Abstract
We tested whether it is possible to selectively block pain signals in the orofacial area by delivering the permanently charged lidocaine derivative QX-314 into nociceptors via TPRV1 channels. We examined the effects of co-applied QX-314 and capsaicin on nociceptive, proprioceptive, and motor function in the rat trigeminal system. QX-314 alone failed to block voltage-gated sodium channel currents (INa) and action potentials (APs) in trigeminal ganglion (TG) neurons. However, co-application of QX-314 and capsaicin blocked INa and APs in TRPV1-positive TG and dental nociceptive neurons, but not in TRPV1-negative TG neurons or in small neurons from TRPV1 knock-out mice. Immunohistochemistry revealed that TRPV1 is not expressed by trigeminal motor and trigeminal mesencephalic neurons. Capsaicin had no effect on rat trigeminal motor and proprioceptive mesencephalic neurons and therefore should not allow QX-314 to enter these cells. Co-application of QX-314 and capsaicin inhibited the jaw-opening reflex evoked by noxious electrical stimulation of the tooth pulp when applied to a sensory but not a motor nerve, and produced long-lasting analgesia in the orofacial area. These data show that selective block of pain signals can be achieved by co-application of QX-314 with TRPV1 agonists. This approach has potential utility in the trigeminal system for treating dental and facial pain.
Keywords: Action potentials, Local anesthetics, QX-314, Trigeminal system, TRPV1, Voltage-gated sodium channels
1. Introduction
Local anesthetics (LAs) abolish the transmission of nociceptive information to the central nervous system (CNS) by blocking voltage-gated sodium channels (VGSCs) and thereby prevent the generation and propagation of action potentials (APs) [6]. Most LAs in clinical use are tertiary amines that under physiological conditions exist in a mixture of protonated and uncharged base forms [5]. The uncharged hydrophobic form of LAs penetrates through the membrane of all neurons, so that in addition to blocking pain signals, LAs produce general numbness from the block of low-threshold sensory nerves, as well deficits in motor function, and a block of autonomic nerves [3,4,15,30].
Selective block of pain signals might be possible either by targeting certain sodium channels that are present only in pain-sensing neurons [7,12,20] or by delivering sodium channel blockers selectively to pain-sensing neurons. One strategy is to deliver a permanently charged sodium channel blocker such as QX-314 (N-ethyl-lidocaine) by entry through large-pore ion channels selectively expressed in nociceptive neurons [33]. The transient receptor potential vanilloid 1 (TRPV1) channel is a primary nociceptive transducer in pain-sensing neurons, activated by noxious heat (>43 °C), capsaicin, protons and endocannabinoids [8,36]. A recent study demonstrated that the pore of TRPV1 channels, when opened by the TRPV1 channel agonist capsaicin, might be large enough to deliver QX-314 or other large molecule selectively into nociceptive neurons [11,22]. Binshtok et al. demonstrated that QX-314, a charged membrane-impermeant lidocaine derivative, can be targeted selectively into nociceptors when co-administrated with capsaicin, resulting in selective blocking of sodium channels and inhibition of excitability in nociceptors [3].
The orofacial area is mainly innervated by the trigeminal nerve that consists of both sensory and motor fibers [18,31]. LAs are widely used in the trigeminal system, including nerve block for general dental treatment, trigeminal neuralgia or trigger point injection for myofacial pain syndrome [32]. However, side-effects such as numbness or systemic toxicity following accidental intravascular injection following LA injection [32] limit the use of current LAs. A strategy for targeting delivery of sodium channel blockers only to nociceptors could minimize many adverse effects. In this study, we tested whether the charged lidocaine derivative QX-314 can be selectively targeted to nociceptors in the trigeminal system via TRPV1 channels, using a combination of in vitro, in vivo and behavioral studies. The trigeminal system lends itself to study the selectivity of nerve block and we found that the effects produced by co-application of QX-314 and capsaicin are restricted to TRPV1-expressing trigeminal nociceptive neurons, with no effect on proprioceptive neurons or motor neurons. We suggest that this approach should be applicable for producing pain-selective local anesthesia in the trigeminal system.
2. Materials and methods
2.1. Animals
All surgical and experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the School of Dentistry, Seoul National University. Animal treatments were performed according to the Guidelines of the International Association for the Study of Pain. Male Sprague–Dawley (SD) rats (OrientBio, Korea) were housed at a temperature of 23 ± 2 °C with a 12-h light–dark cycle (light on 08:00 to 20:00), and fed food and water ad libitum. The animals were allowed to habituate to the housing facilities for 1 week before the experiments, and efforts were made to limit distress to the animals.
2.2. Preparation of TG neurons
TG neurons from adult SD rats (weighing approximately 150–200 g) were prepared as previously described [13]. TG neurons were harvested and transferred to cold (4 °C) HBSS (Life Technologies, USA). The cells were incubated in 3 ml HBSS containing 2.5% trypsin (Sigma–Aldrich, USA) at 37 °C for 45 min. After enzyme digestion, the suspension was washed in DMEM and triturated with a flame-polished Pasteur pipette to separate the cells. Subsequently, the cells were centrifuged through 15% BSA (Sigma–Aldrich, USA), resuspended and plated on coverslips coated with poly-l-ornithine (0.5 mg/ml; Sigma–Aldrich, USA). TG neurons were incubated in Neurobasal medium (Gibco, USA) supplemented with penicillin and streptomycin (1%; Gibco, USA), B-27 supplement (invitrogen, USA), l-glutamine (1 mM; Invitrogen, USA), nerve growth factor (NGF 2.5s, 50 ng/ml; invitrogen, USA), GDNF (2 ng/ml; Sigma–Aldrich, USA) and Ara-C (10 µM; Sigma–Aldrich, USA) at 37 °C under 5% CO2. Electrophysiological recordings were performed within 24 h of plating.
2.3. Retrograde labeling of dental primary afferent neurons and trigeminal motoneurons
Dental primary afferent TG neurons were retrograde labeled with the fluorescent dye, 1,1′-dioctadecyl-3,3,3″3′-teramethylindo-carbocyanine perchlorate (DiI; Molecular Probes, USA) as previously described [27]. Briefly, DiI was placed in the cavity in the upper molar teeth of adult SD rats (150–200 g) under anesthesia with sodium pentobarbital (30 mg/kg body weight, intraperitoneal injection). After 2 weeks, TG neurons were acutely isolated as mentioned above. Trigeminal motoneurons were also retrograde labeled with DiI as previously described [24].
2.4. Electrophysiological recordings
Whole cell voltage and current clamp recording for TG neurons were performed at room temperature to measure INa and APs, respectively, with EPC-9 amplifier and Pulse 8.30 software (both from HEKA, Germany). Patch pipettes were pulled from borosilicate capillaries (Chase Scientific Glass Inc., Rockwood, USA). When filled with the pipette solution, the resistance of the pipettes was 4–7 MΩ (always less than 10 MΩ). The recoding chamber (volume 500 µl) was continuously superfused (1–2 ml/min). Data were low pass filtered at 2 kHz, sampled at 10 kHz, and acquired using Pulse program (HEKA, Germany). The pipette solution for voltage clamp experiments was composed of 110 mM CsCl, 2 mM MgCl2, 1mM CaCl2, 11 mM EGTA, 10 mM HEPES, and pH adjusted to 7.4 with CsOH. The external solution was 60 mM NaCl, 60 mM choline chloride, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 0.1 mM CdCl2, 15mM tetraethylammonium chloride, 5 mM 4-aminopyridine, 10 mM glucose, 10 mM HEPES, and pH adjusted to 7.4 with NaOH. Sodium current was recorded during 30 ms voltage clamp steps from a holding potential of −70 mV to a test potential of 0 mV every 10 s. The pipette solution for current clamp recordings was composed of 135 mM potassium gluconate, 2 mM MgCl2, 6 mM KCl, 10 mM HEPES, 5 mM MgATP, 0.5 mM Li2GTP, and pH adjusted to 7.4 with KOH, and an external solution of 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, 10 mM glucose, and pH adjusted to 7.4 with NaOH. APs were evoked by 3 ms depolarizing current pulses with 250 pA amplitude.
Electrophysiological recordings from trigeminal motoneurons and trigeminal mesencephalic neurons were performed with whole cell patch clamp recording methods similar to the previous studies [16]. Briefly, coronal slices of 200 µm thickness were made from the brain stem of Wistar rats (6–14 days postnatal). The artificial cerebrospinal fluid used as bath solution was composed of 124 mM NaCl, 26 mM NaHCO3, 1.8 mM KCl, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.3 mM MgCl2, and 10 mM Glucose. The internal solution of the patch pipettes had 123 mM K-gluconate, 18 mM KCl, 10 mM NaCl, 3 mM MgCl2, 2 mM ATP-Na2, 0.3 mM GTP-Na3, and 10 mM HEPES, pH 7.4 adjusted with KOH. Mesencephalic trigeminal nucleus neurons and trigeminal motor nucleus neurons were easily recognized under microscope (BX-50WI, Olympus, Japan) by their location and large oval shape. All recordings were performed with Axopatch 200B amplifier and Digidata 1322A data acquisition system (Molecular Devices, CA). Data were low-pass filtered at 5–10 kHz, digitized at a sampling rate of 10 kHz.
