Abstract
Objectives
Amplification is a core component of early intervention for children who are hard of hearing (CHH), but hearing aids (HAs) have unique effects that may be independent from other components of the early intervention process, such as caregiver training or speech and language intervention. The specific effects of amplification are rarely described in studies of developmental outcomes. The primary purpose of this manuscript is to quantify aided speech audibility during the early childhood years and examine the factors that influence audibility with amplification for children in the Outcomes of Children with Hearing Loss (OCHL) study.
Design
Participants were 288 children with permanent hearing loss who were followed as part of the OCHL study. All of the children in this analysis had bilateral hearing loss and wore air-conduction behind-the-ear HAs. At every study visit, hearing thresholds were measured using developmentally-appropriate behavioral methods. Data were obtained for a total of 1043 audiometric evaluations across all subjects for the first four study visits. In addition, the aided audibility of speech through the HA was assessed using probe microphone measures. Hearing thresholds and aided audibility were analyzed. Repeated-measures analyses of variance were conducted to determine if patterns of thresholds and aided audibility were significantly different between ears (left vs. right) or across the first four study visits. Furthermore, a cluster analysis was performed based on the aided audibility at entry into the study, aided audibility at the child’s final visit, and change in aided audibility between these two intervals to determine if there were different patterns of longitudinal aided audibility within the sample.
Results
Eighty-four percent of children in the study had stable audiometric thresholds during the study, defined as threshold changes <10 dB for any single study visit. There were no significant differences in hearing thresholds, aided audibility, or deviation of the HA fitting from prescriptive targets between ears or across test intervals for the first four visits. Approximately 35% of the children in the study had aided audibility that was below the average for the normative range for the Speech Intelligibility Index (SII) based on degree of hearing loss. The cluster analysis of longitudinal aided audibility revealed three distinct groups of children: a group with consistently high aided audibility throughout the study, a group with decreasing audibility during the study, and a group with consistently low aided audibility.
Conclusions
The current results indicated that approximately 65% of children in the study had adequate aided audibility of speech and stable hearing during the study period. Limited audibility was associated with greater degrees of hearing loss and larger deviations from prescriptive targets. Studies of developmental outcomes will help to determine how aided audibility is necessary to affects developmental outcomes in CHH.
INTRODUCTION
Hearing aids (HAs) are provided to children who are hard of hearing (CHH) to increase access to acoustic components of speech and language. However, developmental studies of CHH have generally focused broadly on the positive effects of early intervention, rather than specifically on the effects of amplification. The development of CHH has been studied in relation to the age at which they were enrolled in early intervention services. Specifically, infants who receive early intervention services before 6–9 months of age have better outcomes across a range of communication skills (Moeller, 2000; Yoshinaga-Itano, 2003; Kennedy et al. 2006; Watkin et al. 2007; Fulcher et al. 2012; Vohr et al. 2012; Ambrose et al. 2014). Amplification is a core component of early intervention for CHH, but HAs may have unique effects that are independent from other components of the early intervention process, such as caregiver training or speech and language intervention. The specific effects of amplification are rarely analyzed separately from other components of early intervention in studies of developmental outcomes. This manuscript will quantify aided speech audibility over time and the factors that influence audibility with amplification during the Outcomes of Children with Hearing Loss (OCHL) study. Later in this volume, longitudinal effects of amplification on developmental outcomes are examined (Tomblin et al. this issue, pp. XXXX; McCreery et al. this issue, pp. XXXX).
The influence of HAs on developmental outcomes is frequently considered to be constant or represented with variables that reflect multiple aspects of early intervention, rather than the specific effects of amplification. Prior studies in CHH have usually relied on the age that the child was first fitted with HAs as a predictor of developmental outcomes (Sininger et al. 2010; Wake et al. 2005; Ching et al. 2013). It is noteworthy that the effect of age of amplification on outcomes is not consistent across these studies. For example, Sininger and colleagues (2010) reported that the age of amplification in a group of 44 CHH influenced speech perception and speech production outcomes at 3 years of age, but did not have an effect on other developmental outcomes. In contrast, studies by Wake et al. (2005) of children with hearing loss at 7–8 years of age and Ching et al. (2013) of children with hearing loss at 3 years of age did not find consistent relationships between the age at HA fitting and a range of communication and developmental outcomes in CHH. Therefore, conclusions about the influence of age at amplification on outcomes for children do not appear to be uniform based on previous studies.
The lack of coherence in the effects of age of amplification may be related to several different limitations. First, age of amplification is strongly related to other intervention milestones, such as the age of enrollment in intervention. Although the age of the HA fitting and age at enrollment in early intervention often are easy to document, both are dependent on the age of identification with hearing loss (Holte et al. 2012). Age at amplification may not be adequate to represent the specific effects of amplification independently from other core parts of early intervention such as caregiver training on providing language input and consistent HA use for their child or speech and language therapy.
A second limitation of age of amplification is the reduction in variability in this milestone as a result of the widespread implementation of universal newborn hearing screening (UNHS) in the United States. Currently, amplification is initiated at earlier and more consistent ages across children than in the era prior to UNHS (Holte et al. 2012). Although early age of amplification is positive, the limited variability in the distribution of ages at which amplification is provided may have statistical properties that are not ideal when this variable is used as a predictor of outcomes. Children who pass their newborn hearing screening and are identified and fit with hearing aids at a later ages may have late-onset hearing loss or milder degrees of hearing loss. The heterogeneity of these factors further complicates comparisons of children who receive amplification earlier to those who receive amplification later in the post-UNHS era (Walker et al. 2014). Therefore, the age at which the child receives amplification may not be a strong predictor of individual differences across children or within children over time, when amplification is provided reliably during early infancy or when children receive amplification at later ages due to a range of confounding factors.
A third limitation is that previous studies use age of amplification to predict developmental outcomes without considering sources of variability, such as the amount of aided audibility and consistency of hearing aid use, which could influence the benefits of amplification among children. The variability in aided audibility of speech or the child’s HA use among children who have similar ages of identification has not been directly evaluated in large scale studies. Cross-sectional data suggest that the audibility provided by the HA varies considerably across children due to a number of factors. For example, the degree to which speech audibility can be restored by amplification has been shown to depend on the severity of hearing loss (Sininger et al. 2010; Bagatto et al. 2011) and the proximity of the fitting to prescriptive targets (Strauss & van Dijk, 2008; McCreery, Bentler & Roush, 2013). Children with greater degrees of hearing loss and fittings below prescriptive targets had poorer audibility. Using age of amplification as a predictor of developmental outcomes does not account for the fact that speech audibility may be different across children or over time based on degree of hearing loss or the proximity of the individual HA fitting to prescriptive targets.
Studies of the effects of variability in amplification on outcomes are lacking, which may be due, at least in part, to the complexity of evaluating changes in amplification longitudinally. Multiple inter-related factors will influence the amount of amplification and audibility that a child has access to over time. Changes in hearing thresholds (Pittman & Stelmachowicz, 2003) and ear-canal acoustics (Bagatto et al. 2002; 2005) can lead to variability in the amount of speech audibility children receive from amplification over time. Specifically, children who have progression of hearing loss will have more limited audibility for speech if amplification is not promptly adjusted. Similarly, temporary decreases in hearing due to otitis media or middle ear effusion may impact audibility. Typical ear canal growth during early childhood leads to increased ear canal volume. If the amount of amplification provided by the child’s HA remains constant as the ear canal grows, the sound level in the ear canal will decrease due to the increase in canal volume. These acoustic changes in the ear canal are reflected by changes in the real-ear-to-coupler difference (RECD; Bagatto et al. 2005; Bingham et al. 2009). For these reasons, guidelines for audiological management of pediatric amplification recommend frequent audiometric assessment and verification of HA output to account for these changes during early childhood (Bagatto et al. 2010; King et al. 2010; American Academy of Audiology, 2013). The effects of changes in threshold and hearing aid fitting over time on aided audibility have yet to be documented.
In order to determine the longitudinal effects of amplification on developmental outcomes for CHH who participated in the OCHL study, variability in aided speech audibility across study visits and the factors that affect speech audibility with amplification first must be quantified. The aim of this manuscript is to answer two specific research questions:
How do the characteristics of hearing thresholds and amplification in CHH change during early childhood? Children are expected to experience changes in behavioral thresholds and amplification over time, which is expected to influence aided audibility.
What audiological factors influence aided audibility in CHH during early childhood? Changes in ear canal acoustics, hearing thresholds, and the gain provided by the HA are predicted to influence the consistency of audibility during the early childhood years.
The data from the current study will further the theoretical understanding of methods for modeling the effects of amplification in future studies of developmental outcomes in children and provide support for clinical recommendations for management of pediatric amplification in children.
METHOD
Participants
Comprehensive demographic data for the sample are provided in Tomblin et al. (this issue, pp. XXXX ). The analysis presented in this paper includes audiometric and hearing aid data from 288 children with permanent hearing loss who were followed as part of the OCHL study. All of the children in this analysis had bilateral hearing loss and wore air-conduction behind-the-ear HAs. Children without amplification (n=7), children who wore bone-conduction devices (n= 7) and children who received cochlear implants (CIs) because of progression of hearing loss or limited progress in development during the study (n=10) were excluded. The number of children in the sample at each study visit interval is reported in Table 1 by type of hearing loss. Two hundred and fifteen children (74.7%) did not pass newborn hearing screen. The mean age at confirmation of hearing loss was 7.32 months (SD = 12.09) and the mean age at fitting of amplification was 11.20 months (SD = 14.44) for children who did not pass newborn hearing screening. Seventy-four children were identified with hearing loss after birth, due to passing the NHS or not being screened (one child’s NHS status was unknown). Of the 74 children who were not identified through newborn hearing screening, the mean age at confirmation of hearing loss was 29.18 months (SD = 17.61) and the mean age at fitting of amplification was 31.03 months (SD = 17.63). The mean better-ear PTA for the for all children reported in this analysis at the first visit was 48.22 dB HL (SD = 13.72).
