Abstract
The purpose of this study was to compare the effects of short-term low-fat (LF) and high-fat (HF) diets on fed-state hepatic triacylglycerol (TAG) secretion, the content of proteins involved in TAG assembly and secretion, fatty acid oxidation (FAO), and the fatty acid profile of stored TAG. Using selectively bred obese-prone Sprague–Dawley rats, we directly measured fed-state hepatic TAG secretion, using Tyloxapol (a lipoprotein lipase inhibitor) and a standardized oral mixed meal (45% carbohydrate, 40% fat, 15% protein) bolus in animals fed a HF or LF diet for 2 weeks, after which the rats were maintained on their respective diet for 1 week (washout) prior to the liver being excised to measure protein content, FAO, and TAG fatty acid profiles. Hepatic DGAT-1 protein expression was ~27% lower in HF- than in LF-fed animals (p < 0.05); the protein expression of all other molecules was similar in the 2 diets. The fed-state hepatic TAG secretion rate was ~39% lower (p < 0.05) in HF- (4.62 ± 0.18 mmol·h−1) than in LF- (7.60 ± 0.57 mmol·h−1) fed animals. Hepatic TAG content was ~2-fold higher (p < 0.05) in HF- (1.07 ± 0.15 nmol·g−1 tissue) than in LF- (0.50 ± 0.16 nmol·g−1 tissue) fed animals. In addition, the fatty acid profile of liver TAG in HF-fed animals closely resembled the diet, whereas in LF-fed animals, the fatty acid profile consisted of mostly de novo synthesized fatty acids. FAO was not altered by diet. LF and HF diets differentially alter fed-state hepatic TAG secretion, hepatic fatty acid profiles, and DGAT-1 protein expression.
Keywords: lipid metabolism, liver, diet composition, obese prone, very-low-density lipoprotein, fatty acids
Introduction
Elevated postprandial triacylglycerol (TAG) concentrations are associated with an increased risk of cardiovascular disease (CVD) and death in humans (Nordestgaard et al. 2007). Experimental models suggest that excessive TAG concentrations are proatherogenic and contribute to CVD through direct and indirect mechanisms. Experimental studies have shown that excessive TAG concentrations directly contribute to CVD by interfering with the function of endothelial progenitor cells (Liu et al. 2009), inducing apoptosis in endothelial cells (Shin et al. 2004), increasing inflammation (Norata et al. 2007), and increasing circulating endothelial cell microparticles (increase endothelial cell dysfunction) (Ferreira et al. 2004). In addition, excessive TAG concentrations contribute indirectly to CVD by suppressing the protective effect of high-density-lipoprotein cholesterol (Patel et al. 2009). In the post-prandial period, the increase in TAG concentrations is attributed mostly to an increase in endogenous very-low-density lipoprotein TAG particles (Nakajima et al. 2011). Given that dietary interventions are often the first line of defense against CVD, it is important to examine the mechanisms that control postprandial endogenous TAG concentrations so that effective therapies can be identified.
Previous research in humans (Brons et al. 2009; Gardner et al. 2007; Harber et al. 2005; Mittendorfer and Sidossis 2001; Roberts et al. 2008) and rodents (Abumrad et al. 1978; Boivin and Deshaies 1995; Cahova et al. 2012), comparing the effects of short-term (2 days to 6 weeks) high-fat (HF) diets (40%–60% fat) and low fat (LF) diets (≤35% fat), has demonstrated that HF diets reduce fasting plasma TAG concentrations. These changes occur during energy deficit, energy balance, and energy surplus conditions prior to the development of metabolic disease. The mechanism responsible for these paradoxic effects of diet composition on fasting plasma TAG concentrations is mediated by alterations in fasting hepatic TAG secretion. Short-term HF diets reduce fasting plasma TAG concentrations by reducing the hepatic TAG secretion rate in vivo and ex vivo (Boivin and Deshaies 1995; Cahova et al. 2012; Francone et al. 1992; Kalopissis et al. 1981; Oussadou et al. 1996), whereas LF diets high in simple sugars, particularly fructose, increase fasting plasma TAG by increasing hepatic TAG secretion in vivo (Cahova et al. 2012; Kazumi et al. 1986). Although these studies have established that HF diets, compared with LF diets, reduce fasting-state hepatic TAG secretion in vivo and ex vivo, it is not known if HF diets, compared with LF diets, reduce fed-state hepatic TAG secretion in vivo. Previous research has shown that acute feeding can alter hepatic TAG secretion. For example, in perfused rat livers from LF-fed rats, ex vivo hepatic TAG secretion was greater in fed than in fasted livers (Wilcox and Heimberg 1987). Given that no research has examined how LF and HF diets alter fed-state hepatic TAG secretion in vivo, more research is needed.
Liver TAGs can be synthesized by 1 of the 2 diacylglycerol acyltransferases (DGAT-1 and DGAT-2), which catalyze the esterification of diacylglyceride with long-chain acyl-CoA esters (Wurie et al. 2012). Although both enzymes catalyze the same reaction, they are nonredundant and act in sequence rather than in parallel. The DGAT-1 protein uses exogenous fatty acid in the re-esterification of diacylglycerol and monoacylglycerol generated during intracellular lipolysis (Wurie et al. 2012). In contrast, the DGAT-2 protein acts upstream of DGAT-1, using nascent diacylglyceride and de novo synthesized fatty acids as substrates (Wurie et al. 2012). Both enzymes can modulate hepatic TAG secretion. For instance, hepatic TAG secretion in mice is attenuated when DGAT-2 is knocked down, but not DGAT-1 (Liu et al. 2008), whereas overexpression of DGAT-1 (Liang et al. 2004; Yamazaki et al. 2005), but not DGAT-2 (Yamazaki et al. 2005), increases hepatic TAG secretion. Collectively, DGAT-1 and -2 can modulate hepatic TAG secretion. However, it is not known if LF and HF diets differentially alter the protein expression of hepatic DGAT-1 or -2.
The liver secretes TAGs as part of very-low-density lipoprotein (VLDL)-TAG. It is not completely clear how VLDL-TAGs are assembled, but it has been proposed that the assembly of VLDL-TAGs occurs in 2 distinct steps (Olofsson et al. 2000). In step 1, the microsomal TAG transfer protein (MTP) cotranslationally lipidates apolipoprotein B (apoB) in the endoplasmic reticulum to form a pre-VLDL particle. This step is critical because the knockout of MTP in mice significantly reduces VLDL-TAG secretion (Raabe et al. 1999). In addition to MTP, the sortilin-1 protein is a critical regulator of VLDL-TAG secretion and is involved in trafficking the apoB protein toward the lipidation pathway (Ai et al. 2012). In step 2, TAGs are added to the pre-VLDL particle, ultimately forming mature VLDL-TAG particles for secretion. The effect of a short-term LF or HF diet on hepatic MTP, sortilin-1, and apoB-48 or −100 is not known.