2.5. Immunofluorescent staining
Following whole cell patch clamp recordings, DiI-labeled TG neurons were washed in PBS and then kept in blocking solution containing 5% normal donkey serum, 2% BSA, 2% FBS, and 0.1% Triton X-100 for 1 h at RT. The cells were incubated overnight at 4 °C with a mixture of guinea pig anti-TRPV1 antibody (1:500; Chemicon, USA) and washed in PBS. The slides were then incubated for 1 h at RT with FITC-conjugated guinea pig IgG antibody (1:200; Jackson ImmunoResearch, USA). For the staining of Vmot, the rats were perfused with physiological saline and sequentially with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Whole rat brain was removed and immersed in the post-fixative at 4 °C overnight and then transferred to 10% and 30% sucrose in PB for 48 h. Serial frozen transverse sections (30 µm thickness) were collected in cold PBS. Free-floating sections were incubated with guinea pig anti-TRPV1 antibody (1:500; Chemicon, USA) and processed as described above. All immunohistochemical procedures were performed at room temperature unless otherwise stated. The slices were mounted with VectaShield (Vector Laboratories, USA) and visualized using a confocal microscope using appropriate filter sets (FV-300, Olympus, Japan). To study the expression of TRPV1 in the inferior alveoloar nerve, the nerve was double immunostained for TRPV1 and PGP9.5, a marker for both myelinated and unmyelinated nerve fibers. The rats were anesthetized with ethyl ether and perfused with heparinized saline followed by 4% paraformaldehyde in phosphate buffer (PB, 0.1 M, pH 7.4). The inferior alveolar nerve was removed, post-fixed for 3 h in the same fixative, and cryoprotected in 30% sucrose in PB. Fifteen micrometer thick longitudinal sections of the nerves were cut on a cryostat, blocked with 10% normal donkey serum (NDS, Jackson Immunolabs, USA) in phosphate-buffered saline (PBS, 0.01 M, pH 7.2) for 10 min, and incubated overnight with primary antibodies. For double immunofluorescence, the sections were incubated with a mixture of polyclonal rabbit anti-human PGP 9.5 (1:500; UltraClone, UK) and goat anti-TRPV1 (1:1000; Neuromics, USA). The sections were rinsed and incubated in 2% NDS for 10 min and then incubated with a combination of Cy3-conjuaged donkey anti-goat (1:200, Jackson Immunolabs, USA) and Alexa 488-conjugated donkey anti rabbit (1:200, Invitrogen, USA) in PBS for 3 h. After several rinses, the sections were mounted on slides, coverslipped with VectaShield (Vector laboratories, USA), and observed on a Leica DMR microscope. Images were acquired with a CCD camera (Fluoview, SIS, Germany) attached to the microscope, and contrast and brightness were adjusted with Photoshop (CS2, Adobe Systems Inc., USA).
2.6. dEMG recording of jaw-opening reflex
The rats, weighing between 250 and 300 g, were initially anesthetized with ethyl ether, followed by urethane solution (120 mg/100 g body weight, intraperitoneal injection). Once the rats were stably anesthetized, a dental cavity was made on the left mandibular incisor by using a dental drill. For electrical stimulation, one of the stimulating wires (Belden, USA) was inserted into the cavity made on the left mandibular incisor and sealed with acrylic dental cement and the other reference wire was hypodermically located near the lip. Another pair of copper wires was placed in the left anterior belly of the digastric muscle. Electrical stimulation, 300 µs in duration, was generated with a stimulator (Pulse train module 1831 connected to Pulse interval module 1830, World Precision Instruments, USA) at 30 s intervals. Once the threshold was determined, EMG amplitudes to the electrical nociceptive stimulation (two and a half times to the threshold, ×2.5T) were recorded from the digastric muscle. The EMG leads were connected to an amplifier (DAM80, World Precision Instruments, USA), and then monitored and recorded with IGOR Pro (Ver. 4.0, WaveMetrics Inc., USA). Drugs were applied onto either the exposed IAN or the mylohyoid motor nerve. Capsaicin (0.5 µg/µl) was prepared with 20% ethanol, 5% Tween 20 and 75% normal saline solution and QX-314, and lidocaine was directly dissolved in physiological saline. The solutions were freshly made on the day of the experiment and solubilized in an ultrasonic vibrator (Model 3510, Branson Ultrasonic Co., USA) for 30 min prior to application.
2.7. Behavioral studies
For behavioral observation, the rats (weighing between 250 and 300 g) were placed in a customized cylinder-type acrylic rodent restrainer for evaluation of thermal pain nociceptive response as described previously [26]. The cage has a hole in the top so that the head could receive thermal stimulation and produce withdrawal action. Each cage was placed in a darkened and noise-free room and the animals were habituated for at least 30 min before experiment. Applications of heat stimuli were performed by infrared thermal stimulator (Infrared Diode Laser, LVI-808-10, Korea). Power and current of thermal stimulator were adjusted at 11W and 18.1 A, respectively. This intensity of thermal stimuli produced stable head flick or head withdrawal response with a distance of 10 cm from heat source to vibrissa pad. Each rat received 2 stimuli and the interstimulus interval for each trial was at least 2 min. A cut-off time of 20 s was used in the experiments to prevent possible tissue damage. Before drug injection, latencies of withdrawal responses were determined from all animals. The vehicle and drugs were administered subcutaneously into the left vibrissa pad followed by capsaicin injection (1 µg/10 µl, n = 11) 10 min later, latencies of withdrawal responses were determined at 10, 30, 60, 120, 180, 240, 300, 360 and 420 min after injection.
