Abstract
Objective
Resistance to emtricitabine plus tenofovir disoproxil fumarate (FTC/TDF) or TDF alone used as pre-exposure prophylaxis (PrEP) has been detected in individuals who initiated PrEP during unrecognized acute HIV infection and, rarely, in PrEP breakthrough infections. PrEP-selected resistance could alter future treatment options, and therefore we sought to determine how long resistance persisted after PrEP cessation.
Methods
The Partners PrEP Study was a randomized placebo-controlled trial of FTC/TDF or TDF as PrEP for HIV prevention. We previously reported that PrEP-related mutations (K65R, K70E or M184IV) were detected by 454 sequencing following seroconversion in 9 individuals who acquired HIV during the Partners PrEP Study. In the current study, we used 454 sequencing to detect and quantify PrEP-related mutations in HIV RNA-positive plasma samples prior to seroconversion, as well as in plasma from 6, 12, and 24 months after PrEP cessation from these 9 individuals.
Results
HIV RNA-positive, antibody-negative samples were available prior to seroconversion for 4 of 9 individuals with resistance detected at seroconversion. In all 4 cases, K65R, K70E and M184IV were not detected prior to seroconversion, suggesting PrEP-related resistance was selected and not transmitted. All PrEP-selected mutations were no longer detectable by 6 months after PrEP cessation and remained undetectable at 12 and 24 months in the absence of antiretroviral therapy.
Conclusion
Using highly sensitive assays, PrEP-selected resistance in plasma decays below detection by six months following drug cessation and remains undetectable for ≥24 months. Even high levels of resistance mutations during acute infection decay rapidly in the absence of ongoing PrEP exposure.
Keywords: HIV, pre-exposure prophylaxis, HIV drug resistance, antiretroviral therapy, heterosexual transmission, HIV prevention
Introduction
Resistance to emtricitabine (FTC) or tenofovir (TDF) used as pre-exposure prophylaxis (PrEP) has been detected in individuals who initiated PrEP during unrecognized acute HIV infection and, very rarely, in individuals that acquired HIV while taking PrEP [1-10]. When PrEP-related resistance does occur, it is important to understand whether it persists after PrEP cessation, which could impact subsequent antiretroviral treatment in these individuals and/or result in the transmission of drug resistance to their subsequent partners.
Standard clinical laboratory testing for HIV drug resistance relies on consensus sequencing that can only detect resistance mutations present at high frequencies (>20%) within the viral population. The use of highly sensitive assays, such as 454 ultra-deep sequencing, can detect resistance mutations present at very low frequencies (<1%). Previous studies used highly-sensitive assays and revealed that resistance detected at frequencies as low as 1% following the use of antiretrovirals to prevent mother-to-child transmission can be associated with subsequent treatment failure [11-13] and that resistance may be transmitted even if only present at low frequencies of the viral population [14-18].
Among 121 seroconverters from the Partners PrEP Study, a randomized, placebo-controlled trial of FTC/TDF and TDF as PrEP, we previously reported detection of PrEP-related mutations (K65R, K70E and/or M184IV) by ultra-deep sequencing in 9 individuals at the time HIV seroconversion was first detected [9]. Here we performed highly sensitive resistance testing in longitudinal samples from these 9 seroconverters with PrEP-related resistance. HIV RNA-positive plasma samples available prior to HIV seroconversion, and thus as close as possible to the time of HIV acquisition, were tested to determine whether resistance was selected or transmitted. In addition, plasma samples from 6, 12 and 24 months following detection of seroconversion (when PrEP was withdrawn) were assessed for persistence of PrEP-related resistance mutations.
Methods
Study Population
The Partners PrEP Study was a phase III randomized, double-blind, placebo-controlled trial of oral FTC/TDF and TDF-alone as PrEP for HIV prevention among HIV uninfected persons in HIV serodiscordant partnerships. Details and results from this trial have previously been reported [2,19]. Briefly, 4747 HIV-serodiscordant couples from Kenya and Uganda were enrolled and the HIV-uninfected partner was randomized to FTC/TDF, TDF or placebo. Clinic visits for safety assessments and HIV testing were monthly for up to 36 months. Plasma samples were stored quarterly throughout follow-up. In addition, for those who seroconverted, plasma samples from the visit seroconversion was first detected as well as within 1 month after seroconversion were stored.
HIV Testing
HIV seroconversions were detected by rapid HIV tests conducted at monthly clinic visits and confirmed by Western blot [2]. RT-PCR was used to detect HIV RNA in quarterly archived pre-seroconversion plasma samples. Dates of infection were estimated as 17 days prior to the first HIV RNA-positive antibody-negative visit, or the midpoint between the date seroconversion was first detected and the prior HIV RNA-negative antibody-negative visit.
