Abstract
Background
This study sought to evaluate the impact of atrial fibrillation (AF) on clinical outcomes in patients undergoing transcatheter aortic valve replacement (TAVR).
Methods and Results
Data were evaluated in 1879 patients with baseline and discharge ECGs who underwent TAVR in the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial. A total of 1262 patients manifested sinus rhythm (SR) at baseline/SR at discharge, 113 SR baseline/AF discharge, and 470 AF baseline/AF discharge. Patients who converted from SR to AF by discharge had the highest rates of all-cause mortality at 30 days (p<0.0001 across all groups; 14.2% SR/AF vs. 2.6% SR/SR; adjusted HR=3.41 p=0.0002) and over two-fold difference at 1-year (p<0.0001 across all groups; 35.7% SR/AF vs. 15.8% SR/SR; adjusted HR=2.14, p<0.0001). The presence of AF on baseline and/or discharge ECG was a predictor of 1-year mortality (adjusted HR=2.14 for SR/AF group and HR=1.88 for AF/AF groups, p<0.0001 for both groups vs. SR/SR). For patients discharged in AF, those with lower ventricular response (i.e, <90 bpm) experienced less 1-year all-cause mortality (HR=0.74, p=0.04).
Conclusions
After TAVR, the presence of AF at discharge, and particularly the conversion to AF by discharge and higher ventricular response, are associated with increased mortality. These data underscore the deleterious impact of AF, as well as the need for targeted interventions to improve clinical outcomes, in patients undergoing TAVR.
Keywords: atrial fibrillation, transcatheter aortic valve replacement, mortality
Atrial fibrillation (AF) and atrial flutter are not only common clinical arrhythmias in the general population but are also associated complications of many cardiac procedures.1, 2 AF is especially common in patients undergoing cardiac valve surgery and coronary artery bypass grafting (CABG),3-5 with 5-40% of all patients undergoing open cardiac surgery developing AF.6-8 The development of both preoperative and postoperative AF results in worse outcomes including higher mortality.9-14 With regards to surgical aortic valve replacement (SAVR), postoperative atrial fibrillation is a known risk factor for mortality.15-19 Transcatheter aortic valve replacement (TAVR) has been shown to be an effective and less-invasive alternative to SAVR in elderly and high-risk patients.20-26 The development of AF after TAVR is common, ranging from 6% to 53% in prior reports.22, 27, 28
There are limited data on the association between AF and mortality in patients undergoing TAVR. Thus, the aims of this study are two-fold: 1) to analyze the clinical impact of AF at 30-days and 1-year after TAVR; and 2) to analyze the association between ventricular rate and mortality in AF patients after TAVR in the PARTNER (Placement of AoRTic TraNscathetER Valve) trial.
Methods
A. Study population
The design of the PARTNER trial has been previously described.21, 22 Briefly, it enrolled patients with severe aortic stenosis who were deemed to be either high risk for surgical AVR (cohort A) or non-surgical candidates (cohort B). Patients in cohort A were randomized to either SAVR or AVR by the transfemoral (TF) or transapical (TA) approach. Patients in cohort B with adequate femoral access were randomized to TF TAVR or standard medical therapy. After completion of the randomized trial enrollment, patients were enrolled in a continued access registry for either TA or TF TAVR. All patients who underwent TAVR had implantation of an Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA). The date of data extraction was February 2013.
Patients in the PARTNER Trial had ECG, echocardiogram, and clinical evaluation performed at baseline, discharge, 30 days, 6 months and 1 year post TAVR. Patients were included in this analysis only if they had undergone TAVR as part of either the randomized trial or the non-randomized continued access registry and had baseline and discharge ECGs available for analysis.
The study was approved by the Institutional Review Board at each participating site and all patients provided written informed consent.
B. Endpoints
All baseline, discharge, 30 day, 6 month and 1 year ECG and echocardiograms were interpreted in independent core laboratories. AF was defined as atrial fibrillation or atrial flutter/tachycardia that was present on the baseline and/or discharge ECG. Patients were subdivided into four categories: patients with baseline sinus rhythm (SR) and discharge ECG showing SR, patients with baseline SR/discharge AF, patients with baseline AF/discharge AF, and patients with baseline AF/discharge SR. Clinical outcomes were then compared among the groups; the baseline AF/discharge SR group was not analyzed due to low number of patients in that group. Subgroup analyses were performed based on ventricular rate during AF (<90 bpm vs. ≥90 bpm) and TAVR type (transfemoral vs. transapical).
The primary endpoint of this analysis was overall mortality. Secondary clinical endpoints included: cardiovascular mortality, rehospitalization, stroke/transient ischemic attack (TIA), major bleeding, major vascular event, renal failure requiring dialysis, bradyarrhythmias requiring new pacemaker implantation, and change in 6-minute walk test result (6MWTD, meters), as described in the PARTNER trial protocol.22, 29, 30 All endpoints were measured at 30 days and 1 year. All adverse clinical events were adjudicated by an independent clinical events committee.
C. Statistical Analysis
All analysis was performed on the as-treated population, with results presented as median (25th – 75th percentile) or percentages as appropriate. Continuous variables were compared across groups by the Kruskal-Wallis test, and categorical variables were compared using Chi-square or Fisher's exact test as appropriate. Event rates were reported as Kaplan-Meier estimates at 1 year and compared between groups using the log-rank test. Individual subgroup comparisons are presented if the overall P-value for the comparison across the three groups is less than or equal to 0.05. To adjust for multiple comparisons, a Bonferroni corrected P-value of 0.0167 is used to indicate statistical significance when summarizing pairwise comparisons. Mortality at 30 days and 1-year were modeled using Cox regression. The model included clinically relevant variables such as age, gender, and STS risk score along with bleeding requiring transfusion, bradyarrhythmia, requiring pacemaker implantation, myocardial infarction, renal failure requiring dialysis, and stroke, all defined by discharge. Due to the limited events at day 30, only the variables defined at discharge and STS risk score were included in the model. Landmark analysis was performed using 30-days as Time=0 in order to analyze the association between 30-day survival and 1-year mortality. Statistical analyses were performed using SAS software, versions 9.2 and 9.4 (SAS Institute, Cary, North Carolina).
