Abstract
Genetic aberrations in tumor driver genes provide specific molecular targets for therapeutic intervention, which can greatly improve therapeutic outcomes. Here, we analyzed the mutational frequency of EGFR and KRAS gene, as well as EML4-ALK rearrangement, and summarized the clinicopathological characters of Chinese lung cancer patients. We detected the mutation spectrum of 1033 primary lung cancer patients. The analyzed clinicopathological parameters included gender, age at diagnosis, smoking status, pathological TNM stage, tumor morphology and location, visceral pleural invasion, and histological type. A total of 618 patients had mutations in EGFR or KRAS gene as well as rearrangement of EML4-ALK. Exon 19 deletions and L858R in the EGFR gene were the most frequent mutations. Left-side lung cancer was more common in female patients carrying the KRAS mutation. Rearrangement of EML4-ALK was more common in non-tobacco-using male patients, who also exhibited a higher likelihood of visceral pleura invasion. Elderly females who never smoked and possessed 1–20 mm stage I adenocarcinomas in the right side exhibited a higher frequency of EGFR mutations. Elderly male smokers with right lung tumors were viable candidates for KRAS mutation screening.
The global cancer burden is growing at an alarming rate, emphasizing the need for the urgent implementation of effective prevention strategies. Lung cancer is one of the most critical types, accounting for 13% of cancer diagnoses in 2012. The 5-year relative survival rate of lung cancer patients is gradually improving due to improvements in treatment. However, lung cancer remains the most common cause of cancer death, with 1.6 million patient deaths worldwide in 2012. In China, lung cancer also ranks highest in both cancer prevalence and lethality1. According to histological analyses, lung cancer is classified into non-small cell lung carcinoma (NSCLC), which consists of three main subtypes (adenocarcinoma, squamous cell carcinoma, and large cell carcinoma), and small cell lung carcinoma. Rare subtypes include glandular tumors, carcinoid tumors, and undifferentiated carcinomas.
Recently, a growing number of oncogenic mutations have been identified in lung cancer. Molecular genetic analyses have suggested that anaplastic lymphoma receptor tyrosine kinase (ALK), v-akt murine thymoma viral oncogene homolog 1 (AKT1), B-Raf proto-oncogene, serine/threonine kinase (BRAF), epidermal growth factor receptor (EGFR), v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2), Kirsten rat sarcoma viral oncogene homolog (KRAS) and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) are driver genes in NSCLC2,3,4,5,6. These genetic aberrations provide specific molecular targets for therapeutic intervention, which can greatly improve therapeutic outcomes. Erlotinib and gefitinib, EGFR tyrosine kinase inhibitors (TKIs), and crizotinib, an ALK inhibitor, have generated significant improvements in the objective response rate and resulted in longer progression-free survival7,8.
However, these specific inhibitors only function effectively when used in the correct population. Thus, accurate prevalence, clinical, pathological, and mutational status data are required. In this study, we extended the effort to analyze the phenotype-genotype relationship in Chinese lung cancer patients. We hypothesized that the clinicopathological parameters, including the age, gender, tumor size and site, TNM stage and genotype, of different patients groups are distinct. Based on these results, clinicians can adopt these principles in selecting treatment plans.
Materials and Methods
Ethical approval
This study was conducted in accordance with the amended Declaration of Helsinki. This study was approved by the Institutional Review Board (IRB) of Shanghai Pulmonary Hospital affiliated with Tongji University (20120163). Written informed consent was obtained from all participants. The methods were carried out in accordance with the approved guidelines.
Patients and specimen collection
A total of 1033 consecutive primary lung cancer patients who were admitted into the Shanghai Pulmonary Hospital affiliated with Tongji University from January 2013 to January 2014 were recruited. No choose or correct was performed on patients collection. None of these patients received any anticancer therapies prior to surgery. The recurrent or metastatic patients were excluded. All of the cancer cases were confirmed by pathological tests or/and radical surgical resection. Fresh primary tumor tissues that contained more than 50% tumor cells were collected during surgery.
The clinical and pathological data obtained for analysis included gender, age at diagnosis, smoking status, pathological TNM stage, tumor morphology and location, visceral pleural invasion and histological type. The clinical information was recorded three times by three physicians respectively to ensure the accuracy. Tumors were staged pathologically according to the Union for International Cancer Control (UICC-7) staging system for lung cancer9.
