Abstract
The present study investigated the in vitro and the in vivo interactions among azithromycin, clarithromycin, minocycline, and tigecycline against Pythium insidiosum. In vitro antimicrobial activities were determined by the broth microdilution method in accordance with CLSI document M38-A2, and the antibiotic interactions were assayed using the checkerboard MIC format. In vivo efficacy was determined using a rabbit infection model. The geometric mean MICs of azithromycin, clarithromycin, minocycline, and tigecycline against P. insidiosum were, respectively, 1.91, 1.38, 0.91, and 0.79 μg/ml. By checkerboard testing, all combinations resulted in in vitro synergistic interactions (>60%). Antagonism was not observed. The in vivo studies showed that azithromycin (20 mg/kg/day twice daily) alone or in combination with minocycline (10 mg/kg/day twice daily) significantly decreased the fungal burden. This study demonstrates that azithromycin possesses potent curative efficacy against subcutaneous pythiosis in the rabbit model.
INTRODUCTION
Pythium insidiosum is a eukaryotic organism that grows by forming filamentous structures similar to mycelia of true fungi. This morphological similarity has been targeted for the treatment of many pythiosis cases in humans and other mammals using antifungal drugs (1). Despite a few reports of successful therapy with antifungal drugs (2, 3), many studies have demonstrated their ineffectiveness (1, 4–6). We previously described antibacterial compounds belonging to the glycylcycline, macrolide, and tetracycline classes (7–10) as having potent in vitro activity against P. insidiosum. The present study investigated the in vitro and in vivo interactions among minocycline, azithromycin, clarithromycin, and tigecycline against P. insidiosum.
MATERIALS AND METHODS
Microorganisms and in vitro antimicrobial susceptibility.
The susceptibilities of 28 Brazilian P. insidiosum strains isolated from equine pythiosis lesions (11) and two reference strains, P. insidiosum CBS 101555 and ATCC 58.637, were evaluated. A CLSI M38-A2-based in vitro susceptibility test and combination tests were performed as described previously (9). Azithromycin and minocycline (Pharma Nostra, Rio de Janeiro, Brazil), clarithromycin (Genix, Anápolis, Brazil), and tigecycline (Sigma-Aldrich, St. Louis, MO, USA) were purchased as standard antimicrobial powders.
In vivo animal model.
The efficacies of azithromycin, minocycline, clarithromycin, and tigecycline (Pfizer, New York, NY, USA) alone and in combination were examined in a rabbit subcutaneous infection model (12). Eighty-eight 3-month-old New Zealand rabbits were divided into 16 groups; 8 groups (n = 6) included animals with induced experimental pythiosis treated with minocycline, azithromycin, clarithromycin, tigecycline, minocycline plus azithromycin, minocycline plus clarithromycin, or minocycline plus tigecycline along with a control without antimicrobial treatment. The clinical isolate of P. insidiosum used for the induction of experimental pythiosis (isolate 290 in Table 1) was genotyped and registered under GenBank accession number KJ176713. The remaining eight groups (n = 5) were not infected with the experimental disease and were treated with the same drug regimens established for the infected animals. Treatment with oral azithromycin (20 mg/kg/day twice daily), clarithromycin (20 mg/kg/day twice daily), minocycline (10 mg/kg/day twice daily), or peritoneal tigecycline (1.5 mg/kg/day twice daily) began 21 days after inoculation. All animals were treated for 10 weeks.
TABLE 1.