The experimenter was blind to the treatment group in all behavioral experiments. For behavioral experiments, capsaicin was dissolved in 10% ethanol, 10% Tween 80 and 80% normal saline solution. QX-314 was dissolved in normal saline.
2.8. Chemicals
For in vitro study, capsaicin stock solutions were made in ethanol and stored at −20 °C. QX-314 was dissolved in distilled water and stored at −20 °C. All drugs were purchased from Sigma–Aldrich. The drugs were diluted to their final concentration using the external solution, and then were applied to cells by local perfusion through a capillary tube positioned near the cell of interest. The drug solution flow was driven by gravity (flow rate, 1–2 ml/min) and controlled by miniature solenoid valves.
2.9. Statistics
All data are presented as means ± standard error (SEM). For in vitro studies, an unpaired Student’s t-test was used to determine the differences (SigmaPlot, SPSS, USA). Statistical analyses of thermal hyperalgesia were carried out using a repeated measures ANOVA followed by multiple group comparisons using the LSD post hoc test. Differences were considered to be significant when P value was less than 0.05 or 0.001.
3. Results
3.1. Co-application of QX-314 and capsaicin blocks INa only in TG nociceptor neurons
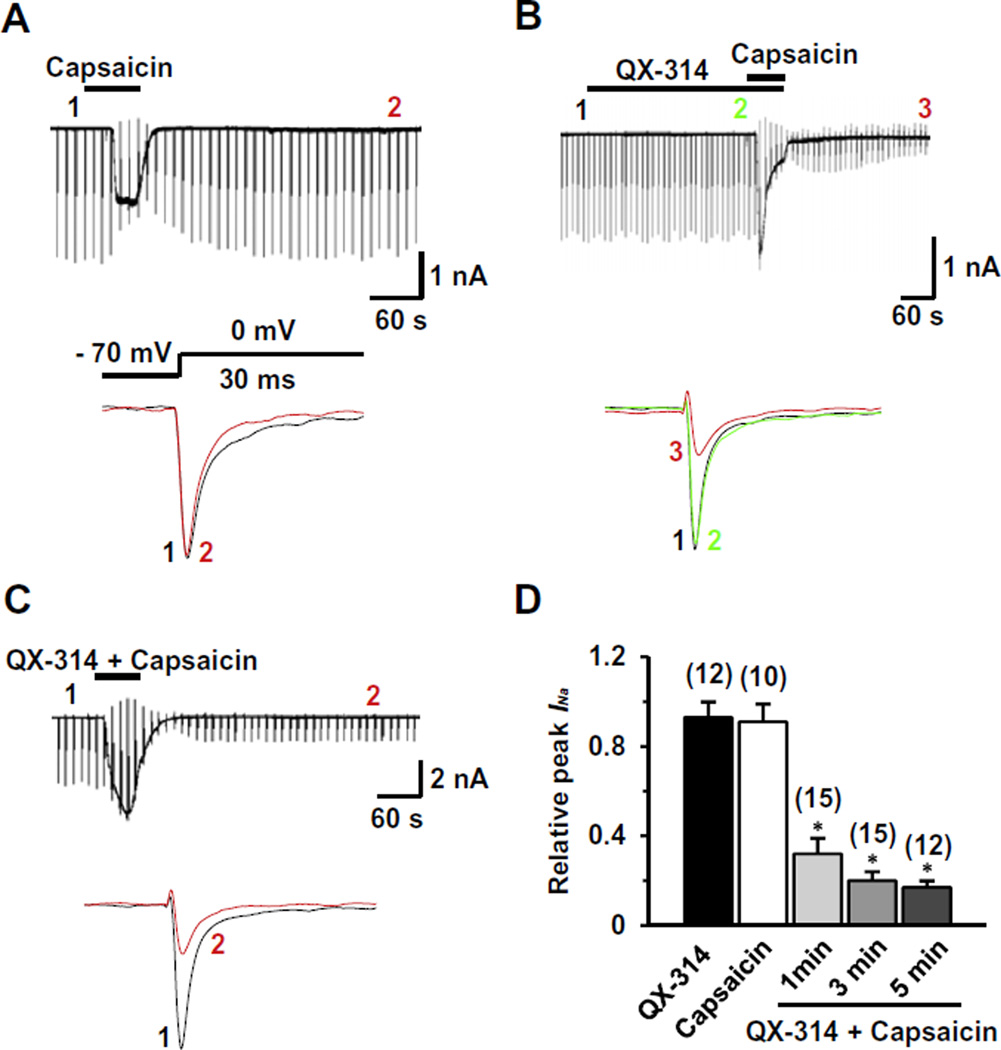
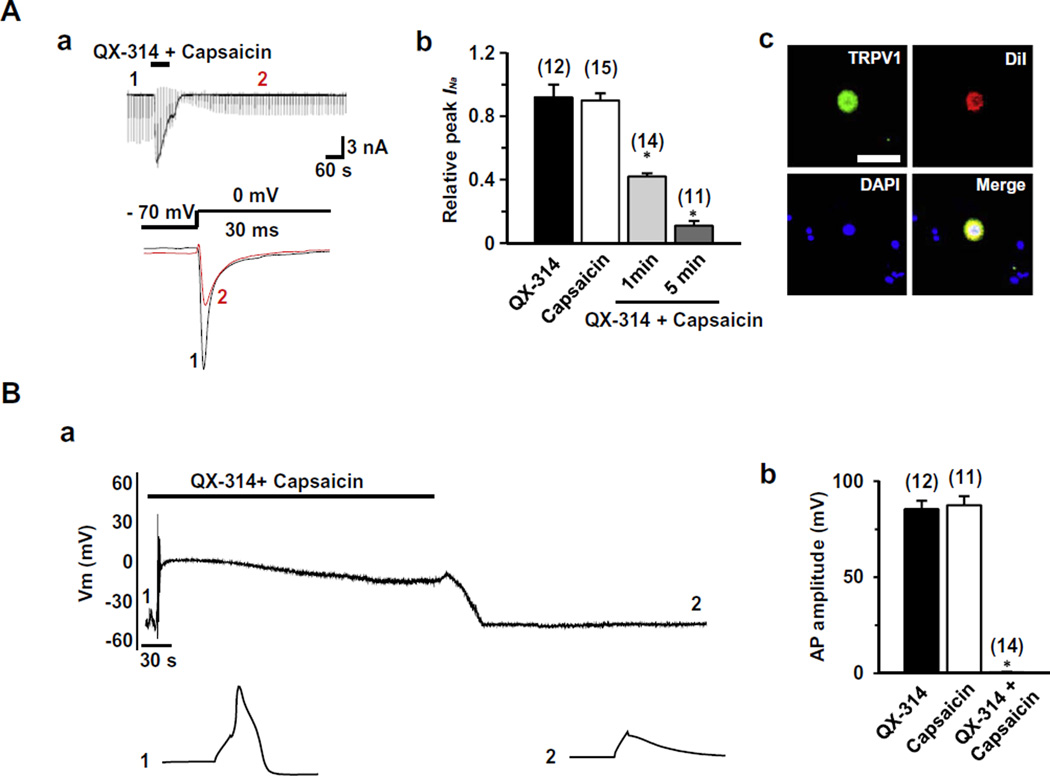
We first tested whether QX-314 applied either alone or together with capsaicin can inhibit voltage-gated sodium current (INa) elicited by a depolarizing step pulse from a holding potential of −70 mV in dissociated TG neurons, a preparation which includes both capsaicin-sensitive and capsaicin-insensitive neurons. To avoid the contamination of capsaicin-induced inward currents during the measurement of INa, we measured INa after the holding current at −70 mV returned to the control level following capsaicin exposure (time points 2 and 3 in Fig. 1A and B). Through this approach, we could also test whether the block persists after washout (5 min) of capsaicin and QX-314, which is expected if the block reflects TRPV1-mediated entry of QX-314 inside the cells. Capsaicin alone (1 µM, 1 min) produced little effect on INa after washout (0.91 ± 0.08, P = 0.6, n = 10) (Fig. 1A); as expected, it evoked inward currents in most small neurons (<25 µm in diameter). However, when QX-314 (5 mM) was applied followed by capsaicin (1 µM, 1 min) or applied together with capsaicin (1 µM, 1 min), INa was dramatically inhibited (0.32 ± 0.07, P < 0.05, n = 15) (Fig. 1B and C) and the inhibitory effects became larger as the application time increased (Fig. 1D). We observed that the blockade of INa was maintained throughout our recordings up to at least 45 min of washout. QX-314 (5 mM, 5 min) applied alone had marginal effects on INa (0.93 ± 0.07, P = 0.1, n = 12), consistent with the previous data obtained from DRG neurons [3] (Fig. 1B and D).
Fig. 1.
Effects of QX-314 and capsaicin on voltage-gated sodium currents (INa) in capsaicin-sensitive TG neurons from adult rats. Representative recordings following the extracellular application of capsaicin alone (1 µM, 1 min) (A), co-application of QX-314 (5 mM) and capsaicin (1 min) after pretreatment by QX-314 alone (B) and co-application of QX-314 and capsaicin (1 min) (C). Upper panel: Long time-base recordings of INa measured during 30-ms voltage steps from a holding potential of −70 mV to a test potential of 0 mV delivered every 10 s. Each vertical deflection indicates INa measured every 10 s. Capsaicin, QX-314 or QX-314 plus capsaicin was applied during the time indicated by the horizontal bar. Lower panel: fast time-base recordings of superimposed sodium current evoked by test pulse at the points indicated in the upper panel. (D) Collected results for the effects of QX-314, capsaicin, or co-application of both on INa in capsaicin-sensitive TG neurons. The numbers in parentheses indicate the number of cells tested. Results are means ± S.E.M. *P < 0.05 compared with the control (INa peak before drug application).
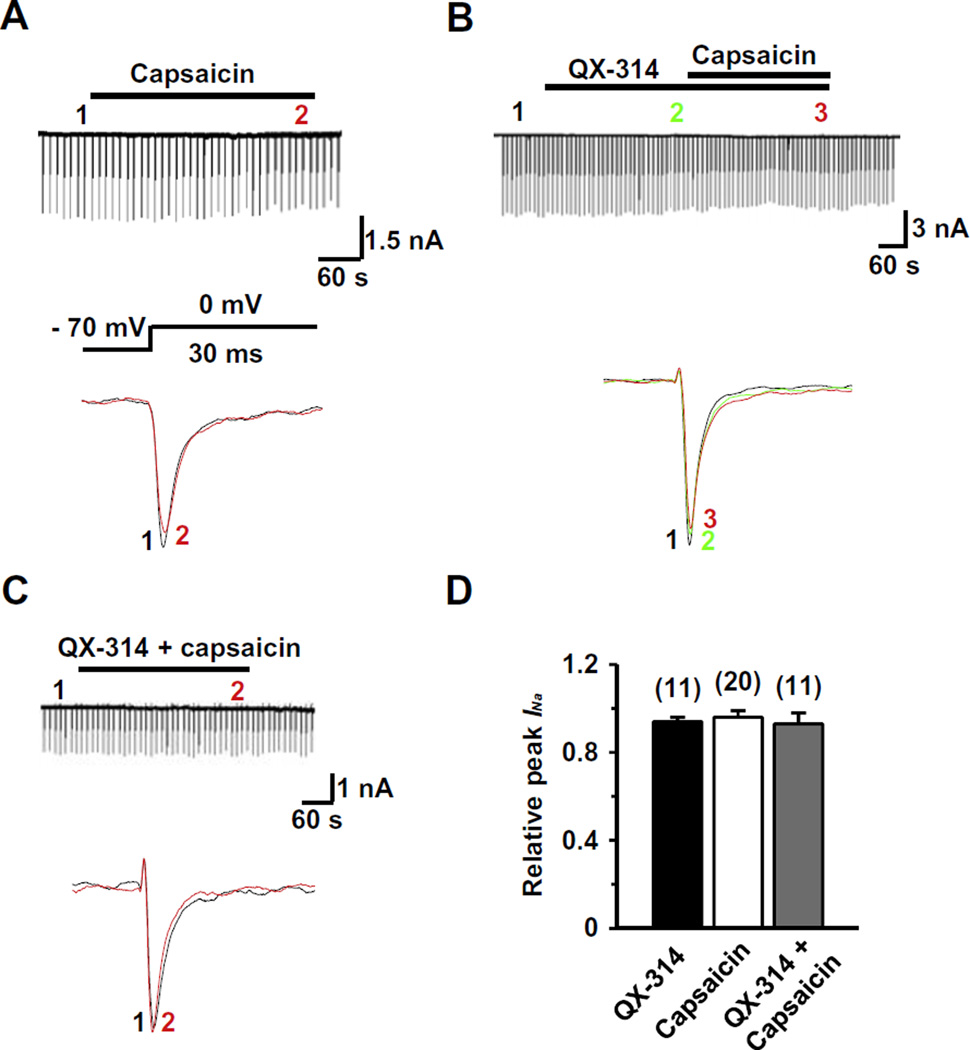
In capsaicin-insensitive TG neurons (>40 µm in diameter), capsaicin neither elicited inward currents (Fig. 2) nor affected INa (Fig. 2A) (0.96 ± 0.03, P = 4.2, n = 20). QX-314, either alone or applied together with capsaicin, also had little effect on INa (0.94 ± 0.02, P = 0.5, n = 11 and 0.93 ± 0.05, P = 0.2, n = 11, respectively) (Fig. 2B–D). Our data suggest that QX-314, entering through TRPV1 channels, blocks INa only in capsaicin-sensitive nociceptive TG neurons and only when co-applied with capsaicin.
Fig. 2.
