recurrence in a series of patients treated in the same manner.7 Similarly, many independent authors have reported a 100% local control rate in the patients that were treated with complete resection of the ABC.8-11
Lampasi et al. also report no recurrences in ABC of the distal fibula after complete resection, but also draw attention to the higher risk for complications with this method compared to intralesional excision, especially in view of the similar final functional outcome.12 Moreover, Flont et al. have published a series of patients where en bloc excision was compared to curettage, and they also claim that en bloc excision is a more demanding procedure that should be considered in the case of recurrent lesions.13 They showed a higher (but not statistically significant) rate of complications with en bloc excisions, which however had a lower (also not statistically significant) risk for relapse. In cases of ABC of the distal fibula, the technique of complete subperiosteal resection has been shown to give excellent local control rate (100% according to Abuhassan et al. and Mostafa et al.), with the advantage of preserving the periosteum and allowing for fast recovery of the patients.14,15
En bloc excision has been advocated by some authors as the most appropriate treatment in the case of ABC of the spine. Wang et al. reported that most patients with ABC of the cervical spine could be efficiently treated with complete surgical excision without any neurological sequelae and no observed recurrences during the follow-up period.16 Perlmutter et al. also reported an uncomplicated postoperative course and no relapses after aggressive resection and fusion for ABC of the cervical region.17 In the same line, Zenonos et al. describe local recurrences after failure of complete removal of the spinal ABC.18
Collectively, complete excision of the ABC is accompanied by an excellent local control rate that approaches 100%. However, the technique is doubtlessly cumbersome and accompanied by a significant risk for complications, such as bleeding, pain and growth disturbances. Furthermore, there is frequently a need for local reconstruction of the resulting skeletal defect. These parameters explain why less radical surgical excision is generally preferred.
Intralesional surgical procedures, with or without use of local adjuvants
The mechanism of action of intralesional excisions is partly the removal of the active component of the cyst, and partly the interference in hemodynamics of the lesion. Indeed, it has been suggested that ABC arise as a cause of hemodynamic disturbances, and this has been base based on manometric pressure studies where increased intracystic pressure was observed.4 The main advantage is the simplicity of the technique, which does not require any sophisticated equipment. Although curettage is a less invasive method than en bloc excision, it also entails open surgery and can cause severe bleeding, or growth disturbances when in the proximity of the physeal plate.
Curettage is associated with an acceptable rate of local control, which is approximately 90% in large series.19,20 However, it should be noted that there is considerable variation between studies, with reported local recurrence rates between in anything between 0% and 100%. The poor performance of the method in certain centers has lead to a widespread use of various adjuvants in an effort to minimize the risk for local recurrence. Common agents used are chemicals such as phenol, bone cement, cryotherapy with liquid nitrogen or the mechanical effect of highspeed burr. In a recent study by Kececi et al. in 85 patients, no significant effect of adjuvant phenol or high-speed burr could be estab-lished.21 The overall local recurrence rate was approximately 12%. On the other hand, Wang et al. reported a superior control rate of about 97% when high-speed burr was used.22 In the same line, Garg et al. demonstrated that the use of a high-speed burr combined to electro-cautery of the lesion significantly reduced the recurrence rate compared to simple curettage.23 Bone cement (polymethylmethacrylate) has proven superior to bone grafting in preventing local relapse.24 Moreover, in a series of 80 patients treated with curettage and cryotherapy, Peeters et al. observed relapses in only 5% of the patients.25 Even more sophisticated techniques have been used in this setting, the most characteristic being argon beam laser therapy. This method relies on a coagulation effect and has been shown to eliminate the risk for local recurrence as compared to simple curettage.20,26 Yet, according to the observations of Steffner et al., the overall complication rate with argon beam laser was 19%, as compared to 6% when high-speed burr was used.20
In summary, it appears that adjuvants can improve local control rate when combined to curettage. Yet, it should be kept in mind that not all of the aforementioned agents are readily available. Whereas most surgeons have access to high-speed burr, phenol, bone cement and electrocautery, cryosurgery and ultimately argon beam coagulation are available only in some centers. Furthermore, their use increases the operative time and it should be noted that similar control rates have been achieved even with less invasive techniques.