In vitro combinations of azithromycin, clarithromycin, minocycline, and tigecycline against 30 Pythium insidiosum isolatesa
| Isolate | MIC (μg/ml) |
Results for drug combinations of: |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZM/CLR |
AZM/MIN |
AZM/TIG |
CLR/MIN |
CLR/TIG |
MIN/TIG |
|||||||||||||||||
| AZM | CLR | MIN | TIG | MICs (μg/ml) |
FICI | INT | MICs (μg/ml) |
FICI | INT | MICs (μg/ml) |
FICI | INT | MICs (μg/ml) |
FICI | INT | MICs (μg/ml) |
FICI | INT | MICs (μg/ml) |
FICI | INT | |
| 118 | 1.00 | 0.50 | 1.00 | 2.00 | 0.25/0.03 | 0.31 | S | 0.06/0.03 | 0.09 | S | 0.03/0.25 | 0.16 | S | 0.03/0.06 | 0.12 | S | 0.03/0.06 | 0.09 | S | 0.03/0.25 | 0.16 | S |
| 135 | 8.00 | 2.00 | 1.00 | 0.50 | 0.12/0.03 | 0.03 | S | 0.03/0.12 | 0.12 | S | 0.03/0.25 | 0.50 | S | 0.06/0.03 | 0.06 | S | 0.03/0.06 | 0.14 | S | 0.12/0.03 | 0.19 | S |
| 137 | 8.00 | 2.00 | 1.00 | 0.25 | 0.50/0.03 | 0.08 | S | 0.03/0.50 | 0.50 | S | 0.03/0.25 | 1.00 | I | 0.25/0.03 | 0.16 | S | 0.03/0.06 | 0.26 | S | 0.50/0.03 | 0.62 | I |
| 138 | 0.50 | 2.00 | 0.50 | 0.50 | 0.06/0.03 | 0.14 | S | 0.12/0.12 | 0.50 | S | 0.03/0.06 | 0.18 | S | 0.03/0.06 | 0.14 | S | 0.03/0.06 | 0.14 | S | 0.12/0.12 | 0.50 | S |
| 156 | 0.50 | 0.50 | 1.00 | 0.50 | 0.03/1.00 | 2.06 | I | 0.06/0.06 | 0.18 | S | 0.03/0.06 | 0.18 | S | 0.03/0.06 | 0.12 | S | 0.06/0.03 | 0.18 | S | 0.06/0.06 | 0.18 | S |
| 178 | 0.12 | 0.25 | 0.50 | 1.00 | 0.03/0.25 | 1.24 | I | 0.03/0.12 | 0.49 | S | 0.06/0.03 | 0.51 | I | 0.03/0.06 | 0.24 | S | 0.06/0.03 | 0.27 | S | 0.12/0.03 | 0.28 | S |
| 187 | 2.00 | 0.50 | 2.00 | 2.00 | 0.25/0.03 | 0.19 | S | 0.03/0.06 | 0.05 | S | 0.03/0.12 | 0.08 | S | 0.03/0.06 | 0.09 | S | 0.06/0.03 | 0.14 | S | 0.06/0.03 | 0.05 | S |
| 198 | 4.00 | 4.00 | 0.25 | 0.50 | 0.03/2.00 | 0.51 | I | 0.12/0.06 | 0.27 | S | 0.03/0.06 | 0.12 | S | 0.06/0.03 | 0.14 | S | 0.03/0.50 | 1.01 | I | 0.06/0.12 | 0.49 | S |
| 210 | 1.00 | 1.00 | 4.00 | 4.00 | 0.03/1.00 | 1.03 | I | 0.03/1.00 | 0.28 | S | 1.00/0.03 | 1.01 | I | 0.03/0.50 | 0.16 | S | 0.50/0.03 | 0.51 | I | 1.00/0.03 | 0.26 | S |
| 219 | 1.00 | 2.00 | 0.25 | 0.25 | 0.03/0.12 | 0.09 | S | 0.03/0.03 | 0.15 | S | 0.03/0.06 | 0.27 | S | 0.12/0.06 | 0.30 | S | 0.03/0.25 | 1.02 | I | 0.03/0.03 | 0.24 | S |
| 223 | 1.00 | 1.00 | 0.25 | 0.25 | 0.25/0.03 | 0.28 | S | 0.06/0.03 | 0.18 | S | 0.03/0.12 | 0.53 | I | 0.12/0.03 | 0.25 | S | 0.03/0.12 | 0.53 | I | 0.03/0.06 | 0.36 | S |
| 227 | 1.00 | 0.50 | 0.25 | 1.00 | 0.03/0.50 | 1.03 | I | 0.25/0.06 | 0.49 | S | 0.06/0.06 | 0.12 | S | 0.06/0.06 | 0.36 | S | 0.12/0.03 | 0.28 | S | 0.06/0.25 | 0.49 | S |
| 232 | 1.00 | 2.00 | 0.25 | 0.25 | 0.03/0.25 | 0.16 | S | 0.12/0.03 | 0.25 | S | 0.03/0.06 | 0.27 | S | 0.06/0.12 | 0.53 | I | 0.03/0.25 | 1.02 | I | 0.03/0.12 | 0.62 | I |
| 247 | 1.00 | 2.00 | 1.00 | 2.00 | 0.03/1.00 | 0.53 | I | 0.03/0.12 | 0.16 | S | 0.03/0.06 | 0.06 | S | 0.06/0.12 | 0.16 | S | 0.06/0.03 | 0.05 | S | 0.12/0.03 | 0.14 | S |
