Abstract
We report the synthesis and characterization of new metal-free organic dyes (namely B18, BTD-R, and CPTD-R) which designed with D-π-A concept to extending the light absorption region by strong conjugation group of π-linker part and applied as light harvester in dye sensitized solar cells (DSSCs). We compared the photovoltaic performance of these dyes in two different photoanodes: a standard TiO2 mesoporous photoanode and a ZnO photoanode composed of hierarchically assembled nanostructures. The results demonstrated that B18 dye has better photovoltaic properties compared to other two dyes (BTD-R and CPTD-R) and each dye has higher current density (Jsc) when applied to hierarchical ZnO nanocrystallites than the standard TiO2 mesoporous film. Transient photocurrent and photovoltage decay measurements (TCD/TVD) were applied to systematically study the charge transport and recombination kinetics in these devices, showing the electron life time (τR) of B18 dye in ZnO and TiO2 based DSSCs is higher than CPTD-R and BTD-R based DSSCs, which is consistent with the photovoltaic performances. The conversion efficiency in ZnO based DSSCs can be further boosted by 35%, when a compact ZnO blocking layer (BL) is applied to inhibit electron back reaction.
Dye sensitized solar cells (DSSCs)1 developed by O’Regan and Grätzel, have attracted considerable attention since 1991, promising to be among the most interesting alternatives to conventional solid–state semiconductor solar cells, being in principle cheap, environmentally compatible and large-area scalable. A typical DSSC is composed of a mesoporous nanostructured titanium oxide thin film, whose surface is covered with a monolayer of dye molecules, a redox -couple electrolyte and a platinized fluorine-doped tin oxide (FTO) glass as counter electrode. For many years, ruthenium polypyridyl complexes were successfully applied as light harvesters, yielding overall conversion efficiency (η) more than 11% under AM 1.5 simulated sunlight (100 mWcm−2)2. Recently, this η set a new record value exceeding 14% by using porphyrin dye molecule together with cobalt-based redox couple electrolyte3,4. Ruthenium complexes are however still the most commonly studied sensitizers for DSSCs, although they are unsuitable for large scale commercialization due to their high cost and scarce availability of ruthenium. To overcome this problem, strong research efforts have been addressed to synthesize ruthenium-free dyes. Metal-free organic dyes have attracted great interest in this respect due to several advantages they offer: (i) high molar extinction coefficients; (ii) easily tunable opto-electronic properties; (iii) enhanced environmental compatibility and abundance of their constituents; (iv) reduced production costs5. The highest η has been achieved with mesoporous TiO2 nanocrystallites film sensitized by different metal-free organic dye molecules: hemicyanine dye (η = 5.1%)6, thienylfluorene dye (η = 5.23%)7, phenothiazine dye (η = 5.5%)8, thienothiophene-thiophenederived dye (η = 6.23%)9, N, N-dimethyl-anilinecyanoacetic acid (η = 6.8%)10, porphyrin dye (η = 7.1%)11, modified D-π-A dye (DEK1, η = 7.17%)12, oligothiophene dye (η = 7.7%)13, coumarin dye (η = 8.2%)14, oligo-phenylene vinylene-unit dye (η = 9.1%)15 and indoline dye (D205, η = 9.52%)16. Recently Demadrille et al. reported new metal free organic dye (RK1) with a photoconversion efficiency as high as 10.2%, which is comparable to N719 dye17.
In metal-free organic dye a donor-acceptor couple, D-π-A, is present, in which the electron donor is not only able to tune the electronic coupling with the acceptor, but also determines the molecule adsorbed state on the titania or zinc oxide nanocrystal in DSSC devices. Several investigations about the donor modification were reported in recent years; in particular, the organic dye D3518 and derivatives (Y12319, LEG420), featuring phenyl extended triphenyl amine donor, gained popularity because of the prominent performance in DSSC devices. It was reported that, when an extended triphenyl amine is used as the donor group, an up-shift of TiO2 conduction band (CB) edge (CBE) can be observed on account of the increased net surface dipole moment of the adsorbed dye molecules.
On the other hand, by inserting specific aromatic units into the π bridge, one can broaden the light-harvesting region of dyes from visible to near-IR region. In this case, several highly efficient dyes were obtained, including the popular LEG-series21, push-pull porphyrins22 and the D-A-π-A WS-series dyes23. Inspired by their pioneer work, we designed and synthesized a series of novel organic dye, namely: (E)-2-(5-((7-(4-(bis(4-(5-(4-(hexyloxy)phenyl)thiophen-2-yl)phenyl)amino)phenyl)-2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (labeled as B18), (E)-2-(5-((6-(4-(bis(4-(5-(4-(hexyloxy)phenyl)thiophen-2-yl)phenyl)amino)phenyl)-4,4-didodecyl-4H-cyclopenta[1,2-b:5,4-b’]dithiophen-2-yl)methylene)-4-oxo-2-thioxotetrahydrothiophen-3-yl)acetic acid (labeled as CPTD-R) and (E)-2-(5-((5-(7-(4-(bis(4-(5-(4-(hexyloxy)phenyl)thiophen-2-yl)phenyl)amino)phenyl)benzo[c] [1,2,5]thiadiazol-4-yl)thiophen-2-yl)methylene)-4-oxo-2-thioxotetrahydrothiophen-3-yl)acetic acid (labeled as BTD-R) featuring with 2-(4-(hexyloxy) phenyl) thiophene extended donor, and EDOT, CPDT, and BTD-thiophene π bridge.
Concerning the electron transport material, intrinsic transport limitations affect TiO2, thus limiting the possibilities of enhancing the photoconversion efficiency of DSSCs exploiting this metal oxide as photoanode24,25,26,27. Other n-type metal oxide semiconductors, such for instance ZnO, SnO2, Nb2O5 and In2O3 can be used as alternative photoanode materials28,29,30,31,32,33,34,35,36, as well as composite systems, e.g. carbon nanotubes37,38 or graphene39 mixed with TiO2 nanoparticles. Among all, ZnO is very promising, due to its electronic band structure (very similar to TiO2)30 and high electron mobility (one order higher than TiO2)40,41,42. In a very recent paper43, Grätzel and co-workers compared ZnO- and TiO2-DSSCs composed of large insulating Al2O3 particles covered with thin layers of either ZnO or TiO2. They pointed out that the performances of the two kinds of cells are similar. However, the higher photogenerated electron transport rate contributes to cell performance for ZnO, while in TiO2 a low recombination rate, combined with higher dye loading and faster electron injection boost η. Due to its large availability and uncountable obtainable low dimension structures, such as nanorods/nanowires20,44, nanotubes45,46, nanosheets47,48, nanoflowers49, tetrapods50,51,52 and hierarchical aggregates53,54,55, ZnO is a very interesting alternative to TiO2 to investigate. Application of these nanostructured ZnO photoanodes in DSSC significantly enhanced η, compared to simple ZnO nanoparticle mesoporous films. To the best of our knowledge, the highest η (7.5%) was reported by hierarchical assembled ZnO nanocrystallites53, which offer large specific surface area for dye loading while poly-dispersed aggregates act as efficient light scatterers, enhancing the probability of photon absorption. Another strategy to boost η in ZnO DSSCs is the application of a ZnO buffer layer (BL) in between the FTO and the ZnO active layer, to inhibit the electron back reaction from the FTO to electrolyte and to enhance the chemical capacitance during the transport and collection processes56,57,58.
Herein we present the details of the synthesis of three new metal-free organic dyes and apply them as light harvesters in both TiO2- and ZnO-based DSSCs. A systematic comparison of the photovoltaic properties of these dyes is carried out on (i) standard TiO2 mesoporous films and (ii) spray deposited hierarchical ZnO nanocrystallites in order to highlights the effect of photoanode materials and we found that all dye molecules outperform in term of current density (Jsc) with hierarchical ZnO nanocrystallites than standard TiO2 mesoporous films. In addition, the effect of BL on the functional performance of ZnO-based DSSCs is also investigated. Charge transport and recombination kinetics in these devices are evaluated by applying transient photocurrent and photovoltage decay (TCD/TVD). The results of this work can contribute to the development of ZnO-based DSSCs by properly designing metal-free organic dyes, which are typically optimized for TiO2, limiting the potential of ZnO photoanodes.