Results
A. Patient Population and Characteristics
The study population included 1879 patients from Cohort A, Cohort B, and the continued access registry of the PARTNER trial (Figure 1), 1097 of whom underwent transfemoral TAVR and 782 of whom underwent transapical TAVR. From this group, 1262 manifested SR at Baseline/SR at Discharge, 113 had SR Baseline/AF Discharge, 470 had AF Baseline/AF Discharge, and 34 had AF Baseline/SR Discharge. The latter group of 34 patients with AF Baseline/SR Discharge were not included in further analyses given their relatively low representation.
Figure 1.
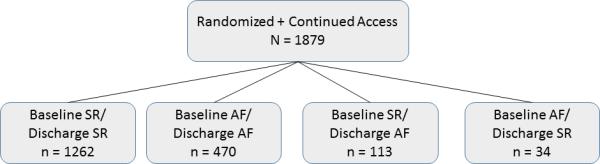
Study population.
Baseline characteristics of each group are included in Table 1. Age ranged from 85 to 86 years across groups. STS score ranged from 10.5 to 11.1, and was highest in the Baseline AF/Discharge AF group. Notably, when compared to Baseline SR/Discharge SR patients, those patients with Baseline AF/Discharge AF were more likely to be male, and had a higher STS score. Patients with Baseline AF/Discharge AF had higher rates of pulmonary hypertension and pacemakers compared to Baseline SR/Discharge SR patients. There were no significant differences among groups for body mass index, diabetes, hypertension, congestive heart failure, prior myocardial infarction, or stroke/TIA.
Table 1.
Baseline Patient Characteristics
| Characteristics | (a) Baseline SR / Discharge SR (n=1262) | (b) Baseline SR / Discharge AF (n=113) | (c) Baseline AF / Discharge AF (n=470) | p-value All Groups | p-value a vs. b | p-value a vs. c | p value b vs. c |
|---|---|---|---|---|---|---|---|
| Age (years) | 85.3 [80.2,89.2] | 86.4 [80.5,90.0] | 86.1 [81.9,89.3] | 0.02 | 0.15 | 0.0009 | 0.92 |
| Male (%) | 46.5 | 47.8 | 57.7 | 0.0002 | 0.79 | <0.0001 | 0.06 |
| STS score | 10.5 [9.1,12.4] | 10.8 [9.7,13.6] | 11.1 [9.6,13.5] | <0.0001 | 0.08 | <0.0001 | 0.45 |
| BMI | 26.1 [22.7,30.0] | 26.3 [22.7,30.8] | 25.2 [22.5,29.3] | 0.21 | |||
| NYHA (%) | 0.22 | ||||||
| Class I-II | 5.7 | 2.7 | 4.9 | ||||
| Class III | 50.6 | 46.0 | 46.8 | ||||
| Class IV | 43.7 | 51.3 | 48.3 | ||||
| Diabetes (%) | 38.4 | 37.2 | 36.0 | 0.64 | |||
| Hypertension(%) | 91.4 | 92.9 | 92.8 | 0.58 | |||
| Prior MI (%) | 26.8 | 22.3 | 23.1 | 0.21 | |||
| Stroke/TIA (%) | 26.5 | 17.4 | 26.7 | 0.11 | |||
| Endocarditis (%) | 0.5 | 1.8 | 0.2 | 0.10 | |||
| Pulmonary Hypertension (%) | 36.2 | 37.4 | 47.8 | 0.0001 | 0.81 | <0.0001 | 0.053 |
| Permanent Pacemaker (%) | 8.5 | 13.3 | 17.9 | <0.0001 | 0.09 | <0.0001 | 0.24 |
| Liver Disease (%) | 2.4 | 1.8 | 3.2 | 0.54 | |||
| COPD (%) | 42.5 | 48.7 | 45.1 | 0.32 | |||
| Rheumatic fever (%) | 1.5 | 1.8 | 1.5 | 0.97 | |||
| Renal disease (Cr ≥2) (%) | 15.8 | 19.5 | 17.7 | 0.44 | |||
| CHA2DS2VASc Score | 5.8 ± 1.4 | 5.6 ± 1.2 | 5.7 ± 1.3 | 0.02 | 0.12 | 0.01 | 0.97 |
Baseline ECG and echocardiogram findings are in Table 2. Notable baseline ECG findings include significantly more 1st degree AV block in the Baseline SR/Discharge AF patients (vs. the Baseline SR/Discharge SR patients). There were no statistically significant differences in rates of interventricular conduction defect, right bundle branch block, or left bundle branch block. Baseline echocardiograms demonstrated a significantly lower LVEF in patients with Baseline AF/Discharge AF (55.0%) vs. the Baseline SR/Discharge SR (57.5%) or the Baseline SR/Discharge AF (58.6%) patients. Compared to the Baseline SR/Discharge SR patients, patients with Baseline AF/Discharge AF were also more likely to have at least moderate mitral regurgitation.
Table 2.
