Abstract
Primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL-LT) is a rare diffuse large B-cell lymphoma confined to the skin of the legs. The typical presentation is characterized by solitary or multiple growing plaques, usually confined to one leg. We report a case of PCDLBCL-LT of activated B-cell subtype characterized by multiple local relapses in the legs, initially, and systemic relapses about seven years after the diagnosis. Local relapses were sensitive to radiation therapy. Cutaneous and systemic relapses responded well to immunomodulatory therapy with lenalidomide followed by Bruton’s tyrosine kinase inhibition with ibrutinib. Ibrutinib is the only treatment that resulted in long-lasting complete remission. Lenalidomide and especially ibrutinib appear to have a significant activity against this lymphoma and should be incorporated in the treatment of this resistant and aggressive lymphoma. To our knowledge, this is the first case of PCDLBCL-LT reported in the literature exhibiting a complete response to ibrutinib.
Key words: Cutaneous diffuse large B-cell lym-phoma, lenalidomide, ibrutinib
Introduction
Primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL-LT), is a rare and aggressive diffuse large B-cell lymphoma (DLBCL). The incidence is about 1 to 4% of all the cutaneous lymphomas.1 It usually affects the elderly patients with a female preponderance.2,3 A smooth violaceous plaque or reddish-bluish nodule on the lower extremities is pro-totypic; however, initial presentations are not necessarily confined to the lower extremities. Unlike primary cutaneous follicular B-cell lymphoma and primary cutaneous B-cell marginal zone lymphoma, the natural history of DLBCL typically includes local cutaneous relapses and extra-cutaneous dissemination.4,5 Overall prognosis is poor, with a 5-year survival rate of only 30-40%, and the optimal systemic treatment is not responsive to chemotherapy, with a high relapse rate of 50%.4 Age over 75, multiple leg lesions, shorter time to progression and extra cutaneous spread portend a worse prog-nosis.2
We report a case of PCDLBCL-LT with shortlived responses to standard systemic DLBCL therapies, including high-dose chemotherapy and autologous stem cell transplantation. We achieved an excellent response to immunomodulatory therapy with lenalidomide followed by a long-lasting complete remission to Burton’s tyrosine kinase inhibition with ibrutinib. These agents alone or in combination with chemotherapy may have a novel role in the management of this aggressive primary cutaneous B-cell lymphoma.
Case Report
A 62-year-old male with a history of neu-rosarcoidosis and longstanding history of lower extremity paralysis presented with a 3cm unilateral smooth nodular lesion on the right shin. Skin biopsy revealed a diffuse dermal infiltrate of large pleomorphic mononuclear cells positive for CD79A, CD20, BCL2, and MUM1, but negative for CD10 or BCL6 (Figure 1), consistent with activated B-cell subtype of DLBCL (ABC-DLBCL). Initial staging evaluation did not show any evidence of disease elsewhere. A diagnosis of PCDLBCL-LT was made. The patient was treated with 6 cycles of rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone (R-CHOP), resulting in a complete response lasting only for 4 months. In April 2006, he developed a nodule proximal to the presenting site and was treated with radiation, 4000 cGy at 200 cGy per fraction for 20 fractions, with complete clearance. Soon thereafter, he developed multiple smooth papular lesions on both bilateral lower extremities which again demonstrated the same lymphoma. Due to the extensive multifocal cutaneous involvement, he was treated with three cycles of rituximab, iphosphamide, carbo-platin, and etoposide (RICE) chemoim-munotherapy (April 2007) followed by a combination of iodine-131 tositumomab (Bexxar) radioimmunoconjugate therapy and high-dose carmustine, etoposide, cytarabine, melphalan chemotherapy (Bexxar-BEAM therapy) with autologous stem cell transplantation (August 2007). He achieved a complete response to RICE chemotherapy and was in remission at the time of transplant, but experienced a relapse in the right lower extremity 6 months post-transplant. Restaging scans were other-wise negative for systemic disease and the recurrent nodule was treated with local radiation. He developed multiple local recurrences confined to the legs for the next 4 years and was managed with local radiation until reaching a maximum of 15 treatments. Localized cutaneous involvement persisted with eventual systemic involvement of the right lower lung and mediastinal and hilar lymphadenopathy (Figure 2A) that was treated with radiation followed by lenalidomide 25 mg per os daily for 1 to 21 days of every 28-day cycle. Most skin lesions regressed completely after two cycles (Figure 2B), but continued use of lenalidomide was precluded due to neutropenia and infectious complications. Due to multifocal recurrences in the legs, multiple radiation treatments leading to radiation necrosis and soft tissue infections, he underwent bilateral above-knee amputation. A follow-up positron emission tomography/computed tomography (PET/CT) scan in November 2013 showed widespread relapse with lesions in the femur and scalp, abdominal nodules and bulky retroperitoneal lymphadenopathy (Figure 2C). Ibrutinib 560 mg qd per os was started in December 2013 with rapid improvement resulting in complete remission (CR). He remains in CR at the time of this writing (Figure 2D).