Results and Discussion
Dye molecules (B18, BTD-R and CPTD-R) characterization
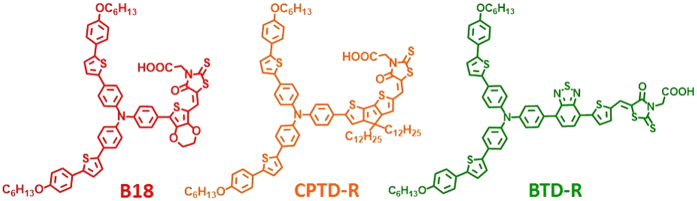
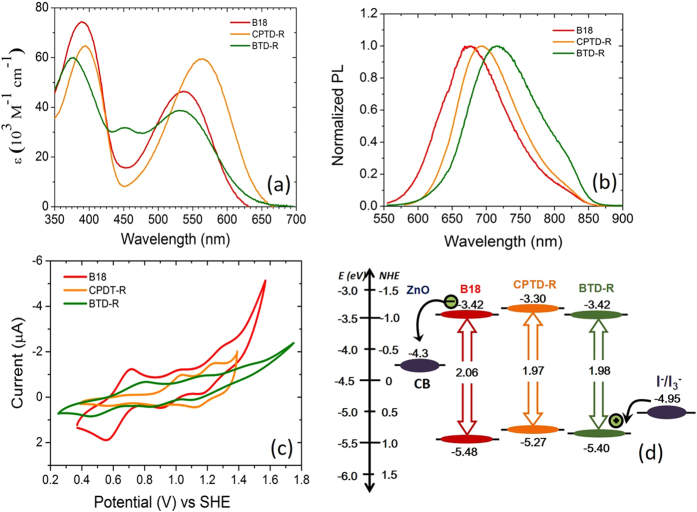
Synthetic procedures used for the preparation of B18, CPRD-R and BTD-R dyes are reported in Supporting Information and the structures of dyes are reported in Fig. 1. The dyes contain a rhodanine-3-acetic acid as anchoring group and triphenyl amine as the donor centrum. Figure 2 shows a systematic comparison of molecule (B18, BTD-R and CPTD-R) optical and electrochemical characterizations and corresponding values are summarized in Table 1. Figure 2 (a) shows the absorption spectra of the dye molecules in CHCl3 solution. All molecules feature absorption in a rather broad range, between 350 and 650 nm, which is a typical characteristic of the π-conjugated donor-acceptor type chemical architecture. The longer wavelength absorption band is mainly due to the π-π* charge transfer transitions from the donor to the cyanoacrylic acid acceptor of the dye molecule. B18, CPDT-R and BTD-R molecules exhibit absorption peaks in the visible region of the spectrum at 537, 565 and 530 nm respectively. The significant red-shift of the absorption peak from 537 to 565 nm in case of CPTD-R with respect to B18 dye is due to presence of a cyclopentadithiopene (CPDT) unit as the π−conjugation. BTD-R molecule, due to the presence of a D-A-π–A configuration, shows a much broader absorption band from 500 to 650 nm compared to B18 and CPTD-R. Moreover, very good extinction coefficients, in the order of 104 M−1 cm−1, are identified for all dyes.
Figure 1. Molecular structures of the B18, CPTD-R and BTD-R organic dyes.
Figure 2.
Characterization of dye molecules: (a) absorption and (b) emission spectra of B18, CPTD-R and BTD-R in CHCl3 solution. (c) Cyclic voltammograms of B18, CPTD-R and BTD-R, obtained in in freshly distilled CHCl3 using 0.5 M Tetrabutylammonium hexafluorophosphate (TBAPF6) as supporting electrolyte in a three–electrode configuration. (d) Schematic of potential levels of B18, CPTD-R and BTD-R with HOMO and LUMO levels showing electron injection to CB of ZnO and dye regeneration by I−/I3− electrolyte.
Table 1. Absorption maxima (103/M−1 cm−1), emission maximums and electrochemical characteristics of B18, CPTD-R and BTD-R dyes.
| Dyes | Absorption λmaxa/nm (103/M−1 cm−1) | Emissionb λmax/nm | Potentials and energy level |
||
|---|---|---|---|---|---|
| Eox/Vc (vs. NHE) | E0–0/Vd(vs. NHE) | Eox–E0–0 /V | |||
| B18 | 537(46.4) | 677 | 0.98 | 2.06 | −1.07 |
| CPTD-R | 565(59.5) | 694 | 0.77 | 1.97 | −1.20 |
| BTD-R | 530(38.7) | 715 | 0.9 | 2.98 | −1.08 |
aAbsorption and emission data were measured in CHCl3 at 25 °C; Electrochemical measurements were performed at 25 °C with each dye (0.5 mM) in CHCl3/0.1 M TBAPF6/N2, Pt disk working and Pt counter electrodes, Ag/AgCl reference electrode, scan rate = 50 mV s−1.
bExcitation wavelength/nm: B18, 537; CPTD-R, 565; BTD-R, 530.
cFirst oxidation values.
dEstimated from the intersection wavelengths of the normalized UV-vis absorption and the fluorescence spectra.
Emission spectra of dye molecules are shown in Fig. 2(b) and corresponding data are listed in Table 1. Fluorescence spectrum of B18 exhibits a broad emission peak at 677 nm, while CPDT-R and BTD-R feature the emission maximum at 694 nm and 715 nm, respectively. This shift of the maximum fluorescence emission to longer wavelengths is observed due to the introduction of D-A-π–A configuration for BTD-R. We employed cyclic voltammetry (CV) to investigate the reduction potentials of the organic dyes (Fig. 2(c) and Table 1). All dyes exhibit reversible oxidation curves, which are ascribed to the removal of an electron from the amine segment. The first oxidation potentials (Eox) of the dyes correspond to the highest occupied molecular orbital (HOMO) energy. Reduction potentials are obtained from the equation Ered = Eox–E0–0, in which E0–0 is the zero–zero excitation energy obtained from the intersection of absorption and emission spectra at the absorption edge. Energy alignment of the organic dye molecules with the device components can be obtained, which is presented in Fig. 2(d), along with the position of the conduction band (CB) of ZnO and the standard redox potential of the electrolyte used in devices (I−/I3−). Figure 2(d) shows the lowest unoccupied molecular orbital (LUMO) levels of B18, CPDT-R and BTD-R, positioned at −3.42, −3.30, and −3.42 eV, respectively, which are higher than the CB of ZnO (−4.3 eV). The driving forces for hot-electron injection are thus 0.88, 1.0, and 0.88 eV, respectively. On the other hand, the HOMO levels are located at −5.48, −5.27, and −5.40 eV, and the driving forces for oxidized dye regeneration reaction are 0.53, 0.32, and 0.45 eV, which are also satisfying for the regeneration reaction by the applied electrolyte.
Photovoltaic performance with standard mesoporous TiO2
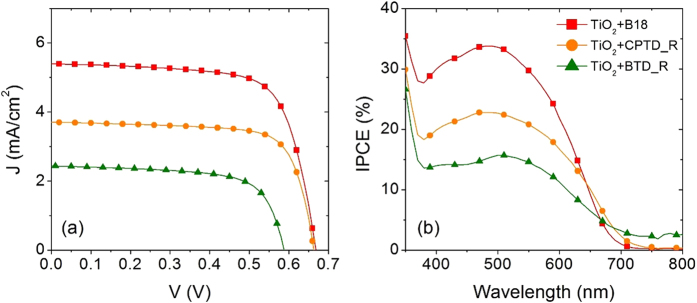
Figure 3(a) shows the comparison of current density vs photovoltage (J-V) characteristics of standard mesoporous TiO2 film sensitized by the three metal-free organic dyes. The corresponding photovoltaic parameters, namely short circuit current densities (Jsc), open circuit voltages (Voc), fill factor (FF) and photoconversion efficiencies (η), are reported in Table 2. Photocurrent densities (Jsc) and open circuit voltages (Voc) for these devices follow the trend B18 > CPTD-R > BTD-R. Since all the other components of the cells (photoanode, electrolyte and counter electrode) are the same, the difference in functional performances is mainly dependent on the properties of dye molecules such as: (i) electron injection efficiency (position of LUMO level with respect to the CB of semiconductor); (ii) oxidized dye regeneration efficiency (position of HOMO level with respect to standard redox potential of I−/I3−); (iii) light harvesting efficiency (γLHE). To enhance device performances, dye molecules should have optimized electron injection, fast oxidized dye regeneration reaction and high γLHE. Among all dyes molecules, CPTD-R dye molecule has higher potential for electron injection compared to other two dyes, whereas B18 has higher driving force for oxidized dye regeneration than CPTD-R and BTD-R.
Figure 3. Photovoltaic properties of standard mesoporous TiO2 DSSCs sensitized with the three different metal free organic dyes.
(a) Current density vs photovoltage curves under 1 sun illumination (AM 1.5 G, 100 mW cm−2); (b) IPCE spectra.