Baseline ECG/Echocardiographic Findings
| ECG/Echo findings | (a) Baseline SR / Discharge SR (n=1262) | (b) Baseline SR / Discharge AF (n=113) | (c) Baseline AF / Discharge AF (n=470) | p-value All Groups | p-value a vs. b | p-value a vs. c | p value b vs. c |
|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | 72.0 [63.0,81.0] | 68.0 [62.0,76.0] | 75.5 [66.0,86.0] | <0.0001 | 0.01 | <0.001 | <0.01 |
| 1st degree AV Block (%) | 19.2 | 27.1 | - | <0.0001 | 0.05 | - | - |
| IVCD (%) | 5.2 | 5.6 | 5.8 | 0.88 | |||
| LBBB (%) | 9.7 | 7.5 | 7.0 | 0.23 | |||
| RBBB (%) | 14.4 | 21.5 | 16.3 | 0.12 | |||
| LVEF (%) | 57.5 [48.8,60.0] | 58.6 [50.1,62.0] | 55.0 [44.4,60.0] | <0.0001 | 0.58 | <0.0001 | 0.002 |
| Mitral Regurgitation (%) | <0.0001 | ||||||
| - None | 4.2 | 1.8 | 1.1 | ||||
| - Trace | 28.5 | 31.8 | 16.7 | ||||
| - Mild | 49.3 | 41.8 | 55.9 | ||||
| - Moderate | 15.6 | 21.8 | 22.9 | ||||
| - Severe | 2.5 | 2.7 | 3.4 | ||||
B. Clinical outcomes
30-day outcomes are listed in Table 3. Both all-cause mortality (14.2%) and cardiovascular mortality (8.3%) were highest in the Baseline SR/Discharge AF group. There were no significant differences in other endpoints including rehospitalization, stroke/TIA, major bleeding, and major vascular complications. Requirement for permanent pacemaker placement were highest at 12.7% in the Baseline SR/Discharge AF group. Patients discharged in AF had a longer length of stay than patients who presented and remained in SR by discharge (6 vs. 7 days, p=0.0004 across all groups).
Table 3.
Outcomes at 30-Days
| Outcome (%): | (a) Baseline SR / Discharge SR | (b) Baseline SR / Discharge AF | (c) Baseline AF / Discharge AF | p-value All Groups | p-value a vs. b | p-value a vs. c | p value b vs. c |
|---|---|---|---|---|---|---|---|
| Mortality | |||||||
| - All Cause | 2.6 | 14.2 | 3.6 | <0.0001 | <0.001 | 0.27 | <0.001 |
| - Cardiovascular | 1.6 | 8.3 | 2.8 | <0.0001 | <0.001 | 0.11 | 0.007 |
| Rehospitalization | 5.3 | 5.8 | 7.2 | 0.36 | |||
| Stroke/TIA | 3.9 | 7.4 | 3.2 | 0.16 | |||
| Major Bleeding | 7.3 | 9.9 | 7.9 | 0.58 | |||
| Major Vascular Event | 5.5 | 4.4 | 5.8 | 0.86 | |||
| Renal Failure requiring dialysis | 1.5 | 6.3 | 3.2 | 0.001 | 0.0003 | 0.02 | 0.13 |
| New Pacemaker | 5.2 | 12.7 | 5.1 | 0.004 | 0.001 | 0.96 | 0.004 |
| LVEF | 58.7 [50.0,63.4] | 59.1 [54.9,62.6] | 55.0 [49.2,60.0] | 0.0001 | 0.78 | <0.0001 | 0.02 |
| Change in 6MTWD (mean in meters) | 21.8 | −4.2 | 12.1 | 0.07 | |||
| Length of Stay in Hospital after TAVR (days) | 6.0 [4.0,7.0] | 7.0 [5.0,8.0] | 7.0 [5.0,8.0] | 0.0004 | 0.002 | 0.005 | 0.09 |
| On Aspirin | 89.6 | 85.3 | 84.2 | 0.008 | 0.19 | 0.003 | 0.80 |
| On Clopidogrel | 58.6 | 30.9 | 27.5 | <0.0001 | <0.0001 | <0.0001 | 0.51 |
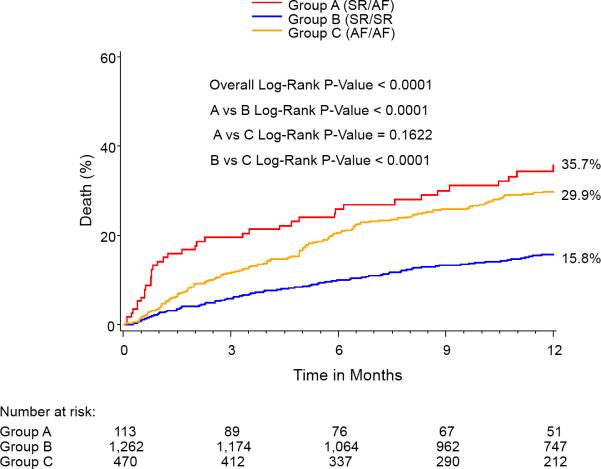
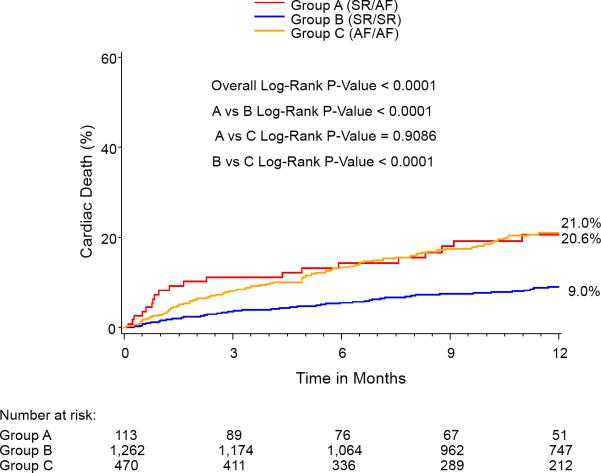
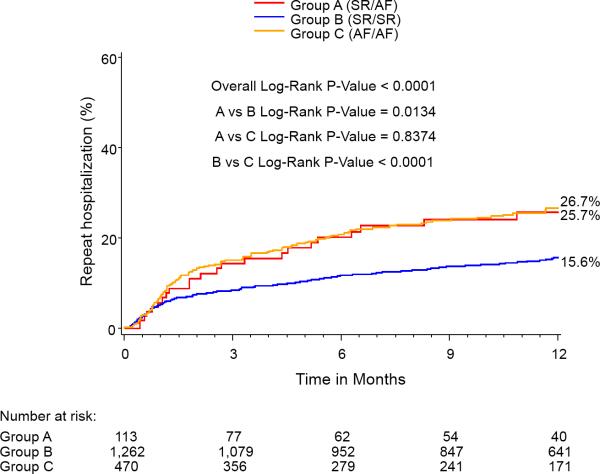
At 1-year, the percent of patients in AF for each group was: SR/SR=5.2%, SR/AF=27.9%, AF/AF=79.8%, p<0.0001 for all comparisons. One year outcomes based on Kaplan-Meier analysis demonstrated significantly higher mortalities in the Baseline SR/Discharge AF (35.7%) group and the Baseline AF/Discharge AF group (29.9%) compared with the Baseline SR/Discharge SR group (15.8%) (Figure 2). The Baseline AF/Discharge AF group had the highest 1-year CV mortality (21%) and rehospitalization rate (26.7%) while the Baseline SR/Discharge SR group had the lowest rates of all-cause mortality, CV mortality, and rehospitalization. In landmark analysis that included those patients surviving at 30 days, rhythm status remained a predictor of outcomes.