| 252 | 2.00 | 0.50 | 2.00 | 1.00 | 0.25/0.03 | 0.19 | S | 0.03/0.12 | 0.08 | S | 0.25/0.03 | 0.16 | S | 0.03/0.12 | 0.12 | S | 0.03/0.25 | 0.31 | S | 0.12/0.03 | 0.09 | S |
| 253 | 4.00 | 4.00 | 0.25 | 1.00 | 0.03/1.00 | 0.26 | S | 0.12/0.03 | 0.15 | S | 0.03/1.00 | 1.01 | I | 0.50/0.03 | 0.25 | S | 0.06/0.12 | 0.14 | S | 0.03/0.12 | 0.25 | S |
| 254 | 1.00 | 0.50 | 0.50 | 0.25 | 0.03/0.25 | 0.53 | I | 0.03/0.12 | 0.28 | S | 0.03/0.06 | 0.27 | S | 0.03/0.06 | 0.18 | S | 0.06/0.03 | 0.24 | S | 0.12/0.03 | 0.37 | S |
| 255 | 4.00 | 2.00 | 2.00 | 0.25 | 0.50/0.03 | 0.14 | S | 0.03/1.00 | 0.51 | I | 0.03/0.25 | 1.01 | I | 0.06/0.03 | 0.05 | S | 0.03/0.50 | 2.02 | I | 1.00/0.03 | 0.62 | I |
| 260 | 4.00 | 2.00 | 4.00 | 4.00 | 1.00/0.03 | 0.27 | S | 0.03/0.50 | 0.12 | S | 0.50/0.03 | 0.12 | S | 0.03/0.12 | 0.05 | S | 0.25/0.03 | 0.12 | S | 0.50/0.03 | 0.12 | S |
| 264 | 4.00 | 1.00 | 0.50 | 0.25 | 0.25/0.03 | 0.09 | S | 0.06/0.06 | 0.14 | S | 0.03/0.12 | 0.51 | I | 0.06/0.06 | 0.18 | S | 0.03/0.12 | 0.53 | I | 0.06/0.06 | 0.36 | S |
| 271 | 2.00 | 4.00 | 2.00 | 2.00 | 0.03/2.00 | 0.52 | I | 0.03/0.50 | 0.27 | S | 0.50/0.03 | 0.27 | S | 1.00/0.03 | 0.27 | S | 0.03/1.00 | 0.51 | I | 0.50/0.03 | 0.27 | S |
| 273 | 2.00 | 0.50 | 1.00 | 1.00 | 0.25/0.03 | 0.19 | S | 0.03/0.12 | 0.14 | S | 0.03/0.25 | 0.27 | S | 0.03/0.06 | 0.12 | S | 0.06/0.03 | 0.15 | S | 0.12/0.03 | 0.16 | S |
| 274 | 1.00 | 2.00 | 4.00 | 4.00 | 0.03/0.50 | 0.28 | S | 0.03/0.50 | 0.16 | S | 0.05/0.03 | 0.06 | S | 0.03/0.12 | 0.05 | S | 0.25/0.03 | 0.12 | S | 0.50/0.03 | 0.12 | S |
| 282 | 2.00 | 4.00 | 4.00 | 4.00 | 0.03/1.00 | 0.27 | S | 0.50/0.03 | 0.26 | S | 0.05/0.03 | 0.03 | S | 0.25/0.03 | 0.07 | S | 0.03/0.25 | 0.07 | S | 0.03/0.50 | 0.12 | S |
| 290 | 2.00 | 4.00 | 4.00 | 4.00 | 0.03/0.50 | 0.14 | S | 0.03/0.25 | 0.08 | S | 0.50/0.03 | 0.26 | S | 0.03/0.50 | 0.12 | S | 0.06/0.03 | 0.02 | S | 0.25/0.03 | 0.07 | S |
| 291 | 4.00 | 0.50 | 2.00 | 0.50 | 0.25/0.03 | 0.12 | S | 0.03/0.06 | 0.04 | S | 0.25/0.03 | 0.12 | S | 0.03/0.06 | 0.09 | S | 0.03/0.06 | 0.18 | S | 0.06/0.03 | 0.09 | S |
| 292 | 8.00 | 2.00 | 2.00 | 0.25 | 1.00/0.03 | 0.14 | S | 0.03/0.12 | 0.07 | S | 0.03/0.12 | 0.50 | S | 0.03/0.12 | 0.08 | S | 0.03/0.50 | 2.02 | I | 0.12/0.03 | 0.18 | S |
| 294 | 8.00 | 4.00 | 0.50 | 0.50 | 2.00/0.03 | 0.26 | S | 0.03/0.12 | 0.25 | S | 0.03/1.00 | 2.00 | I | 0.25/0.03 | 0.12 | S | 0.03/0.25 | 0.51 | I | 0.12/0.03 | 0.31 | S |
| 296 | 2.00 | 8.00 | 0.50 | 0.25 | 1.00/0.03 | 0.50 | S | 0.03/0.06 | 0.14 | S | 0.03/0.06 | 0.26 | S | 0.25/0.03 | 0.09 | S | 0.03/0.12 | 0.50 | S | 0.06/0.03 | 0.24 | S |
| ATCC | 4.00 | 0.50 | 0.25 | 1.00 | 1.00/0.03 | 0.31 | S | 0.12/0.03 | 0.15 | S | 0.03/1.00 | 1.01 | I | 0.25/0.03 | 0.62 | I | 0.03/0.50 | 0.56 | I | 0.03/0.12 | 0.25 | S |
ATCC, ATCC 58.637; AZM, azithromycin; CLR, clarithromycin; MIN, minocycline; TIG, tigecycline; FICI, fractional inhibitory concentration index; INT, interpretation; S, synergism; I, indifference.