Figure 1.
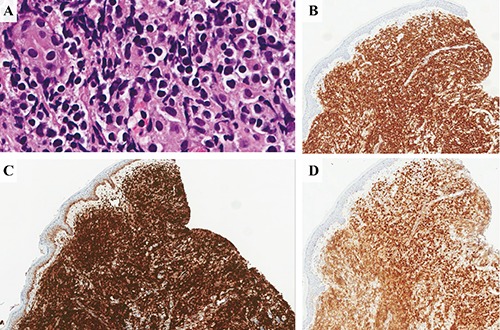
Immunohistochemical analysis. A) Hematoxylin & Eosin 40×; B) CD20 8×, C) BCL2 8×; D) MUM1 8×.
Figure 2.
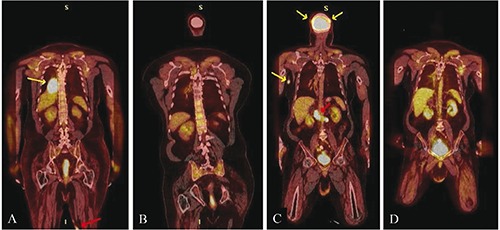
A) Pre-lenalidomide-fused coronal F-18 fluorodeoxyglucose (FDG) PET/CT from February 2012 demonstrates hypermetabolic subcutaneous nodules on the postero-medial left thigh (red arrow) and an extremely hypermetabolic posteromedial right pleural based mass (yellow arrow). B) Post-lenalidomide-fused coronal F-18 FDG PET/CT from July 2012 demonstrates resolution of hypermetabolic right pleural based mass. Subcutaneous hypermetabolic left thigh nodules had also resolved, but are not visualized on this image. C) Pre-ibrutinib-fused coronal F-18 FDG PET/CT from November 2013 demonstrates new hypermetabolic subcutaneous nodules on the medial right upper arm and scalp (yellow arrows) and extensive hypermetabolic retroperitoneal lymphadenopathy (red arrow). D) Post-ibrutinib-fused coronal F-18 FDG PET/CT from March 2014 demonstrates resolution of hypermetabolic subcutaneous nodule on the medial right upper arm and extensive hypermetabolic retroperitoneal lymphadenopathy. Hypermetabolic scalp nodules have also resolved (anatomy not included in image).
Discussion
Our case of PCDLBCL-LT has many interesting features. First of all, it developed in a patient with paralysis secondary to neurosarcoidosis. The development of PCDLBCL-LT in paralyzed legs certainly raises the possibility of an etiologic link between the two conditions. We were not able to find a similar case in our literature search; however, we cannot rule out this possibility. The lymphoma kept relapsing with recurrent tumors confined to the skin of the legs for about seven years. PCDLBCL-LT is confined initially to the cutaneous compartment of lower extremities, but can spread to sites outside of the legs as it progresses. As such, preferential localization and development of this lymphoma in the legs suggest that the microenvironment of legs is important for this lymphoma. This issue needs to be further examined. Our hypothesis is that chronic anti-genic stimulation related to frequent injuries and infections may play a role. Finally, lymphoma appeared to become independent of the microenvironment in the legs and disseminated in a widespread manner outside the legs.
