Abstract
Objective
To evaluate the safety of in utero antiretroviral (ARV) exposure in children born to mothers with HIV, using a trigger-based design.
Design
The Surveillance Monitoring of ART Toxicities Study is a prospective cohort study conducted at 22 US sites to evaluate safety of in utero ARV drug exposure in HIV-uninfected children born to HIV-infected mothers. Children meeting pre-defined clinical or laboratory thresholds have more intensive evaluations to determine whether they meet criteria for adverse events (AEs).
Methods
AE “cases” were defined for the following domains: growth, hearing, language, neurology, neurodevelopment, metabolic, hematologic/clinical chemistry and blood lactate. We used adjusted log-binomial models to calculate relative risks (RR) of case status overall and within individual domains for various ARV exposures during pregnancy.
Results
Among 2680 youth enrolled between 2007 and 2012 (48% female, 66% black, 33% Hispanic), 48% met a trigger and 25% were defined as a case in at least one domain. Language (13.2%) and metabolic (11.4%) cases were most common. After adjustment for birth cohort and other factors, there was no association of any ARV regimen, drug class, or individual drug with meeting overall case criteria (case in any domain). Within individual domains, zidovudine (74% exposed) was associated with increased risk of metabolic case (RR=1.69, 95% CI:1.08–2.64) and didanosine plus stavudine (<1% exposed) with increased risk of both neurodevelopmental (RR=12.4, 95% CI:5.29–29.08) and language (RR=4.84, 95% CI:1.14–20.51) cases.
Conclusions
Our findings support current recommendations for combination ARV therapy during pregnancy, although higher risk of metabolic disorder with zidovudine exposure warrants further study.
Keywords: Antiretroviral, HIV-exposed, safety, infants, mitochondrial dysfunction
INTRODUCTION
Despite the success of combination antiretroviral (ARV) regimens used during pregnancy in reducing HIV transmission,1–4 concerns remain regarding adverse consequences of in utero exposure to ARVs.5–7 Numerous manifestations of ARV toxicity have been reported in adults and children with HIV; however, there are fewer reports of toxicities in infants born to mothers with HIV infection and exposed to ARVs through transplacental passage of the drugs.8 Mitochondrial toxicity is postulated to be one possible mechanism for these events in exposed infants, and can manifest as a variety of clinical and laboratory abnormalities, including hematologic and liver function, myopathy, and central nervous system disorders.8–17 Drug-specific disorders such as anemia after exposure to maternal zidovudine or combination ARV regimens have also been reported.7,18–20 Serious adverse effects have been uncommon and may be difficult to distinguish from pregnancy complications such as pre-eclampsia or prematurity,21 or other maternal exposures such as illicit drugs, alcohol, tobacco, or HIV infection itself.22
Current treatment guidelines for children with in utero or neonatal ARV exposures recommend that those who develop clinical problems of unknown etiology be evaluated for potential mitochondrial dysfunction, and that follow-up of children with exposure to ARVs continue into adulthood because of concerns of carcinogenicity of nucleoside analogue ARV drugs.4 Thus, it is important to provide long-term follow-up of children exposed in utero to ARVs in order to systematically examine a wide spectrum of clinical and laboratory outcomes.
In order to conduct safety monitoring in a rigorous yet cost-effective manner, the Pediatric HIV/AIDS Cohort Study (PHACS) network established the Surveillance Monitoring of ART Toxicities (SMARTT) Study, to identify potential adverse effects of ARV exposures in infants born to HIV-infected women, and to evaluate associations with ARV combinations and specific ARV drugs in order to help inform treatment guidelines for HIV-infected pregnant women.23,24 The SMARTT study uses a trigger-based design, which provides efficient use of study and patient resources. This design provides greater precision of estimated adverse event rates by concentrating on those children most likely to have adverse events, as compared to randomly selecting a subgroup of children to study with detailed assessments.24
In the SMARTT study, potential adverse effects in multiple domains, such as growth and neurological outcomes, were selected based on literature review and clinical experience. For each domain, trigger thresholds were defined which dictated additional pre-specified evaluations. The results of these evaluations were reviewed by an group of clinicians and epidemiologists to determine whether the child met the definition of a “case”, a condition in one or more domains that could result from intrauterine exposure to ART. In this paper, we evaluated the association of ARV exposures with overall case status as well as within specific domains which may reflect adverse effects of ARV exposures.
METHODS
Description of Protocol and Study Population
The PHACS SMARTT study includes two cohorts: the Static and Dynamic Cohorts. Static Cohort children and their mothers (or caregivers) were eligible if the youth were age 1 to 12 years at entry and had detailed information on maternal ARV use during pregnancy and infant outcomes from prior prospective studies (PACTG 219C 25 and the Women and Infants Transmission Study26). The Dynamic Cohort of HIV-infected women and their infants enrolled between 22 weeks gestation and 1 week after delivery. Both cohorts opened to participating sites in the United States including Puerto Rico in March 2007. The Static Cohort completed enrollment in 2010 whereas the Dynamic Cohort continues to enroll. The SMARTT protocol was approved by Human Subject research review boards at each of the participating sites and at the Harvard T. H. Chan School of Public Health. Written informed consent was obtained from the parent or legal guardian at each research site.
At study entry, clinical diagnoses and dates of prenatal ARV use were obtained from medical charts and participant interview. Birth characteristics (gestational age, birth weight, and mode of delivery) were abstracted and maternal HIV disease characteristics were collected both early during pregnancy (earliest available) and prior to delivery, including plasma HIV RNA concentration (viral load, VL), absolute CD4+ lymphocyte (CD4) cell counts, and CD4%. Trimester-specific information on substance use during pregnancy was obtained by self-reported questionnaire, including alcohol, tobacco, marijuana, opiates, and other substances, as previously described.22 Caregivers of participating children completed questionnaires on household composition, education and income levels, past history of psychiatric diagnoses or substance use, and other information related to family environment. After enrollment, children and their mothers or caregivers were followed at annual study visits. A complete physical examination was conducted including anthropometric assessments [height, weight, body mass index (BMI), and skinfold measurements], abstraction of any new diagnoses or illnesses obtained from the medical chart or interview, and collection of limited laboratory measurements including point-of-care capillary blood lactate assessments27. Cognitive, hearing, and language assessments were conducted at specified ages.
The study team defined multiple domains reflecting potential adverse effects of ARV exposure during pregnancy based on expert input and existing literature. These included low growth, neurology, neurodevelopment, language, hearing, metabolic, basic hematology and chemistry studies (including liver and renal function tests), and blood lactate. For each domain, a study “trigger” was established using a non-invasive or inexpensive laboratory or clinical evaluation. For example, a metabolic trigger was defined as a BMI exceeding the age- and sex-specific 95th percentile for children aged 2 years or older. The criteria for triggers are provided in Table 1. As described previously24, trigger thresholds were chosen to achieve high sensitivity at the expense of lower specificity for identifying potential adverse events (AEs) since, as a safety study, it was desirable to capture all children who might have AEs related to ARVs.
Table 1.