The subcutaneous nodular areas of lesions (square centimeters) in infected groups were measured (the longitudinal and transverse lesion lengths) every 7 days after the beginning of treatment using a caliper. Blood samples from all animals were collected from the lateral saphenous vein before inoculation (day 0) and before the start of treatment (day 21), as well as 30 and 60 days after the start of treatment. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, and urea were determined by a semiautomatic analyzer (BA-88A; Mindray, China) using commercial kits (Labtest Diagnóstica S.A., Brazil). A hematological analysis/complete blood count (BC-2800 vet auto hematology analyzer; Mindray, China) was carried out. An enzyme-linked immunosorbent assay (ELISA) (13) was performed to determine the anti-P. insidiosum antibody levels.
At the end of the experiment, all surviving animals were euthanized through deepening of anesthesia using thiopental (60 mg/kg), and representative tissues from the subcutaneous tumor-like lesion region, lung, and kidney were subjected to further microbiological culture in Sabouraud dextrose agar, direct detection of P. insidiosum DNA by a nested PCR (nPCR) assay (11), and histopathological analysis. To quantify the fungal load, tissue slices were stained with methenamine silver stain and scored as 0 (if no hyphae were visible) or 1, 2, 3, or 4 proportional to increasing tissue burdens. The inflammatory responses were observed by hematoxylin-eosin (H&E) stain. The animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources, Federal University of Santa Maria, Brazil.
Statistical analysis.
All data are presented as means ± standard errors of the mean (SEM) unless otherwise indicated and were subjected to logarithmic transformation to obtain homogeneity between groups, and two-way analysis of variance (ANOVA) followed by the Holm-Sidak test was performed to calculate the significant difference between groups. All analyses were performed using Sigma Plot software version 12.5. A significant difference was established at a P value of <0.05.
Nucleotide sequence accession number.
Nucleotide data for P. insidiosum isolate 290 were deposited in GenBank under accession number KJ176713.
RESULTS
In vitro susceptibility.
The results of the in vitro susceptibility and combination tests are shown in Table 1. The MICs (geometric mean MICs) ranged from 0.125 to 8 μg/ml (1.91 μg/ml) for azithromycin, from 0.25 to 8 μg/ml (1.38 μg/ml) for clarithromycin, from 0.25 to 4 μg/ml (0.91 μg/ml) for minocycline, and from 0.25 to 4 μg/ml (0.79 μg/ml) for tigecycline. The MIC90 values were 8 μg/ml for azithromycin and 4 μg/ml for clarithromycin, minocycline, and tigecycline. The interpretation of the drug combinations was based on the lowest fractional inhibitory concentration index (FICI) and demonstrated synergism (percentage of synergic interactions) for the following combinations: minocycline plus azithromycin (93.3%), minocycline plus clarithromycin (93.3%), minocycline plus tigecycline (86.67%), tigecycline plus azithromycin (66.6%), tigecycline plus clarithromycin (60%), and azithromycin plus clarithromycin (70%). Furthermore, antagonistic interactions were not observed.
Experimentally induced pythiosis.
The means and standard errors of subcutaneous lesion nodular areas (square centimeters) of the groups are summarized in Fig. 1. Three animals from the untreated group (n = 6) died in the sixth (n = 2) and ninth (n = 1) weeks. Pythiosis with metastasis to the lungs was considered the causa mortis by necropsy and histopathological analysis. All remaining animals from the untreated and treated groups were followed until the end of the experiment. Clinical cures, i.e., complete remissions of subcutaneous lesions, were observed in animals from the groups given minocycline (one animal after 4 weeks of treatment), minocycline plus clarithromycin (one animal after 7 weeks), minocycline plus tigecycline (one animal after 7 weeks; one animal after 8 weeks), minocycline plus azithromycin (three animals after 7 weeks; one animal after 10 weeks), and azithromycin (one animal after 5 weeks; one animal after 6 weeks; one animal after 8 weeks; two animals after 10 weeks).