Effects of QX-314 and capsaicin on INa in capsaicin-insensitive rat TG neurons. Representative recordings following the extracellular application of capsaicin alone (1 µM) (A), co-application of QX-314 (5 mM) and capsaicin with the pretreatment of QX-314 (B) and co-application of QX-314 and capsaicin (C). Upper panel: Long time-base recordings of INa measured during 30-ms voltage steps from a holding potential of −70 mV to a test potential of 0 mV delivered every 10 s. Each vertical deflection indicates INa measured every 10 s. Capsaicin, QX-314 or QX-314 plus capsaicin was applied during the time indicated by the horizontal bar. Lower panel: fast time-base recordings of superimposed sodium current evoked by test pulse at the points indicated in the upper panel. (D) Collected results for the effects of QX-314, capsaicin, or co-application of both on INa in capsaicin-sensitive TG neurons. The numbers in parentheses indicate the number of cells tested. Results are means ± S.E.M. *P < 0.05 compared with the control (INa peak before drug application).
3.2. Co-application of QX-314 and capsaicin selectively blocks action potentials in TG nociceptor neurons
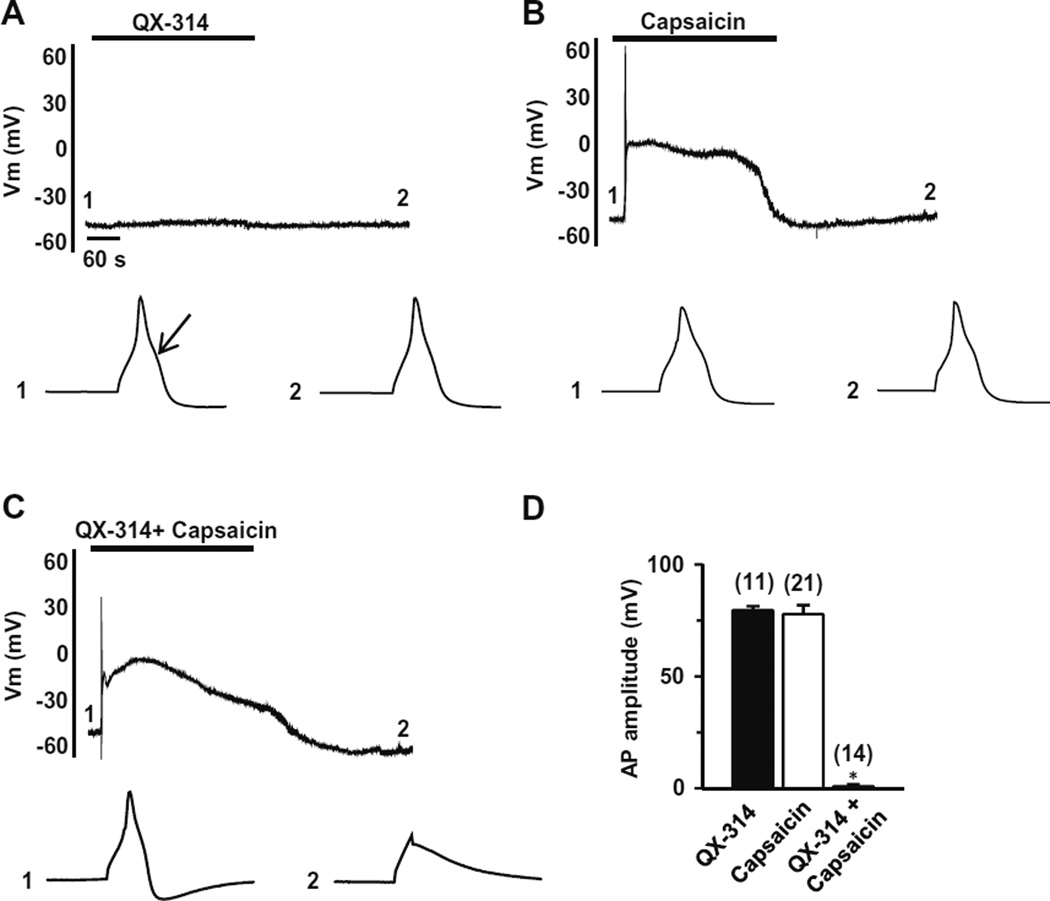
We next examined the effects of QX-314 on action potentials (APs) in TG neurons. As previously reported [26], capsaicin-sensitive neurons had a prominent shoulder in the falling phase of the action potential and in these cells capsaicin elicited a membrane depolarization and then robust AP firing (Fig. 3). Because APs are blocked by capsaicin due to the inactivation of VGSCs following the membrane depolarization elicited by capsaicin (data not shown), we examined APs evoked by current injection (250 pA, 3 ms) 5 min after washout of capsaicin, after the membrane potential returned to baseline (time point 2 in Fig. 3). By this approach, we could again test whether the effect of QX-314 inside the cells remained even after the washout of capsaicin and QX-314. Capsaicin-sensitive TG neurons were small sized (<25 µm in diameter) with an average resting membrane potential (Vrest) of −53.1 ± 1.1 mV (n = 46). QX-314 (5 mM, 5 min) alone had no effect on either Vrest or APs (99.6 ± 2.1%, P = 0.9, n = 11) (Fig. 3A). Capsaicin (1 µM, 5 min) alone decreased AP amplitude during membrane depolarization (data not shown) but APs at 5 min after washout were nearly identical to control (96.3 ± 4.5%, P = 0.4, n = 21; Fig. 3B). However, co-application (5 min) of QX-314 (5 mM) and capsaicin (1 µM) completely abolished the generation of action potential at 5 min after washout (1 ± 0.1%, P < 0.05, n = 14) (Fig. 3C). As in the INa experiments, we again observed that the blockade of AP firing lasted for 45 min of washout. The duration of the effect after washout suggests that the QX-314 remains in nociceptor neurons after its entry via TRPV1 channels.
Fig. 3.
Effects of QX-314 and capsaicin on action potentials in capsaicin-sensitive TG neurons. Representative current-clamp recordings over time following the application of QX-314 (5 mM, 5 min) (A), capsaicin (1 µM, 5 min) (B) or co-application of capsaicin and QX-314 (5 min) (C). Upper panel: action potentials (APs) were elicited by injection of 3-ms depolarizing current pulses with 250 pA amplitude. Note that membrane depolarization and transient action potential discharges were evoked by capsaicin in the capsaicin-sensitive TG neurons. Lower panel: APs recorded at the time points indicated at each upper panel. Arrow indicates the typical hump in the falling phase of AP in the capsaicin-sensitive TG neurons. (D) Collected results for the effects of QX-314, capsaicin, or co-application of both on AP amplitude in capsaicin-sensitive TG neurons. The numbers in parentheses indicate the number of cells tested. Results are means ± S.E.M. *P < 0.05 compared with the control (AP amplitude before drug application).
In capsaicin-insensitive TG neurons (>40 µm in diameter, with Vrest of −53.5 ± 3.4 mV, n = 29), there was no hump in the falling phase of the APs [26] (see Supplementary Fig. 1) and application of either QX-314 or capsaicin alone had no effect on the generation of APs (100.5 ± 1.1%, P = 0.9, n = 8 and 98.8 ± 0.7%, P = 0.8, n = 10, respectively) (Supplementary Fig. 1A and B). Co-application of capsaicin and QX-314 together also had no effect (96.7 ± 3.4%, P = 0.3, n = 11) (Supplementary Fig. 1C). Our data show that the blocking effect of APs by QX-314 and capsaicin, like that on sodium currents, is restricted to capsaicin-sensitive neurons.
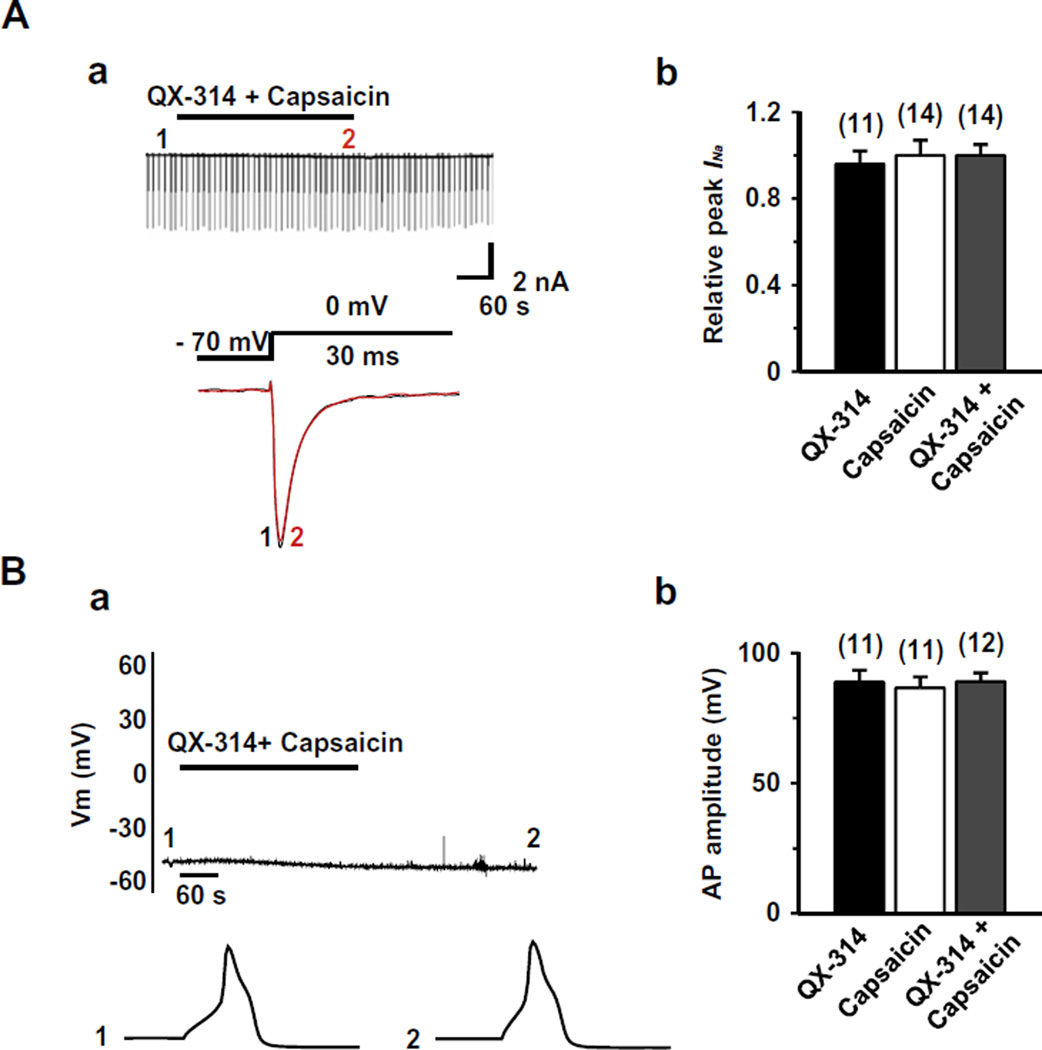
We further tested whether TRPV1 is required for the inhibitory effects of QX-314 on the INa and APs by studying small-sized nociceptive TG neurons (<25 µm in diameter, n = 34) with a hump in the falling phase of the APs in TRPV1 knock-out mice. As shown in Fig. 4, QX-314 applied together with capsaicin consistently failed to affect both INa (0.93 ± 0.05, P = 0.2, n = 14) (Fig. 4A) and APs (93.3 ± 5.8%, P = 0.3, n = 12) (Fig. 4B) in TG neurons from these mice. These results suggest that it is specifically the presence of TRPV1 in nociceptors that is required for QX-314 entry and block.