Minimally invasive surgical techniques
It has been observed that some ABCs heal after a pathological fracture, or after open biopsy. This led Reddy et al. to investigate the outcome of a minimally invasive surgical technique the authors named curopsy, which they described as a percutaneous limited curettage during biopsy, removing the lining membrane from various areas of the lesion. The authors reported that a single such treatment resulted in the healing of 81% of the lesions, somewhat inferior to the success rate after curettage, but on the other hand at a considerably shorter recovery time.19 They concluded that simple, minimally invasive treatments are efficient in the treatment of ABCs. The mechanism of the therapy is not fully understood, but it has been proposed that the destruction of internal cyst architecture during curopsy interferes with the hemodynamic changes and induces healing. Furthermore, in a similar study, Ibrahim et al. reported that percutaneous curettage performed as an outpatient procedure was safe and comparable to open curettage alone regarding functional outcome of the patients.27
Even though such simple treatments appear to work in the majority of cases, it should be noted that ABCs present with a broad spectrum of biological aggressiveness, and these techniques may fail in the case of aggressive lesions. This has been illustrated by Louahem et al., who described the clinical and radiological evolution of ABCs after biopsy.3
Embolization
Embolization has been used for the treatment of ABCs, especially in locations where surgical management is difficult and associated with a substantial risk for complications. Traditionally, embolization as a sole treatment has been applied in ABCs of the axial skeleton. Rossi et al. have reported a series of patients with tumors localized mainly in the axial skeleton, where embolization with N-2-butyl cyanoacrylate had an overall efficacy of 94%, with a relatively low complication rate of 5%.28 Moreover, Amendola et al. had no failures in a series of patients treated with embolization for ABCs of the spine.29 Donati et al. in turn reported a 75% success rate in ABCs of the sacrum treated only with embolization.30
Embolization is also used as a surrogate method to open surgery, in order to minimize the bleeding during excision, and to further reduce the risk for local recurrence.18,31,32 It has also been used in conjunction to sclerotherapy, with good results.33 It has been suggested as a cost-effective technique, which has a primary role in ABCs that are difficult to treat with surgery. It should be mentioned that embolization has its limitations. The absence of feeding vessels that can be catheterized, or the proximity to vessels supplying vital structures such as the spinal cord sometimes makes embolizations impossible. The procedure is technically demanding and complications are not negligible. In a comprehensive retrospective study of more the 400 patients that were treated with selective arterial embolization for neoplastic conditions, including ABC, about a fifth of the patients suffered from post-embolization syndrome and about 10% of them had transient paresthesias. Necrosis and other more serious complications were also observed.34
Sclerotherapy and injection of other drugs
Sclerosants cause damage to the vascular endothelium and cause thrombosis in small vessels, leading to a rather poorly understood cascade of events which results in healing of the lesion. The use of sclerotherapy regiments that relied on alcoholic zein solution was associated with serious adverse effects upon spill-out in the nearby tissues,35-37 as well as documented fatal outcome after injection in an ABC of the cervical spine.38 Modern sclerotherapy treatment for ABCs is polidocanol, which is a safe treatment that generally lacks serious adverse effects (Figure 1). Indeed, in a case series of 72 patients treated with percutaneous intralesional injections of polidocanol,1 Rastogi et al. reported a cure rate of 97%.39 In a prospective study by Varshney et al., scle-rotherapy proved to be equally effective to intralesional excision and was accompanied by less morbidity.40 According to our own experience, the local control rate exceeds 90%, and the method can be used even for the aggressive variant of the tumor.1 Even aggressive ABCs can be cured by sclerotherapy.41 It should be noted that other agents have been injected in ABCs and shown to have effect. Doxycycline, which probably has an analogous mode of action to sclerosants, has been effectively used for percutaneous treatment of ABCs.42 Alcohol injections have a low risk for adverse effects, a good response was however observed in only 59% of the lesions.43 Injection of bone marrow has also been shown to promote healing in the vast majority of patients.44,45
Figure 1.
