Abstract
Merkel cell carcinoma (MCC) is a rare, aggressive skin tumor that mainly occurs in the elderly with a generally poor prognosis. Like all skin cancers, its incidence is rising. Despite the poor prognosis, a few reports of spontaneous regression have been published. We describe the case of a 89-year-old male patient who presented two MCC lesions of the scalp. Following biopsy the lesions underwent complete regression with no clinical evidence of residual tumor up to 24 months. The current knowledge of MCC and the other cases of spontaneous regression described in the literature are reviewed.
Key words: Merkel cell carcinoma, spontaneous regression, immune response
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive skin tumor characterized by frequent local relapses, progression of disease, poor quality of life and short survival.1 MCC was first described by Toker in 1972 as a trabecular carcinoma of the skin,2 and later as neuroendocrine carcinoma of the skin placing it, therefore, in the large family of APUDomas.3
Treatment of MCC is particularly complex: it involves some expertise from the Clinician, and includes both surgical and pharmacological treatments, often combined, in particular in advanced disease.4,5 The spontaneous regression of MCC has been described in a few cases reported in literature. This paper reports a case personally observed by the author, giving final remarks on the possible explanations of this process.
Case Report
Male patient 89-years-old, treated with antiaggregating, diuretics and anti-hypertensive, without any autoimmune disease or other immunocompromized status. From a bit of time, the patient has observed the growth of two distinct lesions of the scalp, on the left temporal site and on the vertex, each of them with a size greater than 4 cm (Figure 1). Approximately two months later the patient undergoes to biopsy of both lesions, and histology shows a Merkel cell carcinoma in both lesions with positive immunohistochemistry for chromogranin A, synaptofisin, CK20, CD117 (spots), neurofilaments (spots), and negative for S100 and TTF1; proliferative index (ki67, MIB1 clone) is equal to 60-70% (Figure 2). Investigations regarding a potential poly-omavirus status was missed. Later, 18F-FDGPET-CT showed uptake on both lesions, and on the left site of the neck, this last considered as metastatic site but without cyto-histological confirma tion. Approximately one month after the biopsy, the two tumoral skin sites spontaneously began to shrink in size, thing that excluded a wider excision. Despite the almost complete remission of the skin lesions, the patient started a palliative radiotherapy on primitive lesions of the scalp and on the left neck with a total dose of 40 Gy in 20 sessions for a period of 30 days. After radiotherapy the patient did not other controls. After 24 months from the biopsy the disease is totally regressed (Figure 3).
Figure 1.
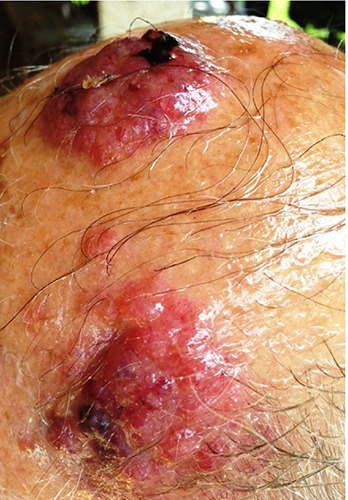
The two lesions before biopsy.
Figure 2.
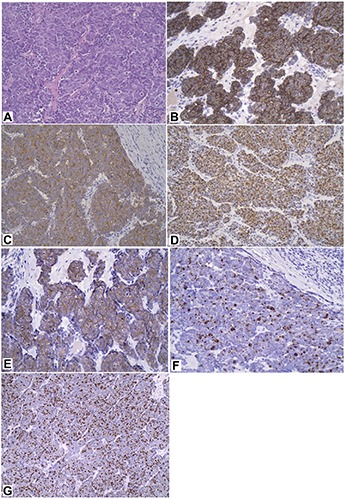
Histological and immunohistochemical pictures of Merkel cell carcinoma. A) Hematoxylin and Eosin staining; B) chromogranin A; C) synaptofisin; D) CK20; E) CD117; F) neurofilaments; G) Ki67-MIB1
Figure 3.