Table 2. Functional performance comparison of three different dyes (B18, CPTD-R and BTD-R) with two metal oxides: Commercial TiO2 nanoparticles and hierarchical assembled ZnO nanoparticles based DSSCs.
| Photoanode | Dyes | Thickness* (μm) | Voc (mV) | Jsc (mA cm−2) | FF (%) | η (%) |
|---|---|---|---|---|---|---|
| TiO2 | B18 | 16.63 | 669 | 5.40 | 71 | 2.56 |
| CPTD-R | 17.17 | 657 | 3.87 | 71 | 1.82 | |
| BTD-R | 16.79 | 590 | 2.44 | 68 | 0.97 | |
| ZnO | B18 | 8.46 | 543 | 8.85 | 56 | 2.68 |
| CPTD-R | 8.69 | 504 | 5.10 | 56 | 1.43 | |
| BTD-R | 9.87 | 542 | 7.14 | 52 | 2.03 |
*Total thickness of the ZnO photoanode, including the BL (800 nm thick).
The device sensitized with B18 dye shows better functional performances in term of Jsc and Voc, hence η, compared to devices exploiting CPTD-R and BTD-R as light harvesters, possibly due to the favorable combination of electron injection and good potential for oxidized dye regeneration. Short circuit photocurrent density, in particular, is enhanced when B18 is used as light harvesters (5.40 mA cm−2), being more than two times higher than that recorded for BTD-R (2.40 mA cm−2) and almost 30% higher that the Jsc obtained for CPTD-r (3.80 mA cm−2). A slight improvement in Voc is observed by comparing devices working with B18 and CPTD-R (669 mV and 657 mV, respectively), while a significant difference in Voc (79 mV) is revealed by analyzing cells sensitized with B18 and BTD-R (669 mV and 590 mV, respectively). Rather good FFs are recorded for all the analyzed devices (around 70%).
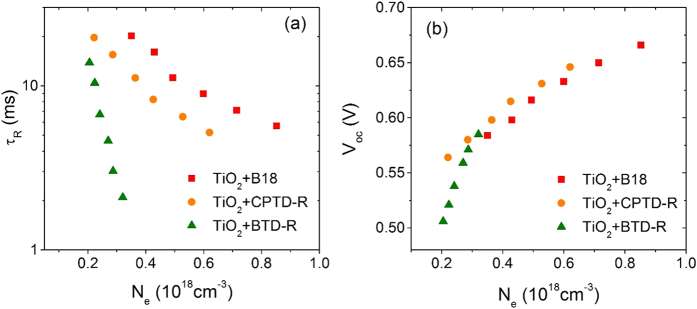
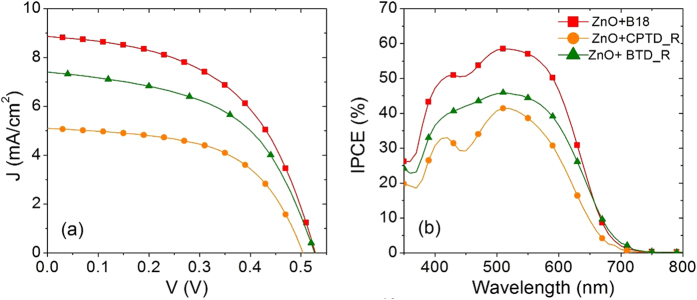
As for the devices with CPTD-R and BTD-R dyes, CPTD-R dye features better photovoltaic performance than BTD-R. The possible reason behind this behaviour is the dominancy of electron injection process over the oxidized dye regeneration process, since CPTD-R has higher electron injection driving force than BTD-R (see Fig. 2(d)). These results are further confirmed by incident photon to current conversion efficiency (IPCE) and TCD/TVD analyses: better IPCE values (see Fig. 3(b)) and longer τR (Fig. 4(a)) were indeed observed for the device sensitized with B18 dye, compared to devices using CPTD-R and BTD-R dyes.
Figure 4.
Comparison of electron-transport kinetics for three different dyes sensitized standard mesoporous TiO2: (a) τR vs Ne; (b) Voc vs Ne (τR: electron lifetime, Voc: open-circuit voltage and Ne: charge density).
The IPCE of the device is a function of light harvesting efficiency of dye molecules (γLHE), quantum yield of electron injection from the LUMO of the excited dye (γin) and collection efficiency (γcol), as follows: IPCE = γLHE × γin × γcol59. IPCE spectra of three different TiO2-based DSSCs are displayed in Fig. 2(b). The highest value of IPCE is: 34% (at 488 nm) for B18, 25% (at 486 nm) for CPTD-R and 16% (at 515 nm) for BTD-R. In the wavelength range 400–600 nm, the IPCE follows the trend: B18 > CPTD-R > BTD-R, consistent with the trend in Jsc (Table 2). All devices are identical in all respect, but for the dye molecules, so differences in IPCE can be reasonably attributed to different dye molecule properties, especially γLHE and γin.
In order to better elucidate the mentioned trend of functional performances, we applied transient current density decay (TCD) and transient voltage decay (TVD) analyses, reported in Fig. 4.
Figure 4(a) displays the plots of electron lifetime (τR) versus charge density (Ne) for TiO2 photoanodes sensitized with the three dye molecules at six different light intensities. At particular value of Ne (0.3 × 1018 cm−3), the τR follow the trend B18 > CPTD-R > BTD-R (see Table S2). This is consistent with the functional performances reported in Table 2, and suggest a reduced charge carrier recombination between the metal oxide CB and the electrolyte, reasonably associated to the observed higher Voc value for the B18 and CPTD-R sensitized devices than BTD-R. No significant difference in TiO2 CBE position is observed (Fig. 4(b)), when compared at particular value of Ne (0.3 × 1018 cm−3) as reported in Table S2, which suggest charge carrier recombination is dominant in determining Voc for devices, when sensitized with different light harvesters.
Overall, low functional performances are identified for TiO2-based DSSCs: investigation of the trend of functional parameters vs photoanode thickness (reported in Figure S4) indicates that, after reaching a maximum at around 11 μm, every functional parameter is decreasing. This is a typical behaviour for TiO2 standard photoanodes and suggest that observed performances are related to dye molecules, which seem not suitable to synergistically work with titanium dioxide to enhances device capability of converting solar energy.
Photovoltaic performance with hierarchical ZnO nanocrystallites
Metal-free dye molecules were also applied as light harvesters in ZnO-based DSSCs, using hierarchical structured ZnO (Figure S1), whose detailed characterization can be found in ref. 53. Briefly, the active layer, composed of polydispersed aggregates with broad size distribution range (between 100 nm and 600 nm), is especially designed for optimization of light managing and charge transport, while maintaining large specific surface area for dye loading. A transparent and compact ZnO BL is deposited in between the FTO glass and the ZnO active layer, aimed at physically insulating the FTO from the electrolyte, thus reducing charge recombination at this interface60. The BL is composed of homogeneously distributed rough lamellae having thickness of a few tens of nanometers and lateral dimensions in the sub-micrometer range and are oriented normal to substrate plane.
Figure 5(a) and Table 2 present the systematic comparison of J-V characteristics of hierarchical ZnO nanocrystallites sensitized by the three different metal-free organic dyes. B18 dye shows the best functional performances also in the case of a ZnO-based electrode, ascribable in particular to a high photocurrent density (as high as 8.85 mA cm−2). No significant differences are observed in Voc between devices sensitized with B18 and BTD-R dyes, while a slightly reduced Voc is recorded for CPTD-R (Fig. 5 (a)).
Figure 5. Photovoltaic properties.
(a) Current density vs photovoltage curves under 1 sun illumination (AM 1.5 G, 100 mW cm−2); (b) IPCE spectra of three different metal free organic dyes sensitized hierarchical structured ZnO DSSCs.
B18 dye has optimized electron injection, fast oxidized dye regeneration reaction and better γLHE compared to other two dyes (BTD-R and CPTD-R), which contributes to better Jsc, hence enhancing η. BTD-R features better photovoltaic performances than CPTD-R dye when applied to hierarchical structured ZnO nanocrystalline, which is opposite to the trend observed in mesoporous standard TiO2. In that case, CPTD-R seems featuring better γin than BTD-R, which is dominant over the oxidized dye regeneration efficiency. This behaviour is mainly attributed to the nature of photoanode material and is consistent with literature, as TiO2 features faster electron injection efficiency compared to ZnO42,61. These results are further supported by IPCE and TCD/TVD measurements in terms of better IPCE values (see Fig. 3(b)) and longer τR (Fig. 6(a)). IPCE values coherently follow the trend observed for Jsc (i.e B18 > BTD-R > CPTD-R) and overall the spectra show greatly enhanced values as compared with those observed in case of TiO2 photoanodes.