Fig. 4.
Effect of co-application of capsaicin and QX-314 on INa and APs in TRPV1 knock-out mice. (A) (a) Upper panel: a representative recording following the application or co-application of QX-314 (5 mM) and capsaicin (1 µM, 5 min) in the TRPV1 knock-out mice TG neurons. Lower panel: superimposed INa evoked by test pulse at the points indicated in upper panel. (b) Summary of the effects of QX-314, capsaicin, or co-application of both on INa in TG neurons from TRPV1 knock-out mice. (B) (a) Upper panel: A representative recording following the application or co-application of QX-314 (5 mM) and capsaicin (1 µM, 5 min). Lower panel: APs recorded at the time points indicated at the upper panel. (b) Collected results for the effects of QX-314, capsaicin, or co-application of both on AP amplitude in TRPV1 knock-out mice. TG neurons were small sized (<25 µm in diameter) with a resting membrane potential (Vres) of −54.2 ± 4.6 mV. The numbers in parentheses indicate the number of cells tested. Results are means ± S.E.M.
3.3. Co-application of QX-314 and capsaicin blocks INa and APs in dental nociceptors
Pain is the only sensation perceived by humans when noxious stimulus is applied to the teeth [34]. Thus, retrograde labeling the cells that innervates tooth pulp identifies a nearly pure population of nociceptors; dental primary afferent neurons [27]. We therefore examined next whether the co-application of QX-314 and capsaicin can block INa and APs in nociceptive dental primary afferent neurons. Consistent with our previous report [27], DiI-labeled neurons represented ~15% of the total cultured TG neurons, and were almost exclusively below 40 µm in diameter. The majority of dental primary afferent neurons responded to capsaicin (n = 37/45), indicating a prominent expression of TRPV1 in dental nociceptive neurons. Co-application (1 min) of capsaicin (1 µM) and QX-314 (5 mM) blocked INa (0.41 ± 0.02, P < 0.05, n = 14) and APs in the DiI-labeled TG neurons (0.6 ± 0.2%, P < 0.05, n = 14) (Fig. 5A and B). Expression of TRPV1 in dental primary afferent neurons was verified by immunoreactivity in DiI-labeled neurons (Fig. 5Ac). We found that only a few dental primary afferent neurons were not responsive to capsaicin (n = 8/45) (data not shown), and these were the only neurons in which the combined application of QX-314 and capsaicin failed to block INa or APs.
Fig. 5.
Effect of co-application of capsaicin and QX-314 on INa and APs in nociceptive dental primary afferent neurons. (A) (a) Upper panel: a representative recordings following the application or co-application of QX-314 (5 mM) and capsaicin (1 µM, 1 min) in dental primary afferent neurons. Lower panel: superimposed INa evoked by test pulse at the points indicated at the upper panel. (b) Collected results for the effects of QX-314, capsaicin, or co-application of both compounds on INa in nociceptive dental primary afferent TG neurons. (c) Dental primary afferent neurons were identified by retrograde labeling with a fluorescent dye, DiI, placed into the molar teeth. The photograph of TG neurons shows TRPV1-immunoreactivity (green), DiI (red), DAPI (blue) and merged. Scale bar = 50 µm. (B) (a) Upper panel: a representative recording following the application or co-application of QX-314 (5 mM) and capsaicin (1 µM, 5 min). Lower panel: APs recorded at the time points indicated at the upper panel. (b) Summary of the effects of QX-314, capsaicin, or co-application of both on AP amplitude in nociceptive dental primary afferent neurons. TG neurons were small sized (<25 µm in diameter) with the resting membrane potential (Vres) of −55.6 ± 3.2 mV. The numbers in parentheses indicate the number of cells tested. Results are means ± S.E.M. *P < 0.05 compared with the control (the AP amplitude before drug application).
3.4. Trigeminal mesencephalic neurons and motoneurons lack functional expression of TRPV1
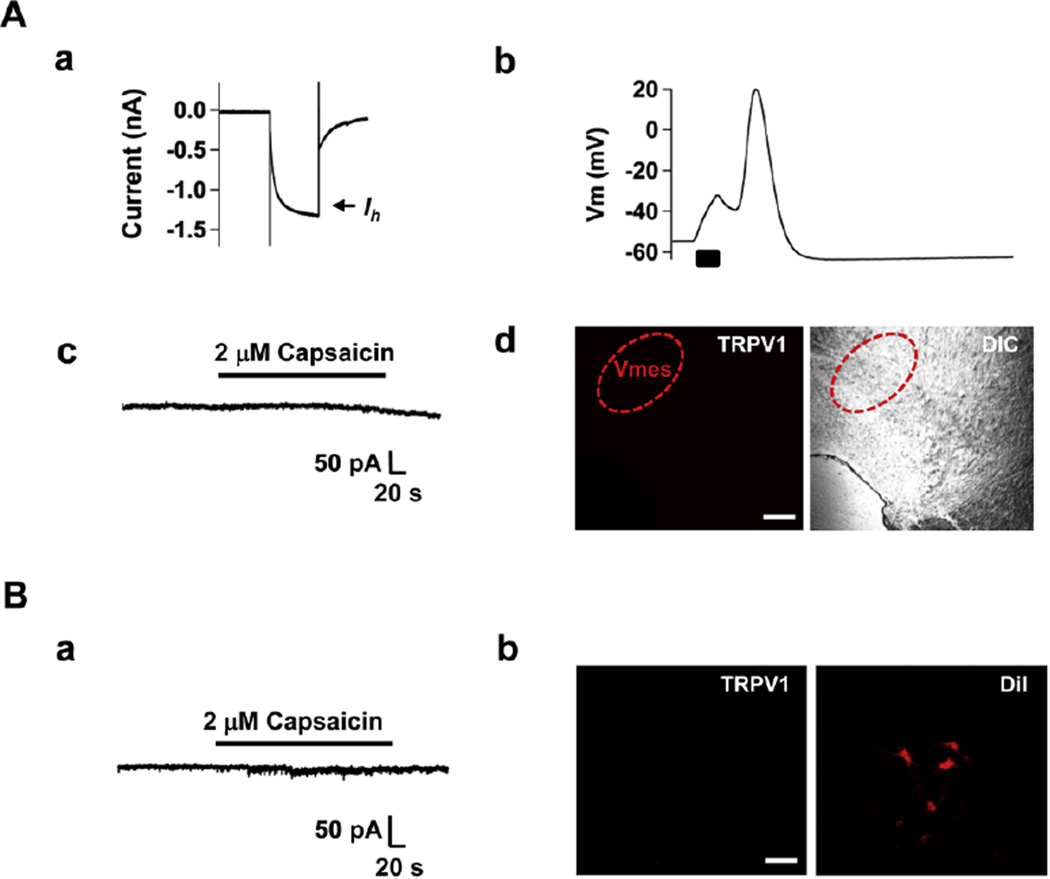
In addition to transmitting pain the trigeminal nerve also conveys proprioceptive sensation to the CNS and has motor axons. Proprioceptive neurons are located in the trigeminal mesencephalic nucleus (Vmes) and motor neurons in the trigeminal motor nucleus (Vmot) [16,21]. To verify the nociceptor-specific effects of the combination of QX-314 and capsaicin, we determined whether TRPV1 is expressed in the trigeminal mesencephalic and motor neurons using slice whole cell recordings and immunohistochemistry. We identified trigeminal mesencephalic neurons by observing large Ih currents (Fig. 6Aa) and typical delayed firing of action potential (Fig. 6Ab) [16]. In these Vmes neurons, the application of capsaicin (2 µM) did not induce inward currents (n = 12) (Fig. 6Ac) and we also could not detect TRPV1-immunoreactivity (Fig. 6Ad). Capsaicin (2 µM) also failed to evoke inward currents (n = 7) (Fig. 6Ba) and TRPV1-immunoreactivity was absent in Vmot neurons identified by retrograde labeling following DiI injection into the masseter muscle (Fig. 6Bb). These results suggest that co-application of QX-314 and capsaicin would not affect proprioceptive sensation or motor function, and is therefore truly pain selective.