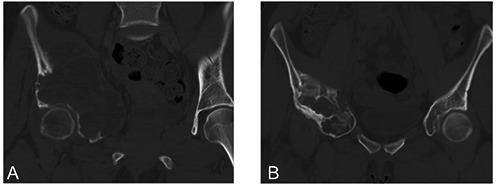
An aneurysmal bone cyst of the pelvis which recurred after intralesional excision and autologous bone grafting (A), showing consolidation after sequential percutaneous injections of polidocanol (B).
In summary, percutaneous administration of polidocanol is a safe and simple procedure with an excellent cure rate. Current evidence favors sclerotherapy as the initial treatment of choice for ABCs. Bone marrow injections can also be considered.
Radiotherapy
Radiotherapy uses ionizing radiation that induces damage to the nuclear DNA in order to induce cell death. It is mainly used in malignant tumors, but has been used by many authorities for the treatment of ABCs, although the molecular mechanisms in this case are poorly understood. Initial reports on the use of radiotherapy for the management of ABCs have shown rather poor results. Indeed, Capanna et al. compared radiotherapy with surgery in the case of ABC of the spine, and showed that radiotherapy when used as a single treatment resulted in a local recurrence in three of six patients, two of them resulting in the death of the patient.46 Marcove et al. on the other hand reported a control rate exceeding 90% when radiotherapy was used, but also reported an irradiation-induced sarcoma in 1 out of 11 patients treated.47 Similarly, Papagelopoulos et al. also described the death of a patient treated with radiotherapy from an irradiation-induced sarcoma.48
Feigenberg et al. did not observe any secondary malignancy in their series of patients with ABC of the spine treated with radiotherapy, but reported other major adverse effects, such as dorsal kyphosis because of vertebral body collapse.49 Similarly, Boriani et al. showed that the combination of curettage and radiotherapy in spinal ABC was accompanied by a high incidence of late axial deformity.11 Marks et al. reported no sequelae after treatment of three adolescents with a dose of 4000 rad.50
Generally, the role of radiotherapy in the treatment of ABC is questionable in view of the considerable risk for serious adverse effects, as the majority of patients are young and even in the phase of skeletal growth. It should be however noted that local radionuclide therapy has emerged as a safe and efficient method, and in a single study resulted in the healing of all lesions without any sequelae.51
Medical treatment
Medical treatment of ABC has relied on the similarities in morphology and biology of ABC to other lesions with increased osteoclastic activity, such as giant cell tumors of the bone. The agents that have been tested thus far inhibit the action of the osteoclasts. In a study where bisphosphonates were used for the treatment of unresectable symptomatic bone tumors, including aneurysmal bone cysts, an effect was observed and there were no adverse effects.52 Bisphosphonates have a molecular structure similar to pyrophosphate, and block signaling pathways that are important in osteoclast activity, such as the Ras, Rac and HMG-CoA pathways. Lange et al. have used denosumab in two patients with spinal ABCs where embolization failed, and reported healing of the lesion and regression of the neurological deficits.53 Denosumab is a monoclonal antibody that blocks RANKL, a ligand that binds to RANK, which is a surface protein that is present on osteoclast progenitors, and promotes their maturation to osteoclasts.
Conclusions
Optimal treatment method of ABCs is still a matter of debate. Various methods are used, and their advantages and drawbacks are summarized in Table 1. Generally, sclerotherapy with polidocanol has proven to be a very good alternative, as it can be done in an outpatient basis and leads to local control comparable to intralesional excision, with probably superior functional outcome. Curettage is still favored by many surgeons, but the risk of complications and the associated morbidity is not negligible. It seems reasonable to suggest that curettage should be reserved for lesions that fail to heal with sclerotherapy, and preferably a high-speed burr should be used in order to minimize the risk for local recurrence. Minimally invasive percutaneous techniques can also be initially considered, especially when the treating physician has to resort to an open biopsy, although the local control rate is inferior to curettage. Embolization of the feeding arteries is often technically demanding and is usually regarded as a supplementary procedure, especially in the cases of ABCs of the axial skeleton. Nowadays, radiation therapy should not be considered as a first-line treatment as it carries a risk for secondary malignancy and has a high failure rate. The outcome after radionuclide therapy is promising but needs to be evaluated in a larger number of patients. Medical treatment has not been properly established in the medical literature and is currently not an option. According to our experience, the vast majority of ABCs can be treated with polidocanol sclerotherapy, which should be the first line of treatment.1 We have seen that the method is simple and safe, and can be performed as a day case surgery. Even aggressive lesions can be treated in this manner.41 The major drawback is multiple treatments are required. We resort to curettage, and fill the cavity with bone cement when sclerotherapy fails.