FIG 1.
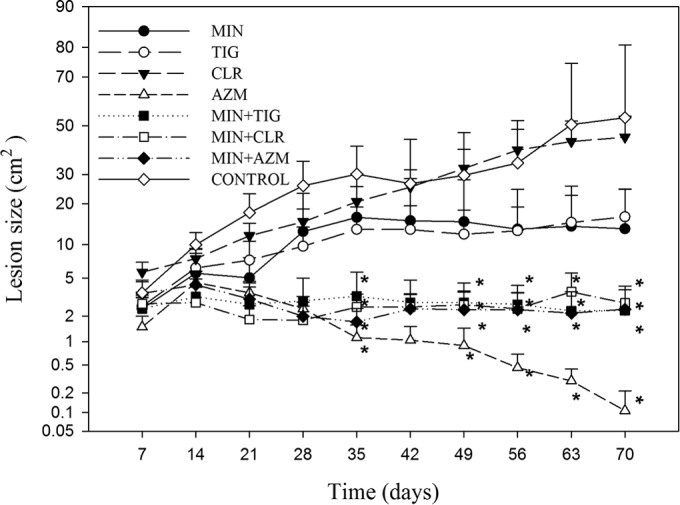
Variation in Pythium insidiosum-induced subcutaneous lesion nodular areas in rabbits receiving monotherapy or combination antibiotic treatment. *Indicates a significant difference compared to the disease control group (P < 0.05). AZM, azithromycin; CLR, clarithromycin; MIN, minocycline; TIG, tigecycline.
Biochemical and hematological analyses.
Rabbits with pythiosis had increased white blood cell (WBC) counts. Interestingly, on the 60th treatment day, the WBC counts from the azithromycin and minocycline-plus-azithromycin groups were significantly different from those for the control group and similar to those of healthy animals. No statistically significant differences were observed in red blood cells (RBC), and despite some intermittent differences, an overview analysis of the hemoglobin concentration and levels of ALT, AST, urea, and creatinine did not reveal any evident alterations or toxicity regarding the disease or treatment.
ELISA, nPCR, and histopathological and morphometric analyses.
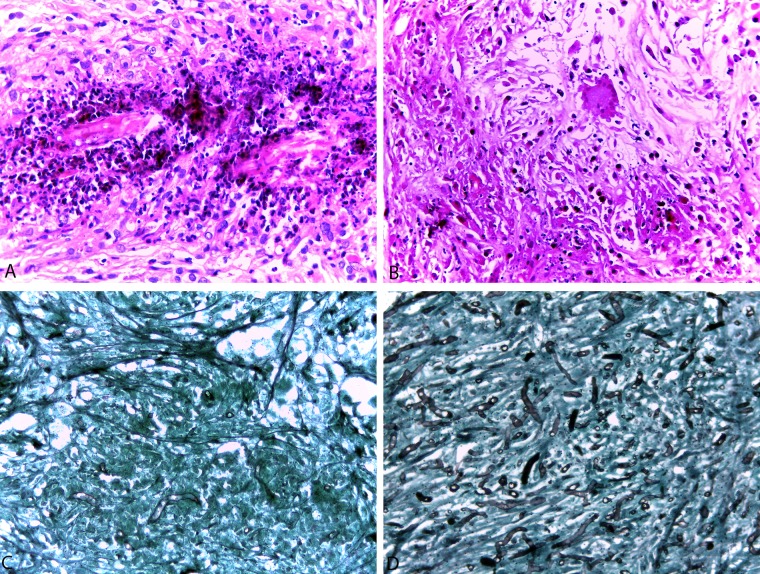
Based on the ELISA results, recovery of microorganisms in culture, nPCR, and blinded histopathological analysis summarized in Table 2, it is evident that the groups treated with azithromycin and minocycline plus azithromycin showed an absence of or significant reduction in fungal burden and a reduced concentration of anti-P. insidiosum antibodies. Although the histological aspects of the lesions were similar in all groups, there was a variation in the number of P. insidiosum hyphae when evaluated by Grocott's stain (Fig. 2). The histopathology of the lesions stained with H&E revealed the presence of multifocal to coalescent necrotic areas, which were delimited by inflammatory infiltrates consisting predominantly of macrophages, lymphocytes, and neutrophil degenerates. Grocott's stain revealed irregular hypha-like structures that were ramified and occasionally septate (Fig. 2). The presence of hyphae was also confirmed by nPCR, which was more sensitive than Grocott's stain or tissue culture. Animals treated with clarithromycin had an increased number of hyphae compared to those in the control group. Pulmonary metastasis in an animal treated with tigecycline was confirmed by hypha detection with Grocott's stain and nPCR tests (Table 2).
