Abstract
Objective
The Johns Hopkins Hospital Emergency Department (JHH-ED) has served as a window on the HIV epidemic for 25 years, and as a pioneer in ED-based screening/linkage-to-care (LTC) programs. We document changes in the burden of HIV and HIV care metrics to the evolving HIV epidemic in inner-city Baltimore.
Design/Methods
We analyzed seven serosurveys conducted on 18,144 adult JHH-ED patients between 1987–2013 as well as our HIV screening/LTC program (2007, 2013) for trends in HIV prevalence, cross-sectional annual incidence estimates, undiagnosed HIV, LTC, antiretrovirals (ARVs) treatment, and viral suppression.
Results
HIV prevalence in 1987 was 5.2%, peaked at >11% from 1992–2003 and declined to 5.6% in 2013. Seroprevalence was highest for black males (initial 8.0%, peak 20.0%, last 9.9%) and lowest for white females. Among HIV+ individuals, proportion of undiagnosed infection was 77% in 1987, 28% in 1992, and 12% by 2013 (p<0.001). Cross-sectional annual HIV incidence estimates declined from 2.28% in 2001 to 0.16% in 2013. Thirty-day LTC improved from 32% (2007) to 72% (2013). In 2013, 80% of HIV+ individuals had ARVs detected in sera, markedly increased from 2007 (27%) (p<0.001). Proportion of HIV+ individuals with viral suppression (<400 copies/ml) increased from 23% (2001) to 59% (2013) (p<0.001).
Conclusions
ED-based HIV testing has evolved from describing the local epidemic to a strategic interventional role, serving as a model for early HIV detection and LTC. Our contribution to community-based HIV-screening and LTC program parallels declines in undiagnosed HIV infection and incidence, and increases in ARV use with associated viral suppression in the community.
Keywords: HIV, emergency department, seroprevalence studies, cascade of care, HIV testing program
Introduction
The clinical presentation of the acquired immunodeficiency syndrome (AIDS) was first recognized in the US in 1981 (1). Two years later, the virus that caused AIDS, the human immunodeficiency virus (HIV) was isolated (2) and commercial antibody tests became available in 1985 allowing for routine laboratory diagnosis. With the advent of these diagnostic tools came the ability to define and track the epidemic that soon evolved into a global pandemic. Thirty-years later, there have been 39 million HIV related deaths, and 35 million people are infected with HIV worldwide (3). The Centers for Disease Control and Prevention (CDC) estimate that more than 1.2 million individuals (age 13 and older) in the US are currently infected with HIV, of which approximately 14% remain unaware of their infection (4).
Emergency Departments (EDs) serve as windows for their communities often revealing the state of public health, as a large portion of the populace visits them. In 2011 there were 136 million visits in the U.S. representing approximately 45 per 100 person-years (5). The uninsured and publically insured are disproportionately represented (6). The Johns Hopkins Hospital Emergency Department (JHH-ED) has served as both an observational window on the HIV-epidemic for over 25 years, and as a pioneer in ED-based testing and linkage to care (LTC) programs (7–14).
Our first probative investigation occurred in 1986 where we studied critically ill and injured patients presenting to our ED with HIV infection (7). The purpose was to gauge the extent of potential health care worker exposure to patients with unknown HIV infection in a high-risk practice setting. This and subsequent studies revealed the relative high rates of undocumented or unknown HIV infection (8–10), as well as other blood-borne infections such as hepatitis B virus, hepatitis C virus (15), and HTLV-I/II infections (16). These seroepidemiological studies yielded critical information on the changing epidemiology of HIV within an inner-city population, and provided opportunity to assess the effectiveness of programmatic interventions critical to HIV diagnosis, LTC, and prevention activities. In this report, we document the changing nature of the local epidemic in Baltimore since 1987 seen through the lens of the JHH-ED, and assess the potential impact of a sustained ED-based testing and LTC program in our ongoing efforts to address the HIV epidemic in Baltimore.
Methods
The Johns Hopkins Hospital is a tertiary care academic hospital located in inner city Baltimore, Maryland. The JHH-ED continues to serve a predominantly indigent population. Annual ED visits have ranged from 60,000 to 70,000 visits during the study periods. The data derived were from two types of data sources. All data reported were either exclusively identity-unlinked, or were concurrent with ED-based HIV screening and LTC programs as described below.
Identity Unlinked Seroprevalence Data
To assess general trends, data were abstracted from seven discrete identity-unlinked serosurveys, between 1987–2013, which included a total 18,144 adult JHH-ED patients in whom excess blood was available for testing. Data included trends in HIV, HIV viral load, HIV cross-sectional annual incidence estimates, and antiretroviral treatment (8–11, 13). Data were abstracted from earlier studies, while some the 2001–2013 data are presented here for the first time. The Johns Hopkins University School of Medicine Institutional Review Board approved all studies.
Methods for the identity-unlinked studies are detailed in previous reports (8–10). Briefly, the identity-unlinked seroprevalence methodology involved, collection of excess sera from all individuals who had blood drawn for any purpose, and assigning of a unique study code, devoid of any patient identifiers from the sera specimen. Patient related data were collected in real time. Chart reviews were undertaken in a specific, structured manner (17). All patient identifiers and other protected health information were irretrievably stripped after relevant patient related data were collected from the chart review and prior to serologic analysis. Results of serologic analysis were merged to identifier-stripped dataset by the unique study code. Thus, no laboratory result could be traced back to any specific patient and was not available in the study database.