Table 1.
Type of hearing loss and age range for each study visit
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Age Range (months) | 5–87 | 11–100 | 17–110 | 23–110 | ||||
| Ear | R | L | R | L | R | L | R | L |
| Sensorineural | 200 | 201 | 214 | 212 | 176 | 175 | 112 | 110 |
| Mixed | 11 | 11 | 6 | 6 | 4 | 3 | 2 | 3 |
| Conductive | 8 | 6 | 7 | 8 | 6 | 6 | 3 | 3 |
| ANSD | 7 | 9 | 6 | 9 | 6 | 8 | 4 | 5 |
| Undetermined | 62 | 61 | 31 | 29 | 16 | 16 | 7 | 7 |
| Total | 288 | 264 | 208 | 128 | ||||
R = Right; L = Left; ANSD = Auditory neuropathy spectrum disorder
Data collection
Children and their families participated in an initial baseline visit, followed by visits twice a year for children under age 2 years and once a year for children older than 2 years for up to four years, or until 9 years of age. Study visits occurred in close proximity to the child’s birthday or two specific age milestones (6 months and 18 months). The period of time for the four visits reported in this study varied from 2 years for the youngest participants to 4 years for children enrolled at older ages. All data collection occurred in a sound-treated audiometric test booth, audiology clinic office or mobile testing van.
Audiometric data
Hearing thresholds were measured using developmentally-appropriate behavioral methods, including visual reinforcement audiometry, conditioned play audiometry, or conventional audiometry. Audiological assessment was completed by an audiologist with experience working with children. Data were obtained for a total of 1043 audiometric evaluations across all subjects for the first four study visits. Octave frequencies from 250 Hz through 8000 Hz were tested and inter-octave frequencies were tested, whenever possible, if there was a difference between octave frequencies of 15 dB or more. The number of test frequencies obtained depended on the child’s attention span and cooperation. Insert earphones (Etymotic Research, ER-3A) were used to assess air conduction thresholds. Supra aural headphones (TDH-49P) were used only in rare cases where inserts were not possible due to ear canal debris or drainage. Bone conduction thresholds were obtained whenever the child’s cooperation permitted. In cases where the audiogram could not be measured at the study visit (Visit 1, n=42, 14.5%; Visit 2, n = 27, 10.2%; Visit 3, n = 14, 6.7%; Visit 4, n= 8, 6.5%), the child’s most recent audiogram was obtained from their clinical audiologist. Tympanometry using a 226 Hz probe tone was assessed at the same time as the study audiogram whenever possible. Table 2 includes the tympanometric classifications across the first four study visits. Normal tympanometry was classified as peak static acoustic admittance > 0.2 mmho and tympanometric peak pressure between +100 and −150 daPa. The presence of tympanostomy tubes was determined by a residual ear canal volume > 2 cm3 and otoscopic inspection of the ear canal.
Table 2.
Classification of tympanometric data by visit
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Ear | R | L | R | L | R | L | R | L |
| Normal | 165 | 163 | 161 | 155 | 116 | 116 | 70 | 70 |
| Abnormal | 45 | 45 | 25 | 33 | 21 | 23 | 9 | 5 |
| Patent tube | 43 | 43 | 33 | 32 | 27 | 20 | 15 | 17 |
| Could not test | 20 | 22 | 17 | 15 | 11 | 15 | 5 | 5 |
| Did not test | 15 | 15 | 28 | 29 | 31 | 31 | 25 | 26 |
| Total | 288 | 288 | 264 | 264 | 206 | 205 | 124 | 123 |
R = Right ear; L = Left ear.
Hearing aid verification data
The aided audibility of speech through the HA was assessed with probe microphone measures at each visit. Individually-measured real-ear-to-coupler difference (RECD) values with the child’s personal earmolds were used whenever possible. If the child would not cooperate with individually-measured RECD, age-related average RECD values were used to simulate in situ measurements of HA output in the 2 cm3 coupler of the Audioscan Verifit or Audioscan RM500 SL HA analyzer. In situ probe microphone measurements of HA output with the HA on the ear were completed with some cooperative older children. Table 3 displays the frequency of each type of verification completed as part of the study visit.
Table 3.
Hearing aid verification method by study visit
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | |
|---|---|---|---|---|
| In situ | 22 (7%) | 21 (8%) | 16 (7%) | 11 (9%) |
| Measured RECD | 160 (57%) | 179 (68%) | 156 (75%) | 108 (84%) |
| Average RECD | 106 (36%) | 64 (24%) | 34 (18%) | 9 (7%) |
| Total | 288 | 264 | 206 | 128 |
RECD = Real-ear-to-coupler difference
Verification was completed for soft (50 or 55 dB SPL) and average (60 or 65 dB SPL, 10 dB higher than the level for soft) input levels using the standard speech stimulus (“carrot passage”) to represent the long-term average speech spectrum. Maximum power output (MPO) was verified using swept pure tone stimuli. For children with nonlinear frequency compression (NFC) activated in their HA (Visit 1 = 76, Visit 2 = 79, Visit 3 = 73, Visit 4 = 53), filtered speech bands with nominal frequencies above the start frequency were measured to estimate the level and frequency location of those bands after lowering. In addition to verification of speech audibility, ANSI (ANSI S3.22–2003) standard measures of HA function were completed to document that the HAs were functioning within manufacturer specifications. If verification indicated that the child’s hearing aids were providing aided audibility less than an SII of 0.25, a letter was provided to the family to follow-up with the child’s dispensing audiologist. Adjustments to the child’s amplification by the research audiologists were not made as part of the study.
Aided audibility calculation
To estimate the aided audibility of speech, a modification of the Speech Intelligibility Index (SII; ANSI S3.5 – 1997, R2007) that permits calculation of audibility with conventional amplification or NFC was applied to verification data obtained for soft and average speech input levels. The audibility of the long-term average speech spectrum was calculated for each listener, ear and input level. The 1/3-octave-band calculation method was used with the average band-importance weighting function from Table 3 of the ANSI SII standard. The calculation assumed a non-reverberant environment. The levels of speech were converted to free-field using the free-field to eardrum transfer function from the SII. Audiometric thresholds were converted from dB HL to dB SPL and then interpolated and extrapolated to correspond with 1/3-octave-band frequencies using a frequency-specific bandwidth adjustment to convert pure tone thresholds to equivalent 1/3-octave-band levels (Pavlovic, 1987). The spectrum levels of speech and threshold-equivalent noise for each child’s audiogram were entered into a spreadsheet to calculate sensation level (SL) for each 1/3-octave band. The sensation level in each band was divided by 30 dB. The SL was multiplied by the importance weight for that band, and the sum of these products for all bands generated the SII for each condition.
For the subgroup of children with NFC signal processing activated in their HAs, the SII estimate of audibility was modified to account for frequency lowering. For these children, the SII calculation was the same as for children with conventional processing, except the SL for each frequency band above the start frequency was calculated at the frequency where the filtered band was measured during the verification process with NFC activated. The outputs of the filtered speech bands above the start frequency were entered into an SII calculator to estimate the amount of information that was accessible with lowering. This method has been used to calculate audibility with NFC in two previous studies (McCreery et al. 2014; Bentler et al. 2014). This modification of the SII for NFC assumes that speech information carries the same importance after frequency lowering and does not account for the potential reduction in spectral distinctiveness that could occur with NFC.
Hearing aid fitting data
The HA verification data obtained at each study visit was also compared to Desired Sensation Level v.5 (DSL; Scollie et al. 2005) prescriptive targets. DSL targets were reported as being used to fit the HA by the child’s dispensing audiologist in all but two cases (McCreery, Bentler & Roush, 2013). Those two children were excluded from the comparisons to prescriptive targets at each study visit. Two estimates of the proximity of the fitting to prescriptive targets were used: root-mean-square (RMS) error and absolute deviation in dB. The RMS error or quadratic mean is calculated by taking the square root of the mean of the squared differences between the HA output and prescriptive target for 500, 1000, 2000 and 4000 Hz. The RMS error criterion has been used to assess proximity to prescriptive targets in previous studies with adults (Byrne & Cotton 1988; Cox & Alexander 1990; Baumfield & Dillon 2001; Moore et al. 2001) and children (McCreery et al. 2013). The RMS provides an overall estimate of the proximity of the fitting to prescriptive targets, but does not indicate the direction of the error (+/−) or frequency. Therefore, the absolute deviation from prescriptive targets was calculated for the same four frequencies used to calculate the RMS error to allow for estimation of deviation from prescriptive target at each frequency.
Statistical analyses
Statistical analyses were completed either using SAS v9.3 or the R software interface (Version 3.0.2; R Core Team, 2014). Descriptive statistics for each outcome measure were calculated to estimate differences in thresholds, ear acoustics and aided audibility at each study visit and as a function of age. Data at each study visit represent children who contributed data at that visit and all previous visits. Analyses of variance were used to analyze mean differences across repeated-measures and between study visits. Linear regression was used to examine predictors of specific outcome measures. All predictor variables were mean-centered to minimize the potential for multicollinearity. Regression assumptions, including normality, were assessed through residual analysis and there was no evidence of violation of the modeling assumptions. Variance inflation factors for all linear regression analyses were less than 5. A linear mixed model with a random intercept for each subject was used to analyze the effects of age, degree of hearing loss, and RMS error across repeated study visits. To analyze different patterns of audibility over the course of the study, a cluster analysis was completed on aided audibility within the sample over time.