Figure 2.

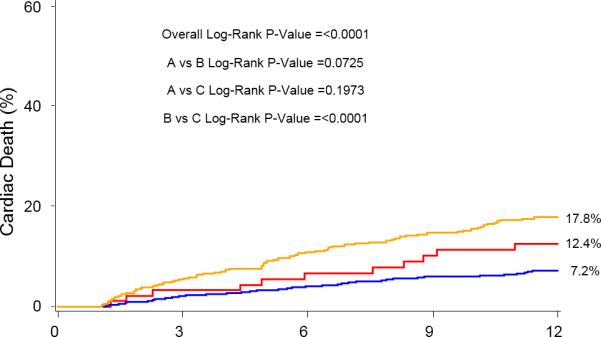
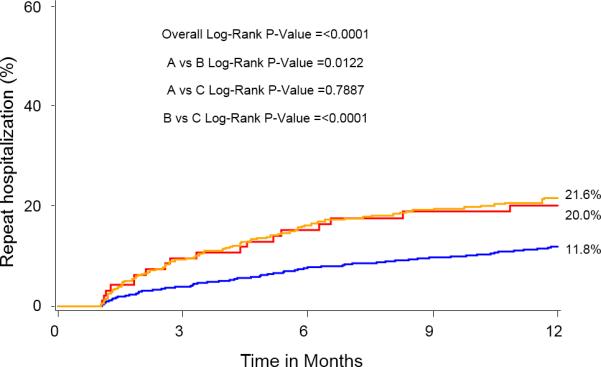
Kaplan-Meier curves for 1-year outcomes for: (A) Mortality, (B) Cardiovascular mortality, and (C) Repeat hospitalization. Figure 2A notes a relatively early divergence for higher rates of mortality and cardiovascular mortality in the SR/AF group. The smaller Kaplan-Meier curves on the right reflect landmark analyses for each outcome performed with Time 0=30 days.
Secondary Outcomes
30 day and 1 year outcomes are presented Tables 3 and 4. Renal failure requiring dialysis and new pacemaker implantations were higher in the SR/AF group at both 30-days and 1-year. There was a non-significant trend for least improvement in 6MWTD in the SR/AF group at both 30-days and 1-year. While rates of Stroke/TIA and major bleeding were not significantly different among groups, there were trends for increased events in the Baseline SR/Discharge AF groups at both 30-days and 1-year.
Table 4.
Outcomes at 1-Year (Based on Kaplan-Meier Analysis)
| Outcome (%): | (a) Baseline SR / Discharge SR | (b) Baseline SR / Discharge AF | (c) Baseline AF / Discharge AF | p-value All Groups | p-value a vs. b | p-value a vs. c | p value b vs. c |
|---|---|---|---|---|---|---|---|
| Mortality | |||||||
| - All cause | 15.8 | 35.7 | 29.9 | <0.0001 | <0.001 | <0.001 | 0.16 |
| - Cardiovascular | 9.0 | 20.6 | 21.0 | <0.0001 | <0.001 | <0.001 | 0.91 |
| Rehospitalization | 15.6 | 25.7 | 26.7 | <0.0001 | 0.01 | <0.001 | 0.84 |
| Stroke/TIA | 6.3 | 10.3 | 9.0 | 0.19 | |||
| Major Bleeding | 11.5 | 16.0 | 16.6 | 0.03 | 0.18 | 0.15 | 0.96 |
| Major Vascular Event | 5.6 | 4.4 | 6.8 | 0.58 | |||
| Renal Failure requiring dialysis | 2.7 | 7.3 | 4.6 | 0.005 | 0.002 | 0.03 | 0.22 |
| New Pacemaker | 6.8 | 16.3 | 7.0 | 0.001 | 0.0004 | 0.96 | 0.002 |
| LVEF | 60.0 [55.0,64.3] | 60.0 [55.0,65.0] | 55.6 [50.0,61.0] | 0.055 | |||
| Change in 6MTWD (mean, in meters) | 33.1 | 8.0 | 38.3 | 0.32 | |||
| On Aspirin | 86.6 | 71.4 | 72.3 | <0.0001 | 0.002 | <0.0001 | 0.89 |
| On Clopidogrel | 32.9 | 27.3 | 16.3 | <0.0001 | 0.39 | <0.0001 | 0.055 |
Multivariable Analysis of Predictors of Mortality
Predictors of 30-day and 1-year mortality are listed in Tables 5 and 6. The development and maintenance of AF by discharge, as opposed to remaining in SR, was associated with higher 30-day mortality (HR = 3.41 [1.78, 6.54], p=0.0002). The development of AF by discharge (SR/AF), as well as presenting and remaining in AF at discharge (AF/AF), were each associated with increased 1-year mortality (HR=2.14 [1.45, 3.10] and HR=1.88 [1.50, 2.36], respectively).
Table 5.
Multivariable Predictors of 30-Day Mortality
| DEATH | p-value: | Hazard Ratio (95% CI): |
|---|---|---|
| Bradyarrhythmic Event Requiring Pacemaker | 0.0301 | 2.57 [1.10,6.03] |
| Bleeding Event Requiring Transfusion | <0.0001 | 3.97 [2.04,7.70] |
| MI | 0.0104 | 4.62 [1.43,14.88] |
| Renal Failure (Dialysis required) | <0.0001 | 8.82 [4.13,18.84] |
| STS Risk Score | 0.0139 | 1.05 [1.01,1.09] |
| Stroke | 0.0216 | 2.99 [1.17,7.62] |
| Baseline SR/Discharge AF (vs. Baseline SR/Discharge SR) | 0.0002 | 3.41 [1.78, 6.54] |
Table 6.