Antiretroviral Resistance Testing
454 sequencing was performed as described previously [9,13,20], with modifications as follows. Total RNA was extracted from 100-500μl plasma, as in Palmer 2005 [21]. For each sample, 4 replicate high-fidelity nested RT-PCR reactions were performed as previously described [9,13,20], with a unique 8bp barcode for each. Input of 400 HIV copies into each replicate (1600 copies per sample) was based on Roche quantitative HIV RNA results. For samples with virus low virus levels (n=5), the maximum possible number of copies was added (range 17-1256 HIV RNA copies). Barcoded PCR products were purified with AMPure XP beads (Agencourt), quantified by Qubit dsDNA HS (Invitrogen), mixed in equimolar concentrations and pyrosequenced in both directions on the 454 GS-Junior platform. 454 data were processed and analyzed using methods as described previously [9].
Results
No evidence of low-frequency PrEP-related resistance prior to seroconversion
Of the 9 individuals in the Partners PrEP Study that had PrEP-related resistance at or within 1 month after detection of seroconversion [9], retrospective testing revealed that 5 individuals did not have HIV RNA detected in a prior archived sample. In 4 individuals, a plasma sample from a clinic visit prior to seroconversion was determined to be HIV RNA-positive. In 3 cases, these samples were from the time of enrollment (prior to randomization) in individuals assigned to active PrEP during unrecognized acute HIV infection (Figure 1). In the fourth case, 2-299, the individual had been assigned to active PrEP and subsequently seroconverted at month 4 after enrollment. Retrospective testing of archived samples from case 2-299 revealed that HIV RNA was first detected at 3 months after enrollment, and that plasma tenofovir levels were undetectable at enrollment and month 3, but were 74ng/ml at month 4.
Figure 1. Pre-seroconversion samples confirm resistance selected by PrEP.
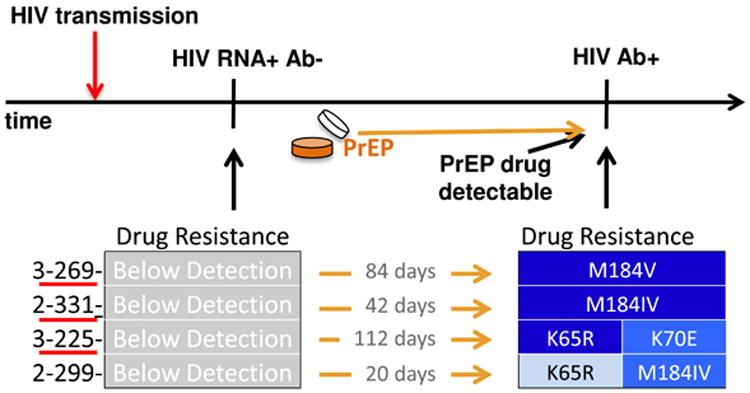
Timeline highlights 4 cases with HIV RNA-positive and HIV antibody-negative samples collected and archived prior to HIV seroconversion. Participants retrospectively found to be HIV infected at enrollment have ID numbers underlined. Three of 4 cases were unrecognized acute infection at study enrollment prior to PrEP drug administration, and the 4th had no evidence of PrEP drug prior to seroconversion. Resistance mutations were not detected in all 4 pre-seroconversion samples, shown on the left in grey. All 4 cases had resistance mutations at seroconversion detection, shown with darker blue denoting higher proportions of HIV variants harboring the noted resistance mutations. Time between the HIV RNA-positive antibody-negative sample and the HIV antibody-positive sample is noted between resistance results.
To determine whether the PrEP-related mutations detected in these individuals at seroconversion were present earlier in infection, prior to detection of PrEP drug, we performed 454 sequencing on the pre-seroconversion HIV RNA-positive plasma samples from these 4 individuals. In all 4 cases, resistance was below detection in HIV RNA from this pre-seroconversion visit, which ranged from 20-112 days prior to seroconversion when PrEP drug was detected (Figure 1).
PrEP-related resistance decays rapidly after PrEP cessation
In individuals in the Partners PrEP Study, PrEP was discontinued immediately upon detection of HIV seroconversion. In the 9 individuals that had PrEP-related mutations at frequencies >1% when seroconversion was detected, we were interested to determine how long that resistance persisted in the absence of antiretroviral therapy. Thus, we performed 454 sequencing on plasma samples from months 6, 12, and 24 following seroconversion, when PrEP or placebo was withdrawn.