Figure 6.
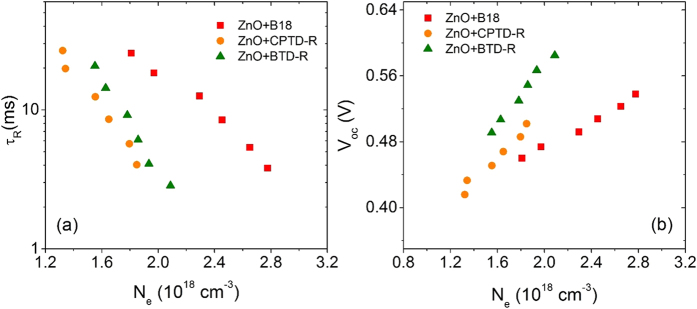
Comparison of electron-transport kinetics for three different dyes sensitized hierarchical ZnO: (a) τR vs Ne; (b) Voc vs Ne; (τR: electron lifetime, Voc: open-circuit voltage, Ne: charge density).
An extension of IPCE spectra, as compared with absorption specta recorded for dye solutions (Fig. 2(a)), from 650 to 700 nm is observed in case of both TiO2 and ZnO devices. This behavior has been already observed in literature30 and can be ascribed to two main reasons. Upon adsorption on metal oxides, conjugation length extension of sensitizers is changed, due to the anchoring on metal oxide by carboxylic groups, which can reflect in IPCE at longer wavelengths. Another reason is the possible J- or H- aggregation of dyes on TiO2: the large amount of adsorbed sensitizers may result in head-to-head or/and face-to-face aggregation on the adsorption state, which is also beneficial to extend the IPCE response of devices.
Voc values of devices follow the trend B18 > BTD-R > CPTD-R, which appears mainly dependent on charge recombination, as indicated by the τR obtained for the corresponding devices and shown in Fig. 6(a). τR values of respectives devices at particular Ne (1.8 × 1018 cm−3) reported in Table S3. The device sensitized with B18 features highest τR and hence possible reduced recombination of the electrons in the CB of the semiconductor with redox couple species of the electrolyte. Sligth differences were observed in the TVD plots (Fig. 6(b), Table S3) and possible shift of the CBE of ZnO upon dye uptake seems negligible, which does not help in enhancing the open circuit voltage of the devices. Similar situation was observed with mesoporous TiO2.
Finally, relatively low FFs were found for ZnO-based DSSCs. Lower FF values for ZnO, as compared with TiO2, have been often reported by previous studies (an overview on ZnO-based solar cells can be found in ref. 62) and mainly attributed to poor injection of photogenerated charges from the dye to the metal oxide. However, numerical simulation on J-V characteristics have also pointed out that series resistances can be also partially responsible of the relatively low FFs recorded in ZnO real devices63.
Comparison of hierarchical ZnO nanocrystallites and standard mesoporous TiO2
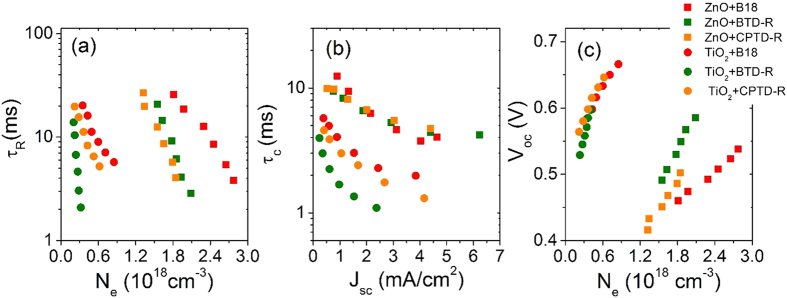
A systematic comparison of the current-density vs voltage and IPCE for hierarchical structured ZnO and standard mesoporous TiO2 film of comparable thickness sensitized by the three different metal-free organic dyes are displayed in Figure S2 and the corresponding functional parameters are reported in Table S1. Voc values are lower for all dyes (20% for B18, 23% for CPTD-R and 11% for BTD-R), when used as light harvesters in ZnO-based DSSCs: TiO2-based devices exhibit systematically higher voltage (Fig. 6 (c)), indicating more negative values of CBE for titanium dioxide. As reported by Hara et al.64, large driving force for back electron transfer (from the metal oxide to the redox couples in the electrolyte) is associated to electrons located in shallow CBE, resulting in short lifetime, which seems confirmed also in our case (Fig. 7 (a)).
Figure 7.
Comparison of electron-transport kinetics for hierarchical structured ZnO and standard mesoporous TiO2 sensitized by B18, CPTD-R and BTD-R based DSSCs: (a) τR vs Ne; (b) τC vs Jsc and (c) Voc vs Ne.
In contrast to Voc, all dyes give higher Jsc in ZnO than in TiO2. This can be explained on the basis of better electron transport properties featured by ZnO (one order of magnitude higher than TiO230) and improved light harvesting in hierarchical assembled ZnO, as we previously reported53.
Effect of blocking layer
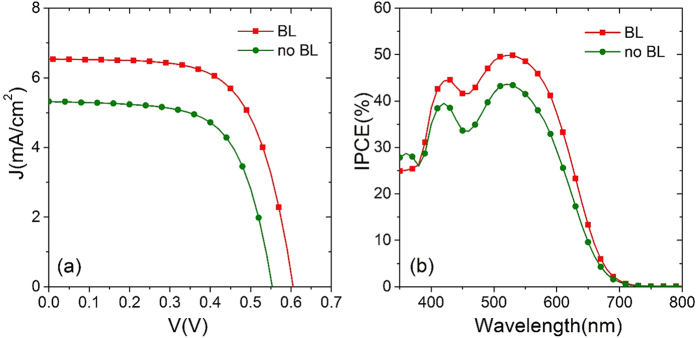
We verified the effect of the application of a compact ZnO blocking layer (BL) between FTO and active layer of hierarchical structured ZnO. The beneficial role of BL in boosting η in ZnO DSSCs was already demonstrated for the commercial dye N71953,59. Here, we extend this concept also to hierarchical structured ZnO photoanodes sensitized with B18 dye. Cell provided with a ZnO BL delivered better functional performances as compared with its counterpart without BL (shown in Fig. 8 and corresponding photovoltaic parameters are reported in Table 3).
Figure 8.
(a) Current density vs photovoltage under 1 sun illumination (AM 1.5 G, 100 mW cm−2); (b) IPCE spectra for hierarchical structured ZnO DSSCs with and without BL sensitized by B18.
Table 3. Effect of BL on the functional performance comparison: B18 dye sensitized hierarchical assembled ZnO nanoparticles based DSSCs with and without the BL.
| Photoanode | BL | Thickness* (μm) | Voc (mV) | Jsc (mA cm−2) | FF (%) | η (%) |
|---|---|---|---|---|---|---|
| ZnO | Yes | 8.30 | 609 | 6.58 | 64 | 2.56 |
| No | 9.04 | 557 | 5.32 | 64 | 1.90 |
*Total thickness of the photoanode, including the BL. 800 nm is the thickness of BL considered in this work.
The highly favorable effect of the BL is clearly visible in J-V curves (see Fig. 8(a)): Jsc was indeed enhanced of about 24% and Voc increased by 52 mV in the device provided by the compact ZnO BL as compared to the device without BL, which result in an overall enhancement of photoconversion efficiency of almost 35%. In a previous work59, investigation through electrochemical impedance spectroscopy demonstrated that the role of BL is especially related to an increased chemical capacitance, resulting in an overall increased τR.
Figure 8(b) shows the beneficial effect of the BL in IPCE spectra, too. Increased IPCE for the cell with BL is consistent with the enhancement in Jsc.