Collectively, short-term HF diets, compared with LF diets, decrease fasting-state hepatic TAG secretion, but it is not clear if this occurs during the fed state in obese-prone Sprague–Dawley rats. In addition, the effect that previous diet (HF vs. LF) has on the protein content of key enzymes involved in TAG assembly and secretion in relation to hepatic TAG content, fatty acid oxidation (FAO), and the fatty acid profile of stored TAG in obese-prone Sprague-Dawley rats is not known. Therefore, the purpose of this study was to examine how LF and HF diets alter fed-state hepatic TAG secretion, the protein expression of key enzymes involved in TAG assembly and secretion, FAO, and the fatty acid profile of stored TAG in obese-prone Sprague–Dawley rats.
Materials and methods
Animals and study design
The Institutional Animal Care and Use Committee at the University of Missouri–Columbia and the Subcommittee for Animal Safety at the Harry S. Truman Memorial VA Hospital approved this animal protocol. Fourteen obese-prone Sprague–Dawley rats from the stock originally created by Dr. Barry Levin (Levin et al. 1997) were purchased from Taconic Farms, Inc. (Hudson, N.Y., USA) when they were 4–5 weeks of age. They were housed in pairs in a temperature- and light-controlled room (12-h light/12-h dark cycle), and had access to standard rodent chow and water ad libitum 24 h a day. At 8–9 weeks of age, the rats were randomized into 1 of 2 diets: a LF diet (energy as 10% fat, 70% carbohydrate (17% fructose), and 20% protein; D12450B, Research Diets, Inc.); or a HF diet (60% fat, 20% carbohydrate (3% fructose), and 20% protein; D12492, Research Diets, Inc.). After 2 weeks on the LF or HF diet, fed-state hepatic TAG secretion was assessed following a mixed meal oral gavage bolus in vivo. After a 1-week washout period, the rats were sacrificed and livers were excised for the measurement of FAO and key proteins involved in TAG assembly and VLDL-TAG secretion. Thus, the animals were on their diet for a total of 3 weeks. Rats were anesthetized (pentobarbital 75 mg·kg−1) and sacrificed by cardiac puncture.
Feeding, food consumption, and body weight
The rats were given ad libitum access to food and water during the acclimation period and during the first week of the experiment. During the second and third weeks of the experiment, the rats were given ad libitum access to food only between 0800 and 2100 h. The food was pulled and the rats were fasted between 2100 and 0800 h to get the rats accustomed to the TAG secretion protocol (described below). During the first 4 days of this feeding regimen, daily food intake decreased, but by the fifth day, the rats became acclimated to the feeding regimen and consumed the same amount of food daily as they did when given ad libitum access to food 24 h per day. Access to water was provided 24 h a day ad libitum throughout the experiment.
Hepatic TAG secretion protocol
After 2 weeks on the HF or LF diet and a 10 h overnight fast, the rats were intravenously injected with 500 mg·kg−1 of the nonionic detergent Tyloxapol, over a 1.0–1.5 min period, in a tail vein. Tyloxapol is a known lipoprotein lipase inhibitor and allowed us to directly assess hepatic TAG secretion after each diet. Thirty minutes after the Tyloxapol injection, the rats were orally gavaged with a liquid meal (described below). Tail vein blood samples were taken at baseline prior to Tyloxapol injection and every hour after the oral gavage for 4 h to assess hepatic TAG secretion. The Tyloxapol injection resulted in a linear increase in serum TAG; the TAG secretion rate was calculated as the slope of the linear increase in TAG (mmol·h−1). The volume of blood obtained did not allow for the separation of endogenous and exogenous TAG with density gradient ultracentrifugation. However, it is assumed that exogenous TAG secretion is similar in the 2 diets because all of the rats received the same amount of exogenous fat in the mixed meal bolus (instead of per body mass). Thus, it is assumed that any differences in TAG secretion observed are because of differences in endogenous hepatic TAG secretion.
Liquid mixed meal
The oral gavage mixed meal was 20 calories, made up of 40% fat, 45% carbohydrate (13.75% fructose, 11.25% glucose, 20.00% maltodextrin), and 15% protein, so that it reflected a typical western diet meal. The meal was made by mixing heavy whipping cream (Wal-Mart Stores, Inc., Bentonville, Ark., USA), fructose (NOW Foods, Bloomingdale, Ill., USA), glucose (NOW Foods), maltodextrin (NOW Foods), and unflavored whey protein isolate (The Isopure Company, LLC, Hauppauge, N.Y., USA) into a 8–10 mL water solution. This was put into a 10 mL syringe for the oral gavage meal. We gave all rats the same standardized mixed meal, instead of adjusting the meal size to body size, for the reasons described above.
Blood and tissue collection, homogenization, and FAO
After anesthetizing the rats, ~6–8 mL of blood was taken from the left ventricle of the heart, added to serum or EDTA tubes, separated by centrifugation (6 min, 8000g, 4 °C), and stored at −80 °C until analysis. The liver homogenization and FAO techniques were performed as described elsewhere (Rector et al. 2008). Liver tissue for FAO (~50–100 mg) was homogenized and kept on ice until oxidation experiments, which were conducted later the same day. Radiolabeled [1-14C] palmitate (American Radiochemicals) was used to measure FAO in fresh liver homogenate. Liver tissue for Western blots and liver TAG were quickly removed from anesthetized rats, flash frozen in liquid nitrogen, and then stored at −80 °C until analysis. Retroperitoneal, mesenteric, and epididymal fat pads were carefully removed, weighed, and flash frozen in liquid nitrogen.
Serum TAG, free fatty acid, and insulin
Serum TAG concentrations were determined using a commercially available colorimetric assay kit (Infinity, Fisher Diagnostics, Middletown, Va., USA). Serum total free fatty acid concentrations were measured with an enzymatic colorimetric assay (Wako Chemicals USA, Inc. Richmond, Va., USA). Serum insulin was determined with an ELISA kit (Comparative Clinical Pathology Services, LLC, Columbia, Mo., USA).