As reported previously, only 5 of the 9 cases of resistance had both evidence of PrEP use (detectable levels of tenofovir) and resistance to the PrEP drugs they were taking (FTC selects for K65R and M184IV; TDF selects for K65R and K70E), suggesting that the resistance mutations detected in these 5 seroconverters were likely due to PrEP selection [9]. This included 1 individual (3-225) randomized to TDF during unrecognized acute infection who had K65R and K70E detected in 56% and 10% of virus variants, respectively, when seroconversion was first detected. The other 4 cases of PrEP-selected resistance were individuals randomized to FTC/TDF (2 individuals with unrecognized acute infection at randomization: 3-269 and 2-331; and 2 individuals who acquired HIV post-randomization: 2-299 and 4-321) who had M184V or M184IV at levels ranging from 1% to 99% at detection of seroconversion. Case 2-299, who also had 1.2% K65R, was lost to follow-up after seroconversion. In follow-up samples from these cases, resistance mutations decayed below detection by 6 months, and remained undetectable at 12 and 24 months after seroconversion (Figure 2).
Figure 2. Levels of K65R, K70E and M184IV after seroconversion and PrEP drug cessation.
Subjects are ordered by levels of resistance from highest to lowest within each treatment arm. An asterisk prior to the identification number indicates subjects with tenofovir detected in plasma during HIV infection. Red underline denotes subjects with unrecognized acute HIV infection at treatment assignment. Samples tested include the time seroconversion was first detected (SC), and months 1, 6, 12 and 24 after seroconversion. Higher levels of resistance denoted by darker blue color. Grey cells with “bd” indicate below detection. Grey cells with “-” indicate sample not available or did not amplify. Abbreviations: FTC, emtricitabine; TDF, tenofovir disoproxil fumarate; bd, below detection.
The remaining 4 cases of resistance were individuals who did not have detectable levels of PrEP drug in any of their HIV RNA or antibody-positive plasma samples (2-283, 6-283, 2-316, the latter two were assigned to the placebo arm), or whose resistance mutation was not associated with their assigned PrEP regimen (2-326 had M184I, which causes resistance to FTC, but this individual was assigned to TDF-alone as PrEP). Case 2-283 had M184V in 16% of virus variants when seroconversion was detected and 1.7% M184V 1 month later, which faded below 1% by 6 months and remained undetectable through 24 months. This individual had no evidence of PrEP drug at clinic visits either before or after seroconversion, and the M184V mutation may have been a result of transmitted resistance [9]. The 3 other cases of resistance (1 TDF: 2-326; 2 placebo: 6-283 and 2-316) had only low levels of M184I at seroconversion ranging from 1-2.5%. M184I, which is naturally polymorphic and known to occur without drug exposure, fluctuated at low levels between below detection and 6% throughout follow-up (Figure 2).
Follow-up after initiation of combination antiretroviral therapy
Follow-up in these 9 cases continued for a median of 40 months (range: 0-51 months) post seroconversion. Only one individual, case 2-331, initiated combination antiretroviral therapy (cART) during follow-up. In this case, cART was initiated at 33 months post-seroconversion with a regimen of FTC/TDF/nevirapine. Viral load from the clinic visit prior to initiation of cART (month 30 post-seroconversion) was 19170 copies per mL, which dropped below detection (<40 copies/mL) at the clinic visit 3 months following initiation of cART (33 months post-seroconversion).
Discussion
In this study we showed that PrEP-selected resistance decays rapidly after PrEP cessation. By 6 months after seroconversion (after PrEP was discontinued), resistance mutations K65R, K70E, and/or M184IV that were present at seroconversion were no longer detected, even with highly sensitive resistance testing. Although fitness assays were not performed on viral isolates from our study, previous studies that have shown that these mutations reduce viral replication fitness compared to wild-type HIV variants [22-25]. Evidence from other studies using highly sensitive resistance testing have suggested that in women and infants exposed to antiretrovirals as prophylaxis to prevent HIV mother-to-child transmission, resistance variants present at >1% of the virus population are associated with an increased risk of subsequent treatment failure [11-13]. Thus, our data suggest that in the rare cases in which resistance is selected by PrEP, resistance rapidly decays to levels that may no longer be clinically relevant.