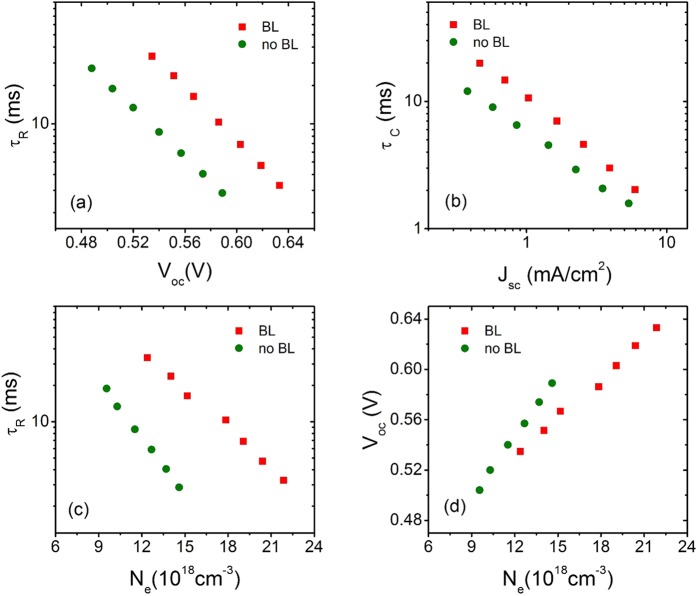
To investigate more in detail about the effect of BL on electron transport and recombination kinetics, we used temporally resolved techniques TCD and TVD under short-circuit and open-circuit conditions, respectively. Figure 9(a) collects the τR versus Voc taken at open circuit condition. The cell with BL has systematically higher τR than cell without BL, when both cells are compared at same Voc level (see Table S6). This reflects the beneficial role of BL to reduce the carrier recombination at the FTO/electrolyte interface by physically insulation of the FTO from electrolyte55,56.
Figure 9.
Comparison of electron-transport kinetics for hierarchical structured ZnO DSSCs with and without BL sensitized by B18: (a) τR vs Voc; (b) τC vs Jsc (c) τR vs Ne and (d) Voc vs Ne.
Figure 9(b) shows plot of electron collection time (τC) versus current density (Jsc) obtained from charge extraction (CE) data under the same condition as mentioned above. In principle, electron collection time for both cells (with and without BL) should be the same, as the active layer of both the cells is the same. The higher value of τC in the cell with BL than without BL at particular value of Jsc (see Table S6) is probably due to better physical contact between the active layer of ZnO nanoparticles and the FTO glass substrate65. So, the longer electron life time and the improved electron collection efficiency are responsible for higher Jsc and Voc (see Table 3) and hence the conversion efficiency of the device with BL.
Figure 9(c) shows plots of τR versus Ne. The τR for the cell with BL and without BL decreases with increase in charge density. At fixed value of Ne, cell with BL has higher value of τR (see Table S6). These results demonstrated that more charge is accumulated for the cell with BL, which explain the trend of Voc with BL > Voc no BL.
Figure 9(d) displays the plots of Voc versus Ne. The Voc for both cells increases with charge density. At fixed value of Ne, the Voc of the cell with BL is higher than the Voc without BL (see Table S6). Typically, two possible reasons exist for this improvement in Voc: one is the reduced recombination between the injected electron and oxidized species of the electrolyte as we already explained in previous section (Fig. 9(c)), and the second one is the change in CB edge position of metal oxide with respect to the redox potential of the electrolyte66. The second possibility in our case is insignificant as we can see in Fig. 9(d) because there is no drastic shift in CB edge positions for the device with and without BL. So, in our case, reduced recombination, which is easily reflected from improved electron life time and collection time, would be responsible for the larger value of Voc in the device with BL.
In order to evaluate the solid demonstration and the reproducibility of BL effects on the device functional parameters, we fabricated two devices per kind (with and without BL), while keeping constant all the other parameters. The results demonstrating the high reproducibility of BL effect and of the device fabrication process are reported in Figure S6, and corresponding functional parameters are reported in Table S6 of the Supporting information.
Conclusions
In summary, we applied three newly synthesized metal-free organic dyes (B18, CPTD-R and BTD-R) as light harvester in DSSCs and carried out systematic comparison of photovoltaic properties of each dye in hierarchical structured ZnO and in benchmarking standard mesoporous TiO2 photoanodes. We demonstrated that B18 dye gives better photovoltaic properties than the other two dyes in both hierarchical structured ZnO and commercial TiO2 due to higher potential of electron injection and better light harvesting. The TCD/TVD results demonstrated that device with B18 dye has better τR and negligible shift in the CB of dye sensitized photoanodes as compared to CPTD-R and BTD-R dyes based devices.
Each dye results in higher Jsc in hierarchical structured ZnO than in standard mesoporous TiO2, mainly due to better electron transport properties featured by hierarchical structured ZnO nanocrystallites compared to TiO2. This behavior is further confirmed by TCD/TVD results, which demonstrated that for each dye, the τR and τc are higher in hierarchical structured ZnO nanocrystallites than in standard mesoporous TiO2.
In addition, we applied ZnO compact BL between the active layer and the FTO glass in ZnO photoanodes, demonstrating a significant improvement in device functional performances. This interpretation is confirmed by the results provided by TCD/TVD such as improved τR and τc for a cell with BL as compared to cell without BL. The results of this work can open a new room for the development of ZnO-based DSSCs by proper combination of well-designed metal-free organic dyes and ZnO nanostructured photoanodes.
Methods
Dyes synthesis
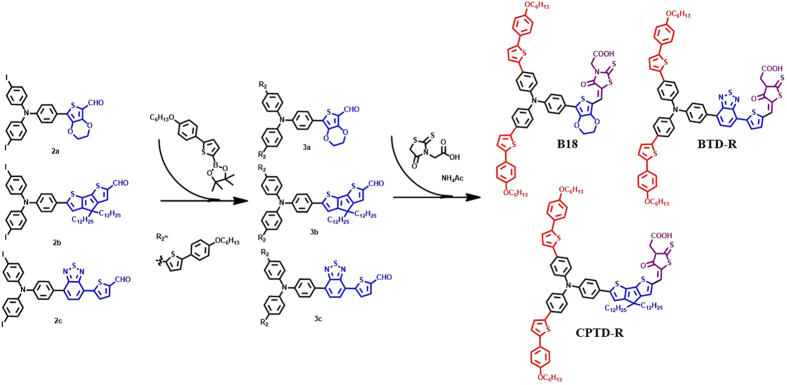
All solvents and reagents, unless otherwise stated, were of analytical grade quality and used as received. Standard Schlenk techniques were employed to manipulate oxygen- and moisture-sensitive chemicals. 7-(4-(bis(4-iodophenyl)amino)phenyl)-2,3-dihydrothieno[3,4-b][1,4]dioxine-5-carbaldehyde (2a), 6-(4-(bis(4-iodophenyl)amino)phenyl)-4,4-didodecyl-4H-cyclopenta[1,2-b:5,4-b′] dithiophene-2-carbaldehyde (2b), 5-(7-(4-(bis(4-iodophenyl)amino)phenyl)benzo[c][1,2,5]thiadiazol-4-yl)thiophene-2-carbaldehyde (2c) were synthesized according to the literature as shown in figure 1067. Tetrahydrofuran (THF) was dried with sodium sand, and benzophenone indicator, dichloromethane (DCM) was dried out with calcium hydride before using. Reactions were carried out under a dry nitrogen atmosphere.
Figure 10. Synthesis of B18, CPTD-R and BTD-R dye molecules.
(E)-2-(5-((7-(4-(bis(4-(5-(4-(hexyloxy)phenyl)thiophen-2-yl)phenyl)amino)phenyl)-2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (B18) synthesis
Compound 2 (93 mg, 0.1 m mol) and rhodanine-3-acetic acid (19 mg, 0.1 m mol), ammonium acetate (3.5 mg, 0.044 m mol) was added into acetic acid (10 ml) under N2. The reaction was stirred at 120 °C for 12 h. The progress of the reaction was monitored with TLC. The solvent was removed under vacuum. The residue was purified on silica chromatograph using DCM/MeOH = 20/1 as eluent. The product was re-crystallized from DCM/MeOH to give red solid of B18 (36 mg, 35%). 1H NMR (CDCl3) δ H 8.02 (s, 1H), 7.72 (d, J = 8.85 Hz, 2H), 7.24 (d, J = 4.00 Hz, 2H), 7.19 (m, 8H), 6.95 (d, J = 4.87 Hz, 4H), 4.92 (s, 2H), 4.41 (d, J = 6.28 Hz, 4H), 4.00 (t, J = 4.45 Hz, 2H), 1.86 (m, 4H), 1.51 (m, 4H), 1.39 (m, 12H), 0.93 (m, 6H). 13C NMR (CDCl3) δ C ppm: 192.0, 166.7, 158.8, 147.4, 146.2, 145.8, 141.9, 137.9, 130.0, 128.0, 127.6, 127.0, 126.9, 126.8, 126.5, 125.7, 125.0,123.4, 123.1, 122.9, 115.6, 115.0, 114.9, 111.0, 68.1, 64.6, 44.4, 31.6, 29.2, 25.7, 22.6, 14.0. IR (KBr, cm−1): 3447, 3208, 2927, 2852, 1770, 1705, 1577, 1497, 1477, 1445, 1402, 1364, 1323, 1247, 1179, 1132, 1080, 1022, 937, 831, 797, 698, 635, 585. MS (MALDI-TOF) m/z: calcd for 1103.458; found 1102.360. Element analysis (%) calcd for C62H58N2O7S5, C, 67.48; H, 5.30; N, 2.54; found C, 67.44, H 5.41; N, 2.60.