Trigger and Case Definitions and Percent Meeting Trigger and Case Status Overall and By Domain
| Domain | Criteria for Meeting Trigger | Criteria for Defining Adverse Event (Case) | Met Trigger Criteria | Met Case Criteriaa
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Among those meeting Triggers | Overall | ||||||||
|
| |||||||||
| Number Evaluated | N (%) | 95% CI | Number Evaluated | N (%) | Cases/Evaluable (%) | 95% CI | |||
| Language Impairment | Language test score >2 SDs below population norms on age-specific testsb | Verification of language scores below threshold | 1499 | 200 (13.3%) | 11.7–15.2% | 200 | 198 (99.0%) | 198/1499 (13.2%) | 11.5–15.0% |
|
| |||||||||
| Metabolic Abnormality | BMI or weight-for-length > 95th percentile, among participants ≥ 2 years of age | Abnormal fasting lipids (cholesterol, HDL, LDL, or TG) or insulin resistance defined by HOMA score | 1790 | 515 (28.8%) | 26.7–30.9% | 398 | 190 (47.7%) | 190/1673 (11.4%) | 9.9–13.0% |
|
| |||||||||
| Impaired Growth | Weight or height <3rd percentile, a drop in weight or height Z-score of >1.3 SDs, or either triceps skinfold or mid-upper arm circumference < 5th percentile | Growth trigger met at 2 or more study visits (consecutive or not) | 2667 | 494 (18.5%) | 17.1–20.1% | 454 | 160 (35.2%) | 160/2097 (7.6%) | 6.5–8.9% |
|
| |||||||||
| Neurologic Diagnosis | Febrile or afebrile seizure, microcephaly, or other neurologic diagnosis | Confirmed neurologic diagnosis based on pediatric neurology consultation and expert review | 2674 | 269 (10.1%) | 8.9–11.3% | 256 | 163 (63.7%) | 163/2661 (6.1%) | 5.2–7.1% |
|
| |||||||||
| Neurodevelopmental Impairment | Score > 2 SDs below population norm on age specific testsc | Verification of scores below age-specific threshold | 1295 | 60 (4.6%) | 3.6–5.9% | 60 | 58 (96.7%) | 58/1295 (4.5%) | 3.4–5.8% |
|
| |||||||||
| Elevated Lactate | Repeatedly elevated point-of-care blood lactate >3 mmol/L (on two assessments at same visit) | Venous lactate > 3 mmol/L and ratio of lactate to pyruvate >20, if obtained | 2576 | 161 (6.3%) | 5.3–7.3% | 128 | 53 (41.4%) | 53/2543 (2.1%) | 1.6–2.7% |
|
| |||||||||
| Chemistry or Hematology Toxicity | Grade ≥ 3 value for target hematologic or clinical chemistry measurement, confirmed by repeat assessmentd | Confirmation of laboratory abnormality on repeat testing without alternative etiology | 2670 | 21 (0.8%) | 0.5–1.2% | 21 | 11 (52.4%) | 11/2670 (0.4%) | 0.2–0.7% |
|
| |||||||||
| Hearing Impairment | Abnormal newborn hearing screen or mixed or sensorineural hearing loss for non-newborns | Confirmation of sensorineural or mixed hearing loss based on audiologic examination and by otolaryngologist, with no alternative explanation for hearing loss. | 1906 | 75 (3.9%) | 3.1–4.9% | 54 | 5 (9.3%) | 5/1885 (0.3%) | 0.1–0.6% |
|
| |||||||||
| Case in any Domain | 2680 | 1281 (47.8%) | 45.9–49.7% | 670/2680 (25.0%) | 23.4–26.7% | ||||
| Case in ≥ 2 Domains | 2680 | 374 (14.0%) | 12.7–15.3% | 137/2680 (5.1%) | 4.3–6.0% | ||||
BMI= body mass index, CI=confidence interval, LDL= low density lipoprotein, HDL=high density lipoprotein, HOMA=homeostatic model assessment, SD=standard deviation Full trigger and case definitions appear in Williams et al.21
Some subjects were evaluated for triggers but pending or indeterminate for case status, including 570 for growth domain (due to lack of at least 2 visits with growth measurements, including 40 who met growth trigger), 117 for metabolic (lacking fasting lipids and insulin measures), 33 for lactate (lacking venous lactate), 21 for hearing (lacking full audiologic exam), and 13 for neurologic domain (lacking follow-up consult or necessary assessments).
Age specific tests include MacArthur-Bates Communicative Development Index at age 1, Ages and Stages at age 2, and Test of Language Development at age 5.
Age specific tests include Bayley III at age 1 and 3, WPPSI at age 5, WISC at age 9 and older.
Specific laboratory tests included white blood count (WBC), polymorphonuclear neutrophil (PMN), lymphocytes, platelets, and hemoglobin for hematology and alanine aminotransferase (ALT), lactate dehydrogenase (LDH), lipase, creatine phosphokinase (CPK), blood urea nitrogen (BUN), creatinine, and glucose for clinical chemistry. Grade 3 or higher toxicity based on NIH Division of AIDS Toxicity tables37
Children who met the study trigger for a particular domain had additional pre-specified assessments, such as laboratory testing and evaluation by an appropriate specialist (Table 1). The results of the triggered evaluations were reviewed by the SMARTT Review Panel, a group of clinical and epidemiological experts, using pre-defined “case” definitions for AEs in each of the target domains. Following these strict criteria, the Panel determined whether each subject who met a trigger also met the corresponding case definition for AEs, while blinded to the specific ARV regimens mothers used during pregnancy. For example, children meeting the metabolic trigger were defined as metabolic “cases” if they had abnormal lipid levels or insulin resistance on further testing. For some domains, a small percentage of participants met a trigger but did not have the follow-up assessments needed to determine case status; these children were not considered evaluable for case determination.
Exposure Measures
The primary exposure of interest was in utero ARV exposure. Children were classified according to exposure to ARV drug classes, to combination ARV regimens including highly active antiretroviral therapy (HAART, defined as ≥3 drugs from ≥2 classes), and to specific ARV agents. The reference group consisted of children unexposed to the specific ARV drug class or drug being considered. Since the critical windows of exposure during pregnancy for most domains are unknown, we also evaluated ARV exposures by trimester of first exposure.
Potential Confounders
We identified potential confounders based on past literature and descriptive statistics from our cohort, using directed acyclic graphs(see Supplemental Figure 1).28–30 We considered the following maternal covariates to be potential confounders: age and race, pre-pregnancy BMI, chronic health conditions such as pre-gestational diabetes, HIV VL and CD4 counts early in pregnancy, and first trimester substance use (including alcohol, tobacco, and illicit drugs22). In addition, socioeconomic measures were evaluated including household income and caregiver education levels. We descriptively summarized pregnancy outcome [low birth weight (LBW, <2500g), preterm birth (<37 weeks gestation) and mode of birth (vaginal or Cesarean delivery)] by case status, but our primary analyses excluded these characteristics and also maternal health measures later in pregnancy, since they may be on the causal pathway given prior evidence of their association with maternal ARVs. 21,23,26
Statistical analysis
Rates of adverse events and exact 95% confidence intervals (CIs) were estimated overall and by domain. Unadjusted and adjusted log-binomial regression models were used to obtain relative risks (RR) for associations between ARV exposures and case status. Separate analyses were also conducted within the following domains: neurologic, neurodevelopmental (ND), a combined neurologic/ND domain (case in either the neurologic or ND domain), metabolic, growth, and language. Other domains (laboratory, hearing, and blood lactate) had too few cases and were not considered separately. Covariates identified a priori as potential confounders and with p<0.20 in unadjusted models for case status were included in initial multivariable models, and those with p<0.10 and/or which changed ARV exposure estimates by >10% were retained in final adjusted models.