Resistance mutations were selected (and not transmitted) in 4 individuals who initiated PrEP during unrecognized acute HIV infection. In these 4 cases, PrEP resistance mutations were not detected with highly sensitive resistance testing prior to evidence of PrEP drug exposure, but resistance was detected at the subsequent visit when both HIV seroconversion and PrEP drug in plasma were first detected (Figure 1). Three of these cases were individuals who tested HIV negative by rapid tests at study enrollment and randomization to FTC/TDF or TDF PrEP, but were retrospectively determined to be HIV RNA positive at enrollment, indicating very early acute HIV infection. The fourth case was an individual who was both HIV antibody and HIV RNA negative at enrollment but had undetectable tenofovir levels indicating no PrEP use until 4 months later, after HIV infection appears to have been acquired; thus, this case is analogous to the 3 individuals who had acute seronegative HIV infection at initial randomization to PrEP. These data help confirm that resistance in these cases was likely not transmitted, but was selected by PrEP drug exposure during acute infection, when HIV replication is high and errors in reverse transcription increase the probability of resistance.
Multiple studies have now shown that the risk of developing resistance from PrEP is very low, but is an important concern for those who initiate PrEP during unrecognized acute infection [1-10]. Our data show that resistance selected in these cases decays rapidly to levels below detection of even highly sensitive assays. However, whether resistance decays to levels that are no longer clinically relevant during cART remains to be determined. Case 2-331 supports this hypothesis, as this individual had M184IV in 48% of viral variants at one month following seroconversion and PrEP cessation, which decayed to below detection and remained below detection through 24 months of follow-up. Initiation of cART after month 30 with FTC/TDF/nevirapine resulted in complete viral suppression within 3 months. Future studies that include follow-up of seroconverters who initiate cART after acquiring HIV when on PrEP will allow us to determine whether the development of PrEP resistance early in infection followed by decay impacts later treatment options.
Acknowledgments
This study would not have been possible with the dedication of the study participants as well as the Partners PrEP Study Team. We also thank Julie Overbaugh for helpful discussions and review of the manuscript.
DAL, JMB and JFW designed and led the study. JFW and DAL designed and conducted the experiments. COM, CW and FAM designed and conducted the bioinformatic analysis of the 454 sequencing data. DAL, JMB, COM, DD, KKT and FAM developed and conducted the statistical analysis of the 454 data. CWH and MAM designed and conducted the pharmacological work. JMB, DD, NM and CC designed and conducted the Partners PrEP Study. DAL wrote the first draft of the manuscript. All authors assisted with the writing of the manuscript.
Funding: This work was supported by grants to DAL: AI104449 from the NIH/National Institute of Allergy and Infectious Disease, www.niaid.nih.gov, and a grant from the University of Washington Center for AIDS Research (CFAR), an NIH funded program under award number P30AI027757. The Partners PrEP Study was supported by grant OOP47674 from the Bill and Melinda Gates Foundation, www.gatesfoundation.org.
Footnotes
Potential conflicts of interest: All authors report no conflict of interest
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin DM, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 5.Chirwa LI, Johnson JA, Niska RW, Segolodi TM, Henderson FL, Rose CE, et al. CD4+ cell count, viral load, and drug resistance patterns among heterosexual breakthrough HIV infections in a study of oral preexposure prophylaxis. AIDS. 2014;28:223–226. doi: 10.1097/QAD.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh UM, Eskay KA, Hardesty R, Kelly C, Margaret C, Molitor C, et al. HIV-1 Resistance Outcomes in Seroconverters from the MTN 003 (VOICE) Study. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. http://croiconference.org/sites/all/abstracts/594.pdf. [Google Scholar]
