Abstract
Objectives
Little cigars and cigarillos are gaining in popularity as cigarette use wanes, mainly due to relaxed regulatory standards that make them cheaper, easier to buy individually, and available in a variety of flavors not allowed in cigarettes. To address whether they should be regulated as strictly as cigarettes, we investigated whether little cigar secondhand smoke (SHS) decreases vascular endothelial function like that of cigarettes.
Methods
We exposed rats to SHS from little cigars, cigarettes, or chamber air, for 10 minutes and measured the resulting acute impairment of arterial flow-mediated dilation (FMD).
Results
SHS from both little cigars and cigarettes impaired FMD. Impairment was greater after exposure to little cigar SHS than by cigarette SHS relative to pre-exposure values, although the post-exposure FMD values were not significantly different from each other.
Conclusions
Exposure to little cigar SHS leads to impairment of FMD that is at least equal to that resulting from similar levels of cigarette SHS. Our findings support the need to prevent even brief exposure to little cigar SHS, and support tobacco control policies that regulate little cigars as strictly as cigarettes.
Keywords: little cigars, filtered cigars, cigarillos, secondhand smoke, vascular endothelial function, flow-mediated dilation
Little cigars (ie, filtered cigars) and cigarillos have been increasing in popularity for at least 2 decades,1 counteracting the success of tobacco control efforts on cigarette smoking. As of October 2015, little cigars and cigarillos are not subject to the same product regulations as cigarettes, including warning labels on packages, minimum pack size, and prohibition of marketing using characterizing flavors other than menthol, and they are often taxed at a lower rate.1,2 Little cigars are also perceived as being less harmful than cigarettes.3 Filtered little cigars in particular are practically identical to cigarettes in size, shape, and filter style4,5 (Figure 1). To avoid being regulated as cigarettes, the small cigar products consist of tobacco wrapped in a tobacco leaf or in paper containing tobacco (cigarettes do not contain tobacco in their wrapper), and cigarillos are heavier than the weight range that defines cigarettes. In addition, the tobacco is of different pH and blend than that in cigarettes.6,7 Nonetheless, unlike conventional cigars, the smoke from little cigars and cigarillos is often inhaled as in cigarette smoking, and secondhand smoke (SHS) poses hazards to bystanders regardless of smoking technique.8 Regulating little cigars as strictly as cigarettes would arguably prevent them from simply replacing cigarettes,9–12 but the relative lack of knowledge about their smoke composition and their health effects1 makes such regulatory expansion difficult to achieve.
Figure 1.
Cigarettes and Little Cigars Used for this Study
Note.
The UPC code and additional ID numbers on one carton of Swisher Sweets filtered little cigars are shown at right, along with the UPC code on individual packs. Little cigars were 100 mm long and needed to be trimmed to fit in our machine optimized for the ~80 mm standard cigarettes. All photographs by M.L. Springer.
Like cigarettes, little cigar smoke contains nicotine and the thousands of chemicals that result from tobacco combustion, and is particularly rich in carbon monoxide, nitrosamines, nitrogen oxides, and ammonia.5–8 Cigar smoke is associated with elevated risk of oral, lung, and esophageal cancers.8 The risk of coronary heart disease is 30% higher for cigar smokers than non-smokers, and doubles for those who inhale the smoke.13 These effects demonstrate the risks associated with long-term use of these products, but the case for regulating them as equivalents of cigarettes would be strengthened by evidence that their immediate health consequences are comparable to those of cigarettes.
One of the most acute health consequences of exposure to cigarette smoke is the immediate impairment of vascular endothelial function, measured as arterial flow-mediated dilation (FMD). FMD is a well-validated marker of cardiovascular risk that is chronically impaired in humans by both active smoking of cigarettes and conventional cigars and by cigarette SHS exposure.14–16 FMD is temporarily impaired in humans by 30 minutes of exposure to SHS or aged sidestream smoke at real-world SHS levels.17–19 Sidestream smoke is smoke from the smoldering tip that comprises ~85% of SHS with the rest being exhaled mainstream smoke.20 Because the sidestream smoke ages in the exposure chamber prior to exposure, like real SHS does in real exposure scenarios, we refer to it here as SHS. Our micro-ultrasound-based approach to measure FMD in living rats yields results whose pharmacological and biophysical effects are similar to those observed in humans.21,22 This rat model showed that impairment of FMD occurred with one minute of exposure to cigarette SHS.23 We report here that brief exposure to little cigar SHS impairs vascular function in rats as least as much as exposure to cigarette SHS.