Fig. 6.
Both trigeminal mesencephalic (proprioceptive) neurons and motoneurons lack functional expression of TRPV1. (A) Trigeminal mesencephalic neurons were identified by their typical Ih current (a) and AP shape (b). Capsaicin failed to activate inward current at Vh, −60 mV (c). Immuno-staining did not detect TRPV1; Mesencephalic trigeminal nucleus visualized under FITC filter and overlay with DIC (d). (B) No currents were evoked by capsaicin (applied at −60 mV) in trigeminal motoneurons (a). Trigeminal motoneurons were identified by retrograde labeling with DiI into the exposed masseter muscle. A representative photograph shows immunoreactivity of TRPV1 (FITC filter, green) and DiI (DiI filter, red) image in motor trigeminal nucleus (b). Scale bar = 200 µm (A); 100 µm (B).
3.5. Co-application of QX-314 and capsaicin inhibits the jaw-opening reflex when applied to sensory but not motor nerves
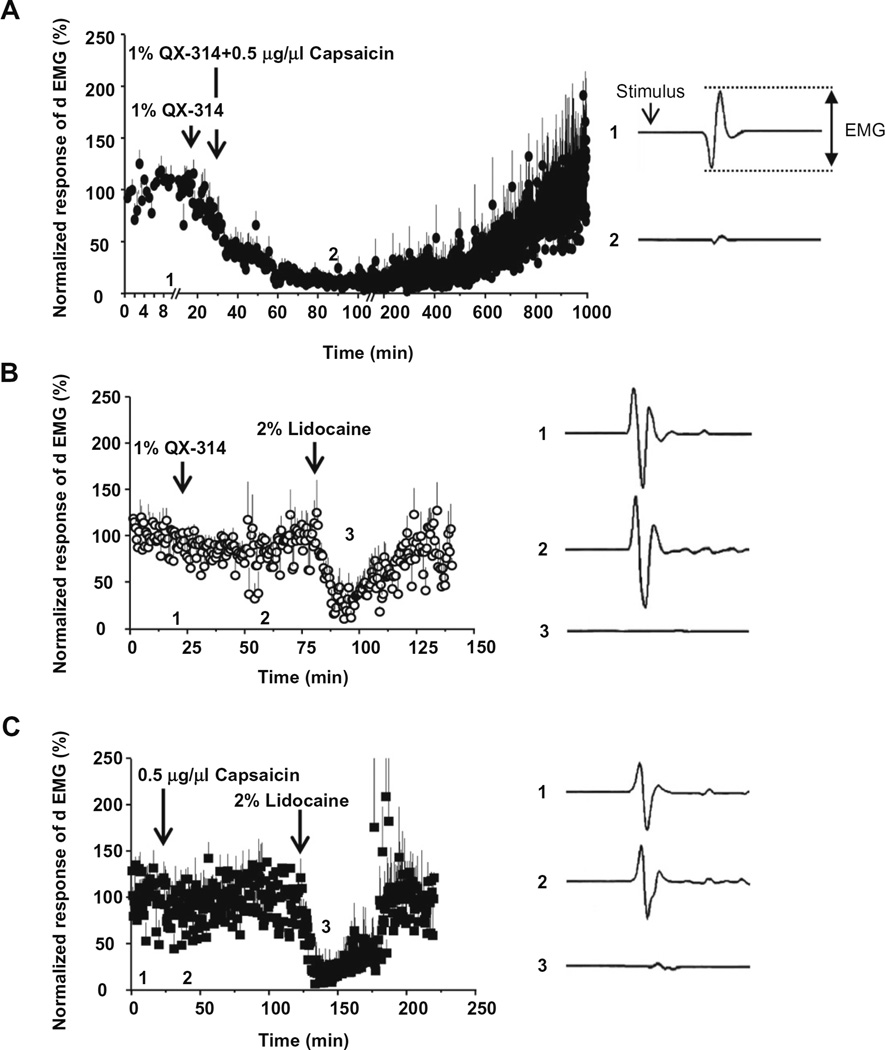
We next examined whether co-application of QX-314 and capsaicin specifically blocks conduction of pain-related signals in vivo by measuring the reflex activation of the digastric muscle in response to electrical stimulation of teeth as part of the nociceptive jaw-opening reflex. Co-application (50 µl) of QX-314 (1%) and capsaicin (0.5 µg/µl) onto the inferior alveolar nerve (the sensory nerve that innervates teeth and is the afferent limb of the reflex) totally blocked the electromyogram of digastric muscle (dEMG) with a slow onset and gradual recovery to base line over several hours (n = 6) (Fig. 7A). Application of either QX-314 (1%) (n = 4) (Fig. 7B) or capsaicin (0.5 µg/µl) alone (n = 4) (Fig. 7C) produced no effect. In contrast, lidocaine alone (2%, 100 µl) completely blocked the dEMG within a few minutes, with a block lasting about an hour (Fig. 7B and C). The inhibition of dEMG produced by co-administration of QX-314 and capsaicin was much longer than that of lidocaine alone (12 h versus 1 h).
Fig. 7.
Effects of QX-314 and capsaicin applied onto sensory nerve tested with jaw-opening reflex. As a measure of jaw-opening reflex, the digastric muscle EMG (dEMG) in response to electrical stimulus (two and a half times to the threshold, ×2.5T) to anterior teeth was measured from the anterior digastric muscle. The drugs were applied onto inferior alveolar nerve. The data points plot normalized amplitudes of dEMG over time (Left panels in A–C). Right panels show dEMGs at the time points (before and after drug administration, respectively) indicated at each left panel. The normalized EMG decreased dramatically (by almost 99%) after the application of 1% QX-314 with 0.5 µg/µl capsaicin (A). However, application of 1% QX-314 or 0.5 µg/µl capsaicin alone produced only modest changes of dEMG amplitudes (approximately −13% in (B) and −20% in C). 2% lidocaine used as a positive control was applied at the end of the experiment.
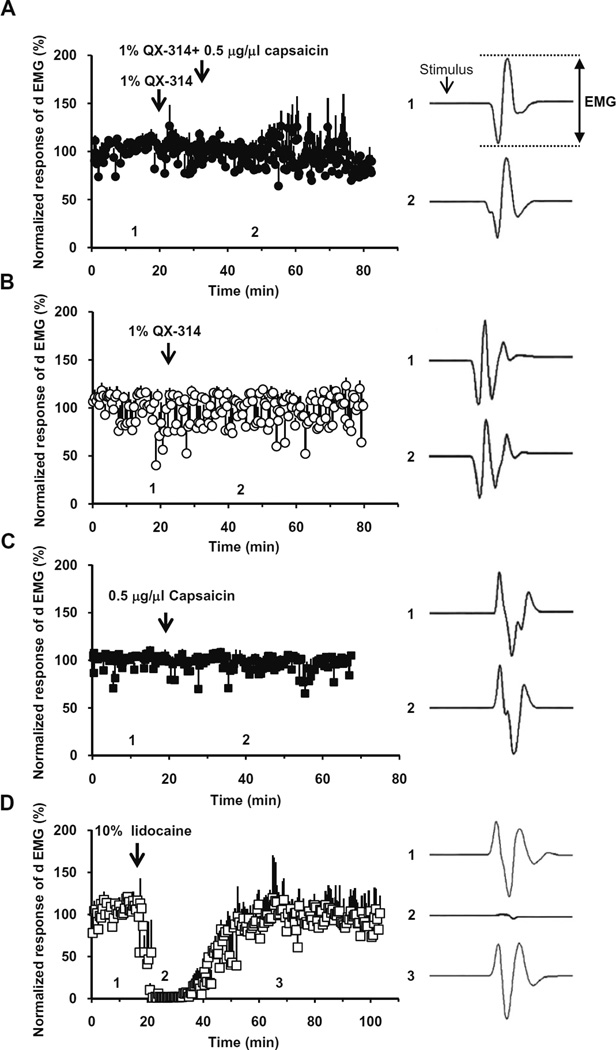
We then examined whether co-application of QX-314 and capsaicin produces a reduction in the reflex activity in the digastric muscle upon application to the mylohyoid nerve, which innervates the anterior belly of the digastric muscle and is the efferent limb of the nociceptive jaw-opening reflex. Application of QX-314 and capsaicin (50 µl), either separately or together, failed to inhibit the reflex (n = 6, 5 and 5, respectively) (Fig. 8A–C). In contrast, lidocaine (10%, 20 µl) administration totally blocked the activity for about an hour in all the rats tested (n = 4) (Fig. 8B and C), as expected for the non-selective blocking action on all types of nerve fibers.
Fig. 8.
Effects of QX-314 and capsaicin applied onto motor nerve tested with jaw-opening reflex. QX-314, capsaicin or both were applied onto mylohyoid nerve, the motor nerve innervating digastric muscle, while measuring dEMGs in response to electrical stimulus (×2.5T). Right panels show the representative dEMGs at the time points indicated in the left panels. The normalized EMG had no significant change in amplitude after 1% QX-314 with 0.5 µg/µl capsaicin (A), application of 1% QX-314 (B) and 0.5 µg/µl capsaicin (C). As a positive control, the dEMG amplitude decreased dramatically upon the application of 10% lidocaine (20 µl) onto mylohyoid nerve (D).