TABLE 2.
Results from histopathological, nested PCR, and culture analysis of kidneys, lungs, and subcutaneous lesions and antibody levels determined by ELISA from Pythium insidiosum-infected rabbitsa
| Group | Mean ± SD fungal load by Grocott's stain (0–4) |
Nested PCR positive (%) |
Culture positive (%) |
Mean ± SD antibody levels by ELISA (optical density) at day: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | L | S | K | L | S | K | L | S | 0 | 30 | 60 | |
| AZM | 0 | 0 ± 0.00A | 0 ± 0.00B | 0 | 0 | 16.7 | 0 | 0 | 0 | 0.165 ± 0.006Aa | 0.139 ± 0.022Ab | 0.112 ± 0.022Bb |
| CLR | 0 | 0 ± 0.00A | 3.17 ± 0.40A | 0 | 0 | 83.3 | 0 | 0 | 66.6 | 0.170 ± 0.011Aa | 0.179 ± 0.037Aa | 0.163 ± 0.038Aa |
| MIN | 0 | 0 ± 0.00A | 1.67 ± 0.42A | 0 | 0 | 66.4 | 0 | 0 | 50.0 | 0.188 ± 0.035Aa | 0.169 ± 0.031Aa | 0.150 ± 0.025Aa |
| TIG | 0 | 0.17 ± 0.17A | 1.50 ± 0.56A | 0 | 16.7 | 50.0 | 0 | 0 | 50.0 | 0.202 ± 0.041Aa | 0.175 ± 0.030Aa | 0.170 ± 0.036Aa |
| MIN + AZM | 0 | 0 ± 0.00A | 0.83 ±0.40B | 0 | 0 | 16.7 | 0 | 0 | 0 | 0.157 ± 0.016Aa | 0.152 ± 0.036Aa | 0.128 ± 0.025Ba |
| MIN + CLR | 0 | 0 ± 0.00A | 1.00 ± 0.26A | 0 | 0 | 83.3 | 0 | 0 | 33.3 | 0.152 ± 0.034Aa | 0.139 ± 0.032Aa | 0.139 ± 0.033Ba |
| MIN + TIG | 0 | 0.33 ± 0.28A | 1.50 ± 0.42A | 0 | 0 | 50.0 | 0 | 0 | 66.6 | 0.196 ± 0.028Aa | 0.171 ± 0.034Aa | 0.158 ± 0.038Aa |
| Disease control | 0 | 1.17 ± 0.60A | 2.50 ± 0.43A | 16.6 | 50.0 | 100 | 0 | 33.3 | 100 | 0.192 ± 0.038Aa | 0.183 ± 0.030Aa | 0.200 ± 0.027Aa |
Superscript letters indicate significant differences. Values not sharing the same superscript letter are significantly different (P < 0.05). Different capital letters indicate significant differences between the groups treated for pythiosis compared to the disease control; different lowercase letters represent significant differences in the same group from day 0. AZM, azithromycin; CLR, clarithromycin; MIN, minocycline; TIG, tigecycline; K, kidney; L, lung; S, subcutaneous lesion; day 0, before antimicrobial treatment started; day 30 and day 60, 30 and 60 days after the start of the antimicrobial treatment regimen.
FIG 2.
(A and B) Tissue sections were stained with hematoxylin-eosin stain (H&E) to examine the inflammatory responses. A histological section of granulomatous tissue demonstrated core areas of necrosis containing the agent, featured as a negative image, surrounded by infiltration of macrophages, lymphocytes, and neutrophil degenerates (A), and observed necrosis areas are associated with the proliferation of fibrous connective tissue and the presence of giant cells (B). (C and D) Quantification of the tissue burden of organisms and inflammatory responses in the granulomatous tissue of animals infected with P. insidiosum. A scoring system of 0 to 4 for each parameter was developed. For quantification of the fungal load, granulomatous tissue slices were stained by methenamine-silver stain and scored as 0 (if no filaments were visible) or as 1 to 4 (panel C represents a score of 1 and panel D represents a score of 4). Magnification, ×400.
DISCUSSION
In the present study, we compared the effects of azithromycin, clarithromycin, minocycline, and tigecycline against P. insidiosum isolates in vitro and in vivo. Our in vitro results showed that all of these antibacterials inhibited in vitro P. insidiosum growth, and all combination treatments resulted in synergistic in vitro interactions (>60%), particularly in the presence of minocycline, for which >86% synergistic interactions were observed. Therefore, minocycline was selected as the main antibiotic for the in vivo studies.