- 8.Liegler T, Abdel-Mohsen M, Atchison R, Mehotra M, Schmidt T, Eden C, et al. Drug resistance and minor drug resistant variants in iPrEx. J Infect Dis. 2014;210(8):1217–27. doi: 10.1093/infdis/jiu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehman DA, Baeten JM, McCoy CO, Weis JF, Peterson D, Mbara G, et al. Risk of Drug Resistance Among Persons Acquiring HIV Within a Randomized Clinical Trial of Single- or Dual-Agent Preexposure Prophylaxis. J Infect Dis. 2015;211(8):1211–8. doi: 10.1093/infdis/jiu677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant RM, Liegler T, Defechereux P, Kashuba ADM, Taylor D, Abdel-Mohsen M, et al. Drug resistance and plasma viral RNA level after ineffective use of oral pre-exposure prophylaxis in women. AIDS. 2015;29:331–337. doi: 10.1097/QAD.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 11.Boltz VF, Zheng Y, Lockman S, Hong F, Halvas EK, McIntyre J, et al. Role of low-frequency HIV-1 variants in failure of nevirapine-containing antiviral therapy in women previously exposed to single-dose nevirapine. Proc Natl Acad Sci USA. 2011;108:9202–9207. doi: 10.1073/pnas.1105688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halvas EK, Wiegand A, Boltz VF, Kearney M, Nissley D, Wantman M, et al. Low Frequency Nonnucleoside Reverse-Transcriptase Inhibitor-Resistant Variants Contribute to Failure of Efavirenz-Containing Regimens in Treatment-Experienced Patients. J Infect Dis. 2010;201(5):672–80. doi: 10.1086/650542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehman DA, Wamalwa DC, Mccoy CO, Matsen FA, Langat A, Chohan BH, et al. Low-frequency nevirapine resistance at multiple sites may predict treatment failure in infants on nevirapine-based treatment. J Acquir Immune Defic Syndr. 2012;60:225–233. doi: 10.1097/QAI.0b013e3182515730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peuchant O, Thiébaut R, Capdepont S, Lavignolle-Aurillac V, Neau D, Morlat P, et al. Transmission of HIV-1 minority-resistant variants and response to first-line antiretroviral therapy. AIDS. 2008;22:1417–1423. doi: 10.1097/QAD.0b013e3283034953. [DOI] [PubMed] [Google Scholar]
- 15.Metzner KJ, Scherrer AU, Preiswerk B, Joos B, Wyl von V, Leemann C, et al. Origin of Minority Drug-Resistant HIV-1 Variants in Primary HIV-1 Infection. J Infect Dis. 2013;208(7):1102–12. doi: 10.1093/infdis/jit310. [DOI] [PubMed] [Google Scholar]
- 16.Lipscomb JT, Switzer WM, Li JF, Masciotra S, Owen SM, Johnson JA. HIV Reverse-Transcriptase Drug Resistance Mutations During Early Infection Reveal Greater Transmission Diversity Than in Envelope Sequences. J Infect Dis. 2014;210(11):1827–37. doi: 10.1093/infdis/jiu333. [DOI] [PubMed] [Google Scholar]
- 17.Truong HHM, Kellogg TA, McFarland W, Louie B, Klausner JD, Philip SS, et al. Sentinel Surveillance of HIV-1 Transmitted Drug Resistance, Acute Infection and Recent Infection. PLoS ONE. 2011;6:e25281. doi: 10.1371/journal.pone.0025281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yerly S, Junier T, Gayet-Ageron A, Amari EBE, Wyl von V, Günthard HF, et al. The impact of transmission clusters on primary drug resistance in newly diagnosed HIV-1 infection. AIDS. 2009;23:1415–1423. doi: 10.1097/QAD.0b013e32832d40ad. [DOI] [PubMed] [Google Scholar]
- 19.Baeten JM, Donnell D, Mugo NR, Ndase P, Thomas KK, Campbell JK, et al. ArticlesSingle-agent tenofovir versus combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2014;14(11):1055–64. doi: 10.1016/S1473-3099(14)70937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronen K, Mccoy CO, Matsen FA, Boyd DF, Emery S, Odem-Davis K, et al. HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women. PLoS Pathog. 2013;9:e1003593. doi: 10.1371/journal.ppat.1003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer S, Kearney M, Maldarelli F, Halvas EK, Bixby CJ, Bazmi H, et al. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J Clin Microbiol. 2005;43:406–413. doi: 10.1128/JCM.43.1.406-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber J, Henry KR, Arts EJ, Quiñones-Mateu ME. Viral fitness: relation to drug resistance mutations and mechanisms involved: nucleoside reverse transcriptase inhibitor mutations. Current Opinion in HIV and AIDS. 2007;2:81–87. doi: 10.1097/COH.0b013e328051b4e8. [DOI] [PubMed] [Google Scholar]
- 23.Paredes R, Sagar M, Marconi VC, Hoh R, Martin JN, Parkin NT, et al. In Vivo Fitness Cost of the M184V Mutation in Multidrug-Resistant Human Immunodeficiency Virus Type 1 in the Absence of Lamivudine. Journal of Virology. 2009;83:2038–2043. doi: 10.1128/JVI.02154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel FA, Invernizzi CF, Oliveira M, Wainberg MA. Diminished efficiency of HIV-1 reverse transcriptase containing the K65R and M184V drug resistance mutations. AIDS. 2007;21:665–675. doi: 10.1097/QAD.0b013e3280187505. [DOI] [PubMed] [Google Scholar]
- 25.White KL, Margot NA, Wrin T, Petropoulos CJ, Miller MD, Naeger LK. Molecular Mechanisms of Resistance to Human Immunodeficiency Virus Type 1 with Reverse Transcriptase Mutations K65R and K65R+M184V and Their Effects on Enzyme Function and Viral Replication Capacity. Antimicrobial Agents and Chemotherapy. 2002;46:3437–3446. doi: 10.1128/AAC.46.11.3437-3446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]