HIV Screening Programmatic Data
A rapid ED-based voluntary HIV-screening and LTC program, restricted only by CDC aged-based recommendations, has been in continuous existence since 2005 (13, 14, 18). Laboratory testing protocols including methods for confirmation of HIV results are detailed in these reports (13, 14, 18). Patients confirmed to be HIV positive were reported to the health department. Longitudinal follow-up regarding long-term retention to care, ARV treatment, and viral suppression on individual HIV-positive patients identified from the screening program were not part of the screening/LTC program.
The LTC program evolved over time and is described in detail in the appropriate respective studies (19, 20) or programs (14, 18, 21, 22). Briefly, prior to 1992 all of our studies were identity unlinked and thus no LTC. Since 1992, all confirmed HIV positive patients were counseled as to options for medical care but a pre-arranged appointment (usually within 1–3 weeks) was arranged by co-operative agreement in the hospitals’ HIV (Moore) clinic. All protocols required trained program staff to carry out testing, counseling, and arrange for follow-up from the ED. ED staff providing care did not participate in LTC arrangements at any time for screened patients. Prior to availability of point of care rapid testing (1992–1995), patients were given a $10 voucher to assist with transportation to return for initial results (19, 20). Beginning in 2005, LTC referred patients received two phone calls reminders from program staff (14, 18, 21). Beginning 2013 patients confirmed positive patients met with a clinical nurse from the HIV clinic while still in the ED, and guaranteed an appointment within one business day (22).
Serologic Analyses
Sera were tested by HIV enzyme immunoassays (ELISA) which differed by survey year as the technology evolved. Testing methodologies used in early surveys are described in previous publications (8–11). For the 2007 and 2013 surveys, the Genetic Systems 3rd generation ELISA (BioRad, Redmond, WA) was used with Western Blot confirmation (BioRad, Redmond WA). For the 2001 to 2013 surveys, samples with indeterminate and positive serologic results were then analyzed for HIV viral load using the Roche Amplicor v1.5 (limit of detection of 400 copies/ml, Roche, Indianapolis, IN).
For both the 2007 and 2013 surveys, antiretroviral (ARVs) testing of serum was performed on all HIV-positive specimens with sufficient volume using HPLC-tandem mass spectrometry (2007) (23) and HPLC-High Resolution Accurate Mass (HRAM) mass spectrometry (2013) (24) by the Clinical Pharmacology Laboratory and Pathology Reference Laboratory, respectively, at our institution. ARVs that were detected included Lamivudine, Emtricitabine, Nevirapine, Abacavir, Tenofovir, Zidovudine, Efavirenz, Raltegravir, Amprenavir, Maraviroc, Darunavir, Nelfinavir, Tipranavir, Indinavir, Lopinavir, Saquinavir, Atazanavir, Ritonavir, and Rilpivirine.
HIV incidence testing was performed on samples from ED surveys between 2001–2013. Testing was performed using an algorithm that included the BED Capture Enzyme Immunoassay Assay (Calypte Biomedical, Portland OR) employing a cut off of <1.5 OD-n), BioRad avidity assay (employing the Genetic Systems 1/2 + O ELISA, BioRad, Redmond WA) (using an avidity index cut off of 40%) and viral load testing >400 copies/ml (25). This testing algorithm has a window period of 101 days and a false recent rate of 0% (25).
Data Analysis
HIV-infected patients were categorized as known HIV-infected if they self-reported infection status or had documented HIV infection in the medical chart at their ED visit. While the presence of ARVs in blood specimens is only available from the 2007 and 2013 serosurveys, it is indicative of HIV diagnosis. However, for consistency across all survey years, these patients were not included in aggregate data, but are noted separately. Patients considered to have undiagnosed HIV infection were those identified HIV positive by the laboratory testing but not known to be HIV-infected.
Seroprevalence of HIV and corresponding 95% confidence intervals for each serosurvey were calculated. Cross-sectional annual HIV incident point estimates and confidence intervals were calculated using the methods previously published (26). Demographic and other characteristic comparisons of seroprevalence, proportion of undiagnosed HIV infection, proportion of HIV viral suppression, and proportion with presence of ARVs was performed across serosurveys by chi-square test or Fisher’s exact test for proportions using SAS 9.4 (SAS Institute Inc., Cary, North Carolina). Mean population HIV viral load for each serosurvey in which it was assayed was calculated as anti-logarithm of the mean of logarithm to the base 10 of each viral load. The comparison of HIV viral load by serosurvey was performed using non-parametric Wilcoxon Rank Sums test. Viral suppression was defined as a viral load < 400 copies/ml, the limit of detection for the assay used. The proportion of infected patients with viral suppression was compared using Cochran Armitage Trend test. LTC was calculated as presence at a follow-up appointment within 30 and 90 days of testing. LTC rates were calculated as a simple proportion.
Results
A total of 18,144 patients were included in the seven identity-unlinked seroprevalence studies between 1987 and 2013. Table 1 shows the demographic characteristics of the population by the study year. The study population was slightly older, had a slightly higher proportion of women, and decreased proportion of black patients in recent years as compared to earlier years (p<0.05, respectively).
Table 1. Demographic Characteristics of the Study Population (1987–2013).