Table 1.
Principal indications, advantages and disadvantages of the most common treatments for aneurysmal bone cysts.
| Treatment | Principal indications | Advantages | Disadvantages |
|---|---|---|---|
| Sclerotherapy | Primary treatment for all ABCs | Can be done in an outpatient basis, cost-effective, good functional outcome | Requires multiple procedures |
| Intralesional excision | All surgically accessible ABCs, especially after failed sclerotherapy | Often curative as a single procedure, cannot be done in an outpatient basis | Morbidity and adverse effects associated with the procedure (bleeding, growth disturbances, risk for infection) |
| En bloc excision | ABCs in expendable bones such as the fibula | Superb local control rate | Morbidity and adverse effects associated with the procedure (bleeding, growth disturbances, risk for infection) |
| Embolization | Often as an adjuvant to surgery or sclerotherapy, difficult-to -access lesions | Good local control | Risk for neurological sequelae, need for equipment and skilled operator |
| Radiotherapy | Lesions that are not amenable to other treatments | Non-invasive | Risk for secondary malignancies, considerable morbidity, growth disturbances |
References
- 1.Brosjö O, Pechon P, Hesla A, et al. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop 2013;84:502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol Off J Am Soc Clin Oncol 2006;24:e1-2. [DOI] [PubMed] [Google Scholar]
- 3.Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop 2012;6:333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopatho-logic study of 66 cases. Cancer 1970;26:615-25. [DOI] [PubMed] [Google Scholar]
- 5.Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg 1988;13:676-83. [DOI] [PubMed] [Google Scholar]
- 6.Burkhalter WE, Schroeder FC, Eversmann WW. Aneurysmal bone cysts occurring in the metacarpals: a report of three cases. J Hand Surg 1978;3:579-84. [DOI] [PubMed] [Google Scholar]
- 7.Jafari D, Jamshidi K, Najdmazhar F, et al. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg 2011;36:648-55. [DOI] [PubMed] [Google Scholar]
- 8.Farsetti P, Tudisco C, Rosa M, et al. Aneurysmal bone cyst. Long-term follow-up of 20 cases. Arch Orthop Trauma Surg 1990;109:221-3. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki T, Halm H, Hillmann A, et al. Aneurysmal bone cysts of the spine. Arch Orthop Trauma Surg 1999;119:159-62. [DOI] [PubMed] [Google Scholar]
- 10.Grzegorzewski A, Pogonowicz E, Sibinski M, et al. Treatment of benign lesions of humerus with resection and non-vascu-larised, autologous fibular graft. Int Orthop 2010;34:1267-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boriani S, De Iure F, Campanacci L, et al. Aneurysmal bone cyst of the mobile spine: report on 41 cases. Spine 2001;26:27-35. [DOI] [PubMed] [Google Scholar]
- 12.Lampasi M, Magnani M, Donzelli O. Aneurysmal bone cysts of the distal fibula in children: long-term results of curettage and resection in nine patients. J Bone Joint Surg Br 2007;89:1356-62. [DOI] [PubMed] [Google Scholar]
- 13.Flont P, Kolacinska-Flont M, Niedzielski K. A comparison of cyst wall curettage and en bloc excision in the treatment of aneurysmal bone cysts. World J Surg Oncol 2013; 11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abuhassan FO, Shannak AO. Subperiosteal resection of aneurysmal bone cysts of the distal fibula. J Bone Joint Surg Br 2009;91:1227-31. [DOI] [PubMed] [Google Scholar]
- 15.Mostafa MF. Subperiosteal resection of fibular aneurysmal bone cyst. Eur J Orthop Surg Traumatol Orthopédie Traumatol 2015;25:443-50. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Liu X, Jiang L, et al. Treatments for primary aneurysmal bone cysts of the cervical spine: experience of 14 cases. Chin Med J (Engl) 2014;127:4082-6. [PubMed] [Google Scholar]