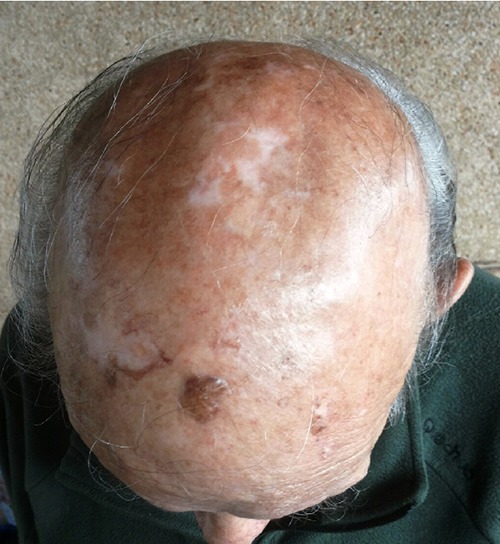
Vertex lesion after 24 months
Discussion
MCC is a highly aggressive skin tumor which rapidly metastasizes with disappointing survival rates close to 50% of cases after 3-year.6 The incidence of MCC is about 2/1.000.000 in Caucasians and 0.1/1.000.000 in black Americans. The average age of onset is the late 60s, with less than 5% occurring in people under the 50 years of age and the highest incidence over the 85-years-old age group.7 About 50% of MCCs occurs on the head and neck, 40% on the extremities, and the remaining on the trunk. Sex incidence appears to be equal.7
Spontaneous regression of MCC is exceedingly rare, and highlights the potentially capricious nature of MCC. As far as we know, a little but not inconsistent number of previous cases has been reported in Literature, so as to speculate that this kind of feature might be not so rare (Table 1).8-29 In fact, considering the prevalence of MCC in over 600 cases reported in literature,30 the estimated prevalence of spontaneous regression appears to be greater than expected (1.7-3% of cases).21 In most cases regression of MCC was preceded by a biopsy of the lesion, as in the present case: it was also suggested a potential stimulation of the immune system.31 It has been reported that dense clusters of lymphocytes are found within tumor nests;10 some reports found that CD4+ and CD8+ cells has heavily infiltrated around the tumor nests, via immunophenotypic analysis of the infiltrated lymphocytes.10,12,16,31 These findings suggest the role of T-cell mediated immune response in the development of tumor regression resulting in apoptosis and cellular necrosis:19 it was also reported that intratumoral CD3+ (and CD8+) cell infiltration is associated with improved overall survival in a Finnish MCC cohort.32 Regarding the role of T-cell mediated immune response in tumor regression, of a certain interest could be the potential role of diet with the aim to enhance the antiproliferative response via an immune-mediated mechanism. Dietary supplements as vitamin C, turmeric, probiotics, cod liver oil, organic vegetables, and mushroom, in particular Trametes versicolor, can elicit Natural Killer activity increasing numbers of CD8+ T-cells and CD19+ B-cells.33 It should be noted that, in the reported case, regression of MCC begins approximately one month after the biopsy, and certainly before radiation treatment. Therefore, we can support that the regression of the lesions is mediated by an immune response stimulated by biopsy.
Table 1.
Complete spontaneous regression in Merkel cell carcinoma described in literature.
| Author | Year | Country | Sex | Age | Primary site | Size (mm) | Follow up |
|---|---|---|---|---|---|---|---|
| O’Rourke et al.8 | 1986 | Australia | F | 90 | Cheek | 20 | 18 m |
| Bayrou et al.9 | 1991 | France | F | 69 | Temple | <20 | 15 y |
| Kayashima et al.10 | 1991 | Japan | F | 68 | Forehead | 16×16 | 11 y |
| Kayashima et al.10 | 1991 | Japan | F | 86 | Cheek | <20 | 36 m |
| Djilali-Bouzina et al.11 | 1992 | France | F | 83 | Cheek | ND | 12 m |
| Duncan et al.12 | 1993 | USA | M | 79 | Scalp | 12×15 | 28 m |
| Hashimoto et al.13 | 1993 | Japan | F | 69 | Cheek | 15×10 | 1 y |
| Tanita et al.14 | 1996 | Japan | F | 75 | Cheek | 30×30 | 1 y |
| Yanguas et al.15 | 1997 | Spain | M | 65 | Ear | 30×30 | 18 m |
| Connelly et al.16 | 1997 | USA | M | 85 | Forehead | 14 | 50 m |
| Satoh et al.17 | 1997 | Japan | M | 87 | Cheek | <20 | 2 m |
| Brown et al.18 | 1999 | USA | M | 89 | Scalp | 9×9 | 33 m |
| Maruo et al.19 | 2000 | Japan | F | 82 | Cheek | 12×10 | 12 m |
| Connelly et al.20 | 2000 | France | F | 71 | Cheek | >20 | 11 m |
| Sais et al.21 | 2002 | Spain | F | 78 | Thigh | 24 | 40 m |
| Junquera et al.22 | 2005 | Spain | F | 79 | Cheek | 30×25 | 3 m |
| Kubo et al.23 | 2007 | Japan | F | 87 | Cheek | 50 | 6 m |
| Vesely et al.7 | 2008 | Canada | F | 67 | Cheek | 29×23 | 6 m |
| Missotten et al.24 | 2008 | Netherlands | M | 90 | Eyelid | <20 | 18 m |
| Karkos et al.25 | 2009 | England | F | 79 | Nose/Neck | 50×50 | 30 m |
| Ciudad et al.26 | 2010 | Spain | F | 86 | Cheek | 22×12 | 18 m |
| Ciudad et al.26 | 2010 | Spain | M | 92 | Scalp | 30×25 | 23 m |
| Hassan et al.27 | 2010 | Ireland | F | 60 | Groin | 30×45 | 4 y |
| Wooff et al.28 | 2010 | Canada | F | 94 | Eyebrow | 40 | 33 m |
| Val-Bernal et al.29 | 2011 | Spain | F | 86 | Forearm | 33×20 | 10 w |
F, Female; M, Male; ND, Not Done; w, week; m, month; y, year. Adapted from Vesley et al.,2008.7
The biggest number of cases of complete spontaneous regression of MCC have been described in the head and neck, mostly with a lesion of size equal or less than 2 cm.7 It was found a significant difference in the incidence of regressing MCC between males and females, with a prevalence for females with 77% of cases.19 Regression, once initiated, was lasting between 1 and 5 months:7 in the present case, regression lasts from 24 months.