METHODS
Animals
We used male Sprague-Dawley rats, 10 weeks old, N = 8 or 9 rats/group. Rats remained anesthetized (ketamine 100 mg/kg, xylazine 5 mg/kg) throughout the experiment and were euthanized immediately afterward. All procedures were approved by the UCSF Institutional Animal Care and Use Committee.
Measurement of Endothelial Function
Flow-mediated dilation was measured in anesthetized (ketamine 100 mg/kg, xylazine 5 mg/kg) rats as we have previously described.22,23 At first, an incision was made to expose the right common iliac artery. Then a suture snare was placed loosely around the common iliac artery to keep the ends of the snare externalized. A baseline ultrasound measurement of femoral artery diameter was taken at diastole with a 35 MHz ultrasound transducer (Vevo660, VisualSonics) system. The artery was occluded for 5 minutes, during which the femoral artery was prevented from moving by a supportive piece of tubing. The snare was released to re-establish perfusion with a rush of blood flow (hyperemia), and ultrasound measurements of femoral artery diameter were performed every 30 seconds for 3 minutes with additional measurements at 4 and 5 minutes. FMD was calculated as % change: (peak diameterpostischemia − diameterbaseline)/diameterbaseline×100.
Exposure to Smoke
Little cigars were Swisher Sweets brand (20/pack) and cigarettes were Marlboro Red brand, neither of which was flavored (Figure 1). We were only able to obtain the little cigars at 100 mm in length (slightly longer than the length of the Marlboro cigarettes typically used in our system, ~80 mm), so their non-filter ends were trimmed to enable them to be lit by our system’s fixed-position automatic lighter coil. The Swisher Sweets product was 1.22 g/stick, or 2.69 lb per thousand sticks, falling within the US Federal Government’s tax definition of a little cigar (maximum of 3 lb per 1000 count).24
Our modified cigarette smoking machine23 uses a single chamber to collect sidestream smoke; the anesthetized rat is exposed by placing its head through a gasket into the chamber. For each experiment, a cigarette or little cigar was pre-humidified overnight by placement over 16% glycerol in distilled water and then was lit and smoked for 3 minutes under well-established research conditions (ISO Standard 3308:2012, one 35 ml puff of 2 sec duration once per minute) and extinguished. Respirable suspended particles <2.5 μm (RSP) were measured with a TSI Sidepak AM510 monitor sampling and returning air from the chamber once per minute. The AM510 was factory calibrated and was then specifically calibrated for tobacco smoke particles in our laboratory by gravimetric sampling of smoke from the exposure chamber, resulting in a calibration factor of 0.3 applied to the raw data. Due to particle adsorption and deposition, particle concentration falls over time (Figures 2 and 3). Particle concentration in the exposure chamber was adjusted by venting until the desired starting concentration was reached. Our target starting concentration was 600 μg/m3 RSP, representative of smoke levels found in restaurants where smoking is allowed,25,26 and similar to the conditions of our earlier study of cigarette SHS.23 The cigarette or little cigar was extinguished, and an individual anesthetized rat, after baseline FMD measurement (denoted as “pre”), was exposed for the specified duration (which was determined in a preliminary experiment; see beginning of Results section) and was then returned to the ultrasound system for post-smoke FMD measurement. Due to technical limitations, the initial post-smoke FMD measurement (“post”) took place roughly 10 minutes after the end of exposure. FMD was measured a third time 30 minutes later to assess recovery (ie, 40 minutes after end of exposure; “recovery”). Exposure to air in the cleaned exposure chamber without tobacco product provided a negative control.
Figure 2.