In terms of pathology, our case is an ABC-DLBCL with strong expression of MUM1 (IRF4). According to a study on 60 patients with PCDLBCL-LT by Grange et al., MUM1 expression was positive in 56.1%,2 suggesting that about half of the cases of PCDLBCL-LT are of ABC subtype according to Hans’ IHC criteria.6 Another study published recently by Wang et al. found a high expression of MUM1 in seven cases of PCDLBCL-LT.7 In a study by Koens et al., the genetic landscape in these patients was found to be similar to the ABCDLBCL with NF-kB activating mutations, and thus, may have a poorer prognosis.8 We plan to undertake a further study to define PCDLBCL-LT based on cell of origin and correlate that with responsiveness to various therapies and prognosis.
As for therapeutic intervention, our case did not respond well to standard therapies for systemic DLBCL, R-CHOP, RICE, and high-dose therapy followed by autologous stem cell transplantation, with the responses lasting only for a few months. The patient responded well to radiation treatments, suggesting that, even with metastatic disease, this lymphoma is still responsive to radiation therapy. Ultimately, the patient developed widespread systemic disease. He had an excellent response to lenalidomide, but could not continue due to neutropenia and recurrent infections in the legs. Fortunately, he tolerated and responded well to ibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor, with the longest-lasting CR. Ibrutinib is a novel inhibitor of Bruton’s tyrosine kinase, a key signaling molecule in B cell receptor signaling pathway, which is constitutively activated in ABC-DLBCL due to genetic mutations. It has been shown to have significant therapeutic activity against ABC-DLBCL.9,10 Our patient went into complete remission with ibrutinib after being refractory to multiple lines of standard therapies, suggesting that B cell receptor signaling plays an essential role in the biology of his ABC-DLBCL. Lenalidomide has been shown to mediate significant cytotoxicity against ABC-DLBCL lymphoma cells via down regulation of IRF4 (MUM1). The response to lenalidomide in this case is likely due to the fact that it is an ABC-DLBCL with expression of MUM1.11 In preclinstudies, lenalidomide has been shown to have a direct effect on DLBCL cell lines with a decrease in the NFkB activity and DNA synthe-sis.12 A phase 2 trial of single agent lenalido-mide in patients with relapsed/refractory aggressive DLBCL demonstrated an overall response rate of 29%, showing that this drug has activity in heavily pretreated patients with DLBCL.13 A significant difference in clinical response was reported by Hernandez et al. to lenalidomide for GCB vs non-GCB patients. The overall response was 52.9% in ABC patients versus 8.7% in patients with GCB type.11,14 As such, response to lenalidomide and ibrutinib is much better for ABC-DLBCL compared to GCB-DLBCL. Pathologic determination of cell of origin of PCDLBCL-LT should be routinely performed for determination of therapeutic options.
Both lenalidomide and ibrutinib have not been approved by the FDA for DLBCL. It may be difficult to get the drugs for the patients. However, it is still possible to get the drug for the patients with the help of drug companies. As lenalidomide and ibrutinib have significant therapeutic activity as single agents, they are being incorporated into the standard regimen for DLBCL (R-CHOP). Addition of lenalidomide to R-CHOP has shown promising results.15 There are ongoing clinical trials evaluating the role of lenalidomide and ibrutinib as single agents and combination therapy for refractory aggressive B cell non-Hodgkin’s lymphoma.16-20
Our opinion is that ABC type of PCDLBCL-LT does not respond well to standard therapeutic options for systemic DLBCL and that lenalido-mide and ibrutinib are quite promising.