Intrahepatic TAG concentrations and the fatty acid profile of hepatic TAG
Intrahepatic TAG concentrations were determined using a commercially available kit (Sigma, F6428), as described elsewhere (Rector et al. 2008). Hepatic fatty acid profiles were determined using a modified version of the chloroform–methanol technique described by Folch et al. (1957). Briefly, liver tissue was homogenized in ice-cold Trizma and EDTA buffer (50 mmol·L−1 Tris and 1 mmol·L−1 EDTA; pH 7.4), and lipids were extracted in chloroform, methanol, and acetic acid (2:1:0.015). Extracted lipids were then run on a thin-layer chromatography silica plate (Sigma Chemical Co., St. Louis, Mo., USA) in a tank containing hexane, diethyl ether, and acetic acid (70:30:1). The chemical 2,7-dichlorofluorscein was used to visualize the individual lipid species on the thin-layer chromatography plate. Triglyceride fractions were scraped from the thin-layer chromatography plate, methylated by incubation with toluene 0.5 mol·L−1 and sodium methoxide (methanol) (1:2) for 1 min, and then separated in isooctane. Fatty acid methyl esters were analyzed with gas chromatography (Agilent Technologies, Wilmington, Del., USA).
Western blot analysis
Liver samples were homogenized using lysis buffer, and Western blot-ready Laemmli samples were created from liver tissue lysates. Samples were separated with SDS–PAGE, transferred to a polyvinyl difluoride membrane, and probed with primary antibodies. Protein bands were quantified using a densitometer (Bio-Rad Laboratories, Hercules, Calif., USA), and protein loading was corrected with 0.1% amido-black (Sigma) staining to determine total protein, as described elsewhere (Rector et al. 2008). The DGAT-1 antibody was purchased from Sigma–Aldrich Co. LLC (St. Louis, Mo., USA). The DGAT-2, sortilin-1, and apoB antibodies were purchased from Abcam (Cambridge, Mass., USA). The MTP antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif., USA).
Statistics
The SPSS statistical software, version 20 (IBM Corporation, Armonk, N.Y., USA), was used to conduct an independent sample t test for each outcome measure. Values are reported as means ± standard error, and significance was set at p ≤ 0.05. Feeding efficiency was calculated by dividing body weight gain (g) by calories of food consumed. Daily fatty acid intake was calculated by multiplying daily fat consumption by the fatty acid content (%) of the diet.
Results
Animal characteristics and diet
The animal characteristics are listed in Table 1. Body weight at baseline, 2 weeks, and 3 weeks was 17%, 20%, and 12% greater, respectively, in the HF-fed animals than in the LF-fed animals (p < 0.05). Although daily food intake was similar in the 2 groups, the higher energy density of the HF diet resulted in increased daily caloric intake and feeding efficiency (32% and 27%, respectively) in HF-fed animals than in LF-fed animals (p < 0.05) during the first 2 weeks of the diet. Fasting serum glucose, lactate, and total free fatty acid concentrations were not different in the 2 groups (p > 0.05), but there was a trend (p = 0.095) for insulin to be lower in the HF group. Table 2 lists the average fatty acid content of each diet and the average fatty acid intake per day.
Table 1.
Animal characteristics.
| Characteristic | Low-fat diet | High-fat diet | p |
|---|---|---|---|
| Body weight at baseline (g) | 324±10 | 379±9 | 0.001 |
| Body weight at 2 wk (g) | 383±13 | 461±7 | <0.001 |
| Final body weight at 3 wk (g) | 413±13 | 464±10 | 0.010 |
| Daily food intake (g) | 23.9±1.0 | 22.9±0.7 | 0.453 |
| Daily caloric intake (calories) | 92.0±3.9 | 120.2±3.8 | <0.001 |
| Feeding efficiency | 0.050±0.004 | 0.064±0.004 | 0.050 |
| Fasting serum glucose (mmol·L−1) | 9.2±0.3 | 8.4±0.4 | 0.151 |
| Fasting serum insulin (ng·mL−1) | 6.1±0.6 | 4.5±0.6 | 0.095 |
| Fasting serum free fatty acid (mEq·L−1) | 0.25±0.04 | 0.27±0.05 | 0.711 |
| Fasting serum lactate (mmol·L−1) | 3.4±0.7 | 2.3±0.2 | 0.161 |
Note: Values are means ± standard error. Feeding efficiency = body weight gain (g)/calories of food consumed.
Table 2.
Dietary fatty acid composition and fatty acid intake.
| Fatty acid | Fatty acid content (%)
|
Fatty acid intake (g·d−1)
|
||
|---|---|---|---|---|
| Low-fat diet | High-fat diet | Low-fat diet | High-fat diet | |
| C 14:0 | 0.5 | 1.1 | 0.01±0.0 | 0.16±0.01* |
| C 16:0 | 14.9 | 19.8 | 0.34±0.03 | 2.81±0.15* |
| C 16:1 | 0.7 | 1.3 | 0.02±0.0 | 0.19±0.01* |
| C 18:0 | 7.1 | 10.6 | 0.16±0.01 | 1.51±0.08* |
| C 18:1n-9 | 28.9 | 34.3 | 0.67±0.06 | 4.88±0.26* |
| C 18:2n-6 | 42 | 28.9 | 0.97±0.08 | 4.12±0.22* |
| C 18:3n-3 | 5 | 2.1 | 0.12±0.01 | 0.29±0.02* |
| 20:1 | 0.2 | 0.6 | 0.01±0.0 | 0.08±0.0* |
| 20:2n-6 | 0.5 | 0.8 | 0.01±0.0 | 0.11±0.01* |
| 20:3n-6 | 0 | 0.1 | 0±0.0 | 0.02±0.0* |
| 20:4n-6 | 0.2 | 0.3 | 0.01±0.0 | 0.04±0.0* |
| 22:5n-3 | 0 | 0.1 | 0±0.0 | 0.01±0.0* |
Note: Values are means ± standard error; n = 4 per group. Fatty acid content in the diet is expressed in % (w/w). Fatty acids not listed were not detected in the diet.
p < 0.01 vs. low-fat-diet group.
