Abstract
GSK1322322 is a novel inhibitor of peptide deformylase (PDF) with good in vitro activity against bacteria associated with community-acquired pneumonia and skin infections. We have characterized the in vivo pharmacodynamics (PD) of GSK1322322 in immunocompetent animal models of infection with Streptococcus pneumoniae and Haemophilus influenzae (mouse lung model) and with Staphylococcus aureus (rat abscess model) and determined the pharmacokinetic (PK)/PD index that best correlates with efficacy and its magnitude. Oral PK studies with both models showed slightly higher-than-dose-proportional exposure, with 3-fold increases in area under the concentration-time curve (AUC) with doubling doses. GSK1322322 exhibited dose-dependent in vivo efficacy against multiple isolates of S. pneumoniae, H. influenzae, and S. aureus. Dose fractionation studies with two S. pneumoniae and S. aureus isolates showed that therapeutic outcome correlated best with the free AUC/MIC (fAUC/MIC) index in S. pneumoniae (R2, 0.83), whereas fAUC/MIC and free maximum drug concentration (fCmax)/MIC were the best efficacy predictors for S. aureus (R2, 0.9 and 0.91, respectively). Median daily fAUC/MIC values required for stasis and for a 1-log10 reduction in bacterial burden were 8.1 and 14.4 for 11 S. pneumoniae isolates (R2, 0.62) and 7.2 and 13.0 for five H. influenzae isolates (R2, 0.93). The data showed that for eight S. aureus isolates, fAUC correlated better with efficacy than fAUC/MIC (R2, 0.91 and 0.76, respectively), as efficacious AUCs were similar for all isolates, independent of their GSK1322322 MIC (range, 0.5 to 4 μg/ml). Median fAUCs of 2.1 and 6.3 μg · h/ml were associated with stasis and 1-log10 reductions, respectively, for S. aureus.
INTRODUCTION
The steady appearance and spread of resistance to marketed antibiotics in bacterial pathogens causing major human diseases have constituted a public health concern for many years (1–3). Moreover, hospitalizations associated with drug-resistant infections have substantial implications for the health care system, such as increased risk of patient mortality, longer stays, and higher hospital costs (4, 5). Although a review of the antibacterial pipeline shows an increase in the number of antibiotic candidates in clinical development (6), with five new FDA approvals since the beginning of 2014 (http://www.pewtrusts.org/antibiotics), there is still a need for novel-acting antimicrobial agents (3, 7). GSK1322322 is a novel inhibitor of peptide deformylase (PDF), an essential metalloprotease that removes the N-formyl group from all nascent polypeptides (8–10) and, so far, a clinically unexploited antibacterial target. GSK1322322 shows good in vitro antibacterial activity against organisms associated with community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI), including strains carrying resistance determinants for commonly used antibacterial agents, with MIC90s of 2 μg/ml against Streptococcus pneumoniae and 4 μg/ml against Haemophilus influenzae and Staphylococcus aureus (11). This compound has also demonstrated good safety, tolerability, and pharmacokinetic (PK) properties in phase I clinical trials (12–14), as well as efficacy in human proof-of-concept clinical studies (15). A considerable number of PDF inhibitors have been discovered over the last decade of research (16), and two, BB-83698 (17) and LBM415 (18), have progressed to phase I clinical trials but have not been further developed. The reasons for the discontinuation of BB-83698 have not been reported, but in the case of LBM415, reversible methemoglobinemia was detected at the highest dose tested in human volunteers (19). These findings were structure based and not related to the compound's mechanism of action (19).
Pharmacokinetic/pharmacodynamic (PK/PD) studies with BB-83698 and LBM415 determined that the area under the concentration-time curve (AUC)/MIC ratio was the parameter which best correlated with efficacy (W. A. Craig and D. R. Andes, presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Chicago, IL, 2001; W. A. Craig and D. R. Andes, presented at the14th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Prague, Czech Republic, 2004). Here we report the characterization of the in vivo PD of GSK1322322 in immunocompetent animal models of infection with S. aureus, S. pneumoniae, and H. influenzae in order to determine the PK/PD parameter and magnitude that best correlates with efficacy in all three organisms.
(The clinical development of GSK1322322 has been terminated at GlaxoSmithKline [GSK] [https://clinicaltrials.gov/ct2/show/NCT01953809].)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used in this study were either clinical isolates obtained from the GSK Microbiology Department Culture Collection or reference strains obtained from the American type Culture Collection (ATCC). S. pneumoniae and S. aureus isolates, including methicillin-, macrolide-, and quinolone-resistant strains, were cultured at 37°C on Trypticase soy agar (TSA) with 5% sheep blood or in cation-adjusted Mueller-Hinton (MH) broth. H. influenzae strains were cultured at 37°C on chocolate agar II plates or in Haemophilus test medium (HTM) broth.
In vitro susceptibility testing of GSK1322322.
PDF inhibitor GSK1322322 was obtained from GlaxoSmithKline Pharmaceuticals (Collegeville, PA) and dissolved in dimethyl sulfoxide (DMSO). MIC endpoints were determined in triplicate by broth microdilution methodology according to Clinical and Laboratory Standards Institute (CLSI) guidelines (20). The MIC was defined as the lowest concentration of compound that inhibited visible growth of the organism.
Animals.
Specific-pathogen-free (SPF) CD1 mice (Charles River, Raleigh, NC) weighing approximately 20 g were used in the respiratory tract infection models. SPF male Cr1:Sprague-Dawley (CD) rats (Charles River) weighing 95 to 105 g were used in the abscess infection models. Animals were allowed access to food and water ad libitum.
All studies, conducted in accordance with the GlaxoSmithKline policy on the care, welfare and treatment of laboratory animals, were reviewed by the Institutional Animal Care and Use Committee at GSK and met or exceeded the standards of the American Association for the Accreditation of Laboratory Animal Care, the U.S. Department of Health and Human Services, and all local and federal animal welfare laws.