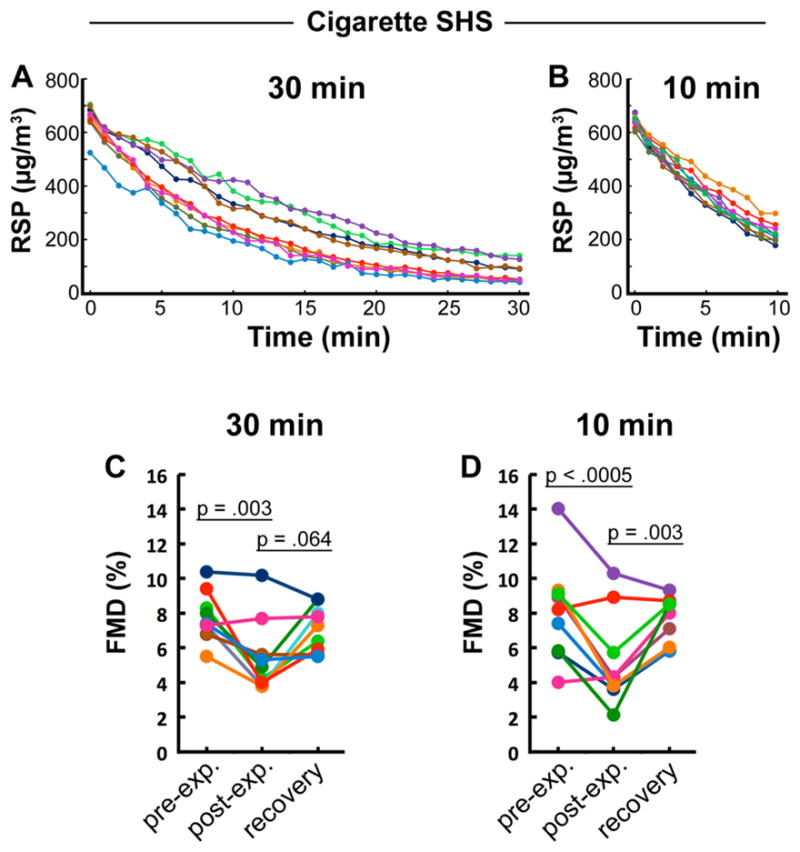
Comparable Impairment of FMD from 30 and 10 Minutes of Exposure to Declining Levels of Cigarette SHS
Note.
Decline of particle concentrations during (a) 30-minute and (b) 10-minute exposure are shown for each rat. (c) FMD response to 30-minute exposure. (d) FMD response to 10 minute exposure. Lines correspond to individual rats; colors track individual animals through smoke and FMD graphs. Thin lines underneath p values denote the data pair being compared.
Figure 3.
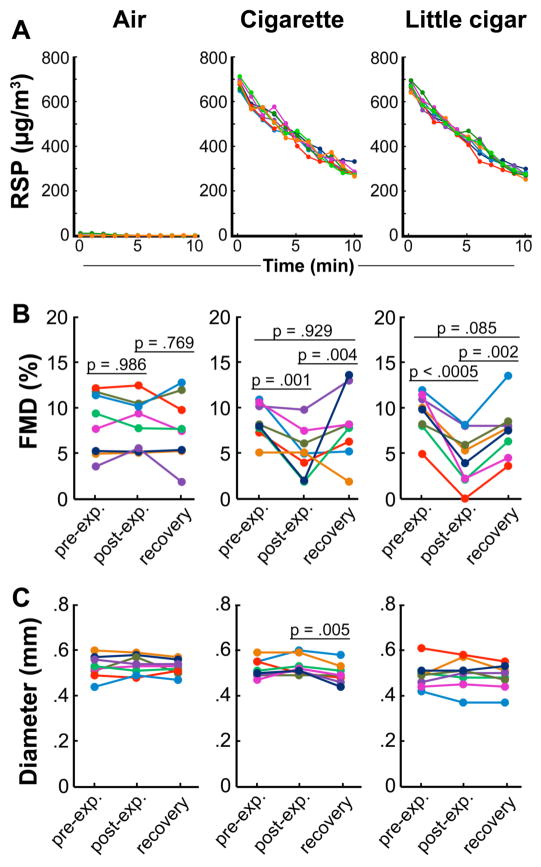
Impairment of FMD from Exposure to Little Cigar SHS
Note.