Sensitivity analyses were conducted further adjusting for LBW to evaluate the impact on findings, and restricting to those who were exposed to HAART during pregnancy to limit potential selection bias. In addition, sensitivity analyses were conducted to account for children with the same mother/caregiver and clustering at the same clinical research site. Analyses were also conducted restricted to the Dynamic Cohort, which includes only those mother/infant pairs followed prospectively since birth. Last of all, while time-to-event models were not appropriate for evaluating overall case status due to differential timing of age-specific tests (in neurodevelopment, language, and hearing domains) relative to age at entry, incidence rate analyses were conducted using Poisson regression models for domains evaluated at every study visit (metabolic from age 2 years, neurologic, and growth).
RESULTS
Characteristics of Study Population
A total of 2680 SMARTT participants (1198 from the Static Cohort and 1482 from the Dynamic Cohort) were enrolled and had trigger data submitted as of December 31, 2012 and were thus considered evaluable (see Table 2). After a median follow of 2.4 years (range 0.1–5.7 years), almost half (47.8%) met a trigger in at least one domain and 25% met the criteria for a case in at least one domain. Overall, 48% of the participants were female, 66% Black, and 33% Hispanic. As reported previously, there is a high rate of prematurity (21%) and LBW (19%) in this population.21 Tobacco and alcohol use during pregnancy were relatively common (19% and 8%, respectively), although hard drug use (cocaine, heroin, or opium) was relatively rare (3%).
Table 2.
Demographic and Maternal Characteristics of SMARTT study participants by Adverse Event Case Status
| Characteristic | Case Status
|
Total (N=2680) | P-Value* | |
|---|---|---|---|---|
| No (N=2010) | Yes (N=670) | |||
| Child Characteristics | ||||
|
| ||||
| Cohort | <0.001 | |||
| Dynamic | 1,193 (59%) | 289 (43%) | 1,482 (55%) | |
| Static | 817 (41%) | 381 (57%) | 1,198 (45%) | |
| Birth Cohort | <0.001 | |||
| < 2002 | 258 (13%) | 133 (20%) | 391 (15%) | |
| 2002–2004 | 244 (12%) | 98 (15%) | 342 (13%) | |
| 2005–2007 | 360 (18%) | 164 (24%) | 524 (20%) | |
| 2008–2009 | 484 (24%) | 192 (29%) | 676 (25%) | |
| 2010–2012 | 664 (33%) | 83 (12%) | 747 (28%) | |
| Female sex | 975 (49%) | 318 (47%) | 1293 (48%) | 0.59 |
| Race/origin | 0.009 | |||
| White | 515 (26%) | 213 (32%) | 728 (27%) | |
| Black/African American | 1,365 (68%) | 406 (61%) | 1,771 (66%) | |
| Puerto Rican | 80 (4%) | 27 (4%) | 107 (4%) | |
| Other | 12 (1%) | 5 (1%) | 17 (1%) | |
| Latino/Hispanic | 636 (32%) | 246 (37%) | 882 (33%) | 0.016 |
| Birth characteristics | ||||
| Low birth weight (<2500g) | 328 (17%) | 168 (25%) | 496 (19%) | <0.001 |
| Preterm birth (<37 weeks gestation) | 380 (19%) | 159 (24%) | 539 (21%) | 0.012 |
| C-section at delivery | 1,073 (55%) | 371 (56%) | 1,444 (55%) | 0.59 |
|
| ||||
| Caregiver/Household Characteristics | ||||
|
| ||||
| Household income<$20,000 | 1,316 (71%) | 429 (68%) | 1,745 (70%) | 0.21 |
| Caregiver not high school graduate | 669 (34%) | 250 (37%) | 919 (35%) | 0.10 |
|
| ||||
| Maternal Characteristics | ||||
|
| ||||
| ARV Regimen during Pregnancy | 0.21 | |||
| Not on HAART | 333 (17%) | 136 (21%) | 469 (18%) | |
| HAART with PI & NNRTI | 127 (7%) | 43 (7%) | 170 (6%) | |
| HAART with PI | 1,315 (68%) | 422 (64%) | 1,737 (65%) | |
| HAART with NNRTI | 171 (9%) | 54 (8%) | 225 (8%) | |
| Age and Marital Status | ||||
| <25 years at birth of child | 650 (33%) | 217 (33%) | 867 (32%) | 0.96 |
| Single, never married | 1,293 (65%) | 400 (60%) | 1,693 (64%) | 0.016 |
| Maternal Immunologic and Virologic Health During Pregnancy | ||||
| VL > 1000 copies/mL at delivery | 297 (16%) | 104 (17%) | 401 (15%) | 0.57 |
| VL > 1000 copies/mL early in pregnancy | 996 (54%) | 343 (56%) | 1,339 (54%) | 0.33 |
| CD4<250 cells/mm3 at delivery | 270 (14%) | 106 (17%) | 376 (15%) | 0.11 |
| CD4<250 cells/mm3 early in pregnancy | 355 (19%) | 118 (19%) | 473 (19%) | 0.95 |
| Maternal Substance Use During Pregnancy | ||||
| Illicit drug use, including hard drugs | 159 (9%) | 55 (9%) | 214 (9%) | 0.74 |
| Hard drug Use (cocaine/opiate) | 49 (3%) | 20 (3%) | 69 (3%) | 0.40 |
| Alcohol use | 156 (8%) | 49 (8%) | 205 (8%) | 0.87 |
| Tobacco use | 336 (18%) | 126 (21%) | 462 (19%) | 0.15 |
| Maternal Sexually Transmitted Infections During Pregnancy | ||||
| Gonorrhea | 54 (3%) | 20 (3%) | 74 (3%) | 0.68 |
| Chlamydia | 178 (9%) | 47 (7%) | 225 (9%) | 0.15 |
| Trichomonas | 228 (13%) | 64 (11%) | 292 (13%) | 0.10 |
| Syphilis | 65 (3%) | 14 (2%) | 79 (3%) | 0.15 |
| Any of above | 419 (22%) | 112 (18%) | 531 (21%) | 0.018 |
HAART=highly active antiretroviral treatment, PI=protease inhibitor, NRTI=nucleoside reverse transcriptase inhibitor, NNRTI=non-nucleoside reverse transcriptase inhibitor.
P-value calculated using Fisher’s Exact Test for binary characteristics and by Chi-Square Test for categorical characteristics.
Data on certain characteristics were not available for some participants, including race (n=57), ethnicity (n=3), birth characteristics (n=41 for birth weight, 63 for gestational age, 70 for delivery by Cesarean section), maternal age at delivery (n=71), marital status (n=35), household income (n=199), caregiver education (n=35), HAART regimen (n=79), maternal CD4 measures (n=182) and HIV RNA viral load (n=215), maternal substance use (n=207), and maternal STIs (n=149–391, depending on STI); percentages are calculated based on those with available data.
There was a significant difference in case status by birth cohort and race, with a higher percentage of cases born in earlier years and among white and Hispanic participants. There were also differences in prevalence of cases by other socio-demographic factors (caregiver marital status and education), but not by maternal HIV status (CD4 or viral load) or substance use. The most commonly met triggers were metabolic (28.8%) and reduced growth (18.5%), and the least common were laboratory triggers (Table 1). Cases were most often met in the language (13.2%) and metabolic (11.4%) domains. Overall, 52% of those meeting a trigger and evaluated for case status met the case definition, ranging from 9%–99% for different domains (Table 1).