Mouse respiratory tract infection (RTI) model.
All S. pneumoniae and H. influenzae isolates were subcultured, respectively, onto TSA plates supplemented with 5% sheep blood, or onto chocolate agar plates, and incubated overnight at 37°C. Colonies were harvested from the agar plates and suspended in phosphate-buffered saline (PBS). Immediately prior to infection, a 5- to 10-fold dilution of the bacterial suspensions was prepared in cooled (approximately 42°C) molten nutrient agar. Mice were anesthetized with 5% isoflurane in 1.5 liter/min of oxygen and infected by intrabronchial instillation with a 20-μl aliquot of the agar suspensions via nonsurgical intubation. The final inocula ranged from 2.3 × 105 to 1.9 × 106 CFU/mouse for S. pneumoniae isolates and from 1.5 × 106 to 2.4 × 106 CFU/mouse for H. influenzae isolates. No correlation was observed between starting inocula and the growth of the strains in the lungs or the efficacy of GSK1322322. Mice used for the PK evaluation of GSK1322322 in this animal model were infected with S. pneumoniae.
Rat abscess infection model.
All S. aureus isolates were subcultured into brain heart infusion broth (BHI) and incubated overnight at 37°C without shaking. Inocula were obtained from the overnight cultures by diluting twice 1:2, and once 1:10, into sterile saline. A final 10-fold dilution was done into 0.6% (wt/vol) semisolid nutrient agar immediately prior to infection. Rats were inoculated with 1 ml of this suspension (1.3 × 106 to 3.2 × 106 CFU/rat) by subcutaneous injection in the groin area. Rats used for the PK evaluation of GSK1322322 in this animal model were infected with S. aureus.
GSK1322322 administration.
Dosing solutions of GSK1322322 were prepared in 20% polyethylene glycol (PEG) immediately prior to each dose and were administered by oral gavage in a volume of 0.4 ml/mouse (RTI model) or 2 ml/rat (abscess model), starting 1 h postinfection. In all studies, additional groups of infected animals were either left untreated (1-h baseline controls) or given a vehicle only and served as 24-h (dose fractionation studies) or 48-h (dose ranging studies) nontreated controls (NTC).
PK/PD index determination.
Dose fractionation studies were performed with two S. pneumoniae isolates (Ery-2 and 1302005S) in the mouse RTI model and two S. aureus isolates (A-24 and 1307005A) in the rat abscess model. Groups of 3 to 5 S. pneumoniae-infected mice were treated with 1, 2, 4, or 8 doses of GSK1322322 over a 24-h period, for a total of 20 dosing regimens, with doses ranging from 20 to 600 mg/kg (of body weight)/day. Groups of 5 S. aureus-infected rats were treated with 1, 2, or 4 doses of GSK1322322 over a 24-h period, for a total of 18 dosing regimens, with doses ranging from 37.5 to 1,200 mg/kg/day.
PK/PD magnitude determination.
Eleven S. pneumoniae isolates and five H. influenzae isolates were used to evaluate the efficacy of GSK1322322 in dose ranging studies in the mouse RTI model (4 or 5 mice/group). The compound was given every 8 h for 2 days at 4 different doses ranging from 18.75 to 300 mg/kg in S. pneumoniae infections and at 4 different doses ranging from 37.5 to 300 mg/kg in H. influenzae infections. Eight S. aureus isolates were used in dose ranging studies in the rat abscess model (5 rats/group), with GSK1322322 administered every 12 h for 2 days at 4 or 5 different doses ranging from 4.7 to 150 mg/kg, depending on the strain.
Animals were euthanized at 1 h (untreated baseline controls), 24 h (dose fractionation studies), or 48 h (dose ranging studies) postinfection. Lungs or abscesses were removed using aseptic technique, placed in bags, and homogenized in 1 ml of PBS (lungs) or sterile saline (abscesses) for 2 min in a Stomacher 80 Biomaster (Seward, Ltd., Worthing, United Kingdom). For enumeration of viable bacteria, 20 μl of 10-fold serial dilutions in PBS (lungs) or sterile saline (abscesses) were plated in triplicate on TSA supplemented with 5% sheep blood (S. aureus and S. pneumoniae infections) or chocolate agar plates (H. influenzae infections) by a modified Miles-Misra technique using the Hamilton Microlab AT-Plus2 liquid handling system. The colonies were counted following overnight incubation at 37°C. The lower limits of quantification were 1.7 log10 CFU/lung and 1.2 log10 CFU/abscess.
Drug PK studies.
Single-dose PK studies were performed in S. pneumoniae Ery-2-infected mice (3 mice/group). Animals were administered oral doses (0.4 ml/mouse) of GSK1322322 at 10, 37.5, 75, 150, 300, or 600 mg/kg at 1 h postinfection. PK parameters could not be obtained from the 10-mg/kg dose, as most of the values were below the limit of quantification (0.05 μg/ml). The PK of GSK1322322 was also evaluated in S. aureus A24-infected rats (3 rats/group). GSK1322322 was administered at 37.5, 75, 150, or 300 mg/kg 1 h postinfection by oral gavage in a volume of 2 ml/rat.
Approximately 30 μl (mice) or 40 μl (rats) of whole blood was collected serially from the lateral tail vein at 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, and 24 h postdose. The lateral tail vein was punctured using a microlancet (mice) or a 23-gauge butterfly needle (rats), and blood was collected into heparin-coated capillary tubes (mice) or into a heparinized Eppendorf tube (rats). The blood was transferred into a microcentrifuge tube, and a 10-μl (mice) or a 25-μl (rats) aliquot was mixed with an equal volume of cold high-performance liquid chromatography (HPLC)-grade water. All samples were frozen immediately on dry ice and maintained at −80°C. Sample analysis using high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) with electrospray ionization, working in multiple-reaction monitoring mode, was performed at GSK (Waters Acquity ultra-HPLC [UHPLC] connected to an API Sciex 4000 tandem quadrupole mass spectrometer). The lower limit of quantification was 0.05 μg/ml.