Shown are the demographic characteristics of 18,144 adult emergency department (ED) patients in seven identity-unlinked seroprevalence studies in an academic inner-city ED, Baltimore, Maryland.
| Characteristic | Demographics of Study Population by Study Year (%)
|
||||||
|---|---|---|---|---|---|---|---|
| 1987 | 1988 | 1992 | 2001 | 2003 | 2007 | 2013 | |
|
| |||||||
| N=2302 | N=2544 | N=1606 | N=1418 | N=2144 | N=3417 | N=4713 | |
| Sex | |||||||
| Male | 51.0 | 48.3 | 52.9 | 47.8 | 45.5 | 46.0 | 45.1 |
| Female | 49.0 | 51.7 | 47.1 | 52.2 | 54.5 | 54.0 | 54.9 |
| Race | |||||||
| Black | 74.0 | 77.3 | 77.7 | 68.0 | 69.0 | 67.3 | 63.1 |
| White | 24.9 | 22.1 | 21.5 | 28.3 | 26.4 | 26.9 | 29.4 |
| Other | 1.0 | 0.7 | 0.8 | 3.7 | 4.6 | 5.9 | 7.6 |
| Age (years) | |||||||
| Mean (SD) | 43.4±18.8 | 43.3±18.9 | 42.6±18.0 | 47.1±17.0 | 48.0±17.1 | 46.3±16.8 | 46.5±17.5 |
| 15–24 | 17.6 | 15.4 | 15.0 | 9.4 | 9.8 | 11.3 | 11.3 |
| 25–34 | 24.0 | 27.1 | 27.5 | 16.4 | 14.0 | 16.5 | 19.4 |
| 35–44 | 16.2 | 17.0 | 20.4 | 24.3 | 22.2 | 21.4 | 15.3 |
| 45–54 | 12.3 | 10.9 | 11.8 | 20.5 | 22.3 | 22.5 | 21.6 |
| ≥55 | 29.9 | 29.6 | 25.3 | 29.5 | 31.7 | 28.2 | 32.3 |
Figure 1 shows HIV seroprevalence between 1987 and 2013. HIV prevalence in 1987 was 5.2%, peaked between 1992–2003 (1992: 11.4%, 2001:11.6%, 2003: 11.4%), and then declined to 7.8% in 2007 and 5.6% in 2013. The highest seroprevalence in all the studies occurred among black patients, with black males and females documented at 20.0% and 11.1%, respectively, in early 2000’s (Supplement Figure 1). Seroprevalence among white males peaked sharply (9.8%) in 1992 and decreased for each study period, returning to 1987 levels by 2013 (3.3% and 3.0%, respectively). Seroprevalence among white females slightly increased from 1.7% in 1987 to 2.1% in 2001, remained stable in 2003 (2.1%) and 2007 (2.2%), and returned to 1987 infection levels by 2013 (1.0%); (2007 versus 2013: p=0.08) (Supplement Figure 1). The proportion of undiagnosed HIV infection among all with serologic confirmation of infection declined from 77% in 1987 to 28% in 1992, with a dramatic nadir of 12% observed in 2013 (p<0.001) (Figure 1). After including ARV use data, the proportion of undiagnosed HIV was 28% in 2007 and 7% in 2013.
Figure 1. Trends in HIV Prevalence and Proportion of Undiagnosed Infections (1987–2013).
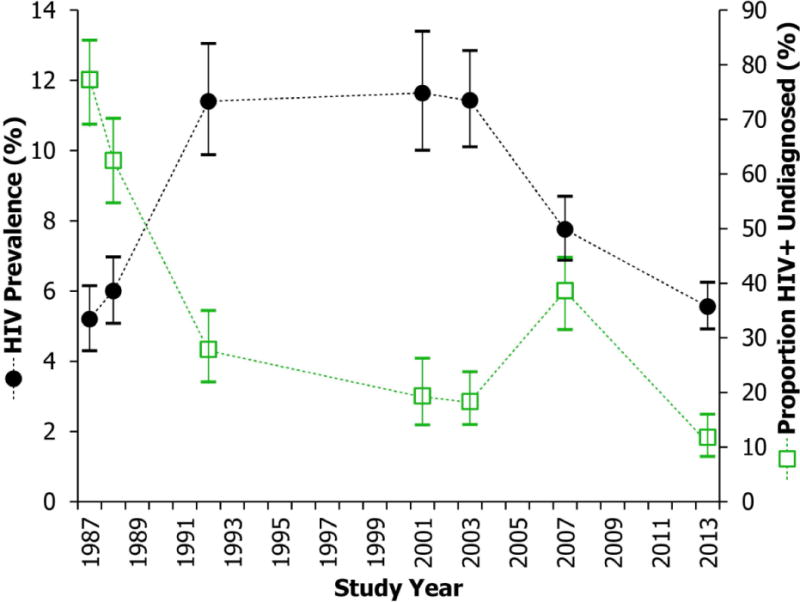
The black circles denote the HIV prevalence during each identity-unlinked serosurvey. The green squares represent the proportion of HIV positive patients in each identity-unlinked serosurvey who were not aware of their HIV positive serostatus. The vertical lines indicate 95% confidence intervals.
Cross-sectional annual HIV incidence estimates in 2001 were 2.28% (95% CI: 0.69%–3.85%) which declined steadily to 0.99% (95% CI: 0.14–1.84%) in 2003, 0.46% (95% CI: 0.01%–0.91%) in 2007, and 0.16% (95% CI: 0%–0.39%) by 2013 (Figure 2).
Figure 2. Proportion of HIV Positive Individuals Virally Suppressed and HIV Incidence Estimates (2001–2013).