Adiposity and hepatic TAG content
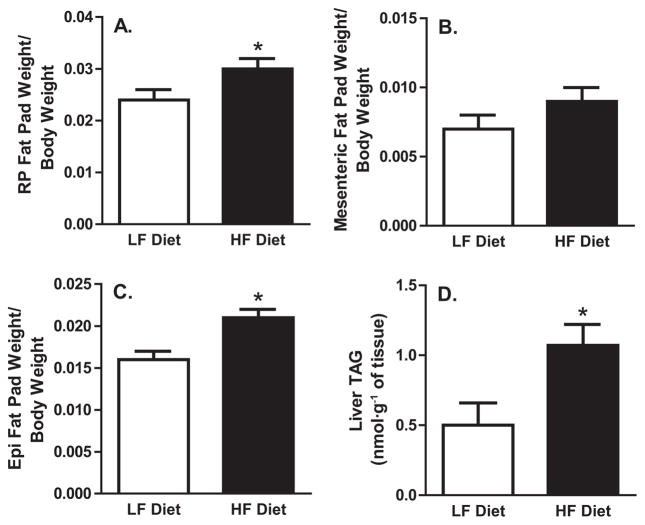
The increased adiposity of obesity is associated with a greater prevalence of liver TAG accumulation, and both pathophysiologies are associated with increased fasting hepatic TAG secretion in humans and rodents. To assess changes in adiposity and liver TAG accumulation, we measured fat pad weight/body weight ratios and intrahepatic TAG content (Fig. 1). The short-term HF diet increased adiposity; the retroperitoneal and epididymal fat pad weight/body weight ratios were 25% and 31% greater, respectively, in the HF group than in the LF group (p < 0.05). Also, the mesenteric fat pad/body weight ratio tended to be higher in HF-fed animals (p = 0.15). Further, the short-term HF diet resulted in a greater than 2-fold increase in intrahepatic TAG accumulation, compared with the LF diet. As in previous research, these data demonstrate that short-term HF feeding is sufficient to produce increased adiposity and steatosis in obese-prone male Sprague–Dawley rats (Jackman et al. 2010; Levin et al. 1997).
Fig. 1.
Adiposity and hepatic triacylglycerol (TAG) content.
(A) Retroperitoneal (RP) fat pad weight/body weight ratio. (B) Mesenteric fat pad weight/body weight ratio. (C) Epididymal (Epi) fat pad weight/body weight ratio. (D) Liver TAG. HF, high fat; LF, low fat. *, p < 0.05 vs. LF-diet group.
Fatty acid profile of hepatic TAG
The fatty acid profile of liver TAG is listed in Table 3. The concentration of every fatty acid in liver TAG was significantly different in the LF- and HF-diet groups (p < 0.05). In particular, the specific fatty acid that was most affected by HF feeding was linoleic acid (18:2n-6), which was ~3.5-fold greater in the HF- than in the LF-diet group. Also largely affected by HF feeding were palmitoleic acid (16:1) and oleic acid (18:1n9), which were ~94% and 31% lower, respectively, in the HF group.
Table 3.
Fatty acid profile in liver triacylglycerol (%).
| Fatty acid | Low-fat diet | High-fat diet | p |
|---|---|---|---|
| 14:0 | 0.7±0.1 | 0.2±0.0 | 0.001 |
| 14:1 | 0.1±0.0 | 0.0±0.0 | 0.005 |
| 16:0 | 29.3±1.2 | 25.0±0.3 | 0.013 |
| 16:1 | 10.7±1.0 | 0.6±0.1 | <0.001 |
| 18:0 | 1.6±0.1 | 3.7±0.1 | <0.001 |
| 18:1n-9 | 39.7±0.9 | 27.4±0.7 | <0.001 |
| 18:1n-7 | 7.4±0.3 | 1.8±0.0 | <0.001 |
| 18:2n-6 | 9.4±1.4 | 33.7±0.2 | <0.001 |
| 20:4n-6 | 0.5±0.1 | 3.9±0.7 | 0.021 |
| 22:4n-6 | 0.2±0.0 | 1.3±0.2 | 0.003 |
| 22:5n-3 | 0.4±0.1 | 2.4±0.3 | 0.002 |
| Total | 100.0 | 100.0 |
Note: Values are means ± standard error; n = 4 per group. Fatty acids not listed were not detected in the liver.
Fasting TAG and the fed-state hepatic TAG secretion rate
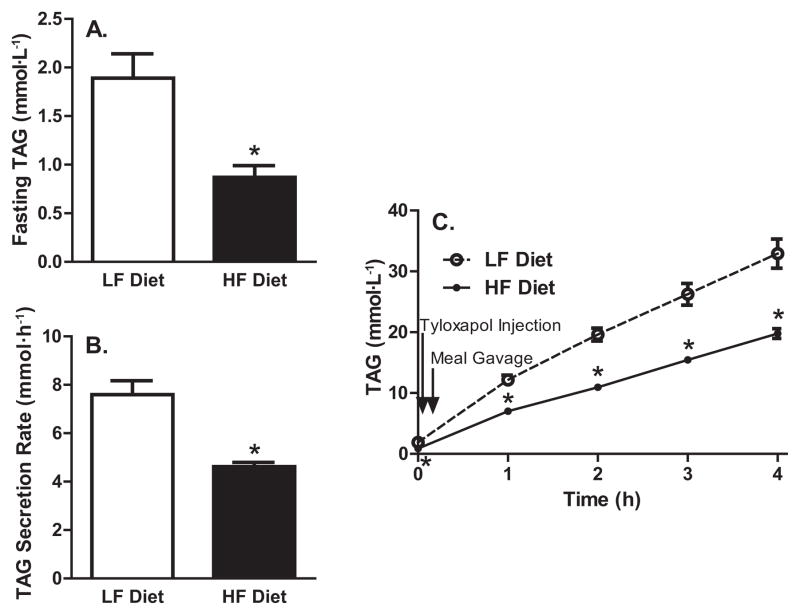
Fasting TAG concentrations and the fed-state hepatic TAG secretion rate are presented in Fig. 2. To assess the effect of a short-term HF diet on steady-state serum TAG concentrations, we assessed serum TAG concentrations after an 11 h fast. The HF-diet group had 54% lower fasting serum TAG concentrations than the LF-diet group (p < 0.05). Increased plasma insulin and reduced lipolysis reduces free fatty acid availability to the liver, thus lowering hepatic TAG secretion in insulin-sensitive animals (Rennie et al. 2000; Zammit et al. 1999). This proposed effect of insulin is complicated by the observed increase in TAG secretion after acute fructose exposure to the liver (Mayes 1993). For the first time, we directly assessed fed-state hepatic TAG secretion (mmol·h−1) in rats orally gavaged with a standardized liquid mixed meal after a Tyloxapol injection. The short-term HF diet resulted in a 39% decrease in the fed-state hepatic TAG secretion rate over the 4 h test, compared with the LF diet. Serum TAG measured 1, 2, 3, and 4 h after Tyloxapol injection were 42%, 44%, 41%, and 40%, respectively, lower in the HF- than in the LF-diet group (p < 0.05). Thus, both fasted and fed-state TAG secretion is lowered by a short-term HF diet.
Fig. 2.