(E)-2-(5-((6-(4-(bis(4-(5-(4-(hexyloxy)phenyl)thiophen-2-yl)phenyl)amino)phenyl)-4,4-didodecyl-4H-cyclopenta[1,2-b:5,4-b′]dithiophen-2-yl)methylene)-4-oxo-2-thioxotetrahydrothiophen-3-yl)acetic acid (CPDT-R) synthesis
The synthesis procedures of CPTD-R dye were according to the literature and it was similar with B18, which is obtained as purple solid. 1H NMR (CD2Cl2) δ H8.01 (s, 1H), 7.60 (m, 10H), 7.34 (s, 1H), 7.28 (d, J = 4.6 Hz, 2H), 7.23 (m, 9H), 6.97 (d, J = 8.2 Hz, 4H), 4.93 (s, 2H), 4.00 (t, J = 4.45 Hz, 2H), 1.86 (m, 4H), 1.51 (m, 4H), 1.39 (m, 12H), 0.93 (m, 6H). 13C NMR (CDCl3) δ C ppm: 192.3, 158.8, 147.1, 146.0, 143.3, 142.0, 137.8, 129.7, 128.9, 126.8, 124.6, 122.9, 115.0, 114.9, 78.2, 68.1, 64.6, 44.4, 31.6, 29.2, 25.7, 22.6, 24.6, 14.1;IR (KBr, cm−1): 3445, 2927, 2849, 1709, 1572, 1493, 1408, 1322, 1293, 1244, 1205, 1112, 1047, 838, 799, 698, 635, 585; MS (MALDI-TOF) m/z: calcd for 1473.647; found 1472.580. Element analysis (%) calcd for C90H107NO5S6, C, 73.28; H, 7.31; N, 0.95; found C, 73.24, H 7.41; N, 0.98.
(E)-2-(5-((5-(7-(4-(bis(4-(5-(4-(hexyloxy)phenyl)thiophen-2-yl)phenyl)amino)phenyl)benzo[c][1,2,5]thiadiazol-4-yl)thiophen-2-yl)methylene)-4-oxo-2-thioxotetrahydrothiophen-3-yl)acetic acid (BTD-R) synthesis
The synthesis procedures of BTD-R dye were according to the literature and it was similar with B18, which is obtained as red solid. 1H NMR (DMSO-d6) δ H8.56 (s, 1H), 8.25 (d, J = 7.7 Hz,2H), 8.04 (d, J = 8.8 Hz, 2H), 7.93 (d, J = 8.2 Hz, 2H), 7.65 (d, J = 9.2 Hz, 4H), 7.57 (d, J = 15.7 Hz, 4H), 7.44 (dd, 4H), 7.23 (d, J = 6.2 Hz, 2H), 7.17 (d, J = 8.3 Hz, 4H), 6.99 (d, J = 9.3 Hz, 4H), 4.91 (s, 2H), 4.00 (t, J = 4.4 Hz, 4H), 1.74 (m, 4H), 1.51 (m, 12H), 0.98 (m, 6H); 13C NMR (CDCl3) δ C ppm: 192.2, 168.8, 158.7, 153.7, 14.2, 146.3, 138.4, 135.5, 133.7, 130.3, 126.8, 123.3, 120.9, 114.8, 78.2, 68.1, 64.3, 44.4, 31.6, 29.2, 24.7, 22.6, 24.6, 14.0; IR (KBr, cm−1): 3438, 3026, 2927, 2852, 2364, 2338, 1705, 1577, 1497, 1434, 1323, 1247, 1192, 1110, 1057, 845, 785, 698, 635, 585; MS (MALDI-TOF) m/z: calcd for 1177.278; found 1176.772. Element analysis (%) calcd for C67H59N3O5S6, C, 68.28; H, 5.05; N, 3.57; found C, 68.24, H 5.12; N, 3.63.
Photoanodes preparation
ZnO BL and active layer were deposited by spray pyrolysis as reported in ref. 53. In brief 0.24 M Zn(CH3COO)2·2H2O (25 mL of methanol/water, 2:1 v/v) is used as precursor for the BL and mixture of ethanolic suspension of commercial ZnO nanoparticles (0.5 g in 15 ml ethanol) and 0.55 M Zn(CH3COO)2·2H2O (40 ml of methanol/water, 3:1 v/v) is used for the active layer. For both the BL and the active layer, the precursors are sprayed using N2 carrier gas at pressure of 0.40 bar on FTO glass (sheet resistance 10 Ω/) kept at 250 °C. Nozzle-to-sample distance: 37 cm for the BL, 25 cm for the active layer. Post deposition annealing is carried out at 450 °C for 30 minutes under ambient conditions.
TiO2 photoanodes of required thickness were prepared on ultrasonically cleaned FTO glass substrate by repetitive screen printing of a transparent layer (15–20 nm sized) and then a scattering layer (150–200 nm sized). Drying process followed for 10 minutes at ambient atmosphere and temperature and then for 6 minutes at 120 °C. Finally all photoanodes were annealed at 500 °C for 30 minutes under ambient conditions.
Thickness of the both photoanodes (ZnO and TiO2) was measured by Dek Tak 150 stylus profiler.
Pt-counter electrodes
The Pt-counter electrode was prepared by spin-coating a solution containing platinum (H2PtCl6 in isopropanol) onto the FTO glass at 2000 rpm for 10 s, then heated at 380 °C for 30 minutes.
Cell fabrication
All photoanodes were treated with ultraviolet ozone (UV-O3) cleaning for 18 minutes to remove the surface contaminants, followed by heating at 200 °C for 15 minutes, then let them cool to 80 °C and keeping at 80 °C before immersion in dye solution. For dye uptake, both ZnO and TiO2 photoanodes were immersed in 0.2 mM dye solution (dye/Chenode-oxycholic acid (CDCA), 1:1 w/w, in mixture of toluene/ethanol, 4:1 v/v) 3 h for B18 and BTD-R and 2 h for CPTD-Rat room temperature. After dye absorption, the photoanodes were washed first with CH2Cl2, then with dry ethanol to remove unabsorbed dye molecules and then dried in hot air. DSSCs were fabricated by sealing the dye sensitized photoanodes and Pt-counter electrodes in a sandwich-type structure with a hot-melt film (SX1170, thickness 60 μm) under thermal compression at 90 °C for 10 s. The space between them was filled by redox couple electrolyte, which is composed of 0.5 M PMII; 0.03 M I2; 0.5 M TBP in a mixture of acetonitrile and valeronitrile (85:15 v/v).
Characterizations
1H NMR and 13CNMR spectra were measured on a Bruker-AF301 AT 400 MHz spectrometer. High resolution mass spectra (HRMS) were measured with a Bruker MALDI TOF mass spectrometer. The UV-visible absorption spectra were observed with a PE950 spectrophotometer and Fluorescent emission spectra were obtained with a Jasco FP-6500 spectrophotometer. FT-IR spectra were recorded on a Bruker VERTEX 70. All cyclic voltammetry measurements were conducted in freshly distilled trichloromethane using TBAPF6 (0.5 M) as supporting electrolyte in a three–electrode system, with each solution being purged with N2 before measurement. The working electrode was a Pt disk; the reference electrode was Ag/AgCl and the counter electrode was a Pt rod. All measurements were made at 23 °C with a CHI660C electrochemical work station. The reduction potentials were calibrated with ferrocene as internal reference. The HOMO and LUMO values were transformed according to the literature68.
The current-voltage (I-V) measurements were carried out with a source meter (Keithley 2400, computer-controlled) under one sun simulated sunlight at AM 1.5G (100 mWcm−2), calibrated with silicon reference cell. The active area of the cells was 0.25 cm2. The incident photon to current conversion efficiency (IPCE) of the devices was measured with a system comprising a Xe lamp (A-1010, PTi, 150 W), monochromator (PTi, 1200 grooves mm−1 blazed at 500 nm), and source meter (Keithley 2400). All measurements were carried by using external shadow mask of area 0.5 × 0.5 cm2.