Data handling and analysis.
Exposure data are presented as the mean (±standard deviation) total concentration in blood (in micrograms per milliliter) from three animals per dose group. PK statistics were calculated nonparametrically. AUC was calculated using the trapezoid rule. The peak concentration (Cmax) was the highest observed concentration of drug in blood. Time above the MIC (T>MIC), the time over which blood concentrations remain above a specific MIC expressed either as hours or as a percentage over a 24-h period (%T>MIC), was calculated by fitting a linear interpolation model between observations and computing the percentage of the line that lies above the MIC. All parameters were calculated based on free (f) drug exposure profiles (fAUC, fAUC/MIC, fCmax/MIC, and %fT>MIC). Protein binding of GSK1322322 was determined in rat and mouse plasma by ultrafiltration at 5 μg/ml.
For repeat dosing, it was assumed that negligible residual concentrations remained at the time the next dose was administered. The outcome measure for comparison of treatments was the number of bacteria isolated from abscesses (log10 CFU/abscess) or lungs (log10 CFU/lungs). Mean log10 CFU from each group were correlated with predicted mean PK parameters using the following Emax model, where c was the PK parameter of interest (dose fractionation studies) or the dose (dose ranging studies), and Emin, Emax, K, and m were coefficients fitted from the data: log drop = Emin + {(Emax − Emin)/[1+ (c/K)m]} + error.
Data from dose fractionation studies with both isolates of each genus were analyzed separately and pooled. A combination of the Emax model and an intermediary model linking PK statistics with dose was used to correlate efficacy with free blood levels for AUC/MIC, Cmax/MIC, and %T>MIC. The coefficient of determination (R2) was used to estimate the variance that could be due to regression with each of the PK/PD indices. The static dose was defined as the dose required to prevent growth over 24 or 48 h; i.e., mean bacterial counts were equivalent to the 1-h NTC. Doses for 1-log10 reductions were defined as those producing mean bacterial counts equivalent to 1 log10 CFU less than the 1-h NTC.
RESULTS
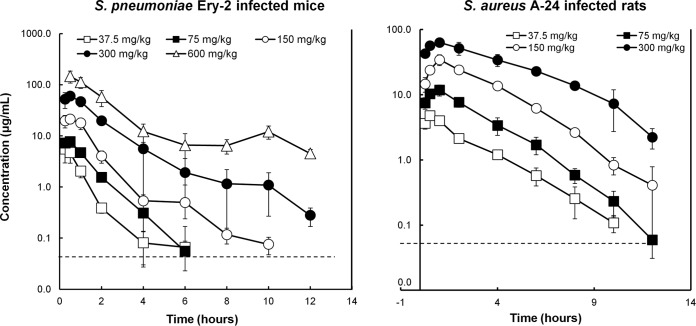
Pharmacokinetics of GSK1322322 in mice and rats.
The PK characteristics of GSK1322322 were determined following single oral dose administration of 37.5, 75, 150, 300, or 600 mg/kg to mice infected with S. pneumoniae Ery-2 (Fig. 1). Time to maximum concentration (Tmax) was observed by 30 min with all doses, with an average free Cmax ranging from 1.8 ± 0.8 to 46.1 ± 12.2 μg/ml (Table 1). Higher-than-dose-proportional increases were observed in the values for free drug AUC from 0 to 12 h (AUC0–12), which ranged from 1.8 ± 0.4 to 101.4 ± 28.6 μg · h/ml; i.e., a 16-fold dose increase resulted in a 56-fold increase in AUC (Table 1). A similar elimination half-life (t1/2) was observed with doses from 37.5 to 300 mg/kg (1.5 to 1.9 h), although it increased to 4 h at the highest dose tested. These PK parameters were also determined in rats infected with S. aureus A-24 after oral administration of single GSK1322322 doses of 37.5, 75, 150, or 300 mg/kg (Fig. 1). Blood concentrations of GSK1322322 increased in a dose-dependent manner across this dose range. The average free Cmax increased with the dose and ranged between 2.3 ± 0.2 and 30.3 ± 4.2 μg/ml, while the Tmax (0.5 to 1 h) was not affected by dose (Table 1). Slightly higher-than-dose-proportional increases were observed in the free drug AUC0–12 values, which ranged from 6.3 ± 0.3 to 150.5 ± 22.5 μg · h/ml. The t1/2 increased slightly with dose, oscillating between 1.5 and 2.3 h. Levels of protein binding of GSK1322322 were 52.6% and 68.8% in rat and mouse plasma, respectively.
FIG 1.
Total concentrations of GSK1322322 in blood after single-dose oral administration to mice with a lung infection caused by S. pneumoniae Ery-2 or to rats with an abscess infection caused by S. aureus A-24. Groups of three animals were used for all data points. The dashed lines represent the lower limits of quantification. The error bars represent standard deviations.
TABLE 1.