Fasting triacylglycerol (TAG) and the fed-state hepatic TAG secretion rate. (A) Fasting serum TAG concentrations. (B) TAG secretion rate. (C) Time course of TAG during Tyloxapol experiment. HF, high fat; LF, low fat. *, p < 0.05 vs. LF-diet group.
Hepatic fatty acid oxidation
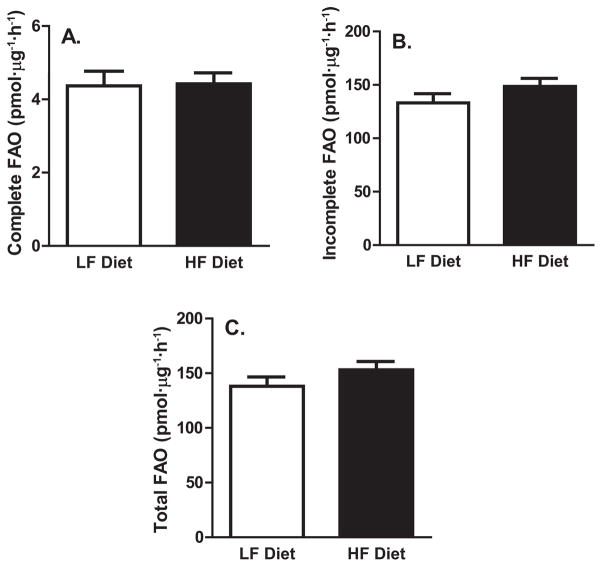
It is possible that alterations in hepatic TAG secretion are mediated by alterations in hepatic FAO through changes in the liver lipid available for packaging as VLDL-TAG. Measures of hepatic complete FAO to CO2, incomplete FAO (acid soluble metabolites), and total FAO (complete + incomplete FAO) are presented in Fig. 3. The short-term HF diet did not result in differences in any liver FAO measures, compared with the LF diet.
Fig. 3.
Complete (A), incomplete (B), and total (C) hepatic fatty acid oxidation (FAO). HF, high fat; LF, low fat.
Protein expression
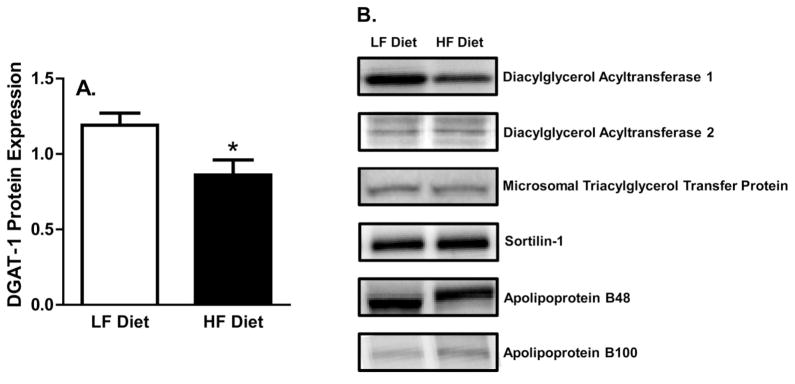
Liver TAGs are synthesized by 1 of the 2 DGAT proteins, and can then either be stored as liver TAG or packaged into a VLDL-TAG particle for secretion; thus, the DGAT proteins play an important role in hepatic TAG secretion. Average DGAT-1 protein expression was ~27% lower in HF- than in LF-fed animals (p < 0.05) (Fig. 4), whereas DGAT-2 protein expression was not altered by diet composition. In addition to the DGAT proteins, hepatic MTP, sortilin-1, and apoB proteins play important roles in the packaging and secretion of hepatic TAG. However, the protein expression of apoB-100, apoB-48, MTP, and sortilin-1 were not altered by diet composition; thus, only representative blots are shown (p > 0.05) (Fig. 4).
Fig. 4.
Western blot protein expression. (A) Diacylglycerol acyltransferase-1 (DGAT-1) protein expression. (B) Protein expression of key proteins involved in hepatic triacylglycerol synthesis and secretion. HF, high fat; LF, low fat. *, p < 0.05 vs. LF-diet group.
Discussion
Abnormally elevated postprandial TAG concentrations, particularly those of endogenous origin, are a major risk factor for the development of CVD (Adiels et al. 2008; Nordestgaard et al. 2007). Dietary intervention is often the first line of defense against CVD, so it is important to understand how diet can affect postprandial endogenous TAG concentrations. It has been established that the composition and duration of dietary interventions can have a profound impact on the rate of hepatic TAG secretion during the fasted state (Abumrad et al. 1978; Boivin and Deshaies 1995; Brons et al. 2009; Cahova et al. 2012; Gardner et al. 2007; Harber et al. 2005; Mittendorfer and Sidossis 2001; Roberts et al. 2008), but the effects of diet composition on fed-state hepatic TAG secretion is less clear. Thus, the aim of this study was to examine how LF and HF diets alter fed-state hepatic TAG secretion, the protein expression of key enzymes involved in TAG assembly and secretion, FAO, and the fatty acid profile of stored TAG in an animal model of obesity (Levin et al. 1997). The main finding of this study is that a short-term HF diet, compared with a LF diet, increases adiposity and hepatic TAG accumulation while simultaneously reducing in vivo hepatic TAG secretion, both during fasting and after a meal delivered by oral gavage. These changes were associated with reduced DGAT-1 protein expression, a critical enzyme in TAG formation. Neither the short-term LF diet nor the HF diet had an impact on hepatic MTP, sortilin-1, or apoB-48 or −100 protein content. In addition, the short-term LF and HF diets did not alter hepatic FAO, but resulted in a differential fatty acid profile of liver TAG.