- 17.Perlmutter DH, Campbell S, Rubery PT, et al. Aneurysmal bone cyst: surgical management in the pediatric cervical spine. Spine 2009;34:E50-3. [DOI] [PubMed] [Google Scholar]
- 18.Zenonos G, Jamil O, Governale LS, et al. Surgical treatment for primary spinal aneurysmal bone cysts: experience from Children’s Hospital Boston. J Neurosurg Pediatr 2012;9:305-15. [DOI] [PubMed] [Google Scholar]
- 19.Reddy KIA, Sinnaeve F, Gaston CL, et al. Aneurysmal bone cysts: do simple treatments work? Clin Orthop 2014;472:190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steffner RJ, Liao C, Stacy G, et al. Factors associated with recurrence of primary aneurysmal bone cysts: is argon beam coagulation an effective adjuvant treatment? J Bone Joint Surg Am 2011;93: e1221-9. [DOI] [PubMed] [Google Scholar]
- 21.Keçeci B, Küçük L, Isayev A, Sabah D. Effect of adjuvant therapies on recurrence in aneurysmal bone cysts. Acta Orthop Traumatol Turc 2014;48:500-6. [DOI] [PubMed] [Google Scholar]
- 22.Wang EHM, Marfori ML, Serrano MVT, Rubio DA. Is curettage and high-speed burring sufficient treatment for aneurysmal bone cysts? Clin Orthop 2014;472: 3483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg S, Mehta S, Dormans JP. Modern surgical treatment of primary aneurysmal bone cyst of the spine in children and adolescents. J Pediatr Orthop 2005;25:387-92. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki T, Hillmann A, Lindner N, Winkelmann W. Cementation of primary aneurysmal bone cysts. Clin Orthop 1997: 240-8. [DOI] [PubMed] [Google Scholar]
- 25.Peeters SP, Van der Geest ICM, de Rooy JWJ, et al. Aneurysmal bone cyst: the role of cryosurgery as local adjuvant treatment. J Surg Oncol 2009;100:719-24. [DOI] [PubMed] [Google Scholar]
- 26.Cummings JE, Smith RA, Heck RK. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop 2010;468:231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim T, Howard AW, Murnaghan ML, Hopyan S. Percutaneous curettage and suction for pediatric extremity aneurysmal bone cysts: is it adequate? J Pediatr Orthop 2012;32:842-7. [DOI] [PubMed] [Google Scholar]
- 28.Rossi G, Rimondi E, Bartalena T, et al. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skeletal Radiol 2010;39:161-7. [DOI] [PubMed] [Google Scholar]
- 29.Amendola L, Simonetti L, Simoes CE, et al. Aneurysmal bone cyst of the mobile spine: the therapeutic role of embolization. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 2013;22:533-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donati D, Frisoni T, Dozza B, et al. Advance in the treatment of aneurysmal bone cyst of the sacrum. Skeletal Radiol 2011;40:1461-6. [DOI] [PubMed] [Google Scholar]
- 31.Boriani S, Lo SL, Puvanesarajah V, et al. Aneurysmal bone cysts of the spine: treatment options and considerations. J Neurooncol 2014;120:171-8. [DOI] [PubMed] [Google Scholar]
- 32.Pearl MS, Wolinsky J P, Gailloud P. Preoperative embolization of primary spinal aneurysmal bone cysts by direct percutaneous intralesional injection of n-butyl-2-cyanoacrylate. J Vasc Interv Radiol 2012;23:841-5. [DOI] [PubMed] [Google Scholar]
- 33.Guarnieri G, Ambrosanio G, Vassallo P, et al. Combined percutaneous and endovas-cular treatment of symptomatic aneurysmal bone cyst of the spine: clinical six months. follow-up of six cases. Neuroradiol J 2010;23:74-84. [DOI] [PubMed] [Google Scholar]
- 34.Rossi G, Mavrogenis AF, Rimondi E, et al. Selective arterial embolisation for bone tumours: experience of 454 cases. Radiol Med (Torino) 2011;116:793-808. [DOI] [PubMed] [Google Scholar]