Blood pharmacokinetic parameters of GSK1322322 following single oral administration to mice (n = 3) with a lung infection caused by S. pneumoniae Ery-2 or to rats (n = 3) with an abscess infection caused by S. aureus A-24
| Infection (organism)a | Dose (mg/kg) | Free Cmaxb (μg/ml) | Tmax (h) | Free AUC0–12b (μg · h/ml) | t1/2b (h) |
|---|---|---|---|---|---|
| Mouse lung (S. pneumoniae Ery-2) | 37.5 | 1.8 ± 0.8 | 0.25 | 1.8 ± 0.4 | 1.8 ± 1.9 |
| 75 | 2.5 ± 0.3 | 0.5 | 3.6 ± 0.1 | 1.6 ± 0.2 | |
| 150 | 6.8 ± 1.1 | 0.5 | 10.9 ± 2.6 | 1.5 ± 0.3 | |
| 300 | 19.3 ± 2.7 | 0.5 | 36.8 ± 5.8 | 1.9 ± 0.3 | |
| 600 | 46.1 ± 12.2 | 0.5 | 101.4 ± 28.6 | 4 ± 0.9 | |
| Rat abscess (S. aureus A-24) | 37.5 | 2.3 ± 0.2 | 0.5 | 6.3 ± 0.3 | 1.8 ± 0.3 |
| 75 | 5.7 ± 1.0 | 1 | 18.1 ± 1.1 | 1.5 ± 0.1 | |
| 150 | 16.4 ± 3.0 | 1 | 58 ± 5.6 | 1.6 ± 0.3 | |
| 300 | 30.3 ± 4.2 | 1 | 150.5 ± 22.5 | 2.3 ± 0.4 |
The levels of protein binding were 68.8% with infection of mice with S. pneumoniae Ery-2 and 52.6% with infection of rats with S. aureus A-24.
Mean values ± standard deviations.
PK/PD index determinations.
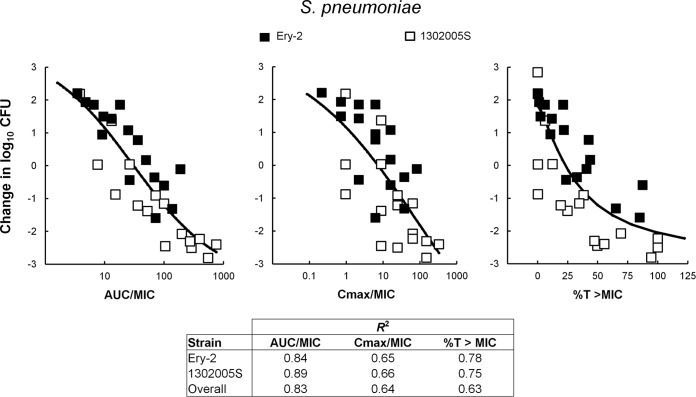
The relationship between the antibacterial effect of 20 different dosing regimens of GSK1322322 after 24 h of treatment with each of the PD indices, fAUC/MIC, fCmax/MIC, and %fT > MIC, was evaluated with two isolates of S. pneumoniae, Ery-2 and 1302005S, in a mouse RTI model. In this model, the organisms grew means of 4.28 and 2.84 log10 CFU/lung, respectively. Data for the two isolates were analyzed separately and combined (Fig. 2). Independently of how the analysis was performed, therapeutic outcome correlated best with the fAUC/MIC index (R2 = 0.84 and 0.89 for S. pneumoniae Ery-2 and 1302005S, respectively; R2 = 0.83 for the two isolates combined). Correlation of in vivo efficacy with the other two parameters was less strong (fCmax/MIC, R2 = 0.65 and 0.66 for S. pneumoniae Ery-2 and 1302005S, respectively, and 0.64 for both isolates combined; %fT>MIC, R2 = 0.78 and 0.75 for S. pneumoniae Ery-2 and 1302005S, respectively, and 0.63 for the two isolates combined). Interestingly, %fT>MIC correlated better with efficacy when each S. pneumoniae isolate was analyzed independently than with the pooled data.
FIG 2.
Relationship between GSK1322322 PK/PD indices and efficacy over 24 h against S. pneumoniae 1302005S and S. pneumoniae Ery-2 in a mouse lung model of infection. Free drug concentrations were used for index calculations. Efficacy is expressed as change in CFU/lung over time compared to that at the start of therapy. Each symbol represents the mean CFU/lung from 3 to 5 mice. The sigmoid line represents the best fit using a combination of the Emax model and an intermediary model linking PK statistics with dose. R2 is the coefficient of determination.
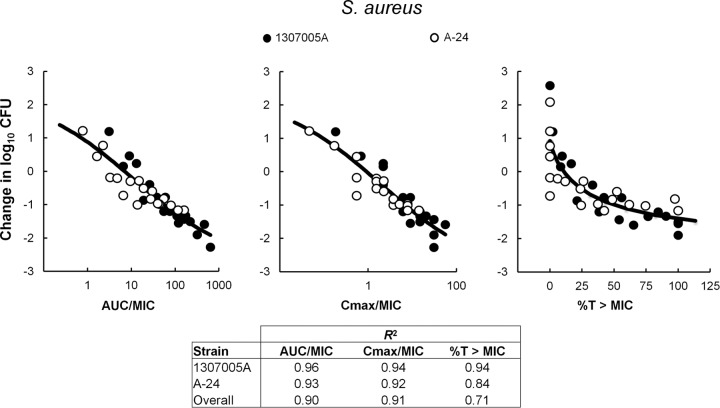
The relationship between the decrease in abscess bacterial counts at the end of 24 h of therapy with 18 different GSK1322322 dosing regimens, and each of the PD indices was also evaluated with two isolates of S. aureus, 1307005A and A-24, in a rat groin abscess model. The organisms grew means of 2.58 and 2.09 log10 CFU/abscess in this model, respectively. Data for the two isolates studied are presented in Fig. 3 and were analyzed both separately and combined. Free AUC/MIC and fCmax/MIC were shown to best correlate with efficacy when the two isolates were analyzed together, with R2 values of 0.9 and 0.91, respectively, versus a coefficient of determination of 0.71 for %fT>MIC (Fig. 3), although correlations for all three parameters were very similar when the two isolates were analyzed separately. This indicates that increasing the number of isolates used in the determination of the PK/PD index could help better discern between them.
FIG 3.