The liver is the only organ that synthesizes and secretes TAG of endogenous origin and, thus, is the primary regulator of blood TAG concentrations. The liver has a remarkable ability to adapt its rate of TAG secretion to diet composition. This is the first study to assess how diet composition alters in vivo fed-state hepatic TAG secretion. Our data show that a HF diet, compared with a LF diet, reduces the fed-state TAG secretion rate by 39%, even when the same mixed meal is ingested. This finding expands on previous research that has shown that HF diets reduce the hepatic TAG secretion rate in fasted animals in vivo and ex vivo (Boivin and Deshaies 1995; Cahova et al. 2012; Francone et al. 1992; Kalopissis et al. 1981; Oussadou et al. 1996). The effect of HF feeding on hepatic TAG secretion might be mediated by altered partitioning of hepatic fatty acid away from VLDL-TAG synthesis and secretion, toward catabolic disposal through FAO. Early work in this area supports this hypothesis. In primary hepatocytes (from rats fed a 60% HF diet), HF feeding inhibited VLDL-TAG secretion by partitioning fatty acid away from lipogenesis and TAG synthesis and secretion, and toward FAO (Francone et al. 1992; Oussadou et al. 1996). However, we did not find any significant differences in FAO between diets. In agreement with our findings, Cahova et al. (2012) demonstrated that HF feeding, compared with standard rodent chow (low in fat), did not significantly alter FAO in the fasted or fed state, although a high-sucrose diet (70% sucrose), compared with standard rodent chow, significantly reduced FAO in both the fasted and fed state. The discrepancy in the effect of HF feeding on hepatic FAO between studies could be due to differences in animal models, diets, and methodologies. For instance, we used obese-prone Sprague–Dawley rats and Cahova et al. (2012) used male hereditary hypertriglyceridemic rats, and both studies found no difference in FAO between HF and LF diets or standard chow diets. However, other investigators used Zucker or Wistar rats and reported that a HF diet increased FAO, compared with a LF diet (Francone et al. 1992; Oussadou et al. 1996). Further, we and others (Cahova et al. 2012) utilized radiolabeled [1-14C] palmitate to assess FAO, whereas previous work utilized radiolabeled [1-14C] oleate (Francone et al. 1992; Oussadou et al. 1996) and different procedures to assess FAO. It is possible that HF feeding increases oleate oxidation without altering palmitate oxidation. This observation warrants further investigation.
For the first time, our data demonstrate that short-term LF and HF diets differentially alter DGAT-1 protein expression without altering DGAT-2, MTP, sortilin-1, or apoB-48 or -100 protein expression, and this was associated with reduced fed-state hepatic TAG secretion. Previous research has shown that complete knockout of the DGAT-1 protein in the liver of mice has no effect on hepatic TAG secretion (Liu et al. 2008), suggesting that lower hepatic DGAT-1 protein expression in HF-fed animals does not contribute to the decrease in hepatic TAG secretion. Instead, we hypothesize that reduced DGAT-1 protein expression might be a response to, rather than a cause of, lower hepatic TAG secretion. Given that the hepatic TAG secretion rate was lower and hepatic TAGs were ~2-fold higher, the need for the machinery (DGAT-1) to maintain the hepatic TAG pool was reduced, which could be fed back and result in a decrease in DGAT-1 protein expression to prevent excessive buildup of hepatic TAG. This hypothesis is corroborated by previous research in mice that demonstrated that hepatic DGAT-1 deficiency resulted in the protection of HF-diet-induced hepatic steatosis (Villanueva et al. 2009). There is some evidence that over-expression of DGAT-1 increases VLDL secretion (Liang et al. 2004; Yamazaki et al. 2005). Thus, in our study, it is possible that the greater DGAT-1 protein expression in LF-fed animals, compared with HF-fed animals, helped contribute to the greater hepatic TAG secretion rate. We hypothesize that the biologic reason for this phenomenon can be explained by the fact that the main component of newly secreted VLDL is TAG, and given that TAG secretion was higher in LF-fed animals, increased DGAT-1 protein expression might be necessary to maintain the intra-luminal endoplasmic reticular TAG pool that is used for the lipidation of nascent VLDL, which would allow the TAG secretion rate to remain high.
It has been hypothesized that the reduction in hepatic TAG secretion in HF-fed animals is not a result of the increase in exogenous fat intake, but rather a result of reduced exogenous carbohydrate intake (Oussadou et al. 1996). Numerous studies have shown that high-carbohydrate diets that contain the simple sugars glucose and fructose can markedly increase hepatic TAG secretion (Boivin and Deshaies 1995; Cahova et al. 2012; Kazumi et al. 1986; Mayes 1993; Mittendorfer and Sidossis 2001; Parks et al. 2008; Roberts et al. 2008), and that this is associated with increased de novo lipogenesis (Faeh et al. 2005; Mayes 1993). These data suggest that hepatic TAG secretion is dependent on an increase in de novo lipogenesis. In further support of this hypothesis, knocking down enzymes involved in hepatic de novo lipogenesis, including DGAT-2 (Liu et al. 2008) and stearoyl-CoA desaturase-1 (SCD-1) (Miyazaki et al. 2007), significantly reduces hepatic TAG secretion. Thus, it is probable that it was really the lowering of carbohydrate intake during the HF diet, rather than the increase in exogenous fatty acids entering the system, that had the most direct impact on modulating fed-state hepatic TAG secretion in our study.
The dietary fatty acid composition of the HF diet (oleic acid > linoleic acid > palmitic acid) closely matched the fatty acid profile of liver TAG (linoleic acid > oleic acid > palmitic acid); this has been documented before (Ciapaite et al. 2011). This close coupling of dietary fatty acid intake with fatty acids stored in liver TAG possibly occurred because dietary fatty acid delivery to the liver exceeded exogenous fatty acid oxidative and secretory capacity, resulting in accumulation of dietary fatty acid in liver TAG. In particular, daily linoleic acid (18:2n-6) ingestion was ~4-fold higher in HF-fed animals than in LF-fed animals, and this was accompanied by a ~3.5-fold higher accumulation of linoleic acid in the hepatic TAG of these animals. In contrast, in the LF diet, dietary fatty acid composition (linoleic acid > oleic acid > palmitic acid) did not match the fatty acid profile of liver TAG (oleic acid > palmitic acid > palmitoleic acid); this has also been documented before (Ciapaite et al. 2011). This difference likely occurred because the fat in the diet is low; thus, most liver TAG was probably derived from de novo synthesis. In our study, oleic acid (18:1n-9) constituted the greatest percentage of fatty acids in the liver TAG of LF-fed animals, because this fatty acid, along with palmitoleic acid (16:1), is the major end product of SCD-1-catalyzed hepatic de novo lipogenesis (Miyazaki et al. 2001). It is possible that the greater incorporation of de novo synthesized oleic or palmitoleic acid in the liver TAG of LF fed animals in our study allowed the greater hepatic TAG secretion induced by LF feeding. This hypothesis is supported by previous research that demonstrated that the knockout of SCD-1 in mouse liver results in lower oleic and palmitoleic acid in the liver TAG of LF-fed animals (Miyazaki et al. 2001). This was accompanied by a reduction in plasma TAG levels, presumably because of reduced hepatic TAG secretion (Miyazaki et al. 2001). In addition, when cultured with exogenous oleate, McA-RH7777 cells (Dolinsky et al. 2004; Sundaram et al. 2010; Tran et al. 2002, 2006; Wang et al. 1997) and Hep G2 cells (Arrol et al. 2000) secrete many more TAG-rich VLDL particles than cultures with no oleate or other fatty acids.