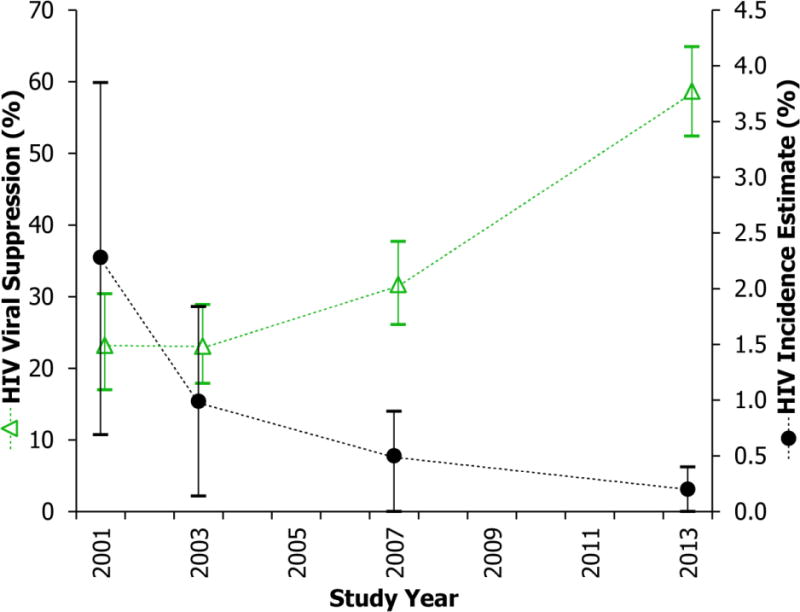
The green triangles denote the proportion of HIV positive patients with an HIV viral load <400 copies/mL in each identity-unlinked serosurvey. The black circles represent cross-sectional HIV incidence estimates determined by a validated multi-assay algorithm with a window period of 101 days and a 0% false-recent misclassification rate. Vertical lines indicate 95% confidence intervals.
Proportion of HIV-infected individuals with viral suppression (<400 copies/ml) increased steadily from 23% in both 2001 and 2003, to 32% in 2007, and 59% by 2013 (p<0.001, Cochran Armitage Trend test) (Figure 2). The mean viral load was 8,128 copies/ml (95% CI: 5,370–12,302) in 2001, increased to 10,964 (95% CI: 7,586–15,849) in 2003, then significantly declined to 4,677 (95% CI: 3,388–6,456) in 2007, and further decreased to 1,318 (95% CI: 933–1,862) in 2013 (p<0.001; Table 2). The temporal trend in decreased viral load was consistent in all demographic subgroups (p<0.05), except those aged 35 and under (p=0.10).
Table 2.
Distribution of HIV Viral Load Levels (2001–2013).
| HIV Viral Loadcopies/mL | 2001 (N=164) |
2003 (N=243) |
2007 (N=265) |
2013a (N=252) |
||||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| ≤ 400b | 23.2 | (17.0, 30.4) | 23.1 | (17.9, 28.9) | 31.7 | (26.1, 37.7) | 58.7 | (52.4, 64.9) |
| 401–1,000 | 7.3 | (3.8, 12.4) | 3.3 | (1.4, 6.4) | 4.9 | (2.6, 8.2) | 4.8 | (2.5, 8.2) |
| 1,001–10,000 | 16.5 | (11.1, 23.0) | 19.8 | (14.9, 25.3) | 19.3 | (14.7, 24.5) | 13.5 | (9.5, 18.3) |
| 10,001–100,000c | 31.7 | (24.7, 39.4) | 26.8 | (21.3, 32.8) | 30.6 | (25.1, 36.5) | 12.3 | (8.5, 17.0) |
| >100,000c | 21.3 | (15.3, 28.4) | 27.2 | (21.7, 33.2) | 13.6 | (9.7, 18.3) | 10.7 | (7.2, 15.2) |
P-value for the distribution of HIV viral load in 2013 compared to previous survey years <0.001.
P-value by the Cochran Armitage Trend test for proportion of HIV viral load ≤ 400 copies/mL (undetectable level) by study year was <0.001
P-value by the Cochran Armitage Trend test for proportion of HIV viral load > 10,000 copies/mL (high viral load level) by study year was <0.001
Consistent with increasing viral suppression, 80% of 214 HIV-infected individuals in 2013 had ARVs detected in their sera, a marked increase from 27% in 2007 (p<0.001). The continuum of care (i.e., diagnosis, to linkage to care, to ARV use and finally, resultant viral suppression) for HIV infected individuals improved significantly from 2007 to 2013 (all p<0.001) (Figure 3).
Figure 3.
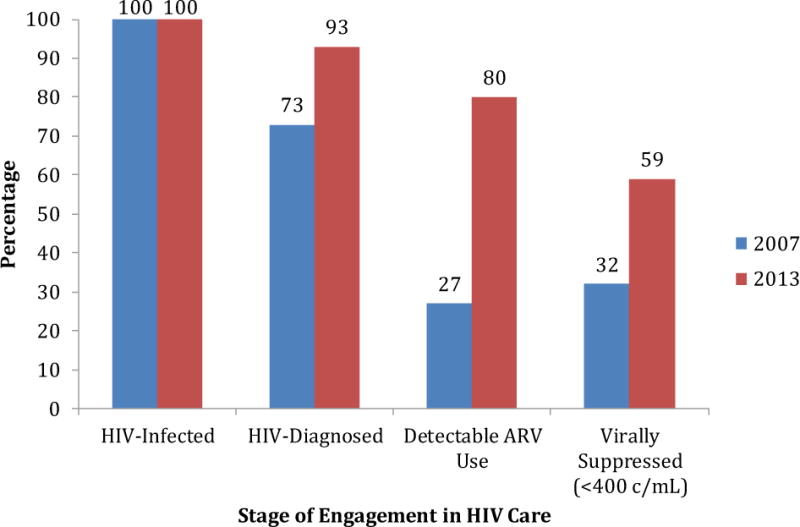
HIV Cascade of Care (2007 and 2013)
Regarding the clinical data from the ED-based HIV testing program from 2005 to 2013, 31,843 ED patients were tested and 179 patients were newly diagnosed with HIV. The rates of HIV testing averaged 80 patients per month in the first year of the program (2005–2006), and increased to an average of 195 per month in 2007 and an average of 566 per month in 2013, with a peak enrolment of over 750 per month. In 2007, 1.1% (25 of 2,342) patient tested were newly diagnosed with HIV and only 7 (32%) of those eligible (3 were not eligible) were linked to care within 30 days, although 77% were linked to care within 90 days. In 2013, 0.4% (25 of 6,797) patients tested were newly diagnosed; 72% and 88% were linked to care within 30 days, and 90 days, respectively.