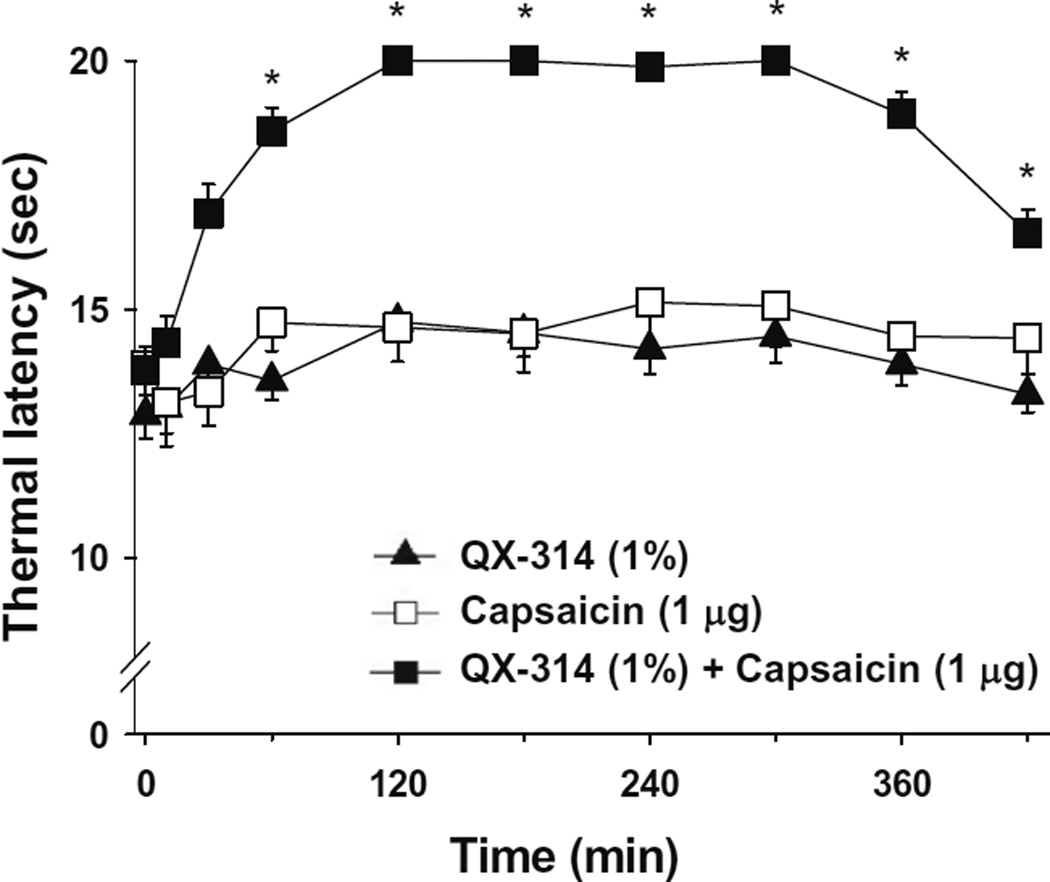
3.6. Co-application of QX-314 and capsaicin inhibits thermal hyperalgesia in the orofacial area
We next investigated whether co-application of QX-314 and capsaicin can reduce orofacial pain. After QX-314, either alone or together with capsaicin, was administered subcutaneously into the left vibrissa pad, we measured latencies of withdrawal responses following thermal stimulation. When QX-314 (1%) was injected together with capsaicin (1 µg), thermal latency significantly increased at 60 min after injection and this anesthetic effect was maintained throughout the full 7 h observation time (F(1, 20) = 33.657, P < 0.001), compared with the absence of any analgesia with QX-314 (1%) or capsaicin (1 µg) alone (Fig. 9).
Fig. 9.
Effects of subcutaneously applied QX-314 and capsaicin on pain behaviors in orofacial area. QX-314 alone, capsaicin alone, or together with QX-314 was administered subcutaneously into the left vibrissa pad and latencies of head withdrawal responses to thermal stimulation were determined at 10, 30, 60, 120, 180, 240, 300, 360 and 420 min. The number of animals was 11. *P < 0.001 versus QX-314.
4. Discussion
Our results suggest that pain-selective local anesthesia can be produced in the orofacial system by the strategy of selectively delivering charged sodium channel blockers into pain-sensing neurons without blocking other types of neurons. The trigeminal nerve, the largest of the cranial nerves, is primarily a sensory nerve in the face and head, but it also has certain motor functions such as mastication and swallowing [31]. The sensory function of the trigeminal nerve is to provide the tactile, proprioceptive and pain sensation of the face and mouth. The trigeminal ganglion (TG), analogous to the dorsal root ganglia (DRG) of the spinal cord, contains the cell bodies of incoming sensory nerve fibers, except for proprioceptor fibers, which have their cell bodies in the trigeminal mesencephalic nucleus (Vmes). Motor branches of the trigeminal nerve are distributed in the mandibular nerve that originates in the motor nucleus of the trigeminal nerve (Vmot) [14,31]. We found that co-administration of QX-314 and capsaicin blocks sodium channels and thereby inhibits excitability only in TRPV1-expressing nociceptive TG neurons and not in Vmes and Vmot neurons, which we also find do not express TRPV1. Consistent with the specificity for action to TRPV1-expressing nociceptive neurons, the jaw-opening reflex, a withdrawal reflex in the orofacial area, was inhibited by the co-application of QX-314 and capsaicin only onto the peripheral sensory nerve, but not onto the peripheral motor nerve. Moreover, from behavioral studies, we found that co-application of QX-314 and capsaicin produced long-lasting anti-nociceptive effects.
Inhibitory effects on INa and APs produced by co-application of QX-314 and capsaicin were restricted to capsaicin-sensitive TG neurons, and neither INa nor APs were blocked by co-application of QX-314 and capsaicin to neurons from TRPV1 knock-out mice. Under in vitro conditions, QX-314 co-applied with capsaicin produced the block of INa or APs in TRPV1-expressing trigeminal ganglion nociceptors within a minute, similar to DRG neurons [11]. Previous experiments in DRG neurons showing block of INa and APs by co-applied QX-314 and capsaicin [3] did not examine the time-course of the effect after washout; we now find that the effects of QX-314 and capsaicin persist for at least 45minutes after washout of both agents, consistent with the idea that QX-314 enters inside the cells through the TRPV1 channel opened by capsaicin and remains inside (blocking sodium channels from the cytoplasmic side) after the TRPV1 channels are no longer open.
Pain is the only sensation perceived by humans when any type of physiologic stimulus is applied specifically to tooth pulp [34]. We previously demonstrated that thermo-TRP channels play a critical role in the transduction of tooth pain [27]. Thus, we used dental primary afferent neurons to determine if co-administration of QX-314 and capsaicin has a selective nociceptor-specific action. As expected, we found that QX-314 applied together with capsaicin blocks both INa and APs in the majority of dental primary afferent neurons but not in Vmes neurons and Vmot neurons. These observations provide the possibility that, provided there are no toxicology issues, a mixture of QX-314 and capsaicin might be useful for producing pain-specific local anesthesia during general dental treatment, while leaving intact other non-painful sensations, motor function, and autonomic signaling.
We further demonstrated nociceptor-specific actions of the co-application of QX-314 and capsaicin using in vivo and behavioral studies. dEMG, which reflects the jaw-opening reflex in response to noxious stimuli, has long been used as an in vivo model of pain withdrawal reflex in the orofacial area [23] and to test the effects of local anesthetics [23,25]. Interestingly, when applied onto the inferior alveolar nerve, the sensory nerve which innervates the teeth, co-application of QX-314 and capsaicin totally abolished dEMG, an effect that lasted several hours. This suggests that TRPV1 channels are present on the axons of the sensory nerve fibers in the inferior alveolar nerve at sufficient density to allow QX-314 entry. TRPV1 is expressed on peripheral somatosensory nerve fibers (e.g. in sciatic nerve) [2], and we also now find that TRPV1 is expressed on the unmyelinated axons of inferior alveolar nerve (see Supplementary Fig. 2). When applied onto the mylohyoid nerve, the motor nerve that innervates the anterior belly of digastric muscle, co-application of QX-314 and capsaicin failed to block dEMG, presumably reflecting the lack of TRPV1 channels in the motor axons. Conduction of C-fiber and, to a lesser extent, Aδ-fiber sensory components of the compound action potential is decreased by perineural application of a high dose of capsaicin (1%) [1,10,28]. We found in the present study that dEMG was not significantly decreased by a much lower concentration of capsaicin (0.05%), when applied by itself.
The blocking effect of co-application of QX-314 and capsaicin applied to the inferior alveolar nerve in dEMG study was much longer than lidocaine. Similarly, co-application of QX-314 and capsaicin produced much longer anti-nociceptive effects in response to thermal stimulation, compared to lidocaine. Thus, the application of QX-314 together with capsaicin may be clinically useful as long-lasting, nociceptive-specific local anesthesia. It seems likely that charged QX-314 remains in the axon longer than lidocaine, which likely readily crosses the membrane in its uncharged (unprotonated) form. Eventually, trapped intracellular QX-314 might be slowly exocytosed from axons or directly metabolised within neurons. In hepatocytes, most lidocaine is N-deethylated via cytochrome P4503A4 (CYP3A4) enzymes [37]. Hence, a metabolic pathway from the quaternary amid QX-314 to lidocaine itself mediated by the same enzyme seems to be plausible. However, nothing is known about CYP-enzymes in sensory neurons or QX-314 metabolism in general.