Association of Demographic and Maternal Characteristics with Case Occurrence
A summary of covariates included in adjusted models for overall case status and for each separate domain is provided in Supplemental Table 1. The final model for overall case status included protective effects of black race or Puerto Rican origin and later birth cohort (2010 or later vs. <2010) and increased risk of case status for those with low caregiver education and maternal tobacco use in the first trimester. LBW was associated with 42% increased risk of case status (95% CI: 22%, 65%) and was included in sensitivity analyses. For models fit within specific domains, first trimester tobacco was associated with higher risk of neurologic, neurologic/ND, and growth cases, low caregiver education was associated with case status in the neurologic/ND and language domains, and pre-gestational diabetes was associated with case status in the neurologic, neurologic/ND, and metabolic domains.
Association of in utero ARV Exposures with Case Occurrence
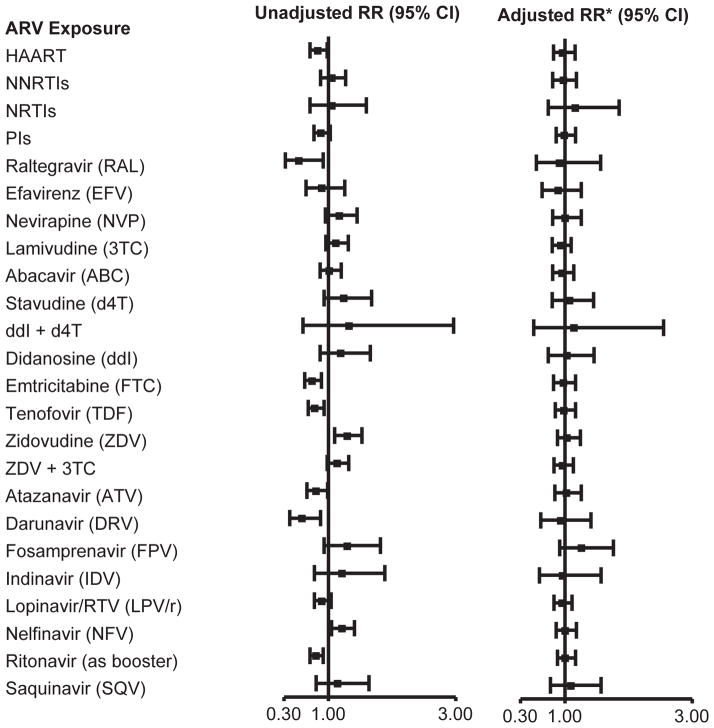
The unadjusted and adjusted associations of in utero ARV exposures with overall case status are summarized in Table 3 and Figure 1 for exposures at any time during pregnancy. In unadjusted models, exposure to a HAART regimen and to specific ARVs including emtricitabine, tenofovir, raltegravir, atazanavir, darunavir, and ritonavir (as a booster) were each associated with protective effects on risk of case status, while exposures to zidovudine and nelfinavir were associated with significantly higher risks. However, after adjustment, there was no association of any ARV regimen, drug class, or individual ARV drug with overall case status, and adjusted RRs were very close to 1 (Figure 1). Findings were similar when evaluated by trimester of the first ARV exposure. HAART and exposure to the same individual ARVs in the first trimester were associated with lower risk of case status in unadjusted models, but not after adjusting for birth cohort and other factors (Supplemental Table 2). Zidovudine (either 1st or 2nd/3rd trimester vs. unexposed) and first trimester exposure to either nelfinavir or stavudine were associated with increased risk in unadjusted models (RRs of 1.31 to 1.47), but not after adjustment. While rarely used, first exposure to fosamprenavir later in pregnancy (2nd or 3rd trimester, 1.2% exposed) was associated with increased risk of overall case status (adjusted RR [aRR]=1.73, 95% CI: 1.23, 2.43).
Table 3.
Association of in utero ARV exposure at Any Time during Pregnancy with Overall Case Status
| Percent Exposed | Percent of Cases | Unadjusted Model (N=2602) | Adjusted Model* (N=2460) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Exposed | Unexposed | RR (95% CI) | P-value | RR (95% CI) | P-value | ||
| By ARV Drug Class or Regimen | |||||||
| HAART | 82.0 | 24.3 | 29.0 | 0.84 (0.72, 0.99) | 0.032 | 0.98 (0.82, 1.16) | 0.77 |
| NNRTIs | 14.3 | 26.3 | 25.0 | 1.05 (0.87, 1.26) | 0.60 | 0.98 (0.81, 1.18) | 0.81 |
| NRTIs | 97.1 | 25.2 | 23.7 | 1.07 (0.71, 1.60) | 0.76 | 1.15 (0.73, 1.82) | 0.54 |
| PIs | 73.8 | 24.5 | 27.1 | 0.90 (0.78, 1.04) | 0.17 | 1.01 (0.86, 1.17) | 0.94 |
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | |||||||
| Lamivudine (3TC) | 73.2 | 26.0 | 23.1 | 1.13 (0.96, 1.31) | 0.14 | 0.94 (0.80, 1.10) | 0.43 |
| Abacavir (ABC) | 20.1 | 25.6 | 25.1 | 1.02 (0.87, 1.20) | 0.82 | 0.96 (0.81, 1.13) | 0.61 |
| Stavudine (d4T) | 4.0 | 31.1 | 24.9 | 1.25 (0.93, 1.67) | 0.15 | 1.07 (0.80, 1.44) | 0.65 |
| Didanosine (ddI) | 3.5 | 30.0 | 25.0 | 1.20 (0.87, 1.66) | 0.27 | 1.02 (0.73, 1.44) | 0.89 |
| Emtricitabine (FTC) | 25.1 | 20.2 | 26.9 | 0.75 (0.63, 0.89) | <0.001 | 0.99 (0.83, 1.17) | 0.88 |
| Tenofovir (TDF) | 29.2 | 21.3 | 26.8 | 0.80 (0.68, 0.93) | 0.004 | 1.00 (0.85, 1.17) | 0.97 |
| Zidovudine (ZDV) | 73.9 | 26.9 | 20.4 | 1.31 (1.11, 1.55) | 0.001 | 1.06 (0.89, 1.25) | 0.51 |
| ZDV + 3TC | 68.5 | 26.3 | 22.8 | 1.15 (0.99, 1.33) | 0.064 | 0.98 (0.84, 1.14) | 0.79 |
| ddI + d4T | 0.5 | 33.3 | 25.1 | 1.33 (0.59, 2.96) | 0.49 | 1.13 (0.51, 2.52) | 0.77 |
| Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | |||||||
| Efavirenz (EFV) | 4.7 | 22.8 | 25.3 | 0.90 (0.65, 1.25) | 0.53 | 0.89 (0.63, 1.24) | 0.48 |
| Nevirapine (NVP) | 9.0 | 29.1 | 24.8 | 1.17 (0.95, 1.45) | 0.14 | 1.02 (0.82, 1.27) | 0.88 |
| Fusion Inhibitors/Integrase Inhibitors | |||||||
| Raltegravir (RAL) | 3.3 | 13.8 | 25.6 | 0.54 (0.32, 0.92) | 0.022 | 0.93 (0.55, 1.56) | 0.78 |
| Protease Inhibitors (PIs) | |||||||
| Atazanavir (ATV) | 15.3 | 20.9 | 26.0 | 0.80 (0.65, 0.98) | 0.035 | 1.02 (0.83, 1.24) | 0.86 |
| Darunavir (DRV) | 5.1 | 15.0 | 25.7 | 0.58 (0.39, 0.88) | 0.010 | 0.93 (0.62, 1.39) | 0.71 |
| Fosamprenavir (FPV) | 2.8 | 32.4 | 25.0 | 1.30 (0.93, 1.82) | 0.13 | 1.28 (0.92, 1.77) | 0.15 |
| Indinavir (IDV) | 1.8 | 30.4 | 25.1 | 1.21 (0.78, 1.89) | 0.39 | 0.96 (0.60, 1.56) | 0.88 |
| Lopinavir (LPV) | 32.6 | 23.6 | 25.9 | 0.91 (0.79, 1.05) | 0.20 | 0.96 (0.83, 1.12) | 0.62 |
| Nelfinavir (NFV) | 22.2 | 29.1 | 24.1 | 1.21 (1.04, 1.40) | 0.012 | 1.00 (0.86, 1.17) | 0.95 |
| Ritonavir (as booster) | 53.3 | 22.9 | 27.8 | 0.82 (0.72, 0.94) | 0.004 | 1.02 (0.89, 1.17) | 0.74 |
| Saquinavir (SQV) | 3.1 | 30.0 | 25.0 | 1.20 (0.85, 1.69) | 0.30 | 1.14 (0.81, 1.60) | 0.47 |
Adjusted model includes black race or Puerto Rican origin, low caregiver education (< high school), 1st trimester maternal tobacco use, and birth cohort (2010+ vs <2010)
Figure 1.