RESULTS
Behavioral audiometric thresholds
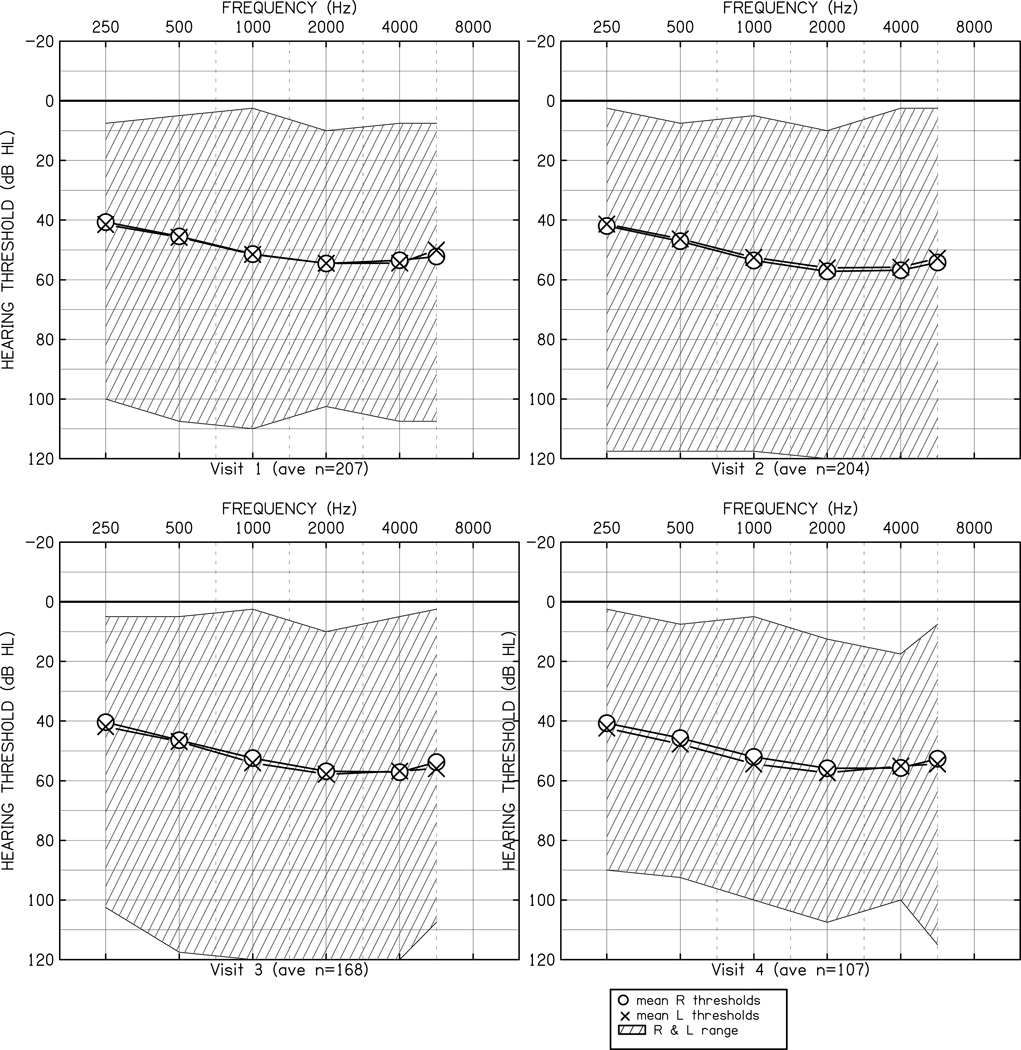
Figure 1 displays the average behavioral audiometric data in dB HL for each study visit, as well as the range of audiometric data as a function of frequency. A repeated-measures analysis of variance was conducted to determine if the pattern of thresholds was significantly different between ears (left vs. right), across test frequencies (250, 500, 1000, 2000, 4000 Hz) or across the first four study visits. The main effect of frequency [F (4, 2468) = 53.76, p < 0.001, η2p = 0.08] was significant, indicating that the average threshold was different as a function of frequency. To examine the pattern of significant differences across frequency, post hoc tests with Tukey’s Honestly Significant Difference (HSD) were conducted with a minimum mean significant difference of 4.1 dB. The average threshold at 250 Hz (41.6 dB) was lower than the 500 Hz threshold (46.2 dB). Both 250 Hz and 500 Hz were lower than the average thresholds at 1000 Hz (53.5 dB), 2000 Hz (56.6 dB) and 4000 Hz (54.9 dB). There were no other significant differences across frequency in the average threshold. The main effects for visit [F(3,617) = .16, p =0.98, η2p = 0.001] and ear [F(1,617) = 2.64, p = 0.11, η2p = 0.004] were not significant. None of the higher-order interactions were significant. The lack of significant main effect for visit indicates that the average behavioral audiometric thresholds did not vary significantly across study visits.
Figure 1.
Audiometric thresholds in dB HL as a function of frequency in Hz. The O (right ear) and X (left ear) symbols represent the mean thresholds at each frequency. The cross-hatched area represents the range of thresholds in the sample at each frequency. Each panel displays thresholds for a specific study visit.
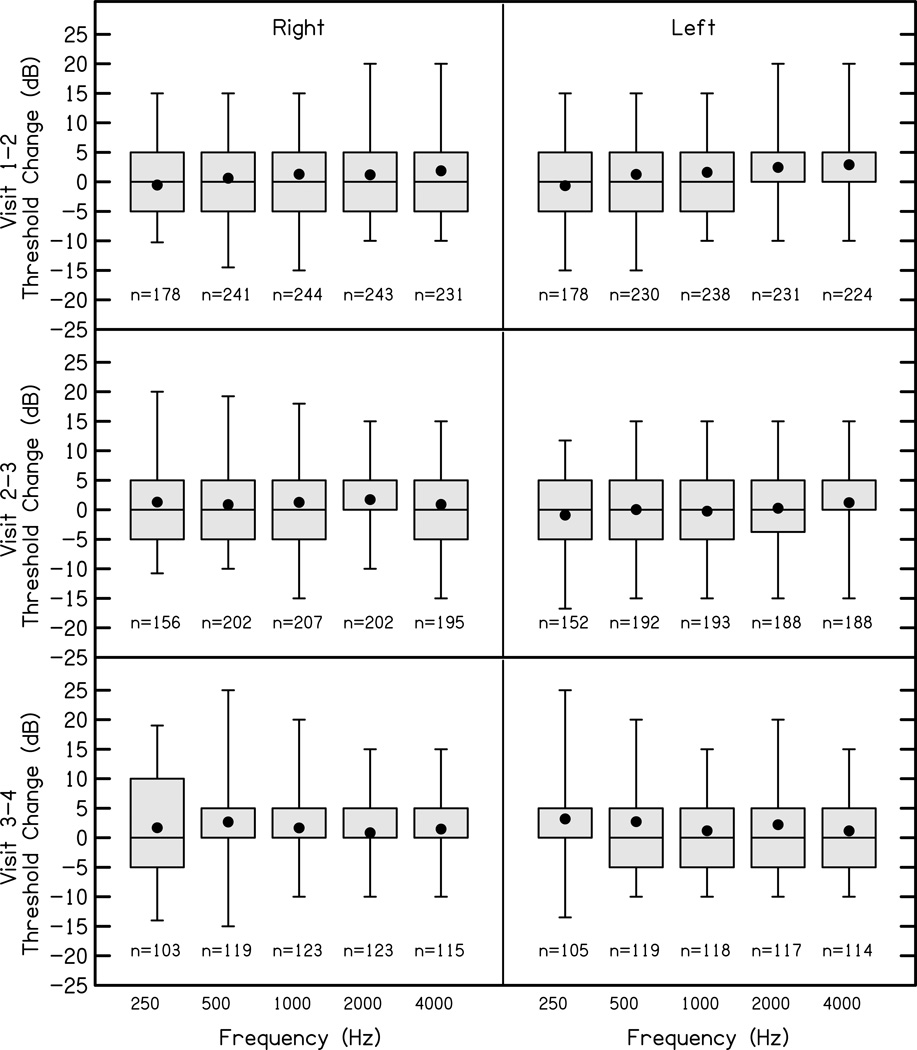
Figure 2 displays the differences between thresholds in dB for each audiometric test frequency across visits for the first four study visits.
Figure 2.
The changes in thresholds between visit intervals (rows) for right (first column) and left (second column) ears at octave frequencies between 250 Hz and 4000 Hz. The boxes represent the 25th-75th percentiles (interquartile range) and the whiskers represent the 5th and 95th percent confidence intervals of the mean. Solid lines are the median and solid circles represent the mean. Positive numbers indicate increased thresholds, and negative number represent decreased thresholds relative to the previous visit.
For all frequencies and intervals between study visits the interquartile range was approximately +/− 5 dB, suggesting that most of the children in the study had stable thresholds during the study period. Importantly, children whose hearing progressed to the point of CI candidacy (n=10) were excluded from this analysis, so these data may underestimate the prevalence and magnitude of thresholds changes in the population of CHH. Changes in better-ear pure tone average between study visits were not associated with age in months (r=0.05, p=0.22) at the earlier visit, indicating that the magnitude of change in threshold did not vary across the age range of the study.
To examine the influence of changes in middle ear status on behavioral thresholds, differences in middle ear status across visits were coded as 1) no change, 2) negative (change from normal or patent tube to abnormal), or 3) positive (change from abnormal to normal or patent tube). The average threshold for the group with no change in middle ear status across 500, 1000, 2000 and 4000 Hz was an increase of 1.28 dB, while the negative change group had a mean increase of 4.5 dB and the positive change group had a mean average threshold improvement of 2 dB. A repeated-measures ANOVA with visit internal (1–2, 2–3, and 3–4), frequency (500 Hz, 1000 Hz, 2000 Hz and 4000 Hz) and middle ear status as factors was conducted to determine if threshold shifts related to middle ear status were statistically significant. None of the mean threshold differences between middle ear status groups were statistically significant between any consecutive visits or audiometric frequencies [F(9,312)=0.58, p = 0.817, η2p=0.02]. Significant individual variability was observed within the groups with middle ear status changes across visits.