Dietary recommendations for adults often suggest diets lower in fat and higher in grains, vegetables, and fruits (carbohydrates) to optimize health. However, our data show that LF and HF diets have pros and cons. For example, although the LF diet did not promote as much weight gain, it resulted in hypertriglyceridemia, whereas the HF diet dramatically reduced TAG concentrations, but at the cost of hepatic steatosis. Given that both of these diets were at the extremes, perhaps a more balanced diet, with the fat and carbohydrate content somewhere in between the 2 diets we studied, would be more suitable for reducing metabolic disease risk.
In conclusion, our data show for the first time that a short-term HF diet, compared with a short-term LF diet, reduces fed-state hepatic TAG secretion and DGAT-1 protein expression in obese-prone Sprague–Dawley rats.
Acknowledgments
This work was supported with resources and the use of facilities at the Harry S Truman Memorial VA Hospital in Columbia, Mo. We would like to thank Grace M. Meers for her technical assistance in the lab. This study was funded by a MU Institute for Clinical and Translational Science Pilot Grant (TDH) and by NIH R01DK088940 (JPT).
Footnotes
Disclosure statement
The authors report no conflict of interest.
Author contributions: Timothy D. Heden: Study conception, study design, data collection, data analysis, interpretation of data, and manuscript preparation. E. Matthew Morris: Study conception, study design, data collection, data analysis, interpretation of data, and manuscript preparation. Monica L. Kearney: Data collection, data analysis, and manuscript preparation. Tzu-Wen Liu: Data collection, data analysis, and manuscript preparation. Young-min Park: Data collection. Jill A. Kanaley: Study conception and study design. John P. Thyfault: Study conception, study design, data collection, data analysis, interpretation of data, and manuscript preparation.
Contributor Information
Timothy D. Heden, Department of Nutrition and Exercise Physiology, University of Missouri, NW502 Medical Science Building, Columbia, MO 65211, USA
E. Matthew Morris, Department of Internal Medicine, Division of Gastroenterology and Hepatology, Columbia, MO 65211, USA; Harry S. Truman Memorial Veterans Medical Center, Columbia, MO 65211, USA.
Monica L. Kearney, Department of Nutrition and Exercise Physiology, University of Missouri, NW502 Medical Science Building, Columbia, MO 65211, USA
Tzu-Wen Liu, Department of Nutrition and Exercise Physiology, University of Missouri, NW502 Medical Science Building, Columbia, MO 65211, USA.
Young-min Park, Department of Nutrition and Exercise Physiology, University of Missouri, NW502 Medical Science Building, Columbia, MO 65211, USA.
Jill A. Kanaley, Department of Nutrition and Exercise Physiology, University of Missouri, NW502 Medical Science Building, Columbia, MO 65211, USA
John P. Thyfault, Department of Nutrition and Exercise Physiology, University of Missouri, NW502 Medical Science Building, Columbia, MO 65211, USA; Department of Internal Medicine, Division of Gastroenterology and Hepatology, Columbia, MO 65211, USA; Harry S. Truman Memorial Veterans Medical Center, Columbia, MO 65211, USA
References
- Abumrad NA, Stearns SB, Tepperman HM, Tepperman J. Studies on serum lipids, insulin, and glucagon and on muscle triglyceride in rats adapted to high-fat and high-carbohydrate diets. J Lipid Res. 1978;19(4):423–432. [PubMed] [Google Scholar]
- Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- Ai D, Baez JM, Jiang H, Conlon DM, Hernandez-Ono A, Frank-Kamenetsky M, et al. Activation of ER stress and mTORC1 suppresses hepatic sortilin-1 levels in obese mice. J Clin Invest. 2012;122(5):1677–1687. doi: 10.1172/JCI61248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrol S, Mackness MI, Durrington PN. The effects of fatty acids on apolipoprotein B secretion by human hepatoma cells (HEP G2) Atherosclerosis. 2000;150(2):255–264. doi: 10.1016/S0021-9150(99)00374-3. [DOI] [PubMed] [Google Scholar]
- Boivin A, Deshaies Y. Dietary rat models in which the development of hypertriglyceridemia and that of insulin resistance are dissociated. Metabolism. 1995;44(12):1540–1547. doi: 10.1016/0026-0495(95)90072-1. [DOI] [PubMed] [Google Scholar]
- Brons C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol. 2009;587(10):2387–2397. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahova M, Dankova H, Palenickova E, Papackova Z, Kazdova L. The opposite effects of high-sucrose and high-fat diet on Fatty Acid oxidation and very low density lipoprotein secretion in rat model of metabolic syndrome. J Nutr Metab. 2012;2012 doi: 10.1155/2012/757205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapaite J, van den Broek NM, Te Brinke H, Nicolay K, Jeneson JA, Houten SM, et al. Differential effects of short- and long-term high-fat diet feeding on hepatic fatty acid metabolism in rats. Biochim Biophys Acta. 2011;1811(7–8):441–451. doi: 10.1016/j.bbalip.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Douglas DN, Lehner R, Vance DE. Regulation of the enzymes of hepatic microsomal triacylglycerol lipolysis and re-esterification by the glucocorticoid dexamethasone. Biochem J. 2004;378(3):967–974. doi: 10.1042/BJ20031320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54(7):1907–1913. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- Ferreira AC, Peter AA, Mendez AJ, Jimenez JJ, Mauro LM, Chirinos JA, et al. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation. 2004;110(23):3599–3603. doi: 10.1161/01.CIR.0000148820.55611.6B. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Francone OL, Griffaton G, Kalopissis AD. Effect of a high-fat diet on the incorporation of stored triacylglycerol into hepatic VLDL. Am J Physiol. 1992;263(4):E615–E623. doi: 10.1152/ajpendo.1992.263.4.E615. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- Harber MP, Schenk S, Barkan AL, Horowitz JF. Alterations in carbohydrate metabolism in response to short-term dietary carbohydrate restriction. Am J Physiol Endocrinol Metab. 2005;289(2):E306–E312. doi: 10.1152/ajpendo.00069.2005. [DOI] [PubMed] [Google Scholar]