Candidate gene mutation analysis
Genomic DNA and total RNA were extracted from fresh tissues using the QIAamp DNA Tissue Kit and RNeasy Kit (Qiagen, Germany), respectively. Mutations in the EGFR and KRAS genes, as well as EML4-ALK rearrangement, were detected using Amoy Diagnostics Kits (Xiamen, China). The kits employ distinctive real-time PCR technology to detect mutations in the target gene. Target DNA is amplified with mutation-specific PCR primers, and the mutant amplicons are detected with a novel fluorescent probe. The test can detect mutations at a sensitivity of 1%. The positive and negative controls were provided by the manufacturer.
Statistical analysis
Χ2 tests were performed to analyze the association between the gene variants and other clinicopathological data. All data were analyzed using the SPSS package for Windows (Version 18.0, Chicago, IL). P values <0.05 was considered statistically significant.
Results
Clinicopathological characteristics
A total of 1033 lung cancer patients were recruited. More than 99% of them are Han people. The mean age of them was 60.68 years old (ranging from 24 to 91). Of these patients, 597 (57.79%) were male, and 624 patients, most of whom were female, had never smoked. 414 (40.08%) and 592 (57.31%) of tumors were located in the left and right sides, respectively. Visceral pleural invasion was observed in 335 patients. Histologically, 759 (73.48%) specimens were lung adenocarcinomas, 189 (18.30%) were squamous carcinoma, 32 (3.10%) were large cell carcinoma, and 15 (1.45%) were small cell carcinoma (Table 1; Fig. 1).
Table 1. The clinicopathologic characters of 1033 Chinese lung cancer patients.
| Number | Percentage | Number | Percentage | |
|---|---|---|---|---|
| Gender | ||||
| Male | 597 | 57.79% | ||
| Female | 436 | 42.21% | ||
| Age | Female | Male | ||
| <30 | 3 | 0.69% | 1 | 0.17% |
| 31–40 | 13 | 2.98% | 9 | 1.51% |
| 41–50 | 67 | 15.37% | 59 | 9.88% |
| 51–60 | 145 | 33.26% | 183 | 30.65% |
| 61–70 | 157 | 36.01% | 242 | 40.54% |
| >70 | 51 | 11.70% | 103 | 17.25% |
| Site | ||||
| Left | 171 | 39.22% | 243 | 40.70% |
| Right | 252 | 57.80% | 340 | 56.95% |
| Bilateral | 13 | 2.98% | 14 | 2.35% |
| TNM stage | ||||
| Stage I | 313 | 71.79% | 359 | 60.13% |
| Stage II | 33 | 7.57% | 81 | 13.57% |
| Stage III | 65 | 14.91% | 133 | 22.29% |
| Stage IV | 25 | 5.73% | 24 | 4.02% |
| Smoking | ||||
| Never smoker | 403 | 92.43% | 221 | 37.02% |
| <100 | 2 | 0.46% | 2 | 0.34% |
| 101–500 | 23 | 5.28% | 139 | 23.28% |
| 501–1000 | 7 | 1.61% | 189 | 31.66% |
| >1000 | 1 | 0.23% | 46 | 7.71% |
| Tumor size (mm) | ||||
| 1–10 | 78 | 17.89% | 79 | 13.23% |
| 11–20 | 138 | 31.65% | 155 | 25.96% |
| 21–30 | 122 | 27.98% | 160 | 26.80% |
| 31–40 | 60 | 13.76% | 88 | 14.74% |
| 41–50 | 13 | 2.98% | 51 | 8.54% |
| 51–60 | 18 | 4.13% | 33 | 5.53% |
| 61–70 | 2 | 0.46% | 14 | 2.35% |
| 71–80 | 1 | 0.23% | 8 | 1.34% |
| 81–90 | 1 | 0.23% | 3 | 0.50% |
| 91–100 | 2 | 0.46% | 4 | 0.67% |
| >100 | 1 | 0.23% | 2 | 0.34% |
| Visceral pleura invasion | ||||
| Yes | 170 | 38.99% | 165 | 27.64% |
| No | 266 | 61.01% | 432 | 72.36% |
Figure 1. The pathologic profiles of the whole population and different mutation subgroups.