Multivariable Predictors of 1-Year Mortality
| DEATH | p-value: | Hazard Ratio (95% CI): |
|---|---|---|
| Male | 0.0026 | 1.40 [1.12,1.73] |
| Renal Failure (Dialysis required) | <0.0001 | 4.13 [2.36,7.24] |
| STS Risk Score | 0.0126 | 1.03 [1.01,1.05] |
| Stroke | 0.0001 | 2.47 [1.57,3.91] |
| Bleeding Event Requiring Transfusion | 0.0029 | 1.89 [1.24,2.87] |
| Baseline SR/Discharge AF (vs. Baseline SR/Discharge SR) | <0.0001 | 2.14 [1.45, 3.10] |
| Baseline AF/Discharge AF (vs. Baseline SR/Discharge SR) | <0.0001 | 1.88 [1.50, 2.36] |
Effect of Ventricular Rate During AF on Outcomes
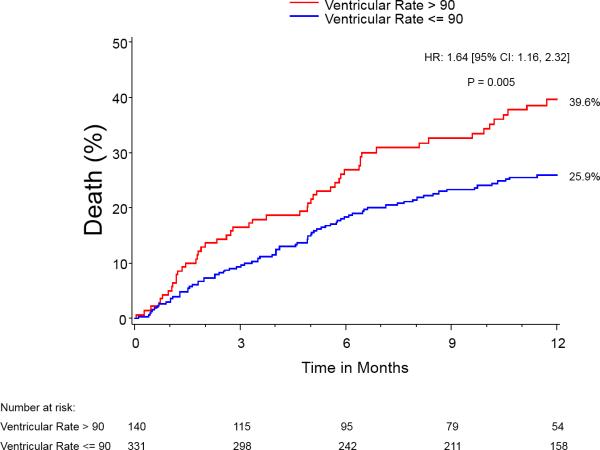
Those patients discharged in AF with lower ventricular response (i.e, <90 bpm) experienced less 1-year all-cause mortality (HR - 0.74 [0.55, 0.99], p=0.04) and CV mortality (HR - 0.55, [0.38-0.79], p=0.0014), compared to those patients with ventricular response ≥90 bpm (Figure 3). There was no statistically significant difference in LVEF at either 30-days (53%) or 1-year (55%) between groups. When comparing patients discharged in AF by ventricular response <90 bpm vs. ≥90 bpm, there were no statistically significant differences in rates of pacemakers at baseline (17.5% for <90 bpm vs. 23.3% for ≥90 bpm, p=0.09) or placement by discharge (7.4% for <90 bpm vs. 5.0% for ≥90 bpm, p=0.22).
Figure 3.
Kaplan-Meier curves for 1–year mortality in patients with baseline AF/discharge AF comparing ventricular response rate at discharge.
Discussion
This study is the largest published analysis of the adverse impact of AF after TAVR on clinical outcomes. The main findings are as follows: 1) patients discharged in AF after TAVR in the PARTNER Trial have increased 30-day and 1-year mortality, cardiovascular mortality, and 1-year repeat hospitalization, particularly in those patients who convert from SR to AF between admission and discharge; 2) patients with AF after TAVR and ventricular response >90 bpm have higher 1-year mortality than those with ventricular response<= 90 bpm; 3) patients with AF after TAVR also have higher rates of renal failure requiring dialysis, permanent pacemaker implantation, and a non-significant increase for stroke/TIA. In particular, there is an early divergence of the survival curves prior to three months, revealing that development of AF by discharge is associated with increased early mortality. These findings provide further evidence that the presence of AF at discharge, particularly if not present at baseline, is not a benign condition in TAVR patients.
Previous studies have shown that AF is generally associated with worse outcomes in many cardiovascular disease (CV) states,3-15 including poorer outcomes in SAVR.16-20 AF is also a known and relatively common complication of TAVR.22, 28, 31, 32 Tanawuttiwat et al. noted an incidence of new AF equal to 53% in a single-center, retrospective study of 231 consecutive patients undergoing transaortic TAVR and 14% after transfemoral TAVR.28 Possible precipitating factors include: cardiovascular and overall physical status related to the aged patient population with AS, such as atrial fibrosis and larger atrial size, and sequelae of the TAVR procedure itself. Although reports of clinical outcomes of AF after TAVR are limited, the absence of AF is associated with better LVEF recovery and improved MR, while the presence of AF is associated with increased stroke risk 22, 27, 28, 33 and mortality.34
The causes of increased mortality in AF patients remain unclear. Although increased thromboembolic risk is often implicated, this study demonstrated AF remained a predictor of mortality even after adjusting for several clinical comorbidities including stroke. Other possible factors besides stroke/TIA may include those associated with cardiovascular as well as overall functional status. With regard to cardiovascular status, this study noted that rapid ventricular response was associated with worse mortality outcomes for patients who developed AF by discharge, with possible reasons for the deleterious effects including reduced cardiac output and heart failure, including systolic as well as diastolic heart failure. Notably, LVEF was worse in patients with AF/AF, and particularly in patients discharged with AF and ventricular response ≥90 bpm, which suggests worsened cardiovascular status. These results argue for prospective studies analyzing the effect of more aggressive rate control on outcomes in such patients. With regard to the impact of permanent pacemaker implantation on TAVR patients, including those with AF, we found that mortality rates in those patients requiring pacemakers were increased more than two-fold at 30-days. These results suggest that co-morbidities associated with conduction abnormalities and pacemaker implantation may be causally related to increased mortality. For example, prior analysis has shown that implantation of new pacemakers is associated with higher rates of repeat hospitalization and mortality or repeat hospitalization at 1 year.35 Further studies examining the role of pacemaker implantation on mortality in TAVR patients are required.