(a) Particle kinetics and quantitation for comparison of cigarette and little cigar sidestream smoke. Each line represents particle measurements over 10 minutes for each rat; errors are SD. (b) Impairment and recovery of FMD after 10 minutes of exposure to air, cigarette SHS, and little cigar SHS. (c) Lack of effects on baseline arterial diameter (pre-occlusion). p > .44 for all sequential diameter measurements unless otherwise noted. Lines correspond to individual rats; colors track individual animals through panels a, b, and c. Thin lines underneath p values denote the data pair being compared.
For each experiment, rats from each group were exposed in a random order, and arterial diameter measurements were obtained by an investigator unaware of the experimental condition.
Statistics
To evaluate differences in FMD or baseline diameter versus times or exposure conditions, we fit a 2-factor (exposure condition and time) repeated measures ANOVA to all data at once using a linear mixed model estimated with restricted maximum likelihood estimation, then tested for differences over time and across exposure conditions using contrasts and pairwise comparisons, adjusted for multiple comparisons using the Šidák method using Stata 13.1. Time was modeled as a repeated effect and the residual covariance structure was independent. Variability in the data are reported as standard error of the mean (SEM) for paired analyses of FMD and diameter results, and standard deviation (SD) for smoke particle concentrations.
RESULTS
Exposure to Cigarette SHS for 30 minutes or 10 minutes Impairs FMD to Comparable Extents
To determine an appropriate exposure time for our primary purpose of studying brief exposure to little cigar SHS, we first validated a hypothesis that a 10-minute exposure would be sufficient in the context of cigarette SHS, with which we had extensive experience. We showed previously that one minute of exposure to cigarette SHS impairs FMD significantly but modestly, and that exposure for 30 minutes substantially impairs FMD, but most of the exposure occurs during the first 10 minutes due to the decline in particle concentration over time in our smoke system.23 Therefore, we directly compared FMD impairment after 30 minutes versus 10 minutes of exposure to cigarette SHS (Figure 2). For 30 minutes: mean starting concentration was 655±53.4 μg/m3, mean concentration over time was 253±59.4 μg/m3, total exposure (area-under-curve) was 7493±1804 μg/m3•min; for 10 minutes: mean starting concentration was 644±23.5 μg/m3, mean concentration over time was 396±27.2 μg/m3, total exposure was 3921±279 μg/m3•min.
Flow-mediated dilation declined after a 10-minute exposure from a mean of 8.0±1.0% to 5.2±0.9% (p < .0005) and after a 30-minute exposure declined from 7.8±0.5% to 5.5±0.7% (p = .003). Recovery of FMD after an additional 30 minutes of exposure (to clean air) in each group reached 7.5±0.5% and 7.1±0.4% in the 10 and 30 min groups (p = .003 and p = .064, respectively, relative to the initial post-exposure values) . There was no significant difference between the initial mean post-exposure impaired FMD values between the 2 exposure time conditions (p = .759), nor between the subsequent mean recovery values (p = .682). This result indicates that exposure to the low levels of smoke remaining during the last 20 minutes of a 30-minute exposure did not decrease FMD further after the higher level exposure during the first 10 minutes. Therefore, a 10-minute exposure to declining SHS levels was used as the standard exposure time for the subsequent experiment comparing SHS from cigarettes and little cigars.
Comparable Impairment of FMD by SHS from Cigarettes and Little Cigars
We performed a direct comparison between little cigar and cigarette SHS, both at starting smoke RSP concentrations of ~670 ug/m3 declining over 10 minutes, with chamber air as a negative control (Figure 3). For air, mean starting concentration was 3±4 μg/m3, mean concentration over time was 1.7±1.3 μg/m3, total exposure was 17±13 μg/ m3•min; for cigarettes, mean starting concentration was 682±20 μg/m3, mean concentration over time was 449±14 μg/m3, total exposure was 4461±138 μg/m3•min; for little cigars, mean starting concentration was 670±19 μg/m3, mean concentration over time was 441±10 μg/m3, total exposure was 4380±104 μg/m3•min.