Association of Overall Case Status with in utero ARV Exposures, unadjusted and adjusted for birth cohort and other potential confounders. *Adjusted model includes black race or Puerto Rican origin, low caregiver education (< high school), 1st trimester maternal tobacco use, and birth cohort (2010+ vs <2010).
Association of in utero ARV Exposures with Case Occurrence in Specific Domains
Across individual domains, few significant associations of any drug class or individual ARV with increased risk of case status were observed in adjusted models (Table 4). However, zidovudine exposure was associated with increased risk of metabolic case in both unadjusted and adjusted models. The combination of didanosine plus stavudine, while now rarely used (<1% exposed), was associated with a 12-fold higher risk of a ND case, and almost 5-fold higher risk of language case. First trimester stavudine exposure was also associated with a two-fold increased risk of a language case, with similar (though non-significant) RR for overall stavudine exposure. When the combined outcome of either neurologic or ND case status was evaluated, there was an increased risk with didanosine exposure (aRR=2.16, 95% CI: 1.14, 4.10) for 1st trimester exposure; aRR=1.75, 95% CI: 0.99, 3.08 for overall exposure), and similar magnitude associations within both individual domains. However, for other ARV drugs, associations were occasionally in opposite directions for these two domains; for example, non-nucleoside reverse transcriptase inhibitors (NNRTIs) were associated with marginally increased risk for neurologic case but decreased risk for ND impairment.
Table 4.
Association of in utero ARV exposure with Case Status in Specific Domains, for exposures with p<0.10 in either unadjusted or adjusted analysis
| Domain/ARV Exposure | Percent Exposed | Percent of Cases | Unadjusted Model | Adjusted Model* | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Exposed | Unexposed | RR (95% CI) | P-value | RR (95% CI) | P-value | ||
| Language Impairment (N=1482 in unadjusted model, N=1474 in adjusted model) | |||||||
| Stavudine (d4T) | 2.5 | 21.6 | 13.0 | 1.66 (0.89, 3.11) | 0.11 | 1.77 (0.95, 3.28) | 0.071 |
| Lopinavir (LPV) | 38.7 | 15.2 | 12.0 | 1.27 (0.97, 1.64) | 0.077 | 1.27 (0.98, 1.64) | 0.074 |
| ddI + d4T | 0.1 | 50.0 | 13.2 | 3.79 (0.94, 15.27) | 0.060 | 4.84 (1.14, 20.51) | 0.032 |
| Stavudine (1st trimester) | 1.6 | 25.0 | 13.0 | 1.92 (0.95, 3.88) | 0.070 | 2.10 (1.05, 4.19) | 0.036 |
| Metabolic Abnormality (N=1640 in unadjusted model, N=1524 in adjusted model) | |||||||
| HAART | 79.0 | 10.0 | 16.0 | 0.62 (0.46, 0.83) | 0.001 | 0.60 (0.44, 0.82) | 0.001 |
| PIs | 70.2 | 9.9 | 14.3 | 0.69 (0.52, 0.91) | 0.009 | 0.69 (0.52, 0.92) | 0.012 |
| Emtricitabine (FTC) | 16.6 | 6.2 | 12.2 | 0.51 (0.31, 0.83) | 0.006 | 0.62 (0.38, 1.00) | 0.049 |
| Tenofovir (TDF) | 21.6 | 7.3 | 12.3 | 0.60 (0.40, 0.89) | 0.011 | 0.71 (0.48, 1.06) | 0.096 |
| Zidovudine (ZDV) | 79.6 | 12.4 | 6.6 | 1.88 (1.23, 2.89) | 0.004 | 1.69 (1.08, 2.64) | 0.022 |
| Lopinavir (LPV) | 28.5 | 5.8 | 13.4 | 0.43 (0.29, 0.64) | 0.001 | 0.46 (0.31, 0.69) | <0.001 |
| Nelfinavir (NFV) | 29.1 | 13.6 | 10.2 | 1.33 (1.00, 1.77) | 0.047 | 1.22 (0.91, 1.64) | 0.18 |
| Ritonavir (as booster) | 43.0 | 7.5 | 14.0 | 0.54 (0.40, 0.73) | 0.001 | 0.59 (0.43, 0.81) | 0.001 |
| Emtricitabine (1st trimester) | 10.2 | 5.4 | 11.9 | 0.45 (0.24, 0.87) | 0.017 | 0.54 (0.28, 1.03) | 0.063 |
| Tenofovir (1st trimester) | 12.8 | 6.7 | 11.9 | 0.56 (0.33, 0.95) | 0.031 | 0.64 (0.38, 1.08) | 0.096 |
| Zidovudine (1st trimester) | 31.2 | 13.7 | 10.1 | 1.35 (1.02, 1.79) | 0.034 | 1.30 (0.98, 1.73) | 0.071 |
| Lopinavir (1st trimester) | 11.4 | 4.8 | 12.0 | 0.40 (0.21, 0.77) | 0.006 | 0.39 (0.20, 0.78) | 0.008 |
| Ritonavir (1st trimester) | 19.3 | 7.0 | 12.2 | 0.57 (0.37, 0.87) | 0.010 | 0.61 (0.40, 0.95) | 0.029 |
| Impaired Growth (N=2062 in unadjusted model, N=1890 in adjusted model) | |||||||
| NRTIs | 97.0 | 7.3 | 14.8 | 0.49 (0.27, 0.92) | 0.027 | 0.48 (0.24, 0.96) | 0.038 |
| Neurologic (N=2582 in unadjusted model, N=2348 in adjusted model) | |||||||
| NNRTIs | 14.4 | 8.3 | 5.8 | 1.43 (0.98, 2.08) | 0.064 | 1.42 (0.97, 2.10) | 0.073 |
| Efavirenz | 4.7 | 9.8 | 6.0 | 1.63 (0.93, 2.86) | 0.085 | 1.68 (0.95, 3.00) | 0.076 |
| Emtricitabine (FTC) | 25.2 | 4.6 | 6.7 | 0.69 (0.47, 1.01) | 0.056 | 0.90 (0.59, 1.35) | 0.60 |
| Tenofovir (TDF) | 29.2 | 4.9 | 6.7 | 0.73 (0.51, 1.04) | 0.082 | 0.88 (0.60, 1.29) | 0.52 |
| Didanosine (1st trimester) | 2.0 | 13.5 | 6.0 | 2.23 (1.10, 4.51) | 0.026 | 2.06 (0.96, 4.38) | 0.062 |
| Emtricitabine (1st trimester) | 15.6 | 4.2 | 6.6 | 0.64 (0.39, 1.05) | 0.078 | 0.79 (0.46, 1.34) | 0.38 |
| Tenofovir (1st trimester) | 17.9 | 4.3 | 6.6 | 0.66 (0.41, 1.04) | 0.070 | 0.73 (0.44, 1.20) | 0.22 |
| Nelfinavir (1st trimester) | 8.4 | 8.8 | 6.0 | 1.48 (0.93, 2.33) | 0.096 | 1.39 (0.88, 2.19) | 0.16 |
| Ritonavir (1st trimester) | 25.6 | 4.4 | 6.8 | 0.64 (0.43, 0.95) | 0.028 | 0.78 (0.53, 1.17) | 0.23 |
| Neurodevelopmental Impairment (N=1276 in unadjusted model, N=1165 in adjusted model) | |||||||
| HAART | 85.1 | 4.0 | 7.9 | 0.50 (0.28, 0.88) | 0.017 | 0.47 (0.27, 0.83) | 0.010 |
| NNRTIs | 16.2 | 2.4 | 5.0 | 0.49 (0.20, 1.20) | 0.12 | 0.38 (0.14, 1.04) | 0.059 |
| Lamivudine (3TC) | 73.0 | 3.6 | 7.0 | 0.52 (0.31, 0.87) | 0.012 | 0.60 (0.36, 1.02) | 0.058 |