Conclusions
PCDLBCL-LT is an aggressive and recalcitrant lymphoma characterized by multiple local recurrences and later widespread systemic dissemination. ABC subtype of PCDLBCL-LT, as in our patient, does not respond well to standard therapeutic options for systemic DLBCL. However, it responds well to immunomodulatory therapy and BTK inhibition. We recommend that these agents should be incorporated into the management of this lymphoma. We believe that these agents have a great potential to improve its abysmal natural history.
References
- 1.Zackheim HS, Vonderheid EC, Ramsay DL, et al. Relative frequency of various forms of primary cutaneous lymphomas. J Am Acad Dermatol 2000;43:793-6. [DOI] [PubMed] [Google Scholar]
- 2.Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol 2007;143:1144-50. [DOI] [PubMed] [Google Scholar]
- 3.Kodama K, Massone C, Chott A, et al. Primary cutaneous large B-cell lym-phomas: clinicopathologic features, classification, and prognostic factors in a large series of patients. Blood 2005;106:2491-7. [DOI] [PubMed] [Google Scholar]
- 4.Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 2008;112:1600-9. [DOI] [PubMed] [Google Scholar]
- 5.Thomas V, Dobson R, Mennel R. Primary cutaneous large B-cell lymphoma, leg type. Proc (Bayl Univ Med Cent) 2011;24:350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Jia L, Liao W, et al. [Primary cutaneous diffuse large B-cell lymphoma, leg type: a study of clinicopathology, immunophenotype and gene rearrangement]. Zhonghua Bing Li Xue Za Zhi 2015;44:100-5. [Article in Japanese] [PubMed] [Google Scholar]
- 8.Koens L, Zoutman WH, Ngarmlertsirichai P, et al. Nuclear factor-kappaB pathway-activating gene aberrancies in primary cutaneous large B-cell lymphoma, leg type. J Invest Dermatol 2014;134:290-2. [DOI] [PubMed] [Google Scholar]
- 9.Brower V. Ibrutinib promising in subtype of DLBCL. Lancet Oncol 2015;16:e428. [DOI] [PubMed] [Google Scholar]
- 10.Young RM, Shaffer AL, 3rd, Phelan JD, Staudt LM. B-cell receptor signaling in diffuse large B-cell lymphoma. Semin Hematol 2015;52:77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 2011;117:5058-66. [DOI] [PubMed] [Google Scholar]
- 12.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lym-phoma. J Clin Oncol 2008;26:4952-7. [DOI] [PubMed] [Google Scholar]
- 13.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lym-phoma. Ann Oncol 2011;22:1622-7. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Girona A, Heintel D, Zhang LH, et al. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol. 2011;154:325-36. [DOI] [PubMed] [Google Scholar]
- 15.Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lym-phoma: a phase II study. J Clin Oncol 2015;33:251-7. [DOI] [PubMed] [Google Scholar]
- 16.Washington University School of Medicine. Brentuximab vedotin and lenalidomide for relapsed or refractory diffuse large B-cell lymphoma. NCT02086604. Last updated: november 2015. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02086604. [Google Scholar]
- 17.National Cancer Institute. Lenalidomide and ibrutinib in treating patients with relapsed or refractory B-cell non-Hodgkin lymphoma. NCT01955499. Last updated: June 2015. Available from: https://clinical-trials.gov/ct2/show/NCT01955499?term=NCT01955499&rank=1. [Google Scholar]
- 18.University of Chicago. Lenalidomide and combination chemotherapy (DA-EPOCHR) in treating patients with MYC-associated B-cell lymphomas. NCT02213913. Last updated: September 2015. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02213913 [Google Scholar]
- 19.The Lymphoma Study Association. Efficacy of lenalidomide in combination with subcutaneous rituximab + miniCHOP in DLBCL patients of 80 y/o or+. NCT02128061. Last updated: November 2015. Available from: https://www.clinical-trials.gov/ct2/show/NCT02128061 [Google Scholar]
- 20.National Cancer Institute. Rituximab, lenalidomide, and ibrutinib in treating patients with previously untreated stage II-IV follicular lymphoma. NCT01829568. Last updated: November 2015. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01829568 [Google Scholar]