Flow-mediated dilation in the group exposed to little cigar SHS declined from 9.4±0.8% before exposure to 4.4±1.0% and recovered to 7.5±1.1% by 30 minutes later. FMD in the group exposed to cigarette SHS declined from 8.5±0.7% before exposure to 5.2±0.9% initially after exposure and recovered to 8.0±1.4% by 30 minutes later. FMD in the air group did not change significantly. Baseline (pre-occlusion) diameters were not affected by smoke exposure and were comparable for all groups from pre- to post-exposure (p > .5), although for cigarettes only, there was a slight but significant (p = .005) reduction in diameter between the initial post-exposure and recovery values, for reasons that remain unknown. The difference between absolute values of post-exposure FMD measurements for the cigarette and little cigar groups did not approach significance (p = .948). We conclude that exposure to little cigar SHS leads to impairment of FMD that is at least comparable to that resulting from the same level of cigarette SHS.
DISCUSSION
A limitation is that the extent of acute endothelial functional impairment was based on response to equivalent smoke particle concentrations from the 2 products, whereas the amount of SHS liberated from each product during real-world use may vary. However, when Swisher Sweets little cigars (100 mm) and Marlboro cigarettes (85 mm) were smoked to completion using laboratory smoking conditions standardized for each product, the little cigars produced roughly 150% as much tar, 250% as much CO, and 350% as much nicotine as the cigarettes,7 indicating that toxicity of little cigar SHS may be even greater relative to that of cigarettes than we have reported here.
We used Marlboro Red cigarettes and Swisher Sweet little cigars because they are representative of commonly-used brands of each product, because we have used Marlboro Red cigarettes in our previous studies,23 and because of the earlier report mentioned above7 that compared the smoke composition from these brands. Given the similarity of effects from exposure to SHS from these 2 products, it is unlikely that other cigarette or little cigar brands would be fundamentally different in terms of effects of SHS on vascular endothelial function.
We conclude that differences between tobacco and rolling paper composition in cigarettes and little cigars do not translate into differences in acute endothelial toxicity. Exposure to little cigar SHS leads to impairment of vascular function that is at least comparable to that resulting from similar levels of cigarette SHS. Our findings support the need to prevent even brief exposure to little cigar secondhand smoke.
IMPLICATIONS FOR TOBACCO REGULATION
Cardiovascular toxicity is a major consequence of active and passive smoking alike,27 and tobacco use causes over 140,000 cardiovascular deaths annually in the United States.28 Cardiovascular toxic effects are rapid and result in increased risk of myocardial infarction and stroke.26,29 Repeated exposure to SHS causes lasting reduction in FMD14 and exposure to SHS during childhood correlates with lower FMD during adulthood.30 For these reasons, acute vascular toxicity is a relevant and important consequence by which to evaluate harmful effects of SHS from little cigars. In our direct comparison of vascular response to similar levels of SHS from little cigars and cigarettes, we observed greater decrease in FMD after exposure to little cigar SHS.
Santo-Tomas et al16 reported that FMD is impaired by active smoking of conventional cigars. Our results extend those findings to passive exposure to SHS from little cigars. The potential public health impact of demonstrating comparable acute harm to vascular function from little cigar and cigarette SHS demonstrates the need not only for regulation but also education about the health effects of little cigars, which are viewed by some smokers as less dangerous than cigarettes.3 These results contribute to tobacco regulation objectives both by countering this impression, and by strengthening the scientific basis for regulatory decisions regarding little cigars. Policies that protect people from even brief exposure to little cigar SHS are well-justified. Little cigars should be regulated like cigarettes and, in the absence of specific evidence to the contrary, regulators should apply knowledge of the cardiovascular effects of cigarettes to little cigars.