| Didanosine (ddI) | 3.8 | 10.4 | 4.3 | 2.41 (1.01, 5.76) | 0.047 | 2.22 (0.93, 5.31) | 0.073 |
| ddI + d4T | 0.2 | 66.7 | 4.4 | 15.15 (6.54, 35.11) | 0.001 | 12.40 (5.29, 29.08) | 0.001 |
| ZDV + 3TC | 68.4 | 3.8 | 6.2 | 0.61 (0.37, 1.01) | 0.055 | 0.70 (0.41, 1.17) | 0.17 |
| Lamivudine (1st trimester) | 32.6 | 3.1 | 5.2 | 0.60 (0.33, 1.09) | 0.095 | 0.64 (0.35, 1.18) | 0.16 |
| Neurologic or Neurodevelopmental (N=2588 in unadjusted model, N=2349 in adjusted model) | |||||||
| Lamivudine (3TC) | 73.1 | 7.4 | 8.5 | 0.87 (0.65, 1.17) | 0.354 | 0.70 (0.52, 0.96) | 0.025 |
| Didanosine (ddI) | 3.5 | 13.3 | 7.5 | 1.78 (1.03, 3.07) | 0.038 | 1.75 (0.99, 3.08) | 0.054 |
| Didanosine (1st trimester) | 2.0 | 17.3 | 7.5 | 2.31 (1.26, 4.25) | 0.007 | 2.16 (1.14, 4.10) | 0.018 |
| Ritonavir (1st trimester) | 25.6 | 5.9 | 8.3 | 0.71 (0.51, 1.00) | 0.047 | 0.83 (0.59, 1.18) | 0.30 |
| Case in Multiple Domains (at least 2) (N=2601 in unadjusted model, N=2369 in adjusted model) | |||||||
| NNRTIs | 14.3 | 3.5 | 5.5 | 0.64 (0.36, 1.12) | 0.12 | 0.50 (0.27, 0.95) | 0.034 |
| NNRTIs (1st trimester) | 8.6 | 2.7 | 5.4 | 0.50 (0.22, 1.11) | 0.089 | 0.44 (0.18, 1.07) | 0.071 |
Adjusted model includes the following covariates for each domain:
Language: low caregiver education and female sex;
Metabolic: pre-gestational diabetes, Latino ethnicity, maternal gonorrhea, and birth cohort (2010+ vs <2010)
Growth: maternal tobacco use during pregnancy, young maternal age (<25 yrs) at delivery, and birth cohort (2010+ vs <2010)
Neurologic: 1st trimester maternal tobacco use, pre-gestational diabetes, toxemia/pre-eclampsia, and birth cohort (2010+ vs <2010)
Neurodevelopment: low household income (<$20K/year)
Neurologic/ND: low caregiver education (< high school) 1st trimester maternal tobacco use, pre-gestational diabetes, toxemia/pre-eclampsia, and birth cohort (2010+ vs <2010)
Multiple Domains: 1st trimester maternal alcohol use, maternal toxemia or pre-eclampsia, and birth cohort (2010+ vs <2010)
Certain individual ARVs were associated with significant protective effects in adjusted models, particularly for metabolic cases (Table 4). Only 4.7% of participants met case criteria in more than one domain. Although this outcome was hypothesized to be a more specific indicator of an ARV-associated adverse outcome, no ARV drug classes or individual drugs were associated with increased risk of case status in multiple domains. Efavirenz was associated with increased risk of neurologic case, and lopinavir with language impairment, although neither attained statistical significance (Table 4).
Sensitivity Analyses
While LBW was strongly associated with case status, further adjustment for LBW in addition to the other covariates yielded results very similar to those in Table 3 (not shown), suggesting that LBW did not play a role as a mediator or confounder. The difference between unadjusted and adjusted RRs was primarily attributable to adjustment for birth cohort.
Analyses restricted to the 2132 HAART-exposed children yielded similar results to those presented in Table 3. The same six ARV drugs showed protective associations in unadjusted but not in adjusted models, and both zidovudine and nelfinavir continued to be associated with increased risk in unadjusted but not in adjusted models. Analyses accounting for clustering of children within the same family or research site also yielded results very similar to those in Table 3, with no associations observed for any ARV drug or drug class with case status. Within the Dynamic cohort, there were again no associations between in utero ARV exposures and case status based in adjusted models controlling for race and birth cohort (Supplemental Table 3).
For individual domains evaluated at each study visit (growth, neurologic, and metabolic), Poisson regression models for comparison of incidence rates (IRs) confirmed previous findings based on RRs (which do not account for follow-up time). More specifically, zidovudine exposure was associated with higher incidence of metabolic cases (IR=6.10 vs. 3.88 cases per 100 person-years for zidovudine-exposed vs. unexposed), which persisted after adjustment (adjusted incidence rate ratio [aIRR]=1.61, 95% CI: 1.01, 2.58, p=0.047; see Supplemental Table 4). HAART and lopinavir exposure were also confirmed to have protective associations with incidence of metabolic cases. Children exposed to efavirenz had higher incidence of neurologic cases (IR=4.89 vs. 2.78 cases per 100 person years), although this difference did not attain statistical significance before or after adjustment (aIRR=1.77, 95% CI: 0.95, 3.28, p=0.069).
DISCUSSION
We developed the SMARTT study as a surveillance system for monitoring potential toxicities related to intrauterine ARV exposures in infants born to mothers with HIV infection. Overall, our findings were very reassuring and suggested no increased risk of AE case status for any ARV drug class or individual drug. Not only were no associations detected, but adjusted risk ratios were very close to 1. From a safety perspective, the lack of association is important; given both the size of this study and the relatively high background rate of adverse outcome (25%), there was high power to detect relatively small differences (5–8% increase in case prevalence at 80% power, depending on percent exposed).