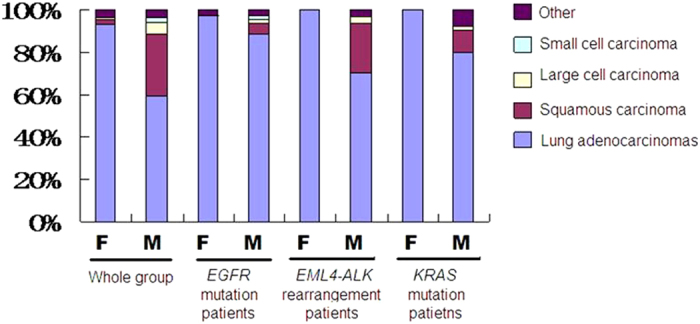
Mutation spectrum
In total, EGFR and KRAS mutations, as well as EML4-ALK rearrangements, were detected in 618 patients. Most of these changes were EGFR mutations. The EGFR mutations identified including exon 19 deletions, exon 20 insertions, G719X, S768I, L858R, etc. (Fig. 2). Exon 19 deletions and L858R were the two most frequent mutations. The mutation frequency of the EGFR and KRAS genes appeared to be affected by gender (P < 0.05). Female patients exhibited a higher rate of EGFR mutations [297 of 436 (68.12%) vs. 215 of 597 (36.01%) male patients), whereas male patients had more KRAS mutations [40 of 597 (6.70%) vs. 13 of 436 (2.98%) female patients]. Moreover, 20 patients exhibited complex mutations, with a combination of exon 19 deletions and L858R.
Figure 2. The EGFR mutations identified in this study.
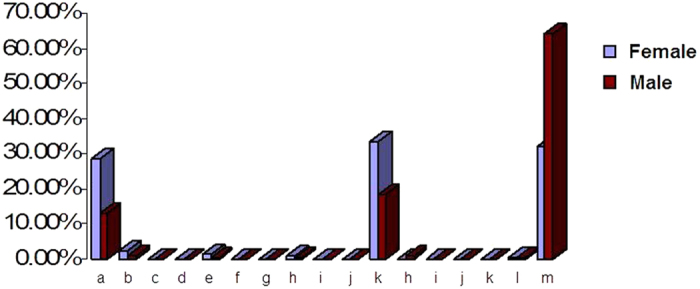
a–m: 19 del; 19-del/L858R; 19-del/20-INS; 19-del/T790M; 20-ins; 20-ins/G719X; 22-ins; L861Q; G719X; G719X/L861Q; S768I; L858R; L858R/S768I; L858R/T790M; L858R/20-ins; L859R; L861Q and WT.
Phenotype-genotype relationships
Nucleotide changes in the EGFR gene accounted for most of the mutations detected. A total of 512 patients, most of whom were middle-aged, had various EGFR mutations. Most EGFR mutation related tumors that were approximately 11–30 mm in diameter were detected in non-tobacco-using patients and in stage I according to the TNM classification; 275 of 297 (92.59%) female patients and 102 of 215 (47.44%) male patients had never smoked. The rest clinicopathological data were not affected by gender in the EGFR mutation patients, except for smoking status.
Compared with the wild-type EGFR patients, the EGFR L858R mutation patients demonstrated clear differences in terms of tumor site, pathological stage and type, tobacco use status, tumor size and visceral pleura invasion status. Most tumors with EGFR L858R mutations were located on the right side, whereas tumors with non-EGFR mutations were on the left side. Although the age profile appeared to be similar between these two groups, slight differences were observed when the age profiles were analyzed by gender. L858R mutation female patients were older than those with non-EGFR mutations.
Tumors with EGFR 19 del mutations were also more likely to occur on the right side. This patient group had more advanced tumors (stage III) compared with the EGFR L858R mutation and wild-type patients. Invasion of the visceral pleura was also common in this group.