Real-ear-to-coupler-difference measures
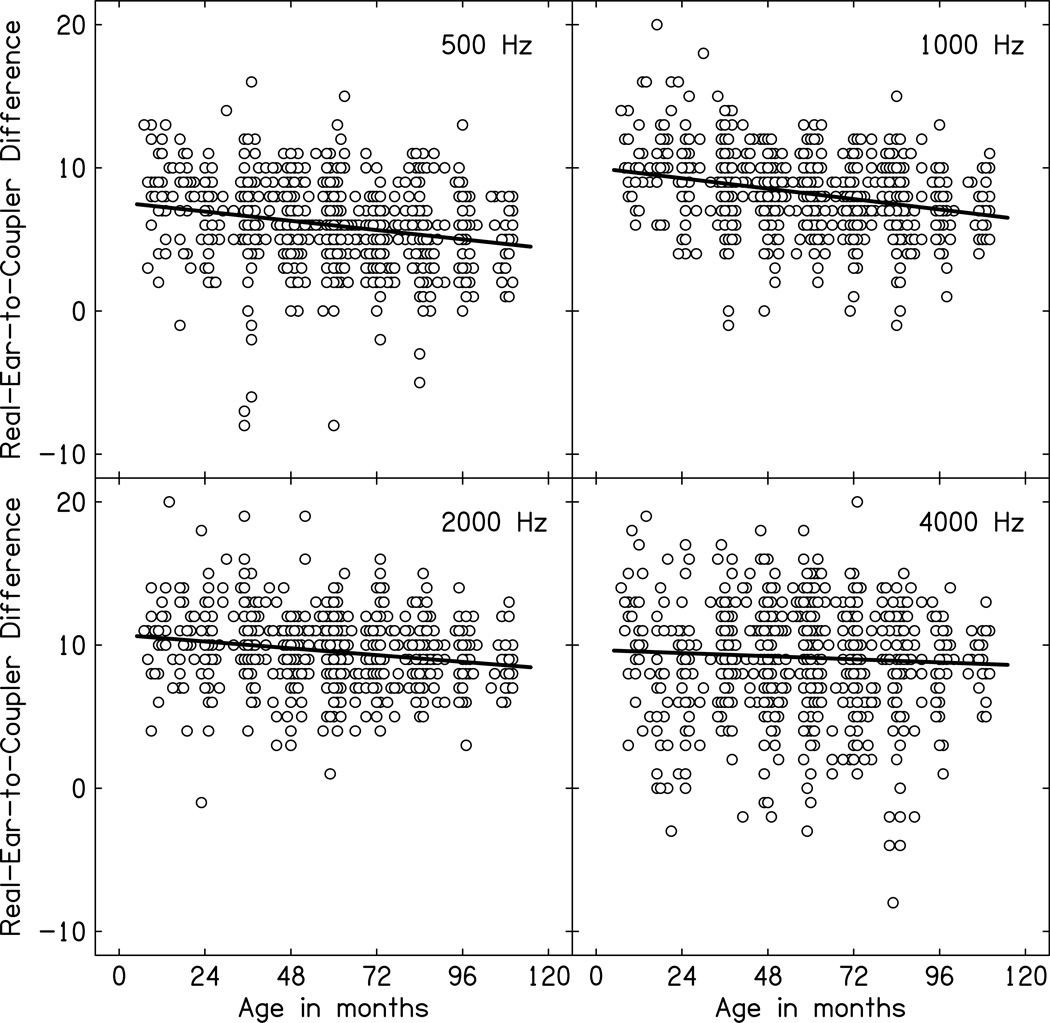
Figure 3 displays the measured RECD data for children with normal middle ear status as a function of age in months with separate panels for each frequency from 500 Hz through 4000 Hz. The relationship between measured RECD and age in months for children with normal middle ear status was evaluated using bivariate correlations, which are displayed in Table 4. RECD decreased for all frequencies as age increased for children with normal middle ear status, consistent with increasing ear canal volume as a function of age.
Figure 3.
Real-ear-to-coupler-differences (RECD) in dB plotted as a function of age in months for children with normal middle ear status (open circles). The solid line represents the linear relationship between RECD and age in months. Each panel represents a different frequency.
Table 4.
Relationship between RECD and age in months for children with normal middle ear status
| Ear | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz |
|---|---|---|---|---|---|
| Right (n = 368) | −0.21* | −0.30* | −0.40* | −0.30* | −0.23* |
| Left (n = 361) | −0.17* | −0.23* | −0.31* | −0.26* | −0.28* |
Values are Pearson correlations (r).
p < 0.001
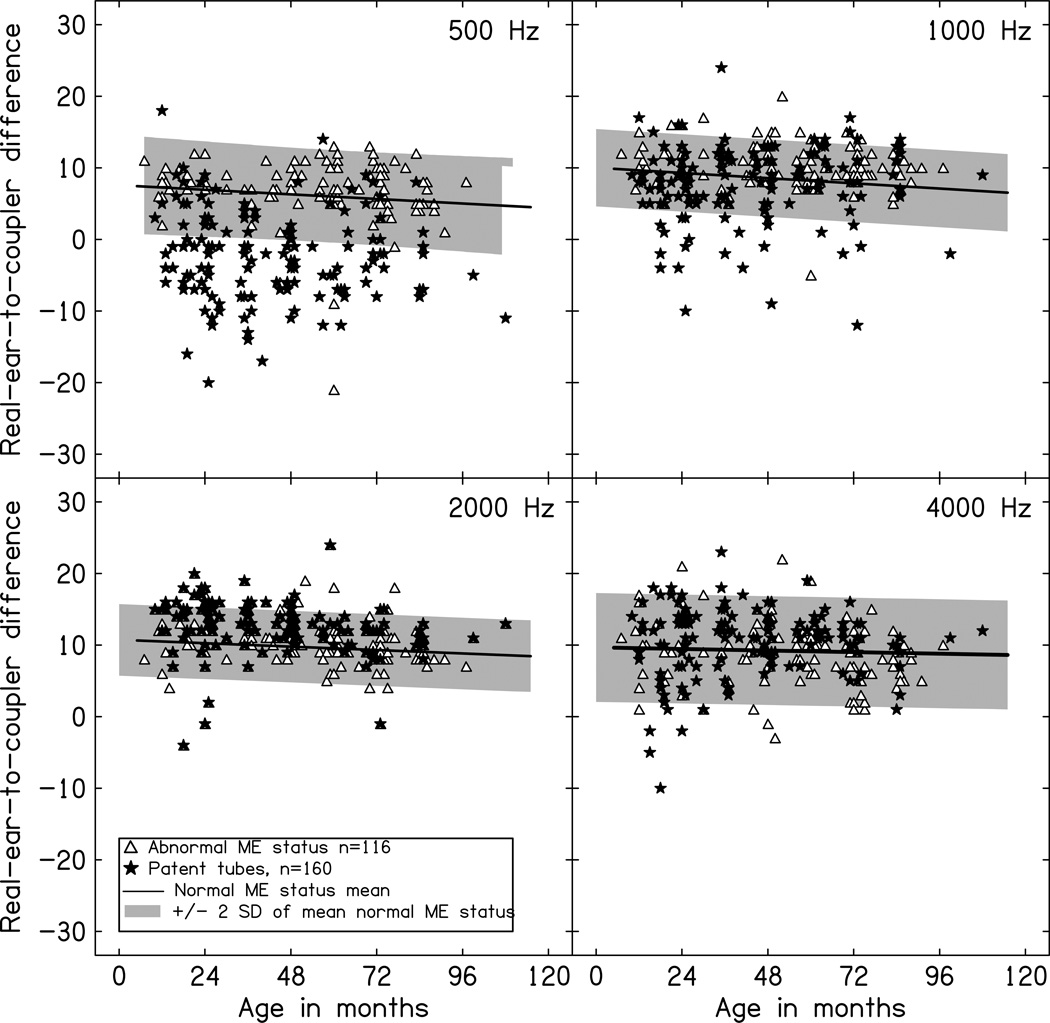
Figure 4 displays the RECD data for children with abnormal middle ear status or patent tympanostomy tubes as a function of age in months. To determine the frequency-specific effects of middle ear status on measured RECD values, a mixed ANOVA was completed with frequency (500, 1000, 2000, 4000 Hz) as a within-subjects factor and middle ear status (normal, abnormal, patent tube) as a between-subjects factor. The two-way interaction between frequency and middle ear status was significant [F(6,948) = 49.09, p<0.001, η2p=0.24], suggesting that RECD values across frequency varied depending on the middle ear status of the child. To evaluate this pattern of significant differences across frequency and middle ear status, post hoc testing using Tukey’s Honestly Significant Difference was completed using a significant minimum mean difference of 1.1 dB. Using this criterion, the children with abnormal middle ear status had higher RECD values at 1000 Hz (10.2 dB) than the children with normal middle ear status (8.0 dB) and children with patent tubes (8.1 dB). Children with abnormal middle ear status and normal middle ear status had higher RECD values at 500 Hz (abnormal = 6.8 dB; normal = 5.8 dB) than the children with patent tubes (500 Hz = −1 dB). None of the other frequencies were significantly different across groups based middle ear status.
Figure 4.
Real-ear-to-coupler-differences (RECD) in dB plotted as a function of age in months for children with patent tubes (filled stars) or abnormal middle ear function (open triangles). The solid line represents the linear relationship between RECD and age in months for normal hearing. The shaded area is the mean +/− 2 standard deviations for children with normal middle ear status from Figure 3. Each panel represents a different frequency. ME= middle ear.