Although no associations with overall case status were observed, some isolated findings for specific outcomes and specific ARV drugs were noted. In particular, didanosine was associated with increased risk of both neurologic and neurodevelopmental cases, whether used early in pregnancy or at any time during pregnancy. In addition, the combination of didanosine plus stavudine, while rarely used (<1%), was associated with greater than a twelve-fold increased risk of a neurodevelopmental case, and stavudine was also associated with a higher risk of language impairment. The high potential for mitochondrial toxicity, including lactic acidosis, during pregnancy with didanosine plus stavudine has led to recommendations against use of this combination.31 Other mechanisms of toxicity, such as epigenetic effects and metabolic toxicities, may also play a role.38–40 While previous animal studies and case reports have noted potential associations of in utero efavirenz exposure with neural tube defects or other neurological outcomes,31–33 we observed only slightly elevated risk of neurological case (9.8% for EFV-exposed vs. 6.0% for EFV-unexposed) which did not attain statistical significance.
Metabolic cases, reflecting both obesity and either dyslipidemia or insulin resistance, were most consistently associated with individual ARV drugs and regimens. Zidovudine was associated with increased risk of metabolic cases while several other NRTIs, PIs as a class, and individual PIs showed protective associations. The protective findings for in utero exposure to PIs and metabolic outcomes are in contrast to the elevated risk of dyslipidemia observed in HIV-infected children treated with PIs.34 Most youth (70%) were exposed to PIs as part of effective combination therapy, and these protective associations may have been partially attributable to residual confounding since mothers not using PIs may have differed in ways not accounted for by our covariate adjustment.
One of the most striking findings of our study was the high background rate of AEs meeting the criteria for “cases” in this population. The most common domains within which cases were observed were metabolic and language, with over 10% of youth affected; the high rates of these conditions may partially reflect other risk factors associated with home environment, socioeconomic status, and nutrition within our cohort, but these rates are likely representative of youth born to HIV-infected mothers in the United States and other high resource settings.
The adverse outcomes of interest in this evaluation did not include pregnancy outcomes such as preterm birth, LBW, and congenital anomalies, which have been addressed in separate reports.21,35,36 A comprehensive safety assessment must consider a wide range of possible adverse outcomes. However, we evaluated multiple domains of interest which could reflect mitochondrial dysfunction, epigenetic effects, or other mechanisms of toxicity related to intrauterine exposures.38–40 A potential limitation of our trigger-based approach is that it may have missed certain AEs, and all domains were treated equally which may not reflect the relative clinical significance of toxicities across different domains. In addition, ARV drugs could have opposing effects on different domains, which would tend to obscure associations with overall case status. Further in-depth evaluations of separate domains are still warranted. In addition, older children typically had longer follow-up and thus greater numbers of trigger evaluations; this may have led to increased risk in unadjusted analyses for “older” ARV drugs like zidovudine and nelfinavir. We accounted for this by controlling for birth cohort and conducting time-to-event analyses for domains evaluated at every visit, which yielded consistent findings. Nonetheless, strengths of our study were the systematic evaluation and classification of adverse outcomes blinded to ARV exposure over multiple domains, the large size and long-term follow-up, and the ability to control for many other potential confounders such as maternal health and substance use. As more women with HIV enter pregnancy already on ARVs based on current recommendations, there is a critical need to identify optimal regimens for both maternal and child safety in surveillance studies such as SMARTT.
Supplementary Material
Acknowledgments
FUNDING:
The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104).
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. We recognize the contributions of the members of the SMARTT Review Panel: Pim Brouwers, Laurie Butler, Lucy Civitello, Marilyn Crain, Carol Elgie, Angela Ellis, Rohan Hazra, Kenneth Rich, George Seage, George Siberry, Russell Van Dyke, Paige Williams, and Cenk Yildirim.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
Footnotes
Author contributions: PLW, RH, RBV, KR, MJC, and GRS conceived the study design. PLW conducted statistical analyses and took the lead role in drafting the paper. CY, AE, and LB prepared data for the statistical analyses. All coauthors reviewed the manuscript and provided critical scientific revisions, and approved the final version of the manuscript.
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2014, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Anna Cintron; Children’s Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Patricia Garvie, James Blood; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Helen Rozelman; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Susan Adubato; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Hermann Mendez, Ava Dennie, Susan Bewley; Tulane University Health Sciences Center: Chi Dola, Robert Maupin, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Newana Beatty, Dan Marullo; University of California, San Diego: Stephen Spector, Jean Manning, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Jenna Wallace, Carrie Chambers, Christine Reed; University of Florida/Jacksonville: Mobeen Rathore, Kristi Stowers, Ann Usitalo; University of Illinois, Chicago: Kenneth Rich, Lourdes Richardson, Renee Smith; University of Miami: Gwendolyn Scott, Claudia Florez, Elizabeth Willen; University of Southern California: Toni Frederick, Mariam Davtyan, Maribel Mejia; University of Puerto Rico Medical Center: Zoe Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
References
- 1.Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz D, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1 infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2005;29(5):484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Suksomboon N, Poolsup N, Ket-Aim S. Systematic review of the efficacy of antiretroviral therapies for reducing the risk of mother-to-child transmission of HIV infection. J Clin Pharm Ther. 2007;32(3):293–311. doi: 10.1111/j.1365-2710.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 3.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother to child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22(8):973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 4.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. [last accessed May 18, 2015];Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2014 May 28; http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf.