Discussion
Since the early days of the HIV epidemic, EDs have often been the point of first medical contact for HIV infected persons, and consequently, have played a key role in strategic initiatives to counter the epidemic. Initially, EDs helped characterize the extent of the epidemic at the local population level. As it became apparent that vulnerable and at risk populations utilized EDs (27, 28) as their sole point of entry into the health system (29), EDs started investigating the feasibility of point of care screening in this busy setting. These early programs demonstrated considerable effectiveness for early detection of HIV, and by 2006, the CDC identified EDs as key sites for early detection of HIV (30). This eventually led to the formation of the National Emergency Department HIV-Testing Consortium, formed to promote ED based HIV screening (31). As a result, HIV screening activity in EDs across the US increased. In 2004, less than 2% of EDs offered HIV screening (32), but by 2009, a quarter of EDs offered some type of organized HIV screening program (33).
In analyzing trends over the last 25 years in our inner-city ED, we have been able to document major changes in HIV prevalence, knowledge of HIV status, improved access to ARVs with enhanced viral suppression and a consequential reduction in incidence among patients from the local community of Baltimore. Simultaneously there has been an observed significant downward trend in number of new HIV cases reported in Baltimore City in the last decade (new reported HIV cases: 1052 in 2007 and 356 in 2013) (34). These positive signs in access to care among HIV positive individuals is in direct contrast to the early days of the epidemic when most individuals did not even know their HIV status. From the 1980’s through the early 1990’s, our ED continuously reported the highest rates of HIV infection among the population it served (8–10). Now, it appears that the overall prevalence of HIV infection peaked a decade ago (Figure 1) and is on the decline. Furthermore, our data on HIV prevalence by gender and race, particularly in black males, mirrors the overall HIV burden over time observed by race and gender in Baltimore City (35), in Maryland, and nationally (36). The declining prevalence and cross-sectional annual incidence of HIV in this ED population observed over the last 10 years (Fig 1 and 3), likely reflects the marked improvement in diagnosis and access to care in the community, the marked increase in ARV use as indicated in our survey, decrease in unrecognized HIV, there were fewer symptomatic HIV individuals attending the ED which would result in a lowering in HIV prevalence, as well as the influence of needle exchange programs and other behavior interventions (37–39). We have also seen a similar decline in HCV prevalence during this time period (unpublished data). These findings are supported by the marked decline in undiagnosed HIV from 77% in 1987 to only 7% in 2013, a remarkable achievement in the promotion of routine screening and early diagnosis, which increased nearly 300% by 2013 for an annualized rate of testing nearly 7000 patients (22).
In addition to screening and early detection, EDs have also seized an important role in the early stages of linking HIV infected patients to care (LTC) by partnering with public health and HIV practitioners. Indeed, Gardner and colleagues identified LTC as integral to a 5-step optimal HIV spectrum of care (HIV diagnosis, LTC, retained in care, treatment with ARVs, viral suppression). Hsieh et al (13), also emphasized the important role of the ED by reestablishing “linkage” for those who originally failed to establish LTC and those who had fallen out of care (40). This entire spectrum of care became known as the HIV Care Continuum Initiative (HIV CCI), after an executive order issued by President Obama 2013 intended to accelerate improvements in HIV prevention and care across the United States (41).
Of particular interest in our population is the proportionate increase of ARV use among HIV infected individuals from 2007 to 2013 (from 27% to 80%). Although improved treatment may be due to a variety of influences, our observed improvement in LTC at 30 and 90 days (from 32% to 72% and 77% to 88%, respectively) might have contributed to increased ARV use, which undoubtedly influenced the improvement in overall viral suppression. Another positive influence in increased ARV treatment in our population was the change in the national guidelines to treat all HIV infected individuals regardless of CD4 counts, recommendations followed by our local referral clinics (42).
Achieving LTC and ultimately viral suppression can be particularly challenging in inner city EDs such as ours. However, with active procedures and leveraging critical infrastructure intrinsic to EDs (e.g., close partnering with on-site clinics with use of on-site case management), we, and others, have achieved LTC rates near, and even over 90% (43–45), which is significantly higher than the national norm of 76% (46). Gardner et al. suggested 90% achievement at each stage as necessary for a meaningful level of intervention, but this would still leave over a third of infected individuals viremic (47). Based on data available prior to 2011, Gardner et al. estimated that, nationally, only 19% of HIV infected individuals reached succeeded in achieving viral suppression. Thus, the achievement of 59% of viral suppression noted here in 2013, (significantly increased from 23% and 32% in 2001 and 2007 respectively), is one of the highest rates of viral suppression reported by any ED and near the best scenario (66%) simulated by Gardner (47).