With regard to overall functional status, several markers were worse in the AF groups, including decreased 6-minute walk test distance (6MWTD), especially in those patients with ventricular rates > 90 bpm, higher rates of renal failure, and bradyarrhythmias requiring pacemakers. These results suggest that physical vitality is reduced in AF patients after TAVR as compared to patients who were in SR at discharge, an hypothesis-generating observation. Further studies of newer-generation TAVRs and techniques are warranted to explore the association between TAVR and procedure type, development of AF, and clinical outcomes.
Frequency of cardiac rhythm monitoring may account for the difference between this study and others reporting stroke and mortality in AF patients after TAVR, as rhythm status in this study was determined with baseline and discharge ECGs. For example, Amat-Santos et al. noted no statistically significant difference in mortality with new-onset AF, which occurred in 31% of patients.31 However, all patients were on continuous cardiac monitors and all but 1 patient experienced spontaneous, electrical, or chemical cardioversion to sinus rhythm during the hospitalization. Greater than 70% of patients experienced AF for less than 24 hours. Thus, patients in this trial would be less likely to be discharged in AF, and possessed different characteristics from those in the present analysis, who were in AF at discharge and likely shouldered a larger AF burden. In another smaller, single-center trial of 389 total patients undergoing TAVR, Stortecky et al. noted increased mortality in 131 AF patients with TAVR (the majority of which were Medtronic CoreValves), most of whom had pre-existing AF.34 Our results differ in noting the highest mortality occurred in patients with baseline SR/discharge AF, findings which may be related to the different burden of AF between study populations.
Clinical and Research Implications
AF is a co-morbidity in TAVR patients that is associated with worse overall outcomes, including mortality. Patients who develop AF by discharge form a problematic group not only with regard to anticoagulation (e.g., whether to anticoagulate, and which medication to use), but also with regard to decisions regarding rate- and rhythm-control strategies. While beta-blockers, amiodarone, and other drug options have shown some promise in treating postoperative AF,36-38 it remains unclear the extent to which these are useful agents in managing TAVR patients.
The maintenance of sinus rhythm suggests a protective effect in TAVR patients. Further research should be directed towards determining the extent to which AF-targeted therapy in TAVR patients can improve outcomes with strategies including: refinement of anticoagulant therapy, rate-control, antiarrhythmic agents, and cardioversion.18, 39
Study Limitations
This study was a post-hoc analysis of a prospective trial with adjudication of ECG and clinical outcome data, and ECGs were analyzed at discrete time points: baseline, discharge, and 1-year. Continuous cardiac rhythm monitoring was not available for this study. Thus, patients with a change in rhythm status or rate between admission and discharge, such as those who developed new onset AF during hospitalization and subsequently converted to SR before discharge, were not included in the group of patients analyzed as having developed AF. Similarly, patients who may have developed AF after discharge were not included in the AF cohort. In addition, this study does not include details regarding history of atrial fibrillation or flutter in any of the patients, nor does it include details of antiarrhythmic or anticoagulant treatment while in the hospital or after discharge. Finally, while adjustments for several covariates were made, the potential for unmeasured confounders exists.
Conclusions
After TAVR, the presence of AF at discharge, and especially the conversion to AF with ventricular rates >90 bpm, is associated with an increase in early and late all-cause and CV mortality. While it is evident that AF is associated with an increase in mortality in patients that undergo TAVR, it remains unclear whether effective treatment of AF rhythm or rate could reduce this increase in mortality. AF patients undergoing TAVR should be further studied for strategies that can improve clinical outcomes.
Supplementary Material
WHAT IS KNOWN
Patients undergoing TAVR have relatively high rates of atrial fibrillation (AF).
Studies in surgical AVR patients have noted that the presence of AF is correlated with worsened outcomes, including higher mortality.
A better understanding of the degree to which AF impacts upon outcomes including mortality would have clinical utility.
WHAT THE STUDY ADDS
For TAVR patients, the presence of AF at discharge, and especially the conversion to AF by discharge, is associated with increased mortality at 30-days and 1-year and repeat hospitalizations at 1-year.
For those TAVR patients with AF, there is an association between higher ventricular rates > 90 bpm and mortality.
Patients with AF after TAVR also have higher rates of renal failure requiring dialysis and permanent pacemaker implantation.
Acknowledgments
Angelo Biviano is supported by National Heart, Lung, and Blood Institute Career Development Award 1K23HL105893. Tamim Nazif has received consulting fees from Edwards Lifesciences. Vasilis Babaliaros has received consulting fees from Direct Flow Medical. Josep Rodes-Cabau is a consultant for Edwards Lifesciences and St. Jude Medical. Wilson Szeto is a consultant for MicroInterventional Devices. William Fearon has received research grant support from St. Jude Medical. Todd Dewey has received consulting fees from Cardiaples and Edwards Lifesciences. Mathew Williams has received consulting fees from Edwards Lifesciences. Michael Mack is an unpaid member of the PARTNER Trial Executive Committee. John Webb is a consultant for Edwards Lifesciences and an unpaid member of the PARTNER Trial Executive Committee. D. Craig Miller is supported by an R01 research grant from the NHLBI #HL67025, has received consulting fees/honoraria from Abbott Vascular, St. Jude Medical, and Medtronic, and is an unpaid member of the PARTNER Trial Executive Committee. Craig Smith and Martin Leon are unpaid members of the PARTNER Trial Executive Committee. Susheel Kodali has received consulting fees from Edwards Lifesciences and is a member of the Scientific Advisory Board of Thubrikar Aortic Valve, Inc.
Footnotes
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00530894.
Disclosures
The other authors report no potential conflicts of interest.