The in vitro susceptibility results in our study were consistent with those of previous studies (7–10). In vivo, the previous studies of induced pythiosis in rabbits demonstrated that caspofungin (1 mg/kg/day) (14), some combinations of terbinafine (125 mg/day) with itraconazole (5 mg/kg/day), fluvastatin (1 mg/kg/day), or caspofungin (1 mg/kg/day) (15), and a vortexed immunotherapy (30 mg at 14-day intervals) (12, 16) presented fungistatic activity, i.e., the progression of subcutaneous lesions was less than that for the untreated group for some therapies, but complete remission of the lesions was not the prevalent observation. In our study, with the exception of clarithromycin monotherapy, all therapies reduced the progression of subcutaneous lesions compared to the control. However, azithromycin was remarkably effective, with complete remission of the lesions in 66.6% and 83.3% of the animals receiving combination therapy with minocycline or monotherapy, respectively.
The results described here suggest that most of the antimicrobial agents evaluated merit attention as new candidates for pythiosis treatment. Nevertheless, it is important to note some limitations of this study. (i) Rabbits are currently the only animals in which experimental pythiosis may be reproduced. However, rabbits are not a natural host of the disease. (ii) All treated animals had lesions with less than 30 days of evolution. Typically, cases of pythiosis respond better to treatment when the lesions are more recent, whereas an unfavorable diagnosis is often observed as the time of disease progression increases. (iii) The prolonged treatment time with antibacterials may be contraindicated due to possible side effects. In this sense, the constant monitoring of hepatic, renal, cardiac, and respiratory function is indicated. (iv) The therapeutic response may be different, depending on the tissue or organ affected by the disease.
It is probable that the mechanism of antimicrobial action of macrolides and tetracyclines against P. insidiosum in this study is similar to that observed against bacteria, i.e., the inhibition of protein synthesis. Interestingly, among the pharmacological characteristics of these compounds (17), therapeutic doses of azithromycin produce tissue concentrations greater than 3 mg/kg in several tissues but low plasma concentrations (0.4 μg/ml) (18).
Further studies, including a larger number of isolates, particularly from genetically diverse P. insidiosum strains, different drug regimen treatments, and associations with other antimicrobials, immunotherapies, and surgery, will provide a better understanding of the therapeutic potential of azithromycin, minocycline, and tigecycline in pythiosis treatment.
To the best of our knowledge, this study represents the first evaluation of the use of antibacterials against P. insidiosum in an experimental disease model. In particular, azithromycin possesses potent in vivo activity against subcutaneous lesions and presents an interesting potential as a novel anti-P. insidiosum therapeutic candidate.
ACKNOWLEDGMENTS
This work was supported by the National Council for Scientific and Technological Development-CNPq (grant 471221/2011-2 to J.M.S.). F.P.K.J. and É.S.L are financially supported by fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES).
REFERENCES
- 1.Gaastra W, Lipman LJA, De Cock AWAM, Exel TK, Pegge RBG, Scheurwater J, Vilela R, Mendoza L. 2010. Pythium insidiosum: an overview. Vet Microbiol 146:1–16. doi: 10.1016/j.vetmic.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Heath JA, Kiehn TE, Brown AE, LaQuaglia MP, Steinherz LJ, Bearman G, Wong M, Steinherz PG. 2002. Pythium insidiosum pleuropericarditis complicating pneumonia in a child with leukemia. Clin Infect Dis 35:E60–E64. doi: 10.1086/342303. [DOI] [PubMed] [Google Scholar]
- 3.Shenep JL, English BK, Kaufman L, Pearson TA, Thompson JW, Kaufman RA, Frisch G, Rinaldi MG. 1998. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin Infect Dis 27:1388–1393. doi: 10.1086/515042. [DOI] [PubMed] [Google Scholar]
- 4.Krajaejun T, Sathapatayavongs B, Pracharktam R, Nitiyanant P, Leelachaikul P, Wanachiwanawin W, Chaiprasert A, Assanasen P, Saipetch M, Mootsikapun P, Chetchotisakd P, Lekhakula A, Mitarnun W, Kalnauwakul S, Supparatpinyo K, Chaiwarith R, Chiewchanvit S, Tananuvat N, Srisiri S, Suankratay C, Kulwichit W, Wongsaisuwan M, Somkaew S. 2006. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin Infect Dis 43:569–576. doi: 10.1086/506353. [DOI] [PubMed] [Google Scholar]