Of the 1033 lung cancer patients, 13 females and 40 males harbored KRAS mutations. Most female patients with KRAS mutations were between 31 and 60 years old, whereas male patients with KRAS mutations were 51 to 70 years old. Left-side cancers were more common in KRAS mutation female patients. Most of these tumors were in stage I, and KRAS mutation female patients demonstrated a lower percentage of stage II and III disease compared with EGFR mutation females (P < 0.05). Only 7 (17.50%) of the KRAS mutation male patients had never smoked. For females, only 1 patient smoked at the time of diagnosis. The tumor volume of the KRAS mutation patients were larger than those of the EGFR mutation patients (P < 0.05). Furthermore, the tumor size of female patients was greater than that of the male patients.
The EML4-ALK rearrangement was observed in 53 patients. Very few differences were observed between female and male patients. For males, the non-tobacco using group exhibited a higher frequency of visceral pleura invasion. Seven (23.33%) of these cases were squamous carcinoma (Table 2).
Table 2. The association between clinicopathologic characters and mutation status of EGFR and KRAS mutation as well as rearrangement of EML4-ALK in 1033 Chinese lung cancer patients.
| EGFR mutateon | EML4-ALK | KRAS mutateon | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 512 | 53 | 53 | |||||||||
| Sex | ||||||||||||
| Male | 215 | 41.99 | 30 | 56.60% | 40 | 75.47% | ||||||
| Female | 297 | 58.01 | 23 | 43.40% | 13 | 24.53% | ||||||
| Age | F1 | M2 | F1 | M2 | F1 | M2 | ||||||
| <30 | 3 | 1.01% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% |
| 31–40 | 10 | 3.37% | 3 | 1.40% | 2 | 8.70% | 0 | 0.00% | 0 | 0.00% | 1 | 2.50% |
| 41–50 | 38 | 12.79% | 20 | 9.30% | 4 | 17.39% | 7 | 23.33% | 3 | 23.08% | 2 | 5.00% |
| 51–60 | 98 | 33.00% | 70 | 32.56% | 10 | 43.48% | 6 | 20.00% | 4 | 30.77% | 15 | 37.50% |
| 61–70 | 109 | 36.70% | 91 | 42.33% | 6 | 26.09% | 13 | 43.33% | 5 | 38.46% | 15 | 37.50% |
| >70 | 39 | 13.13% | 31 | 14.42% | 1 | 4.35% | 4 | 13.33% | 1 | 7.69% | 7 | 17.50% |
| Site | ||||||||||||
| Left | 115 | 38.72% | 77 | 35.81% | 6 | 26.09% | 8 | 26.66% | 7 | 53.84% | 17 | 42.50% |
| Right | 173 | 58.25% | 129 | 60.00% | 16 | 69.57% | 21 | 70.00% | 6 | 46.15% | 23 | 57.50% |
| Bilateral | 9 | 3.03% | 9 | 4.19% | 1 | 4.35% | 1 | 3.33% | 0 | 0.00% | 0 | 0.00% |
| TNM stage | ||||||||||||
| Stage I | 212 | 71.38% | 139 | 64.65% | 17 | 73.91% | 22 | 73.34% | 8 | 61.54% | 26 | 65.00% |
| Stage II | 18 | 6.06% | 12 | 5.58% | 1 | 4.35% | 2 | 6.66% | 4 | 30.76% | 5 | 12.50% |
| Stage III | 50 | 16.84% | 52 | 24.91% | 5 | 21.74% | 6 | 20.00% | 1 | 7.69% | 9 | 22.50% |