A little series of partial spontaneous regression of MCC is reported by Vesely et al., 2008.7 Eleven cases are described in the Literature with the longest follow up of five years. In this series the most common site was the cheek. This series should be separated from cases of complete regressions, in order to avoid confusion and to prevent a wrong estimate of prevalence.
Conclusions
Spontaneous complete regression is a significant event in MCC. A small number of cases of regression has been described in literature, to which we have added a further case. Many of these events began shortly after biopsy, and in some cases lymphocytic infiltration was described. This fact suggests that MCC can be responsive to immunological reactions. In fact, MCC is highly linked with T lymphocyte immune suppression in epidemiologic studies, and CD8+ T cell infiltration into MCC tumors has been shown to be strongly predictive of improved survival. Some Authors have observed, however, that biopsy does not commonly determine the induction of CD8 + lymphocyte infiltration into the tumor, and this fact causes a different survival.34 Further research about mechanism of spontaneous regression, the role of immune response and also of dietary supplements, could contribute to improve the knowledge about biology of MCC in order to offer a better expectation of prognosis.
Acknowledgments
I am grateful to Marco Ungari, MD, pathologist at the Hospital in Cremona, for his valuable contribution.
References
- 1.Duprat JP, Landman G, Salvajoli JV, Brechtbühl ER. A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics 2011;66:1817-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toker C. Trabecular carcinoma of the skin. Arch Dermatol 1972;105:107-10. [PubMed] [Google Scholar]
- 3.Pearse AGE. The neuroendocrine (APUD) cells of the skin. Am J Dermatopathol 1980;2:121-3. [DOI] [PubMed] [Google Scholar]
- 4.Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surgery 2013;2013:850797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirillo F, Vismarra M, Cafaro I, Martinotti M. Merkel cell carcinoma: a retrospective study on 48 cases and review of literature. J Oncol 2012;2012:749030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratner D, Nelson BR, Brown MD, Johnson TM. Merkel cell carcinoma. J Am Acad Dermatol 1993;29:143-56. [DOI] [PubMed] [Google Scholar]
- 7.Vesely MJJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plastic Reconstr Aesthetic Surg 2008;61:165-71. [DOI] [PubMed] [Google Scholar]
- 8.O’Rourke MG, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol 1986;12:994-1000. [DOI] [PubMed] [Google Scholar]
- 9.Bayrou O, Avril MF, Charpentier P, et al. Primary neuroendocrine carcinoma of the skin. Clinicopathologic study of 18 cases. J Am Acad Dermatol 1991;24:198-207. [DOI] [PubMed] [Google Scholar]
- 10.Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol 1991;127:550-3. [PubMed] [Google Scholar]
- 11.Djilali-Bouzina F, Cribier B, Heid E. Regressive neuroendocrine carcinoma after partial biopsy. Nouv Dermatol 1992;11:767-70. [Google Scholar]
- 12.Duncan WC, Tschen JA. Spontaneous regression of Merkel cell (neuroen-docrine) carcinoma of the skin. J Am Acad Dermatol 1993;29:653-4. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto Y, Koike K, Kawagishi N, et al. Merkel cell tumour with spontaneous regression after biopsy. Hifukano Rinsho 1993;35:169-72. [Google Scholar]
- 14.Tanita M, Tabata N, Kato T. Merkel cell carcinoma with spontaneous regression. Skin Cancer 1996;11:70-2. [Google Scholar]
- 15.Yanguas I, Goday JJ, González-Güemes M, et al. Spontaneous regression of Merkel cell carcinoma of the skin. Br J Dermatol 1997;137:296-8. [DOI] [PubMed] [Google Scholar]