Relationship between GSK1322322 PK/PD indices and efficacy over 24 h against S. aureus 1307005A and S. aureus A-24 in a rat abscess model of infection. Free drug concentrations were used for index calculations. Efficacy is expressed as change in CFU/abscess over time compared to that at the start of therapy. Each symbol represents the mean CFU/abscess from five rats. The sigmoid line represents the best fit using a combination of the Emax model and an intermediary model linking PK statistics with dose. R2 is the coefficient of determination.
Dose-response studies with S. pneumoniae isolates.
To determine the magnitude of the PK/PD parameter necessary to achieve efficacy against S. pneumoniae, dose ranging studies were performed against 11 S. pneumoniae isolates, with GSK1322322 MICs of 0.25 to 2 μg/ml (Table 2), in the mouse RTI model. The compound was given every 8 h for 2 days at 4 different doses ranging from 18.75 to 300 mg/kg. The strains grew to a mean of 5.6 to 8.9 log10 CFU/lung in the vehicle-treated controls after 48 h. The mean maximal reduction in bacterial counts after treatment with GSK1322322 was 1.2 to 2.8 log10 CFU/lung (Table 2). The fAUC and fAUC/MIC ratio necessary to achieve a static effect or a 1-log10 kill were determined for each isolate and are summarized in Table 2. For individual line fits, the R2 values ranged from 86% to 100%. Free daily AUC and AUC/MIC values associated with stasis oscillated between 2.0 and 26.5 (mean, 11 ± 7.5; median, 8.2) and 1.5 to 26 (mean, 11 ± 7.9; median, 8.1), respectively (Table 2). For a 1-log10 reduction in bacterial counts, values ranged from 3.6 to 31 (mean, 17 ± 8.2; median, 19) and 3.4 to 60.3 (mean, 19.5 ± 17.8; median, 14.4), respectively (Table 2).
TABLE 2.
In vitro and in vivo activities of GSK1322322 against S. pneumoniae, H. influenzae, and S. aureus isolates
| Organism | MIC (μg/ml) | R2 for line fit (%) | Stasis |
1-log10 reduction |
Max killing (log10 reduction) | ||
|---|---|---|---|---|---|---|---|
| fAUC | fAUC/MIC | fAUC | fAUC/MIC | ||||
| S. pneumoniae | |||||||
| 10127 | 0.25 | 99.9 | 2.0 | 8.1 | 3.6 | 14.4 | 2.8 |
| 1316009S | 0.25 | 96.1 | 6.5 | 26.0 | 15.1 | 60.3 | 1.8 |
| ATCC 10813 | 0.5 | 100 | 8.4 | 16.9 | 22.2 | 44.5 | 1.2 |
| 1307007S | 1 | 95.8 | 16.1 | 16.1 | 20.1 | 20.1 | 2.7 |
| ATCC 6303 | 1 | 99.8 | 19.8 | 19.8 | 24.8 | 24.8 | 2.8 |
| 1629 | 2 | 100 | 15.8 | 7.9 | 21.2 | 10.6 | 2.4 |
| 298443 | 2 | 99.9 | 26.5 | 13.2 | 31.0 | 15.5 | 2.3 |
| 336808 | 2 | 86 | 8.2 | 4.2 | 19.0 | 9.5 | 2.6 |
| 338860 | 2 | 99.5 | 2.9 | 1.5 | 6.7 | 3.4 | 2.8 |
| 340449 | 2 | 94.6 | 8.0 | 4.0 | 14.9 | 7.4 | 2.1 |
| L11259 | 2 | 100 | 7.1 | 3.5 | 9.0 | 4.5 | 2.0 |
| Mean ± SD | NAd | NA | 11.0 ± 7.5 | 11.0 ± 7.9 | 17.0 ± 8.2 | 19.5 ± 17.8 | 2.3 ± 0.5 |
| Median | NA | NA | 8.2 | 8.1 | 19.0 | 14.4 | 2.4 |
| H. influenzae | |||||||
| 1998-100-126H | 1 | 99.7 | 7.3 | 7.3 | 13.4 | 13.4 | 2.9 |
| 503-008H | 1 | 99.8 | 7.1 | 7.1 | 13.9 | 13.9 | 2.7 |
| 08003H | 2 | 99.9 | 14.5 | 7.2 | 16.7 | 8.3 | 2.7 |
| H128 | 2 | 96.6 | 15.3 | 7.6 | 26.0 | 13.0 | 2.7 |
| 19001H | 4 | 91.9 | 14.8 | 3.7 | 37.3 | 9.3 | 3.0 |
| Mean ± SD | NA | NA | 11.8 ± 4.2 | 6.6 ± 1.6 | 21.5 ± 10.2 | 11.6 ± 2.6 | 2.8 ± 0.1 |
| Median | NA | NA | 14.5 | 7.2 | 16.7 | 13.0 | 2.7 |
| S. aureus | |||||||
| 1312007A | 0.5 | 96.6 | 3.9 | 7.8 | 9.0 | 17.9 | 1.8 |
| 1307005Aa | 0.5 | 98.9 | 3.8 | 7.6 | 7.7 | 15.5 | 2.0 |
| X32601a,b,c | 1 | 99.8 | 1.6 | 1.6 | 4.3 | 4.3 | 3.2 |
| 1309006a | 1 | 99.7 | 0.2 | 0.2 | 2.3 | 2.3 | 2.9 |
| PVL-2a,b | 2 | 99.2 | 1.9 | 0.9 | 5.5 | 2.8 | 3.4 |
| PK-2 | 2 | 97 | 2.3 | 1.1 | 14.2 | 7.1 | 2.5 |
| A-24 | 4 | 99.9 | 2.7 | 0.7 | 3.8 | 1.0 | 3.1 |
| T63256a,b,c | 4 | 99.9 | 2.0 | 0.5 | 7.1 | 1.8 | 3.4 |
| Mean ± SD | NA | NA | 2.3 ± 1.2 | 2.6 ± 3.2 | 6.7 ± 3.7 | 6.6 ± 6.6 | 2.8 ± 0.6 |
| Median | NA | NA | 2.1 | 1.0 | 6.3 | 3.5 | 3.0 |
Macrolide resistant.