| Stage IV | 17 | 5.72% | 12 | 5.58% | 1 | 4.35% | 1 | 3.33% | 0 | 0.00% | 3 | 7.50% |
| Smoking | ||||||||||||
| Never smoker | 275 | 92.59% | 102 | 47.44% | 22 | 95.65% | 16 | 53.33% | 12 | 92.31% | 7 | 17.50% |
| <100 | 2 | 0.67% | 1 | 0.47% | 0 | 0.00% | 1 | 3.33% | 0 | 0.00% | 0 | 0.00% |
| 101–500 | 16 | 5.39% | 53 | 24.65% | 1 | 4.35% | 7 | 23.33% | 1 | 7.69% | 9 | 22.50% |
| 501–1000 | 4 | 1.35% | 48 | 22.33% | 0 | 0.00% | 5 | 16.67% | 0 | 0.00% | 20 | 50.00% |
| >1000 | 0 | 0.00% | 11 | 5.12% | 0 | 0.00% | 1 | 3.33% | 0 | 0.00% | 4 | 10.00% |
| Tumor size | ||||||||||||
| 1–10 | 47 | 15.82% | 25 | 11.63% | 4 | 17.39% | 3 | 10.00% | 1 | 7.69% | 4 | 10.00% |
| 11–20 | 95 | 31.99% | 65 | 30.23% | 4 | 17.39% | 11 | 36.67% | 3 | 23.08% | 9 | 22.50% |
| 21–30 | 97 | 32.66% | 73 | 33.95% | 9 | 39.13% | 7 | 23.33% | 1 | 7.69% | 12 | 30.00% |
| 31–40 | 40 | 13.47% | 31 | 14.42% | 3 | 13.04% | 2 | 6.67% | 4 | 30.77% | 3 | 7.50% |
| 41–50 | 8 | 2.69% | 7 | 3.26% | 1 | 4.35% | 2 | 6.67% | 0 | 0.00% | 5 | 12.50% |
| 51–60 | 8 | 2.69% | 6 | 2.79% | 0 | 0.00% | 3 | 10.00% | 2 | 15.38% | 5 | 12.50% |
| 61–70 | 0 | 0.00% | 2 | 0.93% | 2 | 8.70% | 0 | 0.00% | 0 | 0.00% | 2 | 5.00% |
| 71–80 | 1 | 0.34% | 4 | 1.86% | 0 | 0.00% | 2 | 6.67% | 0 | 0.00% | 0 | 0.00% |
| 81–90 | 0 | 0.00% | 1 | 0.47% | 0 | 0.00% | 0 | 0.00% | 1 | 7.69% | 0 | 0.00% |
| 91–100 | 1 | 0.34% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 7.69% | 0 | 0.00% |
| >100 | 0 | 0.00% | 1 | 0.47% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% |
| Pathological type | ||||||||||||
| Lung adenocarcinomas | 289 | 97.31% | 190 | 88.37% | 23 | 100.00% | 21 | 70.00% | 13 | 100.00% | 32 | 80.00% |
| Squamous carcinoma | 0 | 0.00% | 11 | 5.12% | 0 | 0.00% | 7 | 23.33% | 0 | 0.00% | 4 | 10.00% |
| Large cell carcinoma | 0 | 0.00% | 5 | 2.33% | 0 | 0.00% | 1 | 3.33% | 0 | 0.00% | 1 | 2.50% |
| Small cell carcinoma | 0 | 0.00% | 3 | 1.40% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% |
| Other | 8 | 2.69% | 6 | 2.79% | 0 | 0.00% | 1 | 3.33% | 0 | 0.00% | 3 | 7.50% |
| Visceral pleura Invasion | ||||||||||||
| Yes | 129 | 43.43% | 89 | 41.40% | 11 | 47.83% | 17 | 56.67% | 6 | 46.15% | 13 | 32.50% |
| No | 168 | 56.57% | 126 | 58.60% | 12 | 52.17% | 13 | 43.33% | 7 | 53.85% | 27 | 67.50% |
1:Female; 2:Male.
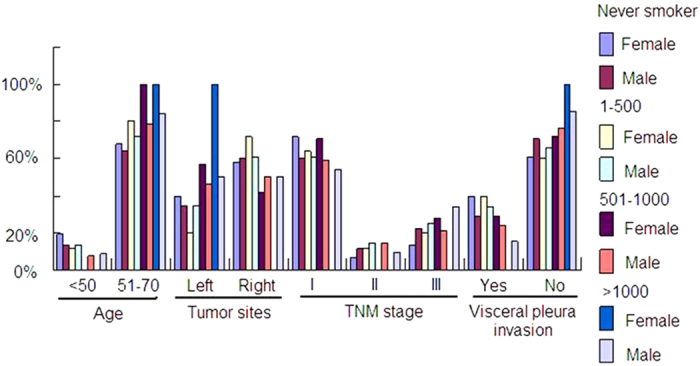
Compare with patients who never smoked, heavy smokers were more likely to be elder and to possess squamous tumors that were larger in size. Most of these tumors were located on the left side and were classified as stage III according to the TNM classification (Fig. 3).
Figure 3. The clinicopathological characteristics of the different smoking status subgroups.