Orofacial pain-selective local anesthesia could be very useful in treating dental pain, trigeminal neuralgia, pain from temporomandibular joint disorder, and other conditions. Capsaicin is probably not an ideal TRPV1 agonist for clinical use, because it activates transient action potential firing in nociceptors. TRPV1 agonists with less pungent or burning characteristics may be better. Recently, we demonstrated that eugenol, which evokes robust TRPV1-mediated inward currents that are blocked by capsazepine, produces a slow depolarization that can fail to trigger APs, probably because of sodium channel inactivation [26]. This result suggests that the initial irritable action of eugenol might be negligible compared to capsaicin, but it may still allow QX-314 to enter nociceptive neurons to block excitability. As recently demonstrated, lidocaine is also a TRPV1 agonist, and can be used therefore in the place of capsaicin to allow the entry of QX-314 through TRPV1 channels [4,17]. QX-314 itself is likely to have little toxicity with local administration; although QX-314 can inhibit cardiac sodium channels acting from the outside of the membrane [29], this effect should be no greater (and probably less) than that produced by systemic availability of lidocaine after local administration.
TRPV1 is not expressed by all nociceptors. Thus, selective entry of QX-314 into TRPV1-expressing nociceptors may not necessarily guarantee total block of pain sensation in surgery or with neuropathic pain conditions. Other channels in addition to TRPV1 may have large enough pores to allow QX-314 entry; recently, TRPA1, but not TRPM8, was demonstrated to undergo pore dilatation when activated by agonists [9,11]. As TRPA1 is also expressed by nociceptors [27], this channel could be another target for QX-314 application. Also, P2X2, receptors expressed by a subpopulation of sensory neurons as heteromers with P2X3 receptors [19] and dilated by the application of appropriate agonists [35], might be another possibility.
Although several issues remain to be addressed before clinical use of this strategy, the delivery of permanently charged local anesthetic compounds into nociceptors by combining them with an agonist for large-pore ion channels specifically expressed on these cells has provided a new possibility for producing pain-selective local anesthesia. Orofacial pain may be an especially attractive target for this strategy since our results show a very high degree of selectivity for the block of TRPV1-expressing nociceptive TG neurons, with no effects on trigeminal proprioceptive sensory neurons and trigeminal motor neurons. In addition to selectivity for pain signals, the much longer lasting (>7 h) block produced by QX-314 when co-applied with TRPV1 agonists compared with lidocaine (~1 h) would be a major advantage in many clinical situations.
Supplementary Material
Acknowledgments
This research was supported by Grant (R0A-2008-000-20101-0) from National Research Laboratory Program and a Grant (2009K001256) from the Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, Republic of Korea. S.B.O was supported by Alice and Joseph Brooks Fund in the Department of Neurobiology, Harvard Medical School and LG Yonam Foundation during part of this work. Drs. Bean and Woolf are inventors on a filed patent related to targeting impermeant sodium channel blockers into nociceptors that has been licensed by Endo Pharmaceuticals from Harvard University.
Abbreviations
- APs
action potentials
- CNS
central nervous system
- dEMG
digastric electromyogram
- DiI
1,1′-dioctadecyl-3,3,3″3′-teramethylindo-carbocyanine perchlorate
- DRG
dorsal root ganglia
- INa
voltage-gated sodium channel currents
- LAs
local anesthetics
- TG
trigeminal ganglion
- TRPV1
transient receptor potential vanilloid 1
- VGSCs
voltage-gated sodium channels
- Vmes
trigeminal mesencephalic nucleus
- Vmot
trigeminal motor nucleus
- Vrest
resting membrane potential.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.pain.2010.02.016.
References
- 1.Baranowski R, Lynn B, Pini A. The effects of locally applied capsaicin on conduction in cutaneous nerves in four mammalian species. Br J Pharmacol. 1986;89:267–276. doi: 10.1111/j.1476-5381.1986.tb10256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardini N, Neuhuber W, Reeh PW, Sauer SK. Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience. 2004;126:585–590. doi: 10.1016/j.neuroscience.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 4.Binshtok AM, Gerner P, Oh SB, Puopolo M, Suzuki S, Roberson DP, Herbert T, Wang CF, Kim D, Chung G, Mitani AA, Wang GK, Bean BP, Woolf CJ. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butterworth J, Oxford GS. Local anesthetics: a new hydrophilic pathway for the drug-receptor reaction. Anesthesiology. 2009;111:12–14. doi: 10.1097/ALN.0b013e3181a91624. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth JFt, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990;72:711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Julius D. Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol. 1999;9:525–530. doi: 10.1016/S0959-4388(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Kim D, Bianchi BR, Cavanaugh EJ, Faltynek CR, Kym PR, Reilly RM. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol Pain. 2009;5:3. doi: 10.1186/1744-8069-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung JM, Lee KH, Hori Y, Willis WD. Effects of capsaicin applied to a peripheral nerve on the responses of primate spinothalamic tract cells. Brain Res. 1985;329:27–38. doi: 10.1016/0006-8993(85)90509-8. [DOI] [PubMed] [Google Scholar]
- 11.Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- 12.Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131:243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang Z, Park CK, Li HY, Kim HY, Park SH, Jung SJ, Kim JS, Monteil A, Oh SB, Miller RJ. Molecular basis of Ca(v)2.3 calcium channels in rat nociceptive neurons. J Biol Chem. 2007;282:4757–4764. doi: 10.1074/jbc.M605248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried K, Bongenhielm U, Boissonade FM, Robinson PP. Nerve injury-induced pain in the trigeminal system. Neuroscientist. 2001;7:155–165. doi: 10.1177/107385840100700210. [DOI] [PubMed] [Google Scholar]
- 15.Gold MS, Reichling DB, Hampl KF, Drasner K, Levine JD. Lidocaine toxicity in primary afferent neurons from the rat. J Pharmacol Exp Ther. 1998;285:413–421. [PubMed] [Google Scholar]
- 16.Kang Y, Saito M, Sato H, Toyoda H, Maeda Y, Hirai T, Bae YC. Involvement of persistent Na+ current in spike initiation in primary sensory neurons of the rat mesencephalic trigeminal nucleus. J Neurophysiol. 2007;97:2385–2393. doi: 10.1152/jn.01191.2006. [DOI] [PubMed] [Google Scholar]
- 17.Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, Gavva NR, Reeh PW, Nau C. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest. 2008;118:763–776. doi: 10.1172/JCI32751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leston JM. Functional anatomy of the trigeminal nerve. Neurochirurgie. 2009;55:99–112. doi: 10.1016/j.neuchi.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 20.McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–856. doi: 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- 21.McDavid S, Verdier D, Lund JP, Kolta A. Electrical properties of interneurons found within the trigeminal motor nucleus. Eur J Neurosci. 2008;28:1136–1145. doi: 10.1111/j.1460-9568.2008.06413.x. [DOI] [PubMed] [Google Scholar]
- 22.Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyoshi T, Aida H, Kaneko Y. Comparative study on anesthetic potency of dental local anesthetics assessed by the jaw-opening reflex in rabbits. Anesth Prog. 2000;47:35–41. [PMC free article] [PubMed] [Google Scholar]
- 24.Oh SB, Piao ZG, Shin SS, Ren D, Park K, Kim JS. GABAergic and serotonergic modulation of calcium currents in rat trigeminal motoneurons. Biochem Biophys Res Commun. 2003;309:58–65. doi: 10.1016/s0006-291x(03)01527-4. [DOI] [PubMed] [Google Scholar]
- 25.Ohkado S, Ichinohe T, Kaneko Y. Comparative study on anesthetic potency depending on concentrations of lidocaine and epinephrine: assessment of dental local anesthetics using the jaw-opening reflex. Anesth Prog. 2001;48:16–20. [PMC free article] [PubMed] [Google Scholar]
- 26.Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain. 2009;144:84–94. doi: 10.1016/j.pain.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Park CK, Kim MS, Fang Z, Li HY, Jung SJ, Choi SY, Lee SJ, Park K, Kim JS, Oh SB. Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem. 2006;281:17304–17311. doi: 10.1074/jbc.M511072200. [DOI] [PubMed] [Google Scholar]
- 28.Petsche U, Fleischer E, Lembeck F, Handwerker HO. The effect of capsaicin application to a peripheral nerve on impulse conduction in functionally identified afferent nerve fibres. Brain Res. 1983;265:233–240. doi: 10.1016/0006-8993(83)90337-2. [DOI] [PubMed] [Google Scholar]
- 29.Qu Y, Rogers J, Tanada T, Scheuer T, Catterall WA. Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na+ channel. Proc Natl Acad Sci USA. 1995;92:11839–11843. doi: 10.1073/pnas.92.25.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigler ML, Drasner K, Krejcie TC, Yelich SJ, Scholnick FT, DeFontes J, Bohner D. Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg. 1991;72:275–281. doi: 10.1213/00000539-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 32.Stanley FM. Handbook of local anesthesia 5e. New York, USA: Elsevier Inc.; 1994. [Google Scholar]
- 33.Suzuki S, Gerner P, Colvin AC, Binshtok AM. C-fiber-selective peripheral nerve blockade. Open Pain J. 2009;2:24–29. [Google Scholar]
- 34.Taddese A, Nah SY, McCleskey EW. Selective opioid inhibition of small nociceptive neurons. Science. 1995;270:1366–1369. doi: 10.1126/science.270.5240.1366. [DOI] [PubMed] [Google Scholar]
- 35.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Woolf CJ. Pain TRPs. Neuron. 2005;46:9–12. doi: 10.1016/j.neuron.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Wang JS, Backman JT, Taavitsainen P, Neuvonen PJ, Kivisto KT. Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos. 2000;28:959–965. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.