- 5.Mofenson LM, Munderi P. Safety of antiretroviral prophylaxis of perinatal transmission for HIV-infected pregnant women and their infants. AIDS. 2002;30(2):200–215. doi: 10.1097/00042560-200206010-00010. [DOI] [PubMed] [Google Scholar]
- 6.European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32(4):380–387. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Thorne C, Newell ML. Safety of agents used to prevent mother-to-child transmission of HIV: is there any cause for concern? Drug Safety. 2007;30(3):203–213. doi: 10.2165/00002018-200730030-00004. [DOI] [PubMed] [Google Scholar]
- 8.Montaner JS, Côté HC, Harris M, Hogg RS, Yip B, Chan JW, Harrigan PR, O’Shaughnessy MV. Mitochondrial toxicity in the era of HAART: evaluating venous lactate and peripheral blood mitochondrial DNA in HIV-infected patients taking antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;34 (Suppl 1):S85–90. doi: 10.1097/00126334-200309011-00013. [DOI] [PubMed] [Google Scholar]
- 9.Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354(9184):1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 10.The Perinatal Antiretroviral Drug Toxicity Working Group. McIntosh K., Chair Mitochondrial toxicity in the offspring of HIV-infected women exposed to antiretroviral drugs: Absence of clear evidence for mitochondrial disease in children who died before five years of age in five United States cohorts. J Acquir Immune Defic Syndr. 2000;25(3):261–268. doi: 10.1097/00126334-200011010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Lindegren ML, Rhodes PH, Gordon L, Fleming P for the Perinatal Safety Review Working Group. Drug safety during pregnancy and in infants. Lack of mortality related to mitochondrial dysfunction among perinatally HIV-exposed children in pediatric HIV surveillance. Ann N Y Acad Sci. 2000;918(1):222–235. [PubMed] [Google Scholar]
- 12.Bulterys M, Nesheim S, Abrams EJ, Palumbo P, Farley J, Lampe M, Fowler MG for the Perinatal Safety Review Working Group. Lack of evidence of mitochondrial dysfunction in the offspring of HIV-infected women. Retrospective review of perinatal exposure to antiretroviral drugs in the Perinatal AIDS Collaborative Transmission Study. Ann N Y Acad Sci. 2000;918(1):212–221. doi: 10.1111/j.1749-6632.2000.tb05491.x. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez K, Bertolli J, Fowler M, Peters V, Ortiz I, Melville S, et al. for the PSD Consortium and the Perinatal Safety Review Working Group. Lack of definitive severe mitochondrial signs and symptoms among deceased HIV-uninfected and HIV-indeterminate children < 5 years of age. Ann N Y Acad Sci. 2000;918(1):236–246. doi: 10.1111/j.1749-6632.2000.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 14.Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, et al. for the French Perinatal Cohort Study Group. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17(12):1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Poirier MC, Divi RL, Al-Harthi L, Olivero OA, Nguyen V, Walker B, et al. for the Women and Infants Transmission Study (WITS) Group. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. 2003;33(2):175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 16.Gerschenson M, Brinkman K. Mitochondrial dysfunction in AIDS and its treatment. Mitochondrion. 2004;4(5–6):763–777. doi: 10.1016/j.mito.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Brogly SB, Ylitalo N, Mofenson LM, Oleske J, Van Dyke R, Crain MJ, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21(8):929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 18.Bae WH, Wester C, Smeaton LM, Shapiro RL, Lockman S, Onyait K, Thior I, Essex M. Hematologic and hepatic toxicities associated with antenatal and postnatal exposure to maternal highly active antiretroviral therapy among infants. AIDS. 2008;22:1633–1640. doi: 10.1097/QAD.0b013e328307a029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacheco SE, McIntosh K, Lu M, Mofenson LM, Diaz C, Foca M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: An analysis of the women and infants transmission study. J Infect Dis. 2006;194:1089–1097. doi: 10.1086/507645. [DOI] [PubMed] [Google Scholar]
- 20.Dryden-Peterson S, Shapiro RL, Hughes MD, Powis K, Ogwu A, Moffat C, Moyo S, Makhema J, Essex M, Lockman S. Increased risk of severe infant anemia after exposure to maternal HAART, Botswana. J Acquir Immune Defic Syndr. 2011;56(5):428–436. doi: 10.1097/QAI.0b013e31820bd2b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watts DH, Williams PL, Kacanek D, Griner R, Rich K, Hazra R, et al. for the Pediatric HIV/AIDS Cohort Study. Combination antiretroviral use and preterm birth. J Infect Dis. 2013;207(4):612–21. doi: 10.1093/infdis/jis728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tassiopoulos K, Read JS, Brogly S, Rich K, Lester B, Spector SA, Yogev R, Seage GR. Substance use in HIV-infected women during pregnancy: self-report versus meconium analysis. AIDS Behav. 2010;14(6):1269–1278. doi: 10.1007/s10461-010-9705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griner R, Williams PL, Read JS, Seage GR, Crain M, Yogev R, et al. for the Pediatric HIV/AIDS Cohort Study. In Utero and postnatal exposure to antiretrovirals among HIV-exposed but uninfected children in the United States. AIDS Patient Care and STDs. 2011 doi: 10.1089/apc.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams PL, Seage GR, Van Dyke RB, Siberry GK, Griner R, Tassiopoulos K, et al. for the Pediatric HIV/AIDS Cohort Study. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol. 2012;175(9):950–61. doi: 10.1093/aje/kwr401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brogly SB, Abzug MJ, Watts H, Cunningham CK, Williams PL, Oleske J, et al. Birth defects among children born to human immunodeficiency virus-infected women. Pediatr Infect Dis J. 2010;29(8):721–727. doi: 10.1097/INF.0b013e3181e74a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz I, Shapiro R, Li D, Govindarajulu U, Thompson B, Watts DH, Hughes MD, Tuomala R. Risk factors for detectable HIV-1 RNA at delivery among women receiving highly active antiretroviral therapy in the women and infants transmission study. J Acquir Immune Defic Syndr. 2010;54(1):27–34. doi: 10.1097/QAI.0b013e3181caea89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crain M, Williams P, Griner R, Tassiopoulos K, Read J, Mofenson L, Rich K for the Pediatric HIV/AIDS Cohort Study. Point-of-care capillary blood lactate measurements in human immunodeficiency virus-uninfected children with in utero exposure to human immunodeficiency virus and antiretroviral medications. Pediatr Infect Dis J. 2011;30(12):1069–74. doi: 10.1097/INF.0b013e318234c886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169(10):1182–90. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 29.Evans D, Chaix B, Lobbedez T, Verger C, Flahault A. Combining directed acyclic graphs and the change-in-estimate procedure as a novel approach to adjustment-variable selection in epidemiology. BMC Med Res Methodol. 2012;156 doi: 10.1186/1471-2288-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology (Cambridge, Mass) 2011 Sep;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. Epub 2011/08/04. eng. [DOI] [PubMed] [Google Scholar]
- 31.Watts DH. Treating HIV during pregnancy: An update on safety issues. Drug Safety. 2006;29(6):467–490. doi: 10.2165/00002018-200629060-00002. [DOI] [PubMed] [Google Scholar]
- 32.Ford N, Alexandra C, Mofenson L. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2011;25(18):2301–4. doi: 10.1097/QAD.0b013e32834cdb71. [DOI] [PubMed] [Google Scholar]
- 33.Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: a systematic review. Exper Opin Drug Saf. 2013;12(6):841–6. doi: 10.1517/14740338.2013.823396. [DOI] [PubMed] [Google Scholar]
- 34.Tassiopoulous K, Williams PL, Seage G, Crian M, Oleske J, Farley J. Association of hypercholesterolemia incidence with antiretroviral treatment including protease inhibitors among perinatally HIV-infected children. Journ Acquir Immune Defic Syndr. 2008;47(5):607–14. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams PL, Crain MJ, Yildirim C, Hazra R, Van Dyke RB, Rich K, Read JS, Stuard E, Rathore M, Mendez HA, Watts DH. Congenital anomalies and in utero antiretroviral exposure in HIV-exposed uninfected infants. JAMA Pediatrics. 2014 doi: 10.1001/jamapediatrics.2014.1889. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siberry GK, Williams PL, Mendez H, Seage GR, III, Jacbson D, Hazra R, Rich K, Griner R, Tassiopoulos K, Kacanek D, Mofenson LM, Miller T, DiMeglio L, Watts DH for the Pediatric HIV/AIDS Cohort Study. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:151–159. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. V1.0, published 12/28/2004, located at http://rcc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS-AE-Grading-Table-v1.0-DEC2004.pdf.
- 38.Marsit CJ, Brummel SS, Kacanek D, Seage GR, III, Spector SA, Armstrong DA, Lester BM, Rich K for the Pediatric HIV/AIDS Cohort Studies Network. Infant peripheral blood repetitive element hypomethylation associated with antiretroviral therapy in utero. Epigenetics. 2015;10(8):708–16. doi: 10.1080/15592294.2015.1060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dyke RB, Wang L, Williams PL. Toxicities associated with dual nucleoside reverse-transcriptase inhibitor regimens in HIV-infected children. J Infect Dis. 2008;198(11):1599–608. doi: 10.1086/593022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curran A, Ribera E. From old to new nucleoside reverse transcriptase inhibitors: changes in body fat composition, metabolic parameters, and mitochondrial toxicity after the switch from thymidine analogs to tenofovir or abacavir. Expert Opin Drug Safety. 2011;10(3):389–406. doi: 10.1517/14740338.2011.542145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.