Aided audibility
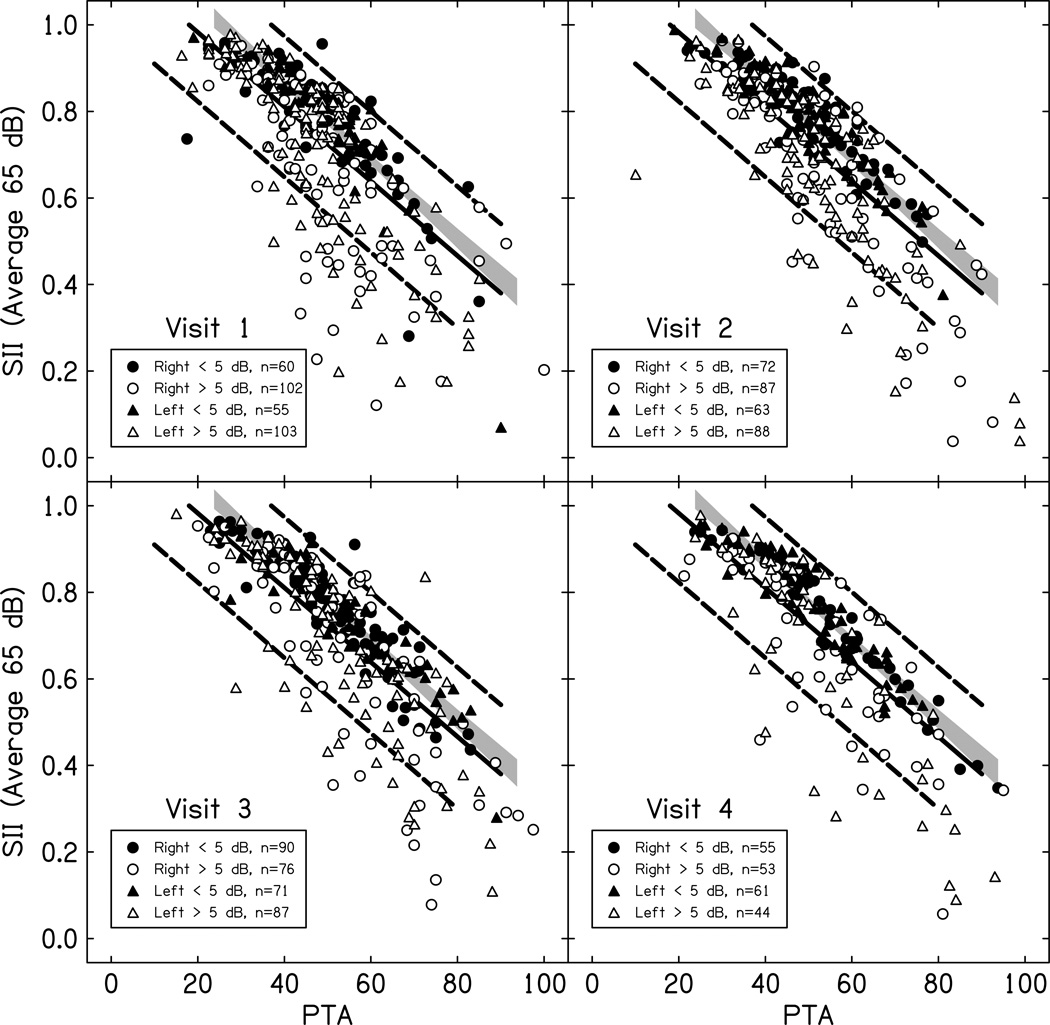
Aided audibility as a function of pure tone average for an average speech input across study visits is plotted in Figure 5. The aided audibility data were compared to a published normative range for children fit to DSL targets (Bagatto et al. 2011). Fittings also were classified based on their proximity to prescriptive targets as measured at the study visit. The comparison of the current results to the normative range allowed for visualization of the effect of proximity to prescriptive targets on audibility across a range of degrees of hearing loss. Across all four visits, the proportion of the sample with fittings that deviated from prescriptive targets by more than 5 dB in at least one ear ranged from 65% (Visit 1) to 42% (Visit 4). Of the CHH who had a fitting that deviated from prescriptive targets by 5 dB, 10% had abnormal middle ear status at the same visit and in the same ear as the deviation > 5 dB. Fittings were also classified into three groups based on their relationship to the normative aided audibility range from Bagatto et al. 2011: greater than or equal to the normative average, less than the normative average and greater than the lower boundary of the 95% confidence interval or less than the 95% confidence interval boundary.
Figure 5.
Aided audibility measured by the SII as a function of pure tone average (PTA). The circles represent right ears and triangles represent left ears for children with normal middle ear (ME) status. Filled symbols have RMS errors from prescriptive targets less than 5 dB and open symbols have RMS errors greater than 5 dB. The solid line is the mean and the dashed lines are the 95% confidence interval of the mean range from Bagatto et al. 2011. Each panel represents a different study visit. The gray shaded region represents the 99% CI of the mean for the children with fittings within 5 dB of prescriptive target.
Table 5 displays the percentage of the sample at each visit that fell into each of these ranges. Although nearly half of the CHH had deviations from prescriptive targets greater than 5 dB at each of the study visits in at least one ear, only 25% had fittings that were below the normative average, but above the 95% confidence interval of the mean for DSL normative data. Another 10% had fittings that were below the 95% CI of the normative mean. Importantly, nearly all of the children who fell below the average for the normative range for aided audibility had fittings that deviated from prescriptive target by 5 dB or more.
Table 5.
Percentage of hearing aid fittings in each part of the normative range for SII by PTA
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Ear | Right | Left | Right | Left | Right | Left | Right | Left |
| ≥ mean | 61.8% | 70.8% | 65.6% | 71.5% | 70.5% | 68.3% | 64.6% | 71.4% |
| < mean | 25.9% | 14.6% | 27.6% | 20.5% | 23.5% | 22.8% | 28.8% | 18.1% |
| < 95% CI | 12.3% | 14.6% | 7% | 8% | 6% | 8.9% | 4.6% | 10.5% |
Mean and confidence intervals (CI) from Bagatto et al. 2010.
Longitudinal changes in aided audibility and deviation from prescriptive targets
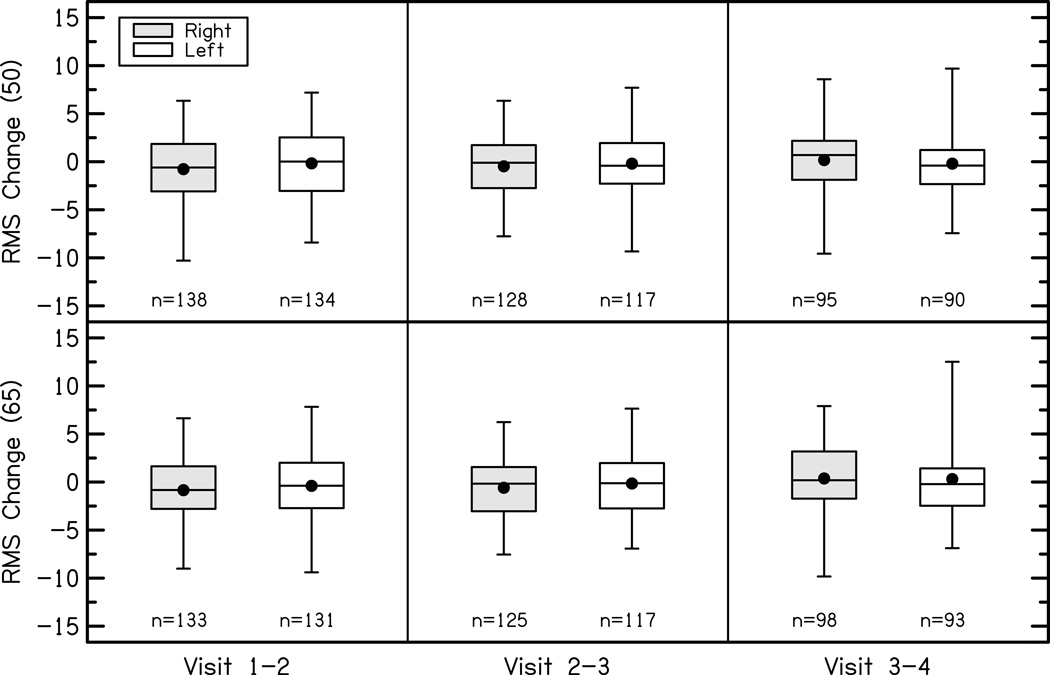
The change in RMS error of the fitting to DSL prescriptive targets at 500, 1000, 2000 and 4000 Hz is plotted in Figure 6. A repeated-measures ANOVA with ear and test interval as factors revealed no significant differences in RMS error between ears [F(1,1387) = 0.77, p = 0.38, η2p = 0.001] or across test intervals for the first four visits [F(2,1387) = 2.31, p = 0.10, η2p = 0.003]. The average RMS error was analyzed instead of the frequency-specific deviations because our previous work with this cohort suggested that the fitting accuracy did not vary as a function of frequency (McCreery et al. 2013). The majority of children in the study had stable RMS error compared to DSL targets across the first four study visits with mean changes of −0.55 dB after visit 1, −0.33 dB after visit 2, and 0.16 after visit 3. Thus, the deviation from prescriptive targets did not change significantly for most subjects between study visits.
Figure 6.
Change in RMS errors for right (shaded) and left (white) ears for soft (top panel) and average (bottom panels) speech input levels between each study visit (columns). The boxes represent the 25th-75th percentiles (interquartile range) and the whiskers represent the 5th and 95th percent confidence intervals of the mean. Solid lines are the median and solid circles represent the mean.