- Jackman MR, MacLean PS, Bessesen DH. Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1097–R1105. doi: 10.1152/ajpregu.00549.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalopissis AD, Griglio S, Malewiak MI, Rozen R, Liepvre XL. Very-low-density-lipoprotein secretion by isolated hepatocytes of fat-fed rats. Biochem J. 1981;198(2):373–377. doi: 10.1042/bj1980373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazumi T, Vranic M, Steiner G. Triglyceride kinetics: effects of dietary glucose, sucrose, or fructose alone or with hyperinsulinemia. Am J Physiol. 1986;250(3):E325–E330. doi: 10.1152/ajpendo.1986.250.3.E325. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273(2):R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Liang JJ, Oelkers P, Guo C, Chu PC, Dixon JL, Ginsberg HN, et al. Overexpression of human diacylglycerol acyltransferase 1, acyl-coa:cholesterol acyltransferase 1, or acyl-CoA:cholesterol acyltransferase 2 stimulates secretion of apolipoprotein B-containing lipoproteins in McA-RH7777 cells. J Biol Chem. 2004;279(43):44938–44944. doi: 10.1074/jbc.M408507200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Millar JS, Cromley DA, Graham M, Crooke R, Billheimer JT, et al. Knockdown of acyl-CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim Biophys Acta. 2008;1781(3):97–104. doi: 10.1016/j.bbalip.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Liu L, Wen T, Zheng XY, Yang DG, Zhao SP, Xu DY, et al. Remnant-like particles accelerate endothelial progenitor cells senescence and induce cellular dysfunction via an oxidative mechanism. Atherosclerosis. 2009;202(2):405–414. doi: 10.1016/j.atherosclerosis.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58(5 Suppl):754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Sidossis LS. Mechanism for the increase in plasma triacylglycerol concentrations after consumption of short-term, high-carbohydrate diets. Am J Clin Nutr. 2001;73(5):892–899. doi: 10.1093/ajcn/73.5.892. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res. 2001;42(7):1018–1024. [PubMed] [Google Scholar]
- Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6(6):484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Nakano T, Tokita Y, Nagamine T, Inazu A, Kobayashi J, et al. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin Chim Acta. 2011;412(15–16):1306–1318. doi: 10.1016/j.cca.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norata GD, Grigore L, Raselli S, Redaelli L, Hamsten A, Maggi F, et al. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studies. Atherosclerosis. 2007;193(2):321–327. doi: 10.1016/j.atherosclerosis.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Non-fasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- Olofsson SO, Stillemark-Billton P, Asp L. Intracellular assembly of VLDL: two major steps in separate cell compartments. Trends Cardiovasc Med. 2000;10(8):338–345. doi: 10.1016/S1050-1738(01)00071-8. [DOI] [PubMed] [Google Scholar]
- Oussadou L, Griffaton G, Kalopissis AD. Hepatic VLDL secretion of genetically obese Zucker rats is inhibited by a high-fat diet. Am J Physiol. 1996;271(6):E952–E964. doi: 10.1152/ajpendo.1996.271.6.E952. [DOI] [PubMed] [Google Scholar]
- Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138(6):1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Puranik R, Nakhla S, Lundman P, Stocker R, Wang XS, et al. Acute hypertriglyceridaemia in humans increases the triglyceride content and decreases the anti-inflammatory capacity of high density lipoproteins. Atherosclerosis. 2009;204(2):424–428. doi: 10.1016/j.atherosclerosis.2008.07.047. [DOI] [PubMed] [Google Scholar]
- Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, Nielsen LB, et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103(9):1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastroin-test Liver Physiol. 2008;294(3):G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- Rennie SM, Park BS, Zammit VA. A switch in the direction of the effect of insulin on the partitioning of hepatic fatty acids for the formation of secreted triacylglycerol occurs in vivo, as predicted from studies with perfused livers. Eur J Biochem. 2000;267(4):935–941. doi: 10.1046/j.1432-1327.2000.01126.x. [DOI] [PubMed] [Google Scholar]
- Roberts R, Bickerton AS, Fielding BA, Blaak EE, Wagenmakers AJ, Chong MF, et al. Reduced oxidation of dietary fat after a short term high-carbohydrate diet. Am J Clin Nutr. 2008;87(4):824–831. doi: 10.1093/ajcn/87.4.824. [DOI] [PubMed] [Google Scholar]
- Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004;109(8):1022–1028. doi: 10.1161/01.CIR.0000117403.64398.53. [DOI] [PubMed] [Google Scholar]
- Sundaram M, Zhong S, Bou Khalil M, Links PH, Zhao Y, Iqbal J, et al. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 2010;51(1):150–161. doi: 10.1194/M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K, Thorne-Tjomsland G, DeLong CJ, Cui Z, Shan J, Burton L, et al. Intracellular assembly of very low density lipoproteins containing apo-lipoprotein B100 in rat hepatoma McA-RH7777 cells. J Biol Chem. 2002;277(34):31187–31200. doi: 10.1074/jbc.M200249200. [DOI] [PubMed] [Google Scholar]
- Tran K, Sun F, Cui Z, Thorne-Tjomsland G, St Germain C, Lapierre LR, et al. Attenuated secretion of very low density lipoproteins from McA-RH7777 cells treated with eicosapentaenoic acid is associated with impaired utilization of triacylglycerol synthesized via phospholipid remodeling. Biochim Biophys Acta. 2006;1761(4):463–473. doi: 10.1016/j.bbalip.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Villanueva CJ, Monetti M, Shih M, Zhou P, Watkins SM, Bhanot S, et al. Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009;50(2):434–442. doi: 10.1002/hep.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, McLeod RS, Yao Z. Normal activity of microsomal triglyceride transfer protein is required for the oleate-induced secretion of very low density lipoproteins containing apolipoprotein B from McA-RH7777 cells. J Biol Chem. 1997;272(19):12272–12278. doi: 10.1074/jbc.272.19.12272. [DOI] [PubMed] [Google Scholar]
- Wilcox HG, Heimberg M. Secretion and uptake of nascent hepatic very low density lipoprotein by perfused livers from fed and fasted rats. J Lipid Res. 1987;28(4):351–360. [PubMed] [Google Scholar]
- Wurie HR, Buckett L, Zammit VA. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS J. 2012;279(17):3033–3047. doi: 10.1111/j.1742-4658.2012.08684.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Sasaki E, Kakinuma C, Yano T, Miura S, Ezaki O. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J Biol Chem. 2005;280(22):21506–21514. doi: 10.1074/jbc.M412989200. [DOI] [PubMed] [Google Scholar]
- Zammit VA, Lankester DJ, Brown AM, Park BS. Insulin stimulates triacylglycerol secretion by perfused livers from fed rats but inhibits it in livers from fasted or insulin-deficient rats implications for the relationship between hyperinsulinaemia and hypertriglyceridaemia. Eur J Biochem. 1999;263(3):859–864. doi: 10.1046/j.1432-1327.1999.00568.x. [DOI] [PubMed] [Google Scholar]