Discussion
Despite the advances achieved in diagnosis and treatment, the overall prognosis of lung cancer remains poor. The 5-year survival rate remains below the expected rate. Efforts to improve the survival of lung cancer patients are currently focused on the development of innovative treatment options, particularly novel target-based therapies directed against key signaling pathways involved in the development and progression of lung cancer10. In the past decades, the application of specifically targeted medication has greatly improved the treatment outcomes of lung cancer patients. TKIs, including gefitinib and erlotinib, have become the standard first-line therapy for patients with advanced NSCLC that harbor activating EGFR mutations11,12, especially for women and patients who have never smoked. This categorization is based on the fairly salient fact that most clinically relevant EGFR mutations in lung cancer patients are either deletions in exon 19 or missense mutations in exon 2113. In the Iressa Pan-Asia Study (IPASS), patients with EGFR mutations exhibited great improvement in the objective response rate and longer progression-free survival after receiving gefitinib compared with patients without EGFR mutations11. Therefore, the molecular profiling of patients is crucial for specific treatments.
The accurate analysis of clinicopathological and molecular genetic characteristics of target patients will help clinicians select an optimal treatment plan. In this study, we analyzed the clinicopathological features of 1033 Chinese lung cancer patients. The incidence of mutation was significantly higher in women. Similar results have been reported by a previous study, which found a higher incidence of lung cancer in female non-smokers. In our study, the detected EGFR gene mutations were also primarily found in patients who had never smoked.
Recently, non-tobacco-using-patients with lung cancers have received considerable attention with the application of EGFR TKIs. These drugs have demonstrated higher responses in specific non-tobacco-using groups: Asian females with adenocarcinoma-type histology and EGFR mutations. Among these clinicopathological factors, the most significant factor is the EGFR gene mutation. Activating EGFR mutations, including exon 19 deletions and a missense mutation (L858R) in exon 21, have been found to be the most powerful biologic predictors of EGFR TKI sensitivity14. However, using EGFR mutational screening to anticipate responses to EGFR TKI treatment is often impractical for clinicians. Therefore, more meticulous and accurate clinical data that can better predict treatment outcomes are urgently required. Our study summarized the characteristics of Chinese lung cancer patients with EGFR mutations. Patients, especially never smoked females, who were 51–70 years old, and had 1–20 mm stage I adenocarcinomas on the right side were more likely to possess EGFR mutations. Patients with these characteristics may benefit from EGFR TKI therapy.
Moreover, 53 of our 1033 patients (5.13%) had an EML4-ALK translocation, which involves the fusion of the N-terminus of echinoderm microtubule associated protein-like 4 (EML4) and the intracellular domain of ALK. The 23 female patients demonstrated features that were similar to those of the entire patient group, whereas the 30 male patients exhibited unique profiles. Although these tumors were of a smaller volume [the diameter of 46.67% (14 of 30) of the samples was approximately 1–20 mm], 56.67% (17 of 30) invaded the visceral pleura. Further, a high proportion (16 of 30, 53.33%) of non-smokers with low-grade (21 of 30, 70%) adenocarcinomas was observed. The difference between the EGFR mutant and EML4-ALK rearrangement patients also explained the clinical observation that tumors with an EML4-ALK translocation did not respond positively to EGFR TKI therapy15. The future development of a targeted agent is required for this patient subgroup.
The frequency of KRAS mutations varies among different ethnic groups, ranging from 19% to 30% in NSCLC16. These mutations, which occurred most frequently in codons 12 and 1317, were more common in female and younger patients as well as patients with adenocarcinoma. KRAS mutations showed close relationship with a history of smoking. However, 6–15% of lung adenocarcinoma patients who had never smoked still harbored a KRAS mutation18.
In our study, the KRAS mutation rate was extremely low in both the entire patient group (53 of 1033, 5.13%) and the adenocarcinoma patient group (45 of 759, 5.93%), which is consistent with a previous conclusion that Asians exhibit a lower frequency of KRAS mutations. Compared with the EGFR mutation and EML4-ALK rearrangement patients, KRAS mutation patients demonstrated unique features in terms of tumor site, pathology stage and smoking status. Tumors occurring on the right side and in male smokers over the age of 50 were more likely to harbor KRAS mutations. Larger tumor size, stage III classification, and reduced visceral pleura invasion were also more frequently observed. This specific group deserves more attention in the development of novel drugs because these patients were not sensitive to current adjuvant chemotherapy17.