Acknowledgments
We thank Neal Benowitz for helpful feedback. Research reported in this publication was supported by grant R01HL120062 from the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH) and the US Food and Drug Administration Center for Tobacco Products (FDA CTP); grant P50CA180890 from the National Cancer Institute at the NIH and FDA CTP; a Special Projects Infrastructure Grant from the California Tobacco-Related Disease Research Program; and a generous donation from the Elfenworks Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA. Some donations to this study were made in memory of Deb O’Keefe.
Footnotes
Human Subjects Statement
Not applicable.
Conflict of Interest Statement
The authors have no competing interests to declare.
Contributor Information
Jiangtao Liu, Visiting Scholar, University of California, San Francisco, Division of Cardiology, San Francisco, CA.
Xiaoyin Wang, Research Specialist, University of California, San Francisco, Cardiovascular Research Institute, San Francisco, CA.
Shilpa Narayan, Staff Research Associate, University of California, San Francisco, Cardiovascular Research Institute, San Francisco, CA; present address Drexel University College of Medicine, Philadelphia, PA.
Stanton A. Glantz, Glantz, Professor, University of California, San Francisco, Division of Cardiology, San Francisco, CA.
Suzaynn F. Schick, Schick, Assistant Professor, University of California, San Francisco, Division of Occupational and Environmental Medicine, San Francisco, CA.
Matthew L. Springer, Springer, Professor, University of California, San Francisco, Division of Cardiology, San Francisco, CA.
References
- 1.American Legacy Foundation. [Accessed April 15, 2015];Tobacco fact sheet: Cigars, cigarillos & little cigars. (revised June 2012). Available at: http://www.legacyforhealth.org/content/download/642/7502/version/2/file/Fact_Sheet-Cigars_Cigarillos_LittleCigars.pdf.
- 2.Kostygina G, Glantz SA, Ling PM. Tobacco industry use of flavours to recruit new users of little cigars and cigarillos. Tob Control. 2014 doi: 10.1136/tobaccocontrol-2014-051830. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn A, Cobb CO, Niaura RS, Richardson A. The other combustible products: prevalence and correlates of little cigar/cigarillo use among cigarette smokers. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv022. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Delnevo CD, Hrywna M. “A whole ‘nother smoke” or a cigarette in disguise: how RJ Reynolds reframed the image of little cigars. Am J Public Health. 2007;97(8):1368–1375. doi: 10.2105/AJPH.2006.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caruso RV, O’Connor RJ, Travers MJ, et al. Design characteristics and tobacco metal concentrations in filtered cigars. Nicotine Tob Res. 2015;11:1331–1336. doi: 10.1093/ntr/ntu341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henningfield JE, Fant RV, Radzius A, Frost S. Nicotine concentration, smoke pH and whole tobacco aqueous pH of some cigar brands and types popular in the United States. Nicotine Tob Res. 1999;1(2):163–168. doi: 10.1080/14622299050011271. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute. [Accessed April 15, 2015];Cigars: Health effects and trends (Smoking and Tobacco Control Monograph No. 9; NIH publication 98-4302) Available at: http://cancer-control.cancer.gov/BRP/tcrb/monographs/9/m9_complete.pdf.