Transient photocurrent and photovoltage decay (TCD and TVD) measurements were carried out with a computer-controlled instrumental setup containing two LED light sources. Six steady-state light intensities were obtained as bias irradiations from a white LED on tuning the driving voltage. A green LED (λ = 532 nm) controlled with a pulse generator (DG535, SRS) generated a perturbation pulse of duration 50 ms. Both the pulsed green light and the steady-state white light irradiated the photoanode side of the cell. The pulsed-probe irradiation was controlled with a LED power supply to maintain the modulated photovoltage less than 5 mV in each measurement. The probe beams generated carriers causing a slightly increased photocurrent (ΔJSC) near JSC of the cell at the short-circuit condition, or a slightly increased photovoltage (ΔVOC) near VOC of the cell at the open-circuit condition, subjected to the white bias light; the current and voltage decays were thereby measured, respectively. The resulting photocurrent and photovoltage transients were recorded on a digital oscilloscope (MSO2014, Tektronix); the signals passed a current preamplifier (SR570, SRS) at a short-circuit condition. For the charge extraction method, white light LED is fired under the open-circuit condition for duration 200 ms and is then turned off at the same time that the system is switched to the short-circuit condition.
Additional Information
How to cite this article: Singh Selopal, G. et al. Metal-free organic dyes for TiO2 and ZnO dye-sensitized solar cells. Sci. Rep. 6, 18756; doi: 10.1038/srep18756 (2016).
Supplementary Material
Acknowledgments
A.V. acknowledges the European Commission for partial funding under the contract F-Light Marie Curie 299490. The authors acknowledge the European Commissions for partial funding under the contract WIROX 295216. I.C. acknowledges Regione Lombardia under X-Nano Project and National Research Council Project for partial funding. G.S.S. acknowledges OIKOS s.r.l. for funding. A.V. acknowledges Kempestiftelserna and Luleå University of Technology Labfonden program for financial support for equipment.
Footnotes
Author Contributions G.S.S., I.C. and A.V. carried out the synthesis and the morphology and structural characterization of ZnO photoanodes. J.L. and M.W. carried out dye synthesis and spectroscopic/optical characterization. G.S.S., H.-P.W., Y.-C.C. and E.W.-G.D. fabricated and characterized the solar cells. G.S.S., H.-P.W., I.C. and A.V. wrote the manuscript. All authors reviewed the manuscript.
References
- O’Regan B. & Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991). [Google Scholar]
- Chen C. Y. et al. Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells. ACS Nano 3, 3103–3109 (2009). [DOI] [PubMed] [Google Scholar]
- Yella A. et al. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 334, 629–634 (2011). [DOI] [PubMed] [Google Scholar]
- Kakiage K. et al. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 15, 15894–15897 (2015). [DOI] [PubMed] [Google Scholar]
- Mishra A., Fischer M. K. R. & Bauerele P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 48, 2474–2499 (2009). [DOI] [PubMed] [Google Scholar]
- Wang Z. S., Li F. Y. & Huang C. H. Photocurrent Enhancement of Hemi-cyanine Dyes Containing RSO3− Group through Treating TiO2 Films with Hydrochloric Acid. J. Phys. Chem. B 105, 9210–9217 (2001). [Google Scholar]
- Thomas K. R. J., Lin J. T., Hsuc Y. C. & Ho K. C. Organic dyes containing thienyl fluorene conjugation for solar cells. Chem. Commun. 32, 4098–4100 (2005). [DOI] [PubMed] [Google Scholar]
- Tian H. et al. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem. Commun. 36, 3741–3743 (2007). [DOI] [PubMed] [Google Scholar]
- Li S.-L., Jiang K.-J., Shao K.-F. & Yang L.-M. Novel organic dyes for efficient dye-sensitized solar cells. Chem. Commun. 26, 2792–2794 (2006). [DOI] [PubMed] [Google Scholar]
- Hara K. et al. Novel Conjugated Organic Dyes for Efficient Dye-Sensitized Solar Cells Adv. Funct. Mater. 15, 246–252 (2005). [Google Scholar]
- Campbell W. M. et al. Highly Efficient Porphyrin Sensitizers for Dye-Sensitized Solar Cells. J. Phys. Chem. C 11, 11760–11762 (2007). [Google Scholar]
- Kozma E. et al. Metal-free organic sensitizers with a sterically hindered thiophene unit for efficient dye sensitized solar cells. J. Mater. Chem. 21, 13785–13788 (2011). [Google Scholar]
- Koumura N. et al. Alkyl-Functionalized Organic Dyes for Efficient Molecular Photovoltaics. J. Am. Chem. Soc. 128, 14256–14257 (2006). [DOI] [PubMed] [Google Scholar]
- Wang Z. S. et al. Thiophene-Functionalized Coumarin Dye for Efficient Dye-Sensitized Solar Cells: Electron Lifetime Improved by Co-adsorption of Deoxycholic Acid. J. Phys. Chem. C 111, 7224–7230 (2007). [Google Scholar]
- Hwang S. et al. A highly efficient organic sensitizer for dye-sensitized solar cells. Chem. Commun. 46, 4887–4889 (2007). [DOI] [PubMed] [Google Scholar]
- Ito S. et al. High-conversion-efficiency organic dye-sensitized solar cells with a novel indoline dye. Chem. Commun. 41, 5194–5196 (2007). [DOI] [PubMed] [Google Scholar]
- Joly D. et al. A Robust Organic Dye for Dye Sensitized Solar Cells Based on Iodine/Iodide Electrolytes Combining High Efficiency and Outstanding Stability. Scientific Reports 4, 4033 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt S. M. et al. Design of organic dyes and cobalt polypyridine redox mediators for high-efficiency dye-sensitized solar cells. J. Am. Chem. Soc. 132, 16714–16724 (2010). [DOI] [PubMed] [Google Scholar]
- Tsao H. N. et al. Cyclopentadithiophene bridged donor-acceptor dyes achieve high power conversion efficiencies in dye-sensitized solar cells based on the tris-cobalt bipyridine redox couple. ChemSusChem. 4, 591–594 (2011). [DOI] [PubMed] [Google Scholar]
- Xu B. et al. Integrated Design of Organic Hole Transport Materials for Efficient Solid-State Dye-Sensitized Solar Cells. Adv. Energy Mater. 5, 1401185 (2015) [Google Scholar]
- Gabrielsson E. et al. Convergent/Divergent Synthesis of a Linker-Varied Series of Dyes for Dye-Sensitized Solar Cells Based on the D35 Donor. Adv. Energy Mater. 3, 1647–1656 (2013). [Google Scholar]
- Li L.-L. & Diau E. W.-G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 42, 291–304 (2013). [DOI] [PubMed] [Google Scholar]
- Chai Q. et al. Rational molecular engineering of cyclopentadithiophene-bridged D-A-π-A sensitizers combining high photovoltaic efficiency with rapid dye adsorption. Sci. Reports 5, 11330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M., Greene L. E., Johnson J. C., Saykally R. & Yang P. Nanowire dye sensitized solar cells. Nat. Mater. 4, 455–459 (2005). [DOI] [PubMed] [Google Scholar]
- Xu C., Wu J., Desai U. V. & Gao D. Multilayer Assembly of Nanowire Arrays for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 133, 8122–8125 (2011). [DOI] [PubMed] [Google Scholar]
- Baxter J. B. & Aydil E. S. Nanowire-based dye-sensitized solar cells. Appl. Phys. Lett. 86, 053114 (2005). [Google Scholar]
- Barnes P. R. F. et al. Re-evaluation of Recombination Losses in Dye-Sensitized Cells: The Failure of Dynamic Relaxation Methods to Correctly Predict Diffusion Length in Nanoporous Photoelectrodes. Nano Lett. 9, 3532–3538 (2009). [DOI] [PubMed] [Google Scholar]
- Kay A. & Gratzel M. Dye-Sensitized Core−Shell Nanocrystals: Improved Efficiency of Mesoporous Tin Oxide Electrodes Coated with a Thin Layer of an Insulating Oxide. Chem. Mater. 14, 2930–2935 (2002). [Google Scholar]
- Guo P. & Aegerter M. A. RU(II) sensitized Nb2O5 solar cell made by the sol-gel process. Thin Solid Films 351, 290–294 (1999). [Google Scholar]