While causation cannot be implied by these data given all the factors that may influence improved HIV incidence, detection and care (e.g. needle exchange programs, primary behavioral intervention programs in the community)(37–39), it is noted that the significantly reduced detection of new infections in this population coincide with the acceptance of ‘opt-in” testing programs, point of care testing in the ED, and ED facilitation of LTC with improved access to ARVs and viral suppression. Of the 93,494 HIV tests from city-funded programs from 2008 to 2013 (H. Ndirangu, MHS, personal communication, June 30, 2014, Baltimore City Health Department, Baltimore, MD), 30.5% were conducted by our program. The JHH-ED program identified more than 200 new diagnoses since 2005. Of these, 139 were from 2008 to 2013, representing a substantial proportion (39%) of the 355 newly diagnosed cases from Baltimore city funded ED-based HIV testing programs during that time frame (H. Ndirangu, MHS, personal communication, June 30, 2014, Baltimore City Health Department, Baltimore, MD). However, to the best of our knowledge the JHH program is the largest and the only sustained program over this time period. Data on ARV treatment is not based on patient reporting, which is subject to bias, but rather is based on a direct measurement of ARVs in sera in all HIV positive individuals. Additional support for LTC and ARV use is the significant increase in viral suppression and ARV use which increased 27% in 2007 to 80% in 2013, i.e., during the same time period. This level of suppression was similar to observations seen in other populations followed at Johns Hopkins Clinics, such as injecting drug users from the ALIVE cohort (48), and patients attending the JHH Moore HIV Care clinic (49).
Our report has several limitations. There were some changes in the demographic distribution of the ED population over time and this may have had some impact on changes in HIV prevalence. Furthermore, data such as sexual behavior or parenteral drug use was not systematically collected over time. Since parenteral drug users and men who have sex with men in Baltimore have been reported to have high prevalence of HIV infection (35), changes in their attendance to the ED would possibly affect our overall prevalence. However, this limitation would not necessarily directly affect our results on LTC, ARV use or viral suppression. As noted, we are not implying causation, but our programmatic efforts parallel the outcomes. It may be argued that the data presented here are not generalizable given some of the unique characteristics of the local population that has among the highest rates of HIV infection. Nonetheless, the overall data parallel national trends, and the role of EDs in high prevalence settings are underscored in the importance of screening and LTC as a critical part of the continuum of care.
With readily available treatment, early diagnosis is the key to initiating the LTC, starting ARVs and ensuring viral suppression that could result in lower transmission rates and ultimately lower incidence of HIV in the population. HIV testing among the ED attendees offer opportunity to provide prevention messages among the HIV negative population, further supporting the national HIV prevention strategy, as is reengaging HIV individuals who failed LTC or left care previously. Collectively, our data on the declining prevalence and incidence, improved knowledge of HIV status, increased presence of ARVs among those infected, and viral suppression in nearly 60% of infected individuals provide encouragement for prevention programs in the community, including the role of EDs in HIV in the continuum of care within Baltimore City and the population that JHH ED serves.
Supplementary Material
Acknowledgments
Funding Source: This work was supported by Division of Intramural Research, National Institute of Allergy and Infectious Diseases at National Institutes of Health; Maryland Department of Health and Mental Hygiene; Baltimore City Health Department; the Gilead Foundation HIV Focus Program; the Emergency Medicine Foundation; and National Institute of Allergy and Infectious Diseases at National Institutes of Health [K01AI100681 to Y-HH].
Footnotes
Potential conflicts of interest
We declare no competing interests.
References
- 1.Centers for Disease Control (CDC) Pneumocystis pneumonia–Los Angeles. MMWR Morb Mortal Wkly Rep. 1981;30(21):250–2. [PubMed] [Google Scholar]
- 2.Barré-Sinoussi F, Chermann J, Rey F, Nugeyre M, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–71. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. HIV/AIDS - Fact Sheet. [cited 2015 January 30]. Available from: http://www.who.int/mediacentre/factsheets/fs360/en/ Last accessed January 30, 2015.
- 4.Centers for Disease Control and Prevention. HIV in the United States: At a glance. [cited 2015 January 30]. Available from: http://www.cdc.gov/hiv/statistics/basics/ataglance.html Last accessed January 30, 2015.
- 5.Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey, 2011 Emergency Department Summary Tables. 2011 [cited 2015 January 30]. Available from: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf Last accessed January 30, 2015.
- 6.Gindi R, Cohen R, Krizinger W. Emergency room use among adults aged 18–64: Early release estimates from the national health interview survey, January–June 2011. 2012 Jan 30; 2015. Available from: http://www.cdc.gov/nchs/data/nhis/earlyrelease/emergency_room_use_january-june_2011.pdf. Last accessed January 30, 2015.