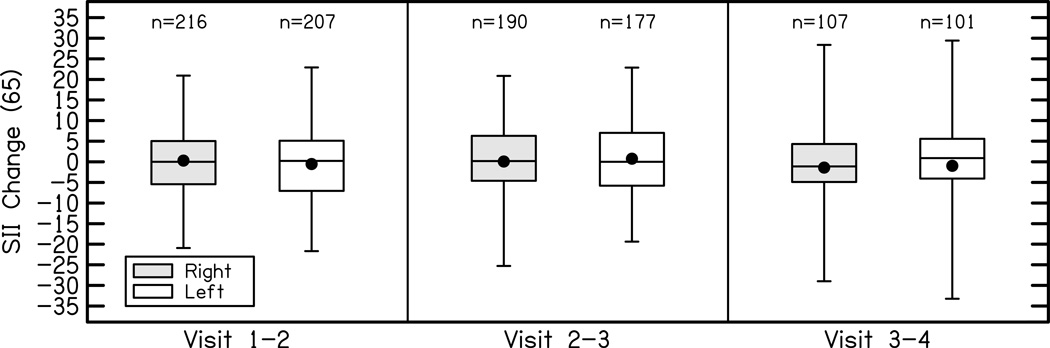
Differences in the aided SII as a function of visit are plotted in Figure 7. To examine the effects of age, degree of hearing loss, and RMS error on aided audibility across study visits, a linear mixed model was completed. Nested within-subjects factors of visit (1–4) and ear (left vs. right) were included in the model. A linear mixed model allows for the type of correlated responses required for this analysis because there are repeated measures both across visits and between ears of the same subject. To account for the correlation, a random intercept term was included in the model which is assumed to follow a normal distribution with mean zero and a variance component for each subject. The estimated subject specific standard deviation is 0.05 with residual standard deviation 0.06. Possible interaction terms were evaluated but were not significant; therefore, they were not included in the final model. The main effects PTA (F = 1861.6, p < .0001) and RMS error (F = 825.9, p < .0001) were significant predictors of aided SII. Lower PTA and lower RMS error were associated with higher aided SII. The main effects of age (F = 0.38, p = .54), ear (F = 0.48, p=0.49) and visit (F = 0.44, p = 0.77) were not related to aided SII after controlling for other factors. The aided SII did not differ between ears or across visits. The aided SII did not appear to vary systematically as a function of age.
Figure 7.
Change in aided audibility for right (shaded) and left (white) ears for average speech input levels between each study visit. The boxes represent the 25th-75th percentiles (interquartile range) and the whiskers represent the 5th and 95th percent confidence intervals of the mean. Solid lines are the median and solid circles represent the mean.
Cluster analysis
Although comparisons of threshold, audibility and RMS error across visits were not significantly different, averaging over the entire sample created the potential to obscure individual differences in these factors within the sample. In order to determine if there were different patterns of longitudinal aided audibility within the sample, a cluster analysis was performed based on the aided audibility at entry into the study, aided audibility at the child’s final visit, and change in aided audibility between these two intervals. Using average input levels (60 or 65 dB SPL) with aided SII as the outcome variable, 160 children had measurements from multiple visits. Because there were no significant differences between ears in previous analyses of audiometric threshold, RMS error or aided SII, the better-ear aided SII was computed for each participant’s first study visit and the last study visit. With these data, children were grouped into clusters based on their baseline aided SII and changes in aided SII across visits. The total amount of change over time was determined by subtracting the initial visit SII from the last visit SII. Each baseline and change in aided SII was used to cluster the participants into three groups using the Ward clustering technique (Ward Jr, 1963). The average baseline and change scores of these groups along with standard deviations are given in and a graphical display of the values are shown in Figure 8. The three clusters represent different levels of aided SII and changes in aided SII during the study. The High cluster includes children with consistently high aided SII during the study (mean aided SII = 0.86); the Decreasing cluster represents children with high baseline aided SII (mean aided baseline SII = 0.78) and decreasing aided SII during the study (mean last visit SII = 0.60); the Low cluster represents children with consistently poor aided SII throughout the study (mean aided SII = 0.55).
Figure 8.
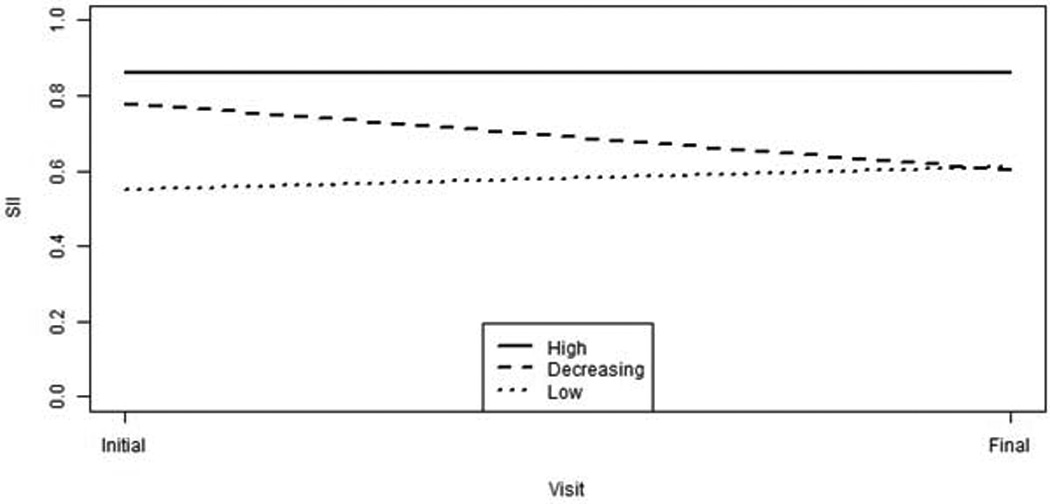
Aided audibility for the initial and final visit for the three cluster groups (High, n=94- solid line; Decreasing, n=30 – dashed line; Low, n =36, – dotted line).
In order to determine which factors predicted cluster membership, a multicategory logistic regression with a generalized logit was used. The number of participants in each cluster is assumed to follow a multinomial distribution. When there are more than two levels of the categorical outcome variable (three clusters), the generalized logit is used. The generalized logit simultaneously uses all pairs of categories by specifying the odds of outcome in one category instead of another. Analyses using baseline RMS error and baseline audiometric thresholds at 250, 500, 1000, 2000, and 4000 Hz indicated RMS error and thresholds at 2000 and 4000 Hz were important predictors of baseline cluster. The High and Decreasing clusters both had high baseline aided SII, so baseline aided SII values did not differentiate between the clusters. The change in RMS error and change in thresholds at 2000 and 4000 Hz from baseline to last visit were included in the model to assess the effect of changes in these variables on cluster membership. Due to missing data, the resulting sample sizes for each cluster in the multicategory logistic regression were 93 (High), 30 (Decreasing), and 36 (Low). The amount of time between the first visit and the last visit did not vary significantly across clusters (High = 42.6 months; Decreasing = 41.6 months; Low 43.1 months).
Overall, the baseline RMS error (p < 0.001) and baseline 2000 Hz threshold (p < 0.001) along with the change in RMS error (p = 0.003) and change in thresholds at 2000 Hz (p < 0.001) and 4000 Hz (p < 0.001) were significant predictors of cluster membership at the 0.05 level. Baseline threshold at 4000 Hz (p = 0.21) was not a significant predictor of cluster. The High cluster had lower RMS error than the Low (OR = 2.34, p < 0.001) and the Decreasing (OR = 2.07, p < 0.001) clusters. The Low and Decreasing clusters did not differ in baseline RMS error (OR = 1.13, p = 0.45). The High cluster had lower baseline 2000 Hz threshold than the Low (OR = 1.39, p < 0.001) and the Decreasing (OR = 1.23, p < 0.001) clusters. The difference in baseline 2000 Hz between the Decreasing and Low clusters was not significant (OR = 1.13, p = 0.08). The High and Low clusters had less change in RMS error than the Decreasing (High vs. Decreasing OR = 1.74, p = 0.001; Low vs. Decreasing OR = 1.33, p = 0.02), but the difference between the High and Low clusters was not significant (OR = 1.31, p = 0.10). The High cluster had smaller changes in 2000 Hz and 4000 Hz thresholds than the Decreasing (2000 Hz OR = 1.23, p < 0.001; 4000 Hz OR = 1.24, p < 0.001) and Low (2000 Hz OR = 1.17, p = 0.03; 4000 Hz OR = 1.35, p = 0.04). Change in threshold at 2000 Hz did not differentiate between the Low and Decreasing clusters (OR = 1.14, p = 0.14), but the Low cluster had smaller less threshold change at 4000 Hz than the Decreasing cluster (OR = 1.31, p < 0.001). CHH with better thresholds and lower RMS errors at baseline and between the first and last study visits were more likely to have high aided audibility throughout the study than CHH with lower or decreasing thresholds and higher or increasing RMS errors.
DISCUSSION
The purpose of this analysis was to examine the longitudinal characteristics of children’s hearing thresholds, ear canal acoustics, and amplification, so that the effects of variations over time in these variables on developmental outcomes can be quantified. Aided audibility of speech is a dynamic characteristic of amplification that is affected by changes in hearing, ear canal growth, and the proximity of amplification to prescriptive targets. The key findings of this study were:
Eighty-four percent of children in the study had stable audiometric thresholds during the study, defined as changes in pure tone average less than 10 dB for any single study visit. Changes in middle ear status due to middle ear dysfunction or the placement of tympanostomy tubes were the most frequent factor associated with positive and negative changes in thresholds.
Children’s ear canal acoustics as measured by longitudinal RECD measures result in a decrease in the relative sound level in the ear canal as age increases, consistent with previous studies of RECD changes as a function of age (e.g., Bagatto et al. 2002).
Over half of the children in the study had an RMS error deviation from prescriptive targets greater than 5 dB at each of the first four study visits. Approximately 35% of the children in the study had aided audibility that was below the average for the normative range for SII based on degree of hearing loss (from Bagatto et al. 2011). Nearly all of the children with below average aided audibility also had fittings with RMS error of greater than 5 dB from prescriptive targets.