- Sayama K., Sugihara H. & Arakawa H. Photoelectrochemical Properties of a Porous Nb2O5 Electrode Sensitized by a Ruthenium Dye. Chem. Mater. 10, 3825–3832 (1998). [Google Scholar]
- Hara K. et al. Highly efficient photon-to-electron conversion with mercurochrome-sensitized nanoporous oxide semiconductor solar cells. Sol. Energy Mater. Sol. Cells 64, 115–134 (2000). [Google Scholar]
- Quintana M., Edvinsson T., Hagfeldt A. & Boschloo G. Comparison of Dye-Sensitized ZnO and TiO2 Solar Cells: Studies of Charge Transport and Carrier Lifetime. J. Phys. Chem. C 111, 1035–1041 (2007). [Google Scholar]
- Martinson A. B. F., McGarrah J. E., Parpia M. O. K. & Hupp J. T. Dynamics of charge transport and recombination in ZnO nanorods arraydye-sensitized solar cells. Phys. Chem. Chem. Phys. 8, 4655–4659 (2006). [DOI] [PubMed] [Google Scholar]
- Keis K., Magnusson E., Lindstrom H., Lindquist S. E. & Hagfeldt A. A 5% efficient photoelectrochemical solar cell based on nanostructured ZnO electrodes. Sol. Energy Mater. Sol. Cells 73, 51–58 (2002). [Google Scholar]
- Hara K. et al. Highly efficient photon-to-electron conversion with mercurochrome-sensitized nanoporous oxide semiconductor solar cells. Sol. Energy Mater. Sol. Cells 64, 115–134 (2000). [Google Scholar]
- Concina I. & Vomiero A. Metal Oxide Semiconductors for Dye- and Quantum-Dot-Sensitized Solar Cells. Small 11, 1744–1774 (2015). [DOI] [PubMed] [Google Scholar]
- Dembele K. T. et al. Effect of multi-walled carbon nanotubes on the stability of dye sensitized solar cells. J. Power Sources 233, 93–97 (2013). [Google Scholar]
- Dembele K. T. et al. Hybrid Carbon Nanotubes–TiO2 Photoanodes for High Efficiency Dye-Sensitized Solar Cells. J. Phys. Chem. C 117, 14510–14517 (2013). [Google Scholar]
- Dembele K. T. et al. Graphene below the percolation threshold in TiO2 for dye-sensitized solar cells. J. Mater. Chem. A 3, 2580–2588 (2015). [Google Scholar]
- Oskam G., Hu Z. S., Penn R. L., Pesika N. & Searson P. C. Coarsening of metal oxide nanoparticles. Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys. 66, 011403 (2002). [DOI] [PubMed] [Google Scholar]
- Ozgur U. et al. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98, 041301 (2005). [Google Scholar]
- Pearton S. J., Norton D. P., Ip K., Heo Y. W. & Steiner T. Recent progress in processing and properties of ZnO. Prog. Mater. Sci. 50, 293–340 (2005). [Google Scholar]
- Chandiran A. K., Abdi-Jalebi M., Nazeeruddin M. K. & Gratzel M. Analysis of Electron Transfer Properties of ZnO and TiO2 Photoanodes for Dye-Sensitized Solar Cells. ACS Nano 8, 2261–2268 (2014). [DOI] [PubMed] [Google Scholar]
- Jimenez-Cadena G., Comini E., Ferroni M., Vomiero A. & Sberveglieri G. Synthesis of different ZnO nanostructures by modified PVD process and potential use for 1dye-sensitized solar cells. Mater. Chem. Phys. 124, 694–698 (2010). [Google Scholar]
- Martison A. B. F., Elam J. W., Hupp J. T. & Pellin M. J. ZnO Nanotube Based Dye-Sensitized Solar Cells. Nano Lett. 7, 2183–2187 (2007). [DOI] [PubMed] [Google Scholar]
- Martison A. B. F. et al. Electron Transport in Dye-Sensitized Solar Cells Based on ZnO Nanotubes: Evidence for Highly Efficient Charge Collection and Exceptionally Rapid Dynamics. J. Phys. Chem. A 113, 4015–4021 (2009). [DOI] [PubMed] [Google Scholar]
- Hosono E., Fujihara S., Honma I. & Zhou H. The Fabrication of an Upright-Standing Zinc Oxide Nanosheet for Use in Dye-Sensitized Solar Cells. Adv. Mater. 17, 2091–2094 (2005). [Google Scholar]
- Hosono E., Mitsui Y. & Zhou H. Metal-free organic dye sensitized solar cell based on perpendicular zinc oxide nanosheet thick films with high conversion efficiency. Dalton Trans. 40, 5439–5441 (2008). [DOI] [PubMed] [Google Scholar]
- Jiang C. Y., Sun X. W., Lo G. Q. & Kwong D. L. Improved dye-sensitized solar cells with a ZnO-nanoflower photoanode. Appl. Phys. Lett. 90, 263501(2007). [Google Scholar]
- Hsu Y. F., Xi Y. Y., Yip C. T., Djurisic. A. B. & Chan W. C. Dye-sensitized solar cells using ZnO tetrapods. J. Appl. Phys. 103, 083114 (2008) [Google Scholar]
- Chiu W.-H. et al. Efficient electron transport in tetrapod-like ZnO metal-free dye-sensitized solar cells. Energy Environ. Sci. 2, 694–698 (2009). [Google Scholar]
- Chen W., Zhang H., Hsing I. M. & Yang S. A new photoanode architecture of dye sensitized solar cell based on ZnO nanotetrapods with no need for calcination. Electrochem. Commun. 11, 1057–1060 (2009). [Google Scholar]
- Zhang Q. F., Chou T. R., Russo B., Jenekhe S. A. & Cao G. Z. Aggregation of ZnO Nanocrystallites for High Conversion Efficiency in Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 47, 2402–2406 (2008). [DOI] [PubMed] [Google Scholar]
- Memarian N. et al. Hierarchically Assembled ZnO Nanocrystallites for High-Efficiency Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 50, 12321–12325 (2011). [DOI] [PubMed] [Google Scholar]
- Vomiero A. et al. ZnO/TiO2 nanonetwork as efficient photoanode in excitonic solar cells. Appl. Phys. Lett. 95, 193104 (2009). [Google Scholar]
- Cameron P. J. & Peter L. M. Characterization of Titanium Dioxide Blocking Layers in Dye-Sensitized Nanocrystalline Solar Cells. J. Phys. Chem. B 107, 14394–14400 (2003). [Google Scholar]
- Kavan L. & Grätzel M. Highly efficient semiconducting TiO2 photoelectrodes prepared by aerosol pyrolysis. Electrochimica Acta 40, 643–652 (1995). [Google Scholar]
- Kruger J., Plass R., Grätzel M., Cameron P. J. & Peter L. M. Charge Transport and Back Reaction in Solid-State Dye-Sensitized Solar Cells: A Study Using Intensity-Modulated Photovoltage and Photocurrent Spectroscopy. J. Phys. Chem. B 107, 7536–7539 (2003). [Google Scholar]
- Grätzel M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 42, 1788−1798 (2009). [DOI] [PubMed] [Google Scholar]
- Selopal G. S. et al. Effect of Blocking Layer to Boost Photoconversion Efficiency in ZnO Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 6, 11236–11244 (2014). [DOI] [PubMed] [Google Scholar]
- Tiwana P., Docampo P., Johnston M. B., Snaith H. J. & Herz L. M. Electron Mobility and Injection Dynamics in Mesoporous ZnO, SnO2, and TiO2 Films Used in Dye-Sensitized Solar Cells. ACS Nano 5, 5158–5166 (2011). [DOI] [PubMed] [Google Scholar]
- Anta J. A., Guillen E. & Tena-Zaera R. ZnO-based dye-sensitized solar cells. J. Phys. Chem. C. 116, 11413–11425 (2012). [Google Scholar]
- Guillén E., Peter L. M. & Anta J. A. Electron Transport and Recombination in ZnO-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 115, 22622–22632 (2001). [Google Scholar]
- Hara K. et al. Highly efficient photon-to-electron conversion with mercurochrome-sensitized nanoporous oxide semiconductor solar cells Sol. Energy Mater. Sol. Cells 70, 151–134 (2001). [Google Scholar]
- Goes M. S. et al. Impedance Spectroscopy Analysis of the Effect of TiO2 Blocking Layers on the Efficiency of Dye Sensitized Solar Cells. J. Phys. Chem. C 116, 12415–12421 (2012). [Google Scholar]
- Schlichthorl G., Huang S. Y., Sprague J. & Frank A. J. Band Edge Movement and Recombination Kinetics in Dye-Sensitized Nanocrystalline TiO2 Solar Cells: A Study by Intensity Modulated Photovoltage Spectroscopy. J. Phys. Chem. B 101, 8141–8155 (1997). [Google Scholar]
- Hagberg D. P. et al. Symmetric and unsymmetric donor functionalization. comparing structural and spectral benefits of chromophores for dye-sensitized solar cells. J. Mater. Chem. 19, 7232–7238 (2009). [Google Scholar]
- Zhou W. et al. Porphyrins modified with a low-band-gap chromophore for dye-sensitized solar cells. Org. Electron. 13, 560–569 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.