References
- 1.Lip GYH. Can we predict stroke in atrial fibrillation? Clin Cardiol. 2012;35:S21–S27. doi: 10.1002/clc.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thacker EL, McKnight B, Psaty BM, Longstreth WT, Jr., Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR. Atrial fibrillation and cognitive decline: A longitudinal cohort study. Neurology. 2013;81:119–125. doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neal WT, Efird JT, Davies SW, Choi YM, Anderson CA, Kindell LC, O'Neal JB, Ferguson TB, Chitwood WR, Kypson AP. Preoperative atrial fibrillation and longterm survival after open heart surgery in a rural tertiary heart institute. Heart Lung. 2013;42:442–447. doi: 10.1016/j.hrtlng.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Siebert J, Anisimowicz L, Lango R, Rogowski J, Pawlaczyk R, Brzezinski M, Beta S, Narkiewicz M. Atrial fibrillation after coronary artery bypass grafting: Does the type of procedure influence the early postoperative incidence? Eur J Cardiothorac Surg. 2001;19:455–459. doi: 10.1016/s1010-7940(01)00621-2. [DOI] [PubMed] [Google Scholar]
- 5.Stamou SC, Dangas G, Hill PC, Pfister AJ, Dullum MK, Boyce SW, Bafi AS, Garcia JM, Corso PJ. Atrial fibrillation after beating heart surgery. Am J Cardiol. 2000;86:64–67. doi: 10.1016/s0002-9149(00)00829-8. [DOI] [PubMed] [Google Scholar]
- 6.Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, Tarazi R, Shroyer AL, Sethi GK, Grover FL, Hammermeister KE. Atrial fibrillation after cardiac surgery: A major morbid event? Ann Surg. 1997;226:501–511. doi: 10.1097/00000658-199710000-00011. discussion 511-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helgadottir S, Sigurdsson MI, Ingvarsdottir IL, Arnar DO, Gudbjartsson T. Atrial fibrillation following cardiac surgery: Risk analysis and long-term survival. J Cardiothorac Surg. 2012;7:87. doi: 10.1186/1749-8090-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostafa A, El-Haddad MA, Shenoy M, Tuliani T. Atrial fibrillation post cardiac bypass surgery. Avicenna J Med. 2012;2:65–70. doi: 10.4103/2231-0770.102280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efird JT, Davies SW, O'Neal WT, Anderson CA, Anderson EJ, O'Neal JB, Ferguson TB, Chitwood WR, Kypson AP. The impact of race and postoperative atrial fibrillation on operative mortality after elective coronary artery bypass grafting. Eur J Cardiothorac Surg. 2014;45:e20–25. doi: 10.1093/ejcts/ezt529. [DOI] [PubMed] [Google Scholar]
- 10.El-Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370–1376. doi: 10.1016/j.jacc.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 11.Horwich P, Buth KJ, Legare JF. New onset postoperative atrial fibrillation is associated with a long-term risk for stroke and death following cardiac surgery. J Cardiac Surg. 2013;28:8–13. doi: 10.1111/jocs.12033. [DOI] [PubMed] [Google Scholar]
- 12.Nardi F, Diena M, Caimmi PP, Iraghi G, Lazzero M, Cerin G, Rossi L, Bongo AS, Cernigliaro C, Lupi A. Relationship between left atrial volume and atrial fibrillation following coronary artery bypass grafting. J Cardiac Surg. 2012;27:128–135. doi: 10.1111/j.1540-8191.2011.01373.x. [DOI] [PubMed] [Google Scholar]
- 13.Saxena A, Dinh DT, Smith JA, Shardey GC, Reid CM, Newcomb AE. Usefulness of postoperative atrial fibrillation as an independent predictor for worse early and late outcomes after isolated coronary artery bypass grafting (multicenter australian study of 19,497 patients). Am J Cardiol. 2012;109:219–225. doi: 10.1016/j.amjcard.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg BA, Zhao Y, He X, Hernandez AF, Fullerton DA, Thomas KL, Mills R, Klaskala W, Peterson ED, Piccini JP. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: Insights from the society of thoracic surgeons caps-care atrial fibrillation registry. Clin Cardiol. 2014;37:7–13. doi: 10.1002/clc.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filardo G, Hamilton C, Hamman B, Hebeler RF, Jr., Adams J, Grayburn P. New-onset postoperative atrial fibrillation and long-term survival after aortic valve replacement surgery. Ann Thorac Surg. 2010;90:474–479. doi: 10.1016/j.athoracsur.2010.02.081. [DOI] [PubMed] [Google Scholar]
- 16.Girerd N, Magne J, Pibarot P, Voisine P, Dagenais F, Mathieu P. Postoperative atrial fibrillation predicts long-term survival after aortic-valve surgery but not after mitral-valve surgery: A retrospective study. Bmj Open. 2011:1. doi: 10.1136/bmjopen-2011-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena A, Dinh D, Dimitriou J, Reid C, Smith J, Shardey G, Newcomb A. Preoperative atrial fibrillation is an independent risk factor for mid-term mortality after concomitant aortic valve replacement and coronary artery bypass graft surgery. Interact Cardiov Th. 2013;16:488–494. doi: 10.1093/icvts/ivs538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena A, Shi WY, Bappayya S, Dinh DT, Smith JA, Reid CM, Shardey GC, Newcomb AE. Postoperative atrial fibrillation after isolated aortic valve replacement: A cause for concern? Ann Thorac Surg. 2013;95:133–140. doi: 10.1016/j.athoracsur.2012.08.077. [DOI] [PubMed] [Google Scholar]