A cluster analysis of longitudinal aided audibility revealed three distinct groups of children: a group with consistently high aided audibility throughout the study, a group with decreasing audibility during the study, and a group with consistently low aided audibility. Children in the Decreasing cluster had increases in thresholds and RMS error during the study, whereas children in the Low cluster had lower thresholds and higher RMS error throughout the study than children in the High cluster.
These findings have important clinical implications for identification of and intervention with children who wear HAs. Some children who wear HAs may be at risk for communication delays due to changes in hearing, poor aided audibility or a combination of these audiological factors.
Audiometric thresholds
Audiometric thresholds for 84% of the cohort were within the range of test-retest reliability for children. Changes in thresholds were not associated with age in months, suggesting that the changes in thresholds that did occur were not likely to occur at a particular age. Changes in behavioral thresholds observed in the cohort often were related to either positive (placement of tympanostomy tubes or resolution of middle ear dysfunction) or negative (middle ear dysfunction) changes in middle ear status. The rate of middle ear problems in this cohort of infants and young children was not found to be related to age with a prevalence ranging from 15% at study visit 1 to 11% at study visit 4. Children who had middle ear problems between study visits that had resolved before subsequent study visits may have experienced changes in hearing that would not be reflected by the sampling frequency used in this study.
The stability of audiometric thresholds across study visits has implications for managing amplification for children and counseling parents about the likelihood of progression of hearing loss. The overall likelihood of significant progression of hearing loss was low, with only 16% of the sample experiencing significant changes in hearing thresholds that were not associated with documented changes in middle ear status, which approximates the 13% reported by Pittman and Stelmachowicz (2003). Importantly, the current sample did not capture children who had progression of hearing loss that resulted in cochlear implantation. The criterion for a significant change in hearing was a shift of 10 dB in the three-frequency pure tone average, which was selected in order to detect children with significant shifts in hearing thresholds between visits. However, this criterion may have failed to detect children with changes at frequencies not included in the pure tone average or changes that were isolated to specific frequencies. Thus, the prevalence of fluctuating or progressive hearing loss is likely to be higher in the general population of CHH than what was observed in this sample.
While the majority of children’s audiometric thresholds remained stable across time, longitudinal changes in thresholds had a significant impact on the aided audibility of speech. Approximately 50% of the children with deviations from prescriptive targets greater than 5 dB also had a change in hearing greater than 10 dB compared to the prior study visit. Only 10% of the children with an RMS error > 5 dB had abnormal middle ear status at the time of their study visit, suggesting that only a small proportion of significant deviations from prescriptive target were the result of transient middle ear conditions that affected audibility. Medical management of middle ear problems in CHH is important for preventing long-term deficits in audibility that result from changes in thresholds. These findings highlight the need for adjustments in amplification when audiometric thresholds change in order to reduce deviations from prescriptive targets. Consistent audiological evaluation allows monitoring of changes in audiometric thresholds, and in turn, adjustment of amplification. Regular audiometric evaluation during infancy and early childhood are particularly important because the changes in hearing thresholds observed in this study were not found to be related to age and did not occur more frequently at particular age intervals.
Ear canal acoustics
In addition to changes related to audiometric thresholds, HA gain must also be adjusted to account for the decline in the sound level in the ear canal as the child experiences normal physical growth. For children with normal middle ear status, the magnitude of the RECD values at each frequency and decreasing RECD as a function of age are in general agreement with previous studies where RECD has been reported for children (Bagatto et al. 2002; Bingham et al. 2009). Similar to audiometric thresholds, middle ear status also affected the RECD. Children with middle ear dysfunction had slightly higher RECD values than children with normal middle ear status, although there was significant overlap in the distributions of data across age and subjects. Children with tympanostomy tubes tended to have lower RECD values than children with normal middle ear status due to the increase in ear canal volume that occurs with the placement of tubes. These findings are consistent with many previous studies on the effect of middle ear status on RECD measurements (Martin, Westwood & Bamford, 1996; Martin, Munro & Langer, 1997). The individual variability in RECD and threshold change in children with changes in middle ear status observed in the current study support the importance of using individually-measured RECD data and adjusting amplification based on changes in thresholds when fluctuations in middle ear status are anticipated to be more than acute, as these changes have an effect on both thresholds and ear canal acoustics, which can alter aided audibilty.
Hearing aid fitting characteristics
Two metrics were used to describe the HA characteristics of children in the study. The deviation from prescriptive target at 500, 1000, 2000 and 4000 Hz was estimated as an RMS error in dB to describe the proximity of the fittings to DSL prescriptive targets. Additionally, aided audibility for speech was estimated using SII and the HA verification data for each child. Over half of the children in the study had an RMS error from prescriptive target of more than 5 dB in at least one ear for each of the first four study visits. Approximately 30% of the children in the study had aided audibility that was below average for the normative range based on degree of hearing loss (Bagatto et al. 2011) for at least one study visit. While not all of the children with RMS errors greater than 5 dB from prescriptive targets fell below the average range in terms of audibility, nearly all of the children who did fall below the average range for audibility had RMS errors from prescriptive targets greater than 5 dB. Children with fittings that were in close proximity to prescriptive targets for their degree of hearing loss had audibility within the normative range. These findings support the importance of using validated prescriptive targets during hearing aid verification to ensure consistency of aided audibility for children with a wide range of degrees of hearing loss. Children with thresholds greater than 50 dB HL and deviations from prescriptive targets > 5 dB RMS error were much more likely to fall outside the normative range for audibility than children with mild hearing loss or smaller deviations from target. Children with moderate or greater degrees of hearing loss have a smaller dynamic range between threshold and levels of discomfort than children with mild hearing loss, which meant that deviations from prescriptive target had a larger impact on audibility for the children with the greatest degrees of hearing loss.
To quantify longitudinal patterns of variability in aided audibility within the sample, a cluster analysis of audibility between the first and last study visits was completed. The cluster analysis revealed three groups of children with different patterns of aided audibility: a group with mean aided audibility of 0.86 at both time points (High cluster), a group with mean aided audibility 0.55 at both time points (Low cluster) and a group that started with mean aided audibility of 0.78 and ended with aided audibility less than .60 (Decreasing Cluster). The High group was characterized by better audiometric thresholds and smaller deviations from prescriptive targets than the Low and Decreasing groups. The Decreasing group was characterized by audiometric thresholds that became poorer and RMS errors that increased during the study. The degree of hearing loss and changes in thresholds over time cannot be treated or prevented. However, the proximity to prescriptive targets can be improved through adjustments based on probe microphone verification by the child’s audiologist. Further analyses will help to determine how differences in audibility affect outcomes (Tomblin et al. this issue, pp. XXXX; McCreery et al. this issue, pp. XXXX). Children with less audibility are predicted to have poorer developmental outcomes than the children with better aided audibility.
Limitations
While this study is the first to document the audiological and amplification characteristics of a large group of infants and children longitudinally, these results should be interpreted in the context of several limitations. Because of the observational design of the study, the audiogram used to fit the HA may have been different than the audiogram used to assess the quality of the HA fitting at the study visit. Although regular audiological monitoring might have resulted in a fitting that was closer to prescriptive targets, the timing of the study visit near the child’s birthday or age milestone did not result in a consistent interval between the child’s last audiological visit and the study visit. Our previous cross-sectional analyses of amplification at entry into the study (McCreery et al. 2013) did not find a systematic relationship between the magnitude of the RMS error and the elapsed time between the last audiological evaluation and the study visit. Additionally, while audibility may be important for predicting developmental outcomes, these findings do not consider if and how much the children in the study wore their HAs. Longitudinal HA use will be explored later in this supplement (Walker et al. this issue, pp. XXXX), as well as the potential interactions of HA use and audibility on developmental outcomes (Tomblin et al. this issue, pp. XXXX, McCreery et al. this issue, pp. XXXX).
Clinical Implications
These results have important clinical implications for HA or amplification management. CHH may experience changes in thresholds during early childhood. Changes in thresholds can alter the amount of aided audibility provided by the child’s HAs, and regular audiological evaluation can help to detect these changes at a time when children may be unable to report these changes to their parents or caregivers. Hearing aid fitting results from this study suggest that both the proximity of the fitting to prescriptive target (RMS error) and normative ranges for audibility may be valuable in assessing the consistency of HA fittings in children. Nearly all of the children with audibility that was less than the average for the normative range had significant deviations from prescriptive targets. The amount of aided audibility that is possible depends on the child’s degree of hearing loss, which is taken into account with the SII normative range as a function of pure tone average. However, the normative range for audibility based on data from Bagatto et al. (2010) was larger than the range in the current data for children with fittings that were within 5 dB of prescriptive targets. Nearly all of the children fit within 5 dB of prescriptive targets were above the average for the normative range, suggesting that the fittings of CHH who fall below the average line could potentially be optimized to provide greater audibility. Minimizing the RMS error of the fitting to prescriptive targets can support higher and more consistent aided audibility.
Summary
The purpose of the current investigation was to characterize the audiometric thresholds, deviations from prescriptive targets for amplification and aided audibility of speech for a large group of infants and young children who wore HAs during a longitudinal study of speech and language development, so that the effects of audibility on outcomes can be characterized. Approximately 65% of CHH in the study had adequate aided audibility of speech, and 86% had stable hearing during the study period. Limited audibility was associated with greater degrees of hearing loss and larger deviations from prescriptive targets, as well as increasing degree of hearing loss and RMS error over the course of the study. Studies of developmental outcomes will help to determine how aided audibility affects developmental outcomes in CHH.
Supplementary Material
Acknowledgements
This research was supported by the following grants from the NIH-NIDCD: R01DC009560 and R01DC013591. The authors wish to thank Marc Brennan and Kris Fernau for their helpful contributions in preparing the manuscript and analyzing data.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to declare.