Methicillin resistant.
Quinolone resistant.
NA, not applicable.
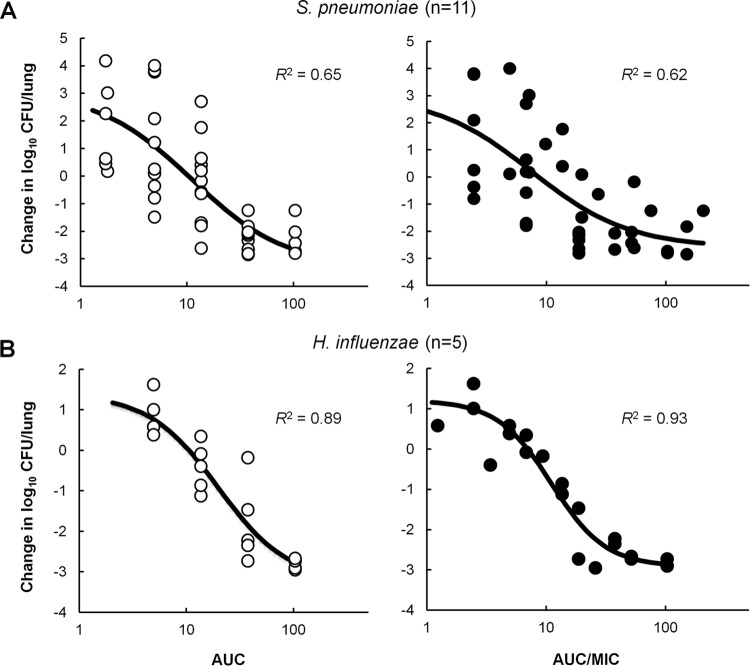
Dose-response studies were also performed against five H. influenzae isolates, with GSK1322322 MICs of 1 to 4 μg/ml, in a mouse RTI model with administration of the compound every 8 h for 2 days at 4 different doses ranging from 37.5 to 300 mg/kg. In the vehicle-treated control groups, organisms grew to a mean of 5.4 to 6.6 log10 CFU/lung after 48 h. GSK1322322 was efficacious against these isolates, with mean maximal reduction in bacterial counts ranging from 2.7 to 3.0 log10 CFU/lung (Table 2). Free daily AUC and AUC/MIC values associated with stasis and 1-log10 kill for the individual isolates are summarized in Table 2. The R2 values for individual line fits ranged from 91.9% to 99.9%. The fAUC necessary to achieve a static effect and a 1-log10 reduction in bacterial counts ranged from 7.1 to 15.3 (mean, 11.8 ± 4.2; median, 14.5) and 13.4 to 37.3 (mean, 21.5 ± 10.2; median, 16.7), respectively, whereas the fAUC/MIC ratios required to reach those endpoints were 3.7 to 7.6 (mean, 6.6 ± 1.6; median, 7.2) and 8.3 to 13.9 (mean, 11.6 ± 2.6; median, 13), respectively (Table 2).
The relationships between the daily free AUC or AUC/MIC and efficacy against both sets of isolates are shown graphically in Fig. 4. Although the exposure-response relationship was very good for individual S. pneumoniae isolates, the correlation was weaker when all data were considered together, irrespectively of relating efficacy to fAUC or to fAUC/MIC, with R2 of 0.65 and 0.62, respectively (Fig. 4). This was not the case for the combined set of H. influenzae isolates, where the correlation between efficacy and fAUC/MIC (R2 of 0.93) was stronger than that observed with fAUC (R2 of 0.89), and both relationships were greater than in the case of S. pneumoniae (Fig. 4).
FIG 4.
Relationship between GSK1322322 free drug 24-h AUC (open circles) or AUC/MIC (filled circles) and efficacy against 11 S. pneumoniae isolates (A) and 5 H. influenzae isolates (B). Each symbol represents the mean CFU/lung from 4 or 5 mice. The sigmoid line represents the best-fit curve using a combination of the Emax model and an intermediary model linking PK statistics with dose. R2 is the coefficient of determination.
Dose-response studies with S. aureus strains.
While both fAUC/MIC and fCmax/MIC correlated well with efficacy against S. aureus, AUC (with or without consideration of MIC) was chosen as the primary PK/PD driver because it demonstrated the most consistent correlation across the organisms tested. Dose ranging studies were performed in the rat groin abscess model of infection against eight S. aureus isolates with GSK1322322 MICs of 0.5 to 4 μg/ml (Table 2). All S. aureus isolates grew well in this nonneutropenic model, to a mean of 6.4 to 8.1 log10 CFU/abscess at 48 h in the vehicle-treated controls. GSK1322322 was administered twice daily for 2 days at 4 or 5 different doses ranging from 4.7 to 150 mg/kg, depending on the isolate. GSK1322322 demonstrated good efficacy against all isolates, with mean maximal reductions in bacterial counts of 1.8 to 3.4 log10 CFU/abscess (Table 2). The free daily AUC and AUC/MIC ratio determined for each isolate are summarized in Table 2. The R2 values for the individual line fits ranged from 96.6% to 99.9%. AUC values associated with stasis and a 1-log10 reduction in bacterial counts oscillated from 0.2 to 3.9 (mean, 2.3 ± 1.2; median, 2.1) and 2.3 to 14.2 (mean, 6.7 ± 3.7; median, 6.3), respectively. Interestingly, the fAUC required to achieve efficacy did not increase with increasing MIC. Consequently, more variability was observed among the fAUC/MIC values, which ranged from 0.2 to 7.8 (mean, 2.6 ± 3.2; median, 1) for a static effect and from 1 to 17.9 (mean, 6.6 ± 6.6; median, 3.5) for a 1-log10 kill (Table 2).