- 35.Falappa P, Fassari FM, Fanelli A, et al. Aneurysmal bone cysts: treatment with direct percutaneous Ethibloc injection: long-term results. Cardiovasc Intervent Radiol 2002;25:282-90. [DOI] [PubMed] [Google Scholar]
- 36.Adamsbaum C, Mascard E, Guinebretière JM, et al. Intralesional Ethibloc injections in primary aneurysmal bone cysts: an efficient and safe treatment. Skeletal Radiol 2003;32:559-66. [DOI] [PubMed] [Google Scholar]
- 37.Topouchian V, Mazda K, Hamze B, et al. Aneurysmal bone cysts in children: complications of fibrosing agent injection. Radiology 2004;232:522-6. [DOI] [PubMed] [Google Scholar]
- 38.Peraud A, Drake JM, Armstrong D, et al. Fatal ethibloc embolization of verte-brobasilar system following percutaneous injection into aneurysmal bone cyst of the second cervical vertebra. AJNR Am J Neuroradiol 2004;25:1116-20. [PMC free article] [PubMed] [Google Scholar]
- 39.Rastogi S, Varshney MK, Trikha V, et al. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polido-canol. A review of 72 cases with long-term follow-up. J Bone Joint Surg Br 2006;88: 1212-6. [DOI] [PubMed] [Google Scholar]
- 40.Varshney MK, Rastogi S, Khan SA, Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop 2010;468:1649-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brosjö O, Tsagozis P. Treatment of an aggressive aneurysmal bone cyst with percutaneous injection of polidocanol: a case report. J Med Case Rep 2014;8:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiels WE, Mayerson JL. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: a preliminary report. Clin Orthop 2013;471:2675-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambot-Juhan K, Pannier S, Grévent D, et al. Primary aneurysmal bone cysts in children: percutaneous sclerotherapy with absolute alcohol and proposal of a vascular classification. Pediatr Radiol 2012;42:599-605. [DOI] [PubMed] [Google Scholar]
- 44.Docquier PL, Delloye C. Treatment of aneurysmal bone cysts by introduction of demineralized bone and autogenous bone marrow. J Bone Joint Surg Am 2005;87: 2253-8. [DOI] [PubMed] [Google Scholar]
- 45.Hemmadi SS, Cole WG. Treatment of aneurysmal bone cysts with saucerization and bone marrow injection in children. J Pediatr Orthop 1999;19:540-2. [DOI] [PubMed] [Google Scholar]
- 46.Capanna R, Albisinni U, Picci P, et al. Aneurysmal bone cyst of the spine. J Bone Joint Surg Am 1985;67:527-31. [PubMed] [Google Scholar]
- 47.Marcove RC, Sheth DS, Takemoto S, Healey JH. The treatment of aneurysmal bone cyst. Clin Orthop 1995:157-63. [PubMed] [Google Scholar]
- 48.Papagelopoulos PJ, Currier BL, Shaughnessy WJ, et al. Aneurysmal bone cyst of the spine. Management and outcome. Spine 1998;23:621-8. [DOI] [PubMed] [Google Scholar]
- 49.Feigenberg SJ, Marcus RB, Zlotecki RA, et al. Megavoltage radiotherapy for aneurysmal bone cysts. Int J Radiat Oncol Biol Phys 2001;49:1243-7. [DOI] [PubMed] [Google Scholar]
- 50.Marks RD, Scruggs HJ, Wallace KM, Fenn JO. Megavoltage therapy in patients with aneurysmal bone cysts. Radiology 1976;118:421-4. [DOI] [PubMed] [Google Scholar]
- 51.Bush CH, Adler Z, Drane WE, et al. Percutaneous radionuclide ablation of axial aneurysmal bone cysts. AJR Am J Roentgenol 2010;194:W84-90. [DOI] [PubMed] [Google Scholar]
- 52.Cornelis F, Truchetet ME, Amoretti N, et al. Bisphosphonate therapy for unresectable symptomatic benign bone tumors: a long-term prospective study of tolerance and efficacy. Bone 2014;58:11-6. [DOI] [PubMed] [Google Scholar]
- 53.Lange T, Stehling C, Fröhlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 2013;22:1417-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