See Supplemental Digital Content, Appendix A List of Acronyms used throughout Outcomes in Children with Hearing Loss Study (http://links.lww.com/EANDH/A206).
References
- Ambrose SE, Berry LMU, Walker EA, Harrison M, Oleson J, Moeller MP. Speech Sound Production in 2-Year-Olds Who Are Hard of Hearing. American Journal of Speech-Language Pathology. 2014;23(2):91–104. doi: 10.1044/2014_AJSLP-13-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Audiology. American Academy of Audiology Clinical Practice Guidelines: Pediatric Amplification. Reston: Virginia; 2013. [Google Scholar]
- ANSI. ANSI S3. 22–2003, Specification of hearing aid characteristics. New York: American National Standards Institute; 2003. [Google Scholar]
- ANSI. S3. 5–1997 R-2007, American national standards methods for the calculation of the articulation index. New York: American National Standards Institute; 1997. [Google Scholar]
- Bagatto MP, Scobie SD, Seewald RC, Moodie KS, Hoover BM. Real-Ear-to-Coupler Difference Predictions as a Function of Age for Two Coupling Procedures. Journal of American Academy of Audiology. 2002;13:407–415. [PubMed] [Google Scholar]
- Bagatto M, Moodie S, Scollie S, Seewald R, Moodie S, Pumford J, Liu KR. Clinical protocols for hearing instrument fitting in the Desired Sensation Level method. Trends in amplification. 2005;9(4):199–226. doi: 10.1177/108471380500900404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatto M, Scollie SD, Hyde M, Seewald R. Protocol for the provision of amplification within the Ontario infant hearing program. International journal of audiology. 2010;49(Suppl 1):S70–S79. doi: 10.3109/14992020903080751. [DOI] [PubMed] [Google Scholar]
- Bagatto MP, Moodie ST, Malandrino AC, Richert FM, Clench DA, Scollie SD. The University of Western Ontario Pediatric Audiological Monitoring Protocol (UWO PedAMP) Trends in amplification. 2011;15(1):57–76. doi: 10.1177/1084713811420304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumfield A, Dillon H. Factors affecting the use and perceived benefit of ITE and BTE hearing aids. Br J Audiol. 2001;35:247–258. doi: 10.1080/00305364.2001.11745243. [DOI] [PubMed] [Google Scholar]
- Bentler R, Walker E, McCreery R, Arenas RM, Roush P. Nonlinear Frequency Compression in Hearing Aids: Impact on Speech and Language Development. Ear and Hearing. 2014;34:535–552. doi: 10.1097/AUD.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham K, Jenstad LM, Shahnaz N. Longitudinal changes in real-ear to coupler difference measurements in infants. Journal of the American Academy of Audiology. 2009;20(9):558–568. doi: 10.3766/jaaa.20.9.4. [DOI] [PubMed] [Google Scholar]
- Byrne D, Cotton S. Evaluation of the National Acoustic Laboratories’new hearing aid selection procedure. J Speech Hear Res. 1988;31:178–186. doi: 10.1044/jshr.3102.178. [DOI] [PubMed] [Google Scholar]
- Ching TYC, Dillon H, Marnane V, Hou S, Day J, Seeto M, Yeh A. Outcomes of Early- and Late-Identified Children at 3 Years of Age: Findings From a Prospective Population-Based Study. Ear and hearing. 2013;34:535–552. doi: 10.1097/AUD.0b013e3182857718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, Alexander G. Evaluation of an in-situ probe microphone method for hearing aid fitting verification. Ear Hear. 1990;11:31–39. doi: 10.1097/00003446-199002000-00008. [DOI] [PubMed] [Google Scholar]
- Fulcher A, Purcell AA, Baker E, Munro N. Listen up: Children with early identified hearing loss achieve age-appropriate speech/language outcomes by 3 years-of-age. International Journal of Pediatric Otorhinolaryngology. 2012;76(12):1785–1794. doi: 10.1016/j.ijporl.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Holte L, Walker E, Oleson J, Spratford M, Moeller MP, Roush P, Tomblin JB. Factors influencing follow-up to newborn hearing screening for infants who are hard of hearing. American Journal of Audiology. 2012;21(2):163–174. doi: 10.1044/1059-0889(2012/12-0016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CR, McCann DC, Campbell MJ, Law CM, Mullee M, Petrou S, Stevenson J. Language ability after early detection of permanent childhood hearing impairment. The New England journal of medicine. 2006;354(20):2131–2141. doi: 10.1056/NEJMoa054915. [DOI] [PubMed] [Google Scholar]
- King AM. The national protocol for paediatric amplification in Australia. International Journal of Audiology. 2010;49(S1):S64–S69. doi: 10.3109/14992020903329422. [DOI] [PubMed] [Google Scholar]
- Martin HC, Munro KJ, Langer DH. Real-ear to coupler differences in children with grommets. British journal of audiology. 1997;31(1):63–69. doi: 10.3109/03005364000000009. [DOI] [PubMed] [Google Scholar]
- Martin HC, Westwood GFS, Bamford JM. Real ear to coupler differences in children having otitis media with effusion. British journal of audiology. 1996;30(2):71–78. doi: 10.3109/03005369609077934. [DOI] [PubMed] [Google Scholar]
- McCreery RW, Bentler RA, Roush PA. Characteristics of Hearing Aid Fittings in Infants and Young Children. Ear and Hearing. 2013;34:701–710. doi: 10.1097/AUD.0b013e31828f1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery RW, Alexander J, Brennan MA, Hoover B, Kopun J, Stelmachowicz PG. The Influence of Audibility on Speech Recognition With Nonlinear Frequency Compression for Children and Adults With Hearing Loss. Ear and hearing. 2014 doi: 10.1097/AUD.0000000000000027. ePub head of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery RW, Walker EA, Spratford M, Oleson J, Bentler R, Holte L, Roush P. Longitudinal auditory development outcomes in children who are hard of hearing. Ear and Hearing. doi: 10.1097/AUD.0000000000000213. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BC, Alcantara J, Marriage J. Comparison of three procedures for initial fitting of compression hearing aids I. Experienced users fitted bilaterally. Br J Audiol. 2001;35:339–353. doi: 10.1080/00305364.2001.11745252. [DOI] [PubMed] [Google Scholar]
- Moeller MP. Early Intervention and Language Development in Children Who Are Deaf and Hard of Hearing. Pediatrics. 2000;106(3):e43–e43. doi: 10.1542/peds.106.3.e43. [DOI] [PubMed] [Google Scholar]
- Pavlovic CV. Derivation of primary parameters and procedures for use in speech intelligibility predictions. The Journal of the Acoustical Society of America. 1987;82(2):413–422. doi: 10.1121/1.395442. [DOI] [PubMed] [Google Scholar]
- Pittman AL, Stelmachowicz PG. Hearing loss in children and adults: audiometric configuration, asymmetry, and progression. Ear and hearing. 2003;24(3):198. doi: 10.1097/01.AUD.0000069226.22983.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollie S, Seewald R, Cornelisse L, Moodie S, Bagatto M, Laurnagaray D, Pumford J. The desired sensation level multistage input/output algorithm. Trends in amplification. 2005;9(4):159–197. doi: 10.1177/108471380500900403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sininger YS, Grimes A, Christensen E. Auditory development in early amplified children: factors influencing auditory-based communication outcomes in children with hearing loss. Ear and hearing. 2010;31(2):166–185. doi: 10.1097/AUD.0b013e3181c8e7b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S, van Dijk C. Hearing instrument fittings of pre-school children: do we meet the prescriptive goals? Int J Audiology. 2008;47(Suppl 1):S62–S71. doi: 10.1080/14992020802300904. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Harrison M, Ambrose SE, Walker EA, Oleson JJ, Moeller MP. Language outcomes in young children with mild to severe hearing loss. Ear and Hearing. doi: 10.1097/AUD.0000000000000219. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Walker EA, McCreery RW, Arenas RM, Harrison M, Moeller MP. Outcomes of children with hearing loss: Data collection and methods. Ear and Hearing. doi: 10.1097/AUD.0000000000000212. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohr B, Topol D, Girard N, St Pierre L, Watson V, Tucker R. Language outcomes and service provision of preschool children with congenital hearing loss. Early human development. 2012;88(7):493–498. doi: 10.1016/j.earlhumdev.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Wake M, Poulakis Z, Hughes EK, Carey-Sargeant C, Rickards FW. Hearing impairment: A population study of age at diagnosis, severity, and language outcomes at 7–8 years. Archives of Disease in Childhood. 2005;90(3):238–244. doi: 10.1136/adc.2003.039354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Holte L, Spratford M, Oleson J, Welhaven A, Harrison M. Timeliness of Service Delivery for Children With Later-Identified Mild-to-Severe Hearing Loss. American Journal of Audiology. 2014;23(1):116–128. doi: 10.1044/1059-0889(2013/13-0031). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, McCreery RW, Spratford M, Oleson JJ, Van Buren J, Bentler R, Roush P, Moeller MP. Trends and predictors of longitudinal hearing aid use for children who are hard of hearing. Ear and Hearing. doi: 10.1097/AUD.0000000000000208. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkin P, McCann D, Law C, Mullee M, Petrou S, Stevenson J, Kennedy C. Language ability in children with permanent hearing impairment: the influence of early management and family participation. Pediatrics. 2007;120(3):e694–e701. doi: 10.1542/peds.2006-2116. [DOI] [PubMed] [Google Scholar]
- Ward JH., Jr Hierarchical grouping to optimize an objective function. Journal of the American statistical association. 1963;58(301):236–244. [Google Scholar]
- Yoshinaga-Itano C. From screening to early identification and intervention: Discovering predictors to successful outcomes for children with significant hearing loss. Journal of deaf studies and deaf education. 2003;8(1):11–30. doi: 10.1093/deafed/8.1.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.