- 19.Schulenberg R, Antonitsis P, Stroebel A, Westaby S. Chronic atrial fibrillation is associated with reduced survival after aortic and double valve replacement. Ann Thorac Surg. 2010;89:738–744. doi: 10.1016/j.athoracsur.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Ghadimi K, Patel PA, Gutsche JT, Sophocles A, Anwaruddin S, Szeto WY, Augoustides JG. Perioperative conduction disturbances after transcatheter aortic valve replacement. J Cardiothorac Vasc Anesth. 2013;27:1414–1420. doi: 10.1053/j.jvca.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB, Investigators PT. Two-year outcomes after transcatheter or surgical aortic-valve replacement. NEJM. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 22.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, Investigators PT. Transcatheter versus surgical aortic-valve replacement in high-risk patients. NEJM. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 23.Clavel MA, Webb JG, Rodes-Cabau J, Masson JB, Dumont E, De Larochelliere R, Doyle D, Bergeron S, Baumgartner H, Burwash IG, Dumesnil JG, Mundigler G, Moss R, Kempny A, Bagur R, Bergler-Klein J, Gurvitch R, Mathieu P, Pibarot P. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928–1936. doi: 10.1161/CIRCULATIONAHA.109.929893. [DOI] [PubMed] [Google Scholar]
- 24.Fairbairn TA, Meads DM, Hulme C, Mather AN, Plein S, Blackman DJ, Greenwood JP. The cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis at high operative risk. Heart. 2013;99:914–920. doi: 10.1136/heartjnl-2013-303722. [DOI] [PubMed] [Google Scholar]
- 25.Lindman BR, Pibarot P, Arnold SV, Suri R, McAndrew TC, Maniar HS, Zajarias A, Kodali S, Kirtane AJ, Thourani VH, Tuzcu EM, Svensson LG, Waksman R, Smith CR, Leon MB. Transcatheter versus surgical aortic valve replacement in patients with diabetes and severe aortic stenosis at high risk for surgery: An analysis of the PARTNER trial. J Am Coll Cardiol. 2014;63:1090–9. doi: 10.1016/j.jacc.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appel CF, Hultkvist H, Nylander E, Ahn H, Nielsen NE, Freter W, Vanky F. Transcatheter versus surgical treatment for aortic stenosis: Patient selection and early outcome. Scand Cardiovasc J. 2012;46:301–307. doi: 10.3109/14017431.2012.699636. [DOI] [PubMed] [Google Scholar]
- 27.Motloch LJ, Reda S, Rottlaender D, Khatib R, Muller-Ehmsen J, Seck C, Strauch J, Madershahian N, Erdmann E, Wahlers T, Hoppe UC. Ann Thorac Surg; Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement.; 2012. pp. 124–131. [DOI] [PubMed] [Google Scholar]
- 28.Tanawuttiwat T, O'Neill BP, Cohen MG, Chinthakanan O, Heldman AW, Martinez CA, Alfonso CE, Mitrani RD, Macon CJ, Carrillo RG, Williams DB, O'Neill WW, Myerburg RJ. New-onset atrial fibrillation after aortic valve replacement: Comparison of transfemoral, transapical, transaortic, and surgical approaches. J Am Coll Cardiol. 2014;63:1510–1519. doi: 10.1016/j.jacc.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 29.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, Investigators PT. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. NEJM. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 30.Green P, Cohen DJ, Genereux P, McAndrew T, Arnold SV, Alu M, Beohar N, Rihal CS, Mack MJ, Kapadia S, Dvir D, Maurer MS, Williams MR, Kodali S, Leon MB, Kirtane AJ. Relation between six-minute walk test performance and outcomes after transcatheter aortic valve implantation (from the PARTNER trial). Am J Cardiol. 2013;112:700–706. doi: 10.1016/j.amjcard.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amat-Santos IJ, Rodes-Cabau J, Urena M, DeLarochelliere R, Doyle D, Bagur R, Villeneuve J, Cote M, Nombela-Franco L, Philippon F, Pibarot P, Dumont E. Incidence, predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59:178–188. doi: 10.1016/j.jacc.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 32.van der Boon RM, Houthuizen P, Nuis RJ, van Mieghem NM, Prinzen F, de Jaegere PP. Clinical implications of conduction abnormalities and arrhythmias after transcatheter aortic valve implantation. Current cardiology reports. 2014;16:429. doi: 10.1007/s11886-013-0429-4. [DOI] [PubMed] [Google Scholar]
- 33.Nuis RJ, Van Mieghem NM, Schultz CJ, Moelker A, van der Boon RM, van Geuns RJ, van der Lugt A, Serruys PW, Rodes-Cabau J, van Domburg RT, Koudstaal PJ, de Jaegere PP. Frequency and causes of stroke during or after transcatheter aortic valve implantation. Am J Cardiol. 2012;109:1637–1643. doi: 10.1016/j.amjcard.2012.01.389. [DOI] [PubMed] [Google Scholar]
- 34.Stortecky S, Buellesfeld L, Wenaweser P, Heg D, Pilgrim T, Khattab AA, Gloekler S, Huber C, Nietlispach F, Meier B, Juni P, Windecker S. Atrial fibrillation and aortic stenosis: Impact on clinical outcomes among patients undergoing transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2013;6:77–84. doi: 10.1161/CIRCINTERVENTIONS.112.000124. [DOI] [PubMed] [Google Scholar]
- 35.Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PS, El-Chami MF, Herrmann HC, Mack M, Makkar RR, Miller DC, Pichard A, Tuzcu EM, Szeto WY, Webb JG, Moses JW, Smith CR, Williams MR, Leon MB, Kodali SK, Office PP. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (placement of aortic transcatheter valves) trial and registry. JACC. Cardiovasc Interv. 2015;8:60–69. doi: 10.1016/j.jcin.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Mayson SE, Greenspon AJ, Adams S, Decaro MV, Sheth M, Weitz HH, Whellan DJ. The changing face of postoperative atrial fibrillation prevention: A review of current medical therapy. Cardiol Rev. 2007;15:231–241. doi: 10.1097/CRD.0b013e31813e62bb. [DOI] [PubMed] [Google Scholar]
- 37.Brooks DC, Schindler JL. Perioperative stroke: Risk assessment, prevention and treatment. Curr Treat Options Cardiovasc Med. 2014;16:282. doi: 10.1007/s11936-013-0282-1. [DOI] [PubMed] [Google Scholar]
- 38.Howard PA, Barnes BJ. Potential use of statins to prevent atrial fibrillation after coronary artery bypass surgery. Ann Pharmacother. 2008;42:253–258. doi: 10.1345/aph.1K590. [DOI] [PubMed] [Google Scholar]
- 39.Saxena A, Dinh D, Dimitriou J, Reid C, Smith J, Shardey G, Newcomb A. Preoperative atrial fibrillation is an independent risk factor for mid-term mortality after concomitant aortic valve replacement and coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2013;16:488–494. doi: 10.1093/icvts/ivs538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.