In this study, we summarized the characteristics of Chinese lung cancer patients, especially in terms of different driver gene mutation subgroups. The distinct features of the different subgroups could help clinicians select more specific and effective treatments for patients. Furthermore, our results demonstrated the necessity to develop novel therapeutic agents for non-EGFR mutation patients. Besides, the small size of the KRAS mutation group prevented us from obtaining detailed clinical information. A further study in a large population would be helpful for developing more productive treatments.
Additional Information
How to cite this article: Yang, Y. et al. Elderly male smokers with right lung tumors are viable candidates for KRAS mutation screening. Sci. Rep. 6, 18566; doi: 10.1038/srep18566 (2016).
Acknowledgments
This work was supported by the Shanghai city health appropriate technology projects (No. 12012111) and the National Natural Scientific Foundation of China (81501750). The funder had no role in study design. Funding: The Shanghai city health appropriate technology projects (No. 12012111).
Footnotes
Author Contributions Y.Y., C.S., W.Y., X.Z., L.Z. and G.N.J. designed the study. Y.Y., C.S., H.S., W.Y., X.Z., L.Z. and G.N.J. performed the study and analyzed the data. Y.Y., W.Y. and C.S. wrote the main manuscript. All authors reviewed the manuscript. L.Z. and G.N.J. approved the manuscript.
References
- Jie H., Ping Z. & Wanqing C. Chinese Cancer Registry Annual Report (2013). Beijing, China, Military Medical Science Press, 2014. [Google Scholar]
- Hammerman P. S. et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 1, 78–89 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard D. A. et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 23, 5900–5909 (2005). [DOI] [PubMed] [Google Scholar]
- Jin G. et al. EML4-ALK fusion gene in Korean non-small cell lung cancer. J Korean Med Sci. 27, 228–230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K. et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 18, 378–381 (2012). [DOI] [PubMed] [Google Scholar]
- Fukui T. et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer, 77, 319–325 (2012). [DOI] [PubMed] [Google Scholar]
- Lynch T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 350, 2129–2139 (2004). [DOI] [PubMed] [Google Scholar]
- Kwak E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 363, 1693–1703 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis E. S. et al. Application of the revised lung cancer staging system (IASLC Staging Project) to a cancer center population. J Thorac Cardiovasc Surg. 138, 412–418 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. J., Stinchcombe T. E., Der C. J. & Socinski M. A. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selectingpatients for epidermal growth factor receptor-targeted therapy? J Clin Oncol. 28, 4769–4777 (2010). [DOI] [PubMed] [Google Scholar]
- Mok T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 361, 947–957 (2009). [DOI] [PubMed] [Google Scholar]
- Kalikaki A. et al. Clinical outcome of patients with non-small cell lung cancer receiving front-line chemotherapy according to EGFR and K-RAS mutation status. Lung Cancer 69, 110–115 (2010). [DOI] [PubMed] [Google Scholar]
- Pao W. et al. Integration of molecular profiling into the lung cancer clinic. Clin Cancer Res. 15, 5317–5322 (2009). [DOI] [PubMed] [Google Scholar]
- Lee Y. J. et al. Impact of environmental tobacco smoke on the incidence of mutations in epidermal growth factor receptor gene in never-smoker patients with non-small-cell lung cancer. J Clin Oncol. 28, 487–492 (2010). [DOI] [PubMed] [Google Scholar]
- Li T., Kung H. J., Mack P. C. & Gandara D. R. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 31, 1039–1049 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. J. & Stinchcombe T. E. KRAS mutation: should we test for it, and does it matter?, J Clin Oncol. 31, 1112–1121 (2013). [DOI] [PubMed] [Google Scholar]
- Laurie S. A. & Goss G. D. Role of epidermal growth factor receptor inhibitors in epidermal growth factor receptor wild-type non-small-cell lung cancer. J Clin Oncol. 31, 1061–1069 (2013). [DOI] [PubMed] [Google Scholar]
- Shepherd F. A. et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRASmutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvantchemotherapy. J Clin Oncol. 31, 2173–2181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