- 8.Baker F, Ainsworth SR, Dye JT, et al. Health risks associated with cigar smoking. JAMA. 2000;284(6):735–740. doi: 10.1001/jama.284.6.735. [DOI] [PubMed] [Google Scholar]
- 9.Freiberg MJ. Federal approaches to the regulation of non-cigarette tobacco products. Am J Prev Med. 2012;43(5 Suppl 3):S249–S254. doi: 10.1016/j.amepre.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen HV, Grootendorst P. Intended and unintended effects of restrictions on the sale of cigarillos to youth: evidence from Canada. Tob Control. 2015;24(4):382–388. doi: 10.1136/tobaccocontrol-2013-051387. [DOI] [PubMed] [Google Scholar]
- 11.Delnevo CD, Giovenco DP, Ambrose BK, et al. Preference for flavoured cigar brands among youth, young adults and adults in the USA. Tob Control. 2015;24(4):389–394. doi: 10.1136/tobaccocontrol-2013-051408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agaku IT, Alpert HR. Trends in annual sales and current use of cigarettes, cigars, roll-your-own tobacco, pipes, and smokeless tobacco among US adults, 2002–2012. Tob Control. doi: 10.1136/tobaccocontrol-2014-052125. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs EJ, Thun MJ, Apicella LF. Cigar smoking and death from coronary heart disease in a prospective study of US men. Arch Intern Med. 1999;159(20):2413–2418. doi: 10.1001/archinte.159.20.2413. [DOI] [PubMed] [Google Scholar]
- 14.Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334(3):150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 15.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 Pt 1):2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 16.Santo-Tomas M, Lopez-Jimenez F, Machado H, et al. Effect of cigar smoking on endothelium-dependent brachial artery dilation in healthy young adults. Am Heart J. 2002;143(1):83–86. doi: 10.1067/mhj.2002.119765. [DOI] [PubMed] [Google Scholar]
- 17.Frey PF, Ganz P, Hsue PY, et al. The exposure-dependent effects of aged secondhand smoke on endothelial function. J Am Coll Cardiol. 2012;59(21):1908–1913. doi: 10.1016/j.jacc.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Heiss C, Amabile N, Lee AC, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51(18):1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Kato T, Inoue T, Morooka T, et al. Short-term passive smoking causes endothelial dysfunction via oxidative stress in nonsmokers. Can J Physiol Pharmacol. 2006;84(5):523–529. doi: 10.1139/y06-030. [DOI] [PubMed] [Google Scholar]
- 20.Taylor AE, Johnson DC, Kazemi H. Environmental tobacco smoke and cardiovascular disease. A position paper from the Council on Cardiopulmonary and Critical Care, American Heart Association. Circulation. 1992;86(2):699–702. doi: 10.1161/01.cir.86.2.699. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Sievers RE, Varga M, et al. Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo. J Appl Physiol. 2013;114(6):752–760. doi: 10.1152/japplphysiol.01302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiss C, Sievers RE, Amabile N, et al. In vivo measurement of flow-mediated vasodilation in living rats using high resolution ultrasound. Am J Physiol Heart Circ Physiol. 2008;294(2):H1086–H1093. doi: 10.1152/ajpheart.00811.2007. [DOI] [PubMed] [Google Scholar]
- 23.Pinnamaneni K, Sievers RE, Sharma R, et al. Brief exposure to secondhand smoke reversibly impairs endothelial vasodilatory function. Nicotine Tob Res. 2014;16(5):584–590. doi: 10.1093/ntr/ntt189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of the Treasury Alcohol and Tobacco Tax and Trade Bureau. [Accessed October 12, 2015];Tobacco products. 2012 Available at: http://www.ttb.gov/tobacco/tobacco-products.shtml.
- 25.California Environmental Protection Agency. Part A: Exposure assessment. San Francisco, CA: California Environmental Protection Agency, Office of Environmental Health Hazard Assessment; 2005. Proposed identification of environmental tobacco smoke as a toxic air contaminant. [Google Scholar]
- 26.Institute of Medicine. [Accessed April 15, 2015];Secondhand smoke exposure and cardiovascular effects: Making sense of the evidence. 2010 Available at: http://www.nap.edu/catalog.php?record_id=12649#toc. [PubMed]
- 27.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111(20):2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services. How Tobacco Smoking Causes Disease: The Biology and Behavioral Basis for Smoking-attributable Disease: A Report of the Surgeon General. Rockville, MD: Office of the Surgeon General; 2010. [Accessed April 15, 2015]. Report number QV 137 H847. Available at: http://www.surgeongeneral.gov/library/reports/tobaccosmoke/index.html. [Google Scholar]
- 29.Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ. 2004;328(7446):977–980. doi: 10.1136/bmj.38055.715683.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juonala M, Magnussen CG, Venn A, et al. Parental smoking in childhood and brachial artery flow-mediated dilatation in young adults: the Cardiovascular Risk in Young Finns Study and the Childhood Determinants of Adult Health Study. Arterioscler Thromb Vasc Biol. 2012;32(4):1024–1031. doi: 10.1161/ATVBAHA.111.243261. [DOI] [PubMed] [Google Scholar]