- 7.Baker J, Kelen G, Sivertson K, Quinn T. Unsuspected human immunodeficiency virus in critically ill emergency patients. JAMA. 1987;257(19):2609–11. [PubMed] [Google Scholar]
- 8.Kelen G, Fritz S, Qaqish B, Brookmeyer R, Baker J, Kline R, et al. Unrecognized human immunodeficiency virus infection in emergency department patients. N Engl J Med. 1988;318(25):1645–50. doi: 10.1056/NEJM198806233182503. [DOI] [PubMed] [Google Scholar]
- 9.Kelen G, DiGiovanna T, Bisson L, Kalainov D, Sivertson K, Quinn T. Human immunodeficiency virus infection in emergency department patients. Epidemiology, clinical presentations, and risk to health care workers: the Johns Hopkins experience. JAMA. 1989;262(4):516–22. doi: 10.1001/jama.262.4.516. [DOI] [PubMed] [Google Scholar]
- 10.Kelen G, Hexter D, Hansen K, Tang N, Pretorius S, Quinn T. Trends in human immunodeficiency virus (HIV) infection among a patient population of an inner-city emergency department: implications for emergency department-based screening programs for HIV infection. Clin Infect Dis. 1995;21(4):867–75. doi: 10.1093/clinids/21.4.867. [DOI] [PubMed] [Google Scholar]
- 11.Laeyendecker O, Rothman R, Henson C, Horne B, Ketlogetswe K, Kraus C, et al. The effect of viral suppression on cross-sectional incidence testing in the johns hopkins hospital emergency department. J Acquir Immune Defic Syndr. 2008;48(2):211–5. doi: 10.1097/QAI.0b013e3181743980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh Y, Gauvey-Kern M, Peterson S, Woodfield A, Deruggiero K, Gaydos C, et al. An emergency department registration kiosk can increase HIV screening in high risk patients. J Telemed Telecare. 2014;20(8):454–9. doi: 10.1177/1357633X14555637. Epub Oct 14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh Y, Kelen G, Laeyendecker O, Kraus C, Quinn T, Rothman R. HIV Care Continuum for HIV-Infected Emergency Department Patients in an Inner-City Academic Emergency Department. Ann Emerg Med. 2015;66(1):69–78. doi: 10.1016/j.annemergmed.2015.01.001. Epub Feb 19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothman R, Kelen G, Harvey L, Shahan J, Hairston H, Burah A, et al. Factors associated with no or delayed linkage to care in newly diagnosed human immunodeficiency virus (HIV)-1-infected patients identified by emergency department-based rapid HIV screening programs in two urban EDs. Acad Emerg Med. 2012;19(5):497–503. doi: 10.1111/j.1553-2712.2012.01351.x. [DOI] [PubMed] [Google Scholar]
- 15.Kelen G, Green G, Purcell R, Chan D, Qaqish B, Sivertson K, et al. Hepatitis B and hepatitis C in emergency department patients. N Engl J Med. 1992;326(21):1399–404. doi: 10.1056/NEJM199205213262105. [DOI] [PubMed] [Google Scholar]
- 16.Kelen G, DiGiovanna T, Lofy L, Junkins E, Stein A, Sivertson K, et al. Human T-lymphotropic virus (HTLV I-II) infection among patients in an inner-city emergency department. Ann Intern Med. 1990;113(5):368–72. doi: 10.7326/0003-4819-113-5-368. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert E, Lowenstein S, Koziol-McLain J, Barta D, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996;27(3):305–8. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh Y-H, Jung J, Shahan J, Pollack H, Hairston H, Moring-Paris D, et al. Outcomes and Cost Analysis of Three Operational Models for Rapid HIV Testing Services in an Academic Inner-City Emergency Department. Ann Emerg Med. 2011;58(Suppl 1):S133–9. doi: 10.1016/j.annemergmed.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Kelen G, Hexter D, Hansen K, Humes R, Vigilance P, Baskerville M, et al. Feasibility of an emergency department-based, risk-targeted voluntary HIV screening program. Ann Emerg Med. 1996;27(6):687–92. doi: 10.1016/s0196-0644(96)70184-1. [DOI] [PubMed] [Google Scholar]
- 20.Kelen G, Shahan J, Quinn T. Emergency department-based HIV screening and counseling: experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33(2):147–55. doi: 10.1016/s0196-0644(99)70387-2. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh Y-H, Jung J, Shahan J, Moring-Parris D, Kelen G, Rothman R. Emergency medicine resident attitudes and perceptions of HIV testing before and after a focused training program and testing implementation. Acad Emerg Med. 2009;16(11):1165–73. doi: 10.1111/j.1553-2712.2009.00507.x. [DOI] [PubMed] [Google Scholar]
- 22.Signer D, Peterson S, Hsieh Y-H, Haider S, Saheed M, Neira P, et al. Scaling Up HIV Testing in an Academic Emergency Department: An Integrated Testing Model with Fourth-Generation and Point-of-Care Testing. Public Health Rep. 2015 doi: 10.1177/00333549161310S110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avery L, Parsons T, Meyers D, Hubbard W. A highly sensitive ultra performance liquid chromatography-tandem mass spectrometric (UPLC-MS/MS) technique for quantitation of protein free and bound efavirenz (EFV) in human seminal and blood plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(31):3217–24. doi: 10.1016/j.jchromb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzinke M, Breaud A, Parsons T, Cohen M, Piwowar-Manning E, Eshleman S, et al. The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta. 2014;433:157–68. doi: 10.1016/j.cca.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookmeyer R, Konikoff J, Laeyendecker O, Eshleman S. Estimation of HIV incidence using multiple biomarkers. Am J Epidemiol. 2013;177(3):264–72. doi: 10.1093/aje/kws436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kassanjee R, McWalter T, Bärnighausen T, Welte A. A new general biomarker-based incidence estimator. Epidemiology. 2012;23(5):721–8. doi: 10.1097/EDE.0b013e3182576c07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohareb A, Rothman R, Hsieh Y. Emergency department (ED) utilization by HIV-infected ED patients in the United States in 2009 and 2010 - a national estimation. HIV Med. 2013;14(10):605–13. doi: 10.1111/hiv.12052. [DOI] [PubMed] [Google Scholar]