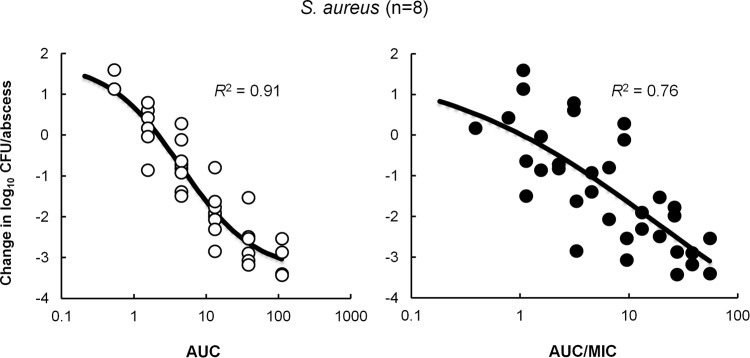
A comparison of the relationships between the daily fAUC or fAUC/MIC and efficacy against the eight S. aureus isolates showed that AUC correlates better with efficacy than AUC/MIC, with R2 of 0.91 and 0.76, respectively (Fig. 5). Recently, we have reported that GSK1322322 could prevent the in vitro growth of S. aureus strains for up to 6 h at concentrations 8- to 32-fold below the MICs and that this sub-MIC effect appeared more substantial on those strains at the higher end of the MIC spectrum (21). Therefore, we investigated the activity of GSK1322322 at concentrations below its MIC against the eight isolates used in this study. As shown in Table 3, a very strong growth inhibition could be observed against all isolates with GSK1322322 MICs of 2 and 4 μg/ml at concentrations of 1/16 its MIC. This could perhaps explain why increases in MIC did not seem to have an effect on the exposure necessary for efficacy against this organism.
FIG 5.
Relationship between GSK1322322 free drug 24-h AUC (open circles) or AUC/MIC (filled circles) and efficacy against eight S. aureus isolates. Each symbol represents the mean CFU/abscess from five rats. The sigmoid line represents the best-fit curve using a combination of the Emax model and an intermediary model linking PK statistics with dose. R2 is the coefficient of determination.
TABLE 3.
In vitro and in vivo characteristics of GSK1322322 against eight S. aureus isolates
| S. aureus isolate | MIC (μg/ml) | Fraction of MIC that inhibits 95% growth for 6 h | fAUC (stasis) |
|---|---|---|---|
| 1312007A | 0.5 | 1/2 | 3.9 |
| 1307005A | 0.5 | 1/4 | 3.8 |
| X32601 | 1 | 1/8 | 1.6 |
| 1309006 | 1 | 1/8 | 0.2 |
| PVL-2 | 2 | 1/16 | 1.9 |
| PK-2 | 2 | 1/16 | 2.3 |
| A-24 | 4 | 1/32 | 2.7 |
| T63256 | 4 | 1/16 | 2.0 |
DISCUSSION
In an era characterized by steady increases in bacterial resistance to most commonly used antibacterial agents combined with a scarcity of new molecules reaching phase III clinical trials, the development of new inhibitors of essential bacterial pathways with acceptable safety, tolerability, and efficacy properties for human use has become a pressing need. Antimicrobial PK/PD studies can help determine the therapeutic potential of a drug by integrating its PK properties, in vitro potency, and in vivo efficacy and can be used to design dosing regimens in humans that balance efficacy and safety, to avoid over- or underdosing and to minimize resistance development (22–27). GSK1322322 is a potent PDF inhibitor that has progressed to phase IIa clinical trials (14). These studies were done to characterize the PK/PD relationship of GSK1322322 against major respiratory and skin pathogens in order to guide the progression of this compound through clinical studies and to inform the development of susceptibility breakpoints.
Oral PK studies over a broad range of doses in two different rodent animal models showed slightly higher-than-dose-proportional pharmacokinetics, with doubling doses resulting in 3-fold increases in AUC. In rodents, the half-life was short (approximately 2 h) and protein binding moderate (50 to 70% bound). GSK1322322 is 66% protein bound in human plasma and has been shown to have rapid absorption (Tmax of 0.5 to 1 h) and nonlinear PK in single-dose oral first-time-in-human (FTIH) studies (12).
Analysis of the fractionated GSK1322322 dosing regimens evaluated in S. pneumoniae- and S. aureus-infected animals suggested that efficacy is dose dependent and independent of the dosing interval. Results with two S. pneumoniae isolates indicated that the daily fAUC/MIC is the most important index for efficacy, irrespective of analyzing the data separately or with both isolates combined. On the other hand, although regression analysis based on the coefficient of determination (R2) suggested a reasonable correlation between all three parameters and in vivo efficacy for each of the S. aureus isolates independently, when data from the two isolates were combined, daily fAUC/MIC and fCmax/MIC became the best indices for predicting efficacy of GSK1322322 against S. aureus. Clearly, increasing the number of isolates used in these dose fractionation studies can help to better differentiate among the indices, which are logically interrelated (28). As the fAUC/MIC ratio demonstrated the best correlation for S. pneumoniae and was equally as predictive as fCmax/MIC for S. aureus, fAUC/MIC was selected from the dose fractionation studies as the key PK/PD parameter for GSK1322322.