- 5.Sathapatayavongs B, Leelachaikul P, Prachaktam R, Atichartakarn V, Sriphojanart S, Trairatvorakul P, Jirasiritham S, Nontasut S, Eurvilaichit C, Flegel T. 1989. Human pythiosis associated with thalassemia hemoglobinopathy syndrome. J Infect Dis 159:274–280. doi: 10.1093/infdis/159.2.274. [DOI] [PubMed] [Google Scholar]
- 6.Permpalung N, Worasilchai N, Plongla R, Upala S, Sanguankeo A, Paitoonpong L, Mendoza L, Chindamporn A. 2015. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: a retrospective study of 18 patients. J Antimicrob Chemother 70:1885–1892. doi: 10.1093/jac/dkv008. [DOI] [PubMed] [Google Scholar]
- 7.Loreto ES, Mario DAN, Denardi LB, Alves SH, Santurio JM. 2011. In vitro susceptibility of Pythium insidiosum to macrolides and tetracycline antibiotics. Antimicrob Agents Chemother 55:3588–3590. doi: 10.1128/AAC.01586-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahl DL, de Jesus FPK, Loreto ES, Zanette RA, Ferreiro L, Ben Pilotto M, Alves SH, Santurio JM. 2012. In vitro susceptibility of Pythium insidiosum isolates to aminoglycoside antibiotics and tigecycline. Antimicrob Agents Chemother 56:4021–4023. doi: 10.1128/AAC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jesus FP, Ferreiro L, Loreto ES, Pilotto MB, Ludwig A, Bizzi K, Tondolo JS, Zanette RA, Alves SH, Santurio JM. 2014. In vitro synergism observed with azithromycin, clarithromycin, minocycline, or tigecycline in association with antifungal agents against Pythium insidiosum. Antimicrob Agents Chemother 58:5621–5625. doi: 10.1128/AAC.02349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loreto ÉS, Tondolo JSM, Pilotto MB, Alves SA, Santurio JM. 2014. New insights into the in vitro susceptibility of Pythium insidiosum. Antimicrob Agents Chemother 58:7534–7537. doi: 10.1128/AAC.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botton SA, Pereira DIB, Costa MM, Azevedo MI, Argenta JS, Jesus FPK, Alves SH, Santurio JM. 2011. Identification of Pythium insidiosum by nested PCR in cutaneous lesions of Brazilian horses and rabbits. Curr Microbiol 62:1225–1229. doi: 10.1007/s00284-010-9781-4. [DOI] [PubMed] [Google Scholar]
- 12.Santurio JM, Leal AT, Leal ABM, Festugatto R, Lubeck I, Sallis ESV, Copetti MV, Alves SA, Ferreiro L. 2003. Three types of immunotherapics against pythiosis insidiosi developed and evaluated. Vaccine 21:2535–2540. doi: 10.1016/S0264-410X(03)00035-5. [DOI] [PubMed] [Google Scholar]
- 13.Santurio JM, Leal AT, Leal ABM, Alves SH, Lubeck I, Griebeler J, Copetti MV. 2006. Indirect ELISA for the serodiagnostic of pythiosis. Pesq Vet Bras 26:47–50. doi: 10.1590/S0100-736X2006000100010. [DOI] [Google Scholar]
- 14.Pereira DIB, Santurio JM, Alves SH, Argenta JS, Potter L, Spanamberg A, Ferreiro L. 2007. Caspofungin in vitro and in vivo activity against Brazilian Pythium insidiosum strains isolated from animals. J Antimicrob Chemother 60:1168–1171. doi: 10.1093/jac/dkm332. [DOI] [PubMed] [Google Scholar]
- 15.Argenta JS, Alves SH, Silveira F, Maboni G, Zanette RA, Cavalheiro AS, Pereira PL, Pereira DIB, Sallis ESV, Potter L, Santurio JM, Ferreiro L. 2012. In vitro and in vivo susceptibility of two-drug and three-drug combinations of terbinafine, itraconazole, caspofungin, ibuprofen and fluvastatin against Pythium insidiosum. Vet Microbiol 157:137–142. doi: 10.1016/j.vetmic.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Pereira DIB, Santurio JM, Alves SH, de Azevedo MI, Silveira F, da Costa FF, Sallis ESV, Potter L, Ferreiro L. 2008. Comparison between immunotherapy and caspofungin as agents to treat experimental pythiosis in rabbits. J Mycol Med 18:129–133. doi: 10.1016/j.mycmed.2008.05.001. [DOI] [Google Scholar]
- 17.Chambers HF, Deck DH. 2009. Tetracyclines, macrolides, clindamycin, chloramphenicol, streptogramins, and oxazolidinones, p 795–806. In Katzung BG, Masters SB, Trevor AJ (ed), Basic and clinical pharmacology, 11th ed McGraw-Hill Medical, New York, NY. [Google Scholar]
- 18.Foulds G, Shepard RM, Johnson RB. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother 25(Suppl A):73–82. doi: 10.1093/jac/25.suppl_A.73. [DOI] [PubMed] [Google Scholar]