- 16.Connelly TJ, Kowalcyk AP. Another case of spontaneous regression of Merkel cell (neuroendocrine) carcinoma. Dermatol Surg 1997;23:588-90. [DOI] [PubMed] [Google Scholar]
- 17.Satoh M, Kikkawa Y, Iwatsuki K. Spontaneous regression of Merkel cell tumour after biopsy. Hifukano Rinsho 1997;39:1449-51. [Google Scholar]
- 18.Brown TJ, Jackson BA, Macfarlane DF, Goldberg LH. Merkel cell carcinoma: spontaneous resolution and management of metastatic disease. Dermatol Surg 1999;25:23-5. [DOI] [PubMed] [Google Scholar]
- 19.Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma - a case showing replacement of tumour cells by foamy cells. Br J Dermatol 2000;142:1184-9. [DOI] [PubMed] [Google Scholar]
- 20.Connelly TJ, Cribier B, Brown TJ, Yanguas I. Complete spontaneous regression of Merkel cell carcinoma: a review of the 10 reported cases. Dermatol Surg 2000;26:853-6. [DOI] [PubMed] [Google Scholar]
- 21.Sais G, Admella C, Soler T. Spontaneous regression in primary cutaneous neuroen-docrine (Merkel cell) carcinoma: a rare immune phenomenon? J Eur Acad Dermatol Venereol 2002;16:82-3. [DOI] [PubMed] [Google Scholar]
- 22.Junquera L, Torre A, Vicente JC, et al. Complete spontaneous regression of Merkel cell carcinoma. Ann Otol Rhinol Laryngol 2005;114:376-80. [DOI] [PubMed] [Google Scholar]
- 23.Kubo H, Matsushita S, Fukushige T, et al. Spontaneous regression of recurrent and metastatic Merkel cell carcinoma. J Dermatol 2007;34:773-7. [DOI] [PubMed] [Google Scholar]
- 24.Missotten GS, de Wolff-Rouendaal D, de Keizer RJ. Merkel cell carcinoma of the eyelid review of the literature and report of patients with Merkel cell carcinoma showing spontaneous regression. Ophthalmology 2008;115:195-201. [DOI] [PubMed] [Google Scholar]
- 25.Karkos PD, Sastry A, Hampal S, Al-Jafari M. Spontaneous regression of Merkel cell carcinoma of the nose. Head Neck 2010;32:411-4. [DOI] [PubMed] [Google Scholar]
- 26.Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in merkel cell carcinoma: report of two cases with a description of dermoscopic features and review of the literature. Dermatol Surg 2010;36:68793. [DOI] [PubMed] [Google Scholar]
- 27.Hassan SJ, Knox M, Griffin M, Kennedy MJ. Spontaneous regression of metastatic Merkel cell carcinoma. Ir Med J 2010;103:21-2. [PubMed] [Google Scholar]
- 28.Wooff JC, Trites JR, Walsh NM, Bullock MJ. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol 2010;32:614-7. [DOI] [PubMed] [Google Scholar]
- 29.Val-Bernal J F, García-Castaño A, García-Barredo R, et al. Spontaneous complete regression in Merkel cell carcinoma after biopsy. Adv Anat Pathol 2011;18:174-7. [DOI] [PubMed] [Google Scholar]
- 30.Haag ML, Glass LF, Fenske NA. Merkel cell carcinoma. Diagnosis and treatment. Dermatol Surg 1995;21:669-83. [DOI] [PubMed] [Google Scholar]
- 31.Takenaka H, Kishimoto S, Shibagaki R, et al. Merkel cell carcinoma with partial spontaneous regression: an immunohisto-chemical, ultrastructural and TUNEL labeling study. Am J Dermatopathol 1997;19:614-8. [DOI] [PubMed] [Google Scholar]
- 32.Sihto H, Bohling T, Kavola H, et al. Tumor infiltrating immune cells and outcome of merkel cell carcinoma: a population-based study. Clin Cancer Res 2012;18:2872-88. [DOI] [PubMed] [Google Scholar]
- 33.Vandeven N, Nghiem P. Complete spontaneous regression of Merkel cell carcinoma metastatic to the liver: did lifestyle modifications and dietary supplements play a role? Glob Adv Health Med 2012;1:20-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS One 2012;7:e41465. [DOI] [PMC free article] [PubMed] [Google Scholar]