Dose ranging studies were undertaken to determine the magnitude of the fAUC/MIC necessary for efficacy of GSK1322322 against 11 S. pneumoniae, 5 H. influenzae, and 8 S. aureus isolates. A comparable analysis using fAUC as the PK/PD parameter was also performed with all three species. Strains with a wide range of GSK1322322 MICs (4- to 8-fold different) and several antibiotic resistance phenotypes (S. aureus isolates) were used for these studies. As expected, given the novel mechanism of action of this compound, GSK1322322 was efficacious against all isolates tested, irrespective of their resistance phenotypes. Moderate variability was observed for the combined dose-response curves of the different S. pneumoniae isolates (R2 of 0.62), and median daily fAUC/MIC values associated with stasis and a 1-log10 kill in this organism were 8.1 and 14.4, respectively. Similar fAUC/MIC values were obtained for H. influenzae, 7.2 and 13, respectively, although in this case the correlation between the magnitude of the PK/PD parameter and efficacy remained strong even when all isolates were combined (R2 of 0.93). No statistical differences were observed in the correlation of fAUC/MIC or fAUC and in vivo antibacterial effect of GSK1322322 in either of these two organisms, with similar mean/median magnitudes and coefficients of determination. PK/PD studies performed with other PDF inhibitors have also shown AUC/MIC to be the parameter that best correlated with efficacy (Craig and Andes, presented at the 41st ICAAC; Craig and Andes, presented at the 14th ECCMID). In fact, a median value of 31.4 was predicted to achieve a static effect with LBM415 against S. pneumoniae isolates in studies performed using a neutropenic mouse thigh infection model (Craig and Andes, presented at the 14th ECCMID). As 4-fold-lower magnitudes were necessary in immunocompetent animals (Craig and Andes, presented at the 14th ECCMID), the fAUC/MIC value would be similar to that obtained with GSK1322322 in the present studies. The results obtained with S. pneumoniae and H. influenzae support the concept, previously demonstrated with other antimicrobial agents, that the magnitude of the PK/PD index required for efficacy is generally similar for different organisms and among drugs within the same antimicrobial class (reviewed in reference 29).
Interestingly, the results obtained in the studies performed with S. aureus isolates were strikingly different in two major aspects. First, the fAUC/MIC necessary to achieve efficacy was much lower, with median values of 1 and 3.5 required for stasis and a 1-log10 kill, respectively (R2 of 0.76). Second, the data suggested that fAUC, rather than fAUC/MIC, was a better predictor of efficacy for this novel class agent against the wild-type S. aureus population, as a stronger correlation was obtained with this parameter (R2 of 0.91). Efficacious AUCs were similar against all isolates, independent of their GSK1322322 MIC, and median fAUCs of 2.1 and 6.3 μg · h/ml were required to achieve stasis and a 1-log10 reduction in bacterial counts, respectively. In vivo studies had already shown better-than-anticipated efficacy of PDF inhibitors against S. aureus, and further in vitro investigation unveiled a pronounced inhibitory effect of PDF inhibitors on the first 6 to 8 h of S. aureus growth at concentrations 8- to 32-fold below the MIC, a property that did not extend to S. pneumoniae or H. influenzae isolates (21). In fact, sub-MICs of GSK1322322 could inhibit ≥95% growth of the eight S. aureus strains used in this study, with the lowest fractions of MIC inhibiting growth of those strains with the highest MICs. This could explain the lack of a linear relationship between the drug exposure required for efficacy and the MIC of the isolate causing the infection, particularly as repeated in vivo administration of the compound (as done in these studies) would result in exertion of this effect at regular intervals. It has already been reported for S. aureus that subinhibitory concentrations of certain antimicrobials can suppress virulence factor production (30), including production of alpha-toxin (31, 32), increase susceptibility to phagocytosis (31), and modulate adherence to fibronectin (33, 34). Given that antibacterial agents are often present at subinhibitory concentrations during the normal course of antibiotic therapy, these types of effects should perhaps be taken into account when evaluating the magnitude of the PK/PD indices against certain organisms.
Much higher fAUC/MIC ratios (median of 57.8) were necessary to achieve efficacy with LBM415 against five S. aureus isolates (Craig and Andes, presented at the 14th ECCMID), although the use of a neutropenic model and higher inocula may have contributed to this discrepancy. We have observed that the presence of neutrophils can reduce the free GSK1322322 AUC necessary to achieve efficacy against S. aureus 6- to 9-fold (data not shown). A very strong impact has also been reported with tedizolid (35, 36), for which it has been speculated that the majority of the bacterial killing in normal animals is due to the effect of the drug mediated through neutrophils (35). In addition, increases in the magnitude of the daily AUC/MIC values required for stasis have been observed with S. aureus for daptomycin and linezolid (4-fold) and for vancomycin (10-fold) when the starting inocula increased from 105 to 107 CFU/thigh in the neutropenic model (37). No differences could be seen between fAUC/MIC and fAUC in the LBM415 study, but four of the five isolates used had LBM415 MICs of 1 μg/ml (Craig and Andes, presented at the 14th ECCMID). This stresses the importance of performing PK/PD studies with strains covering a wide range of MICs, as this phenomenon was noticeable only because strains with 8-fold differences in their GSK1322322 MICs were used.
In conclusion, these studies show that GSK1322322 has dose-dependent antimicrobial activity against several isolates of S. pneumoniae, H. influenzae, and S. aureus, including some resistant to other antibacterial agents. Free AUC/MIC is the PK/PD index that best predicts efficacy against H. influenzae and S. pneumoniae, with median values of ∼8 and 14 required to achieve stasis and 1-log10 killing, respectively. Initial phase I PK studies with repeat oral dosing of GSK1322322 suggest that these values would be achievable in the clinic (14). Of interest, fAUC appears to be a better parameter than fAUC/MIC for predicting efficacy against S. aureus, with magnitudes at least 4-fold lower than those necessary for the other two pathogens. This could be due to the potent effect that subinhibitory concentrations of this compound have on the early growth of S. aureus isolates, a phenomenon that seems more pronounced against those isolates with higher MICs. These findings highlight the importance of performing PK/PD studies with strains that encompass a wide range of MICs and suggest that PK/PD indices and magnitudes could potentially