- 28.Bozzette S, Berry S, Duan N, Frankel M, Leibowitz A, Lefkowitz D, et al. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. N Engl J Med. 1998;339(26):1897–904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 29.Kelen G, Rothman R. Emergency department-based HIV testing: too little, but not too late. Ann Emerg Med. 2009;54(1):65–71. doi: 10.1016/j.annemergmed.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Branson B, Handsfield H, Lampe M, Janssen R, Taylor A, Lyss S, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 31.Lyons M, Lindsell C, Haukoos J, Almond G, Brown J, Calderon Y, et al. Nomenclature and definitions for emergency department human immunodeficiency virus (HIV) testing: report from the 2007 conference of the National Emergency Department HIV Testing Consortium. Acad Emerg Med. 2009;16(2):168–77. doi: 10.1111/j.1553-2712.2008.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres G, Yonek J, Pickreign J, Whitmore H, Hasnain-Wynia R. HIV testing and referral to care in U.S. hospitals prior to 2006: results from a national survey. Public Health Rep. 2009;124(3):400–8. doi: 10.1177/003335490912400309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothman R, Hsieh Y-H, Harvey L, Connell S, Lindsell C, Haukoos J, et al. 2009 US Emergency Department HIV Testing Practices. Ann Emerg Med. 2011;58(Suppl 1):S3–S9.e4. doi: 10.1016/j.annemergmed.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Maryland Department of Health and Mental Hygiene. Maryland HIV/AIDS Quarterly Update - Fourth Quarter 2014.: Center for HIV Surveillance, Epidemiology and Evaluation, Infectious Disease Bureau, Prevention and Health Promotion Administration, Maryland Department of Health and Mental Hygiene. 2015 [cited 2015 August 30]. Available from: http://phpa.dhmh.maryland.gov/OIDEOR/CHSE/SiteAssets/SitePages/statistics/Maryland%20HIV%20AIDS%20Quarterly%20Update-4rd%20Quarter%20(Data%20reported%20through%2012-31-2014%202.pdf). Last Accessed Aug 30, 2015.
- 35.Maryland Department of Health and Mental Hygiene. Baltimore City HIV/AIDS Epidemiological Profile - Fourth Quarter 2012.: Center for HIV Surveillance, Epidemiology and Evaluation, Infectious Disease Bureau, Prevention and Health Promotion Administration, Maryland Department of Health and Mental Hygiene. 2013 [cited 2014 November 18]. Available from: http://phpa.dhmh.maryland.gov/OIDEOR/CHSE/SiteAssets/SitePages/statistics/Baltimore%20City%20HIV%20AIDS%20Epidemiological%20Profile%2012-2012.pdf. Last Accessed Jan 30, 2015.
- 36.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV Incidence in the United States, 2006–2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, Kazatchkine M, Sidibe M, Strathdee S. Time to act: a call for comprehensive responses to HIV in people who use drugs. Lancet. 2000;376(9740):551–63. doi: 10.1016/S0140-6736(10)60928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latkin C, Davey M, Hua W. Needle exchange program utilization and entry into drug user treatment: is there a long-term connection in Baltimore, Maryland? Subst Use Misuse. 2006;41(14):1991–2001. doi: 10.1080/10826080601026027. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen T, Weir B, Des Jarlais D, Pinkerton S, Holtgrave D. Syringe exchange in the United States: a national level economic evaluation of hypothetical increases in investment. AIDS Behav. 2014;18(11):2144–55. doi: 10.1007/s10461-014-0789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyons M, Raab D, Lindsell C, Trott A, Fichtenbaum C. A novel emergency department based prevention intervention program for people living with HIV: evaluation of early experiences. BMC Health Serv Res. 2007;7:164. doi: 10.1186/1472-6963-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The White House Office of National AIDS Policy. Executive Order – HIV Care Continuum Initiative - Accelerating improvements in HIV prevention and care in the United States through the HIV Care Continuum Initiative. Washington, DC: The White House Office of the Press Secretary; 2013. [cited 2014 August 20]. Available from: http://www.whitehouse.gov/the-press-office/2013/07/15/executive-order-hiv-care-continuum-initiative. Last Accessed Jan 30, 2015. [Google Scholar]
- 42.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents.: Department of Health and Human Services. 2012 [updated May 1, 2014; cited 2014 September 3]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Last Accessed Jan 30, 2015.
- 43.Christopoulos K, Zetola N, Klausner J, Haller B, Louie B, Hare C, et al. Leveraging a rapid, round-the-clock HIV testing system to screen for acute HIV infection in a large urban public medical center. J Acquir Immune Defic Syndr. 2013;62(2):e30–8. doi: 10.1097/QAI.0b013e31827a0b0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haukoos J, Hopkins E, Bender B, Sasson C, Al-Tayyib A, Thrun M, et al. Comparison of enhanced targeted rapid HIV screening using the Denver HIV risk score to nontargeted rapid HIV screening in the emergency department. Ann Emerg Med. 2013;61(3):353–61. doi: 10.1016/j.annemergmed.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haukoos J, Hopkins E, Conroy A, Silverman M, Byyny R, Eisert S, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010;304(3):284–92. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 46.Marks G, Gardner L, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS. 2010;24(17):2665–78. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 47.Gardner E, McLees M, Steiner J, Del Rio C, Burman W. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westergaard R, Hess T, Astemborski J, Mehta S, Kirk G. Longitudinal changes in engagement in care and viral suppression for HIV-infected injection drug users. AIDS. 2013;27(16):2559–66. doi: 10.1097/QAD.0b013e328363bff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore R, Bartlett J. Dramatic decline in the HIV-1 RNA level over calendar time in a large urban HIV practice. Clin Infect Dis. 2011;53(6):600–4. doi: 10.1093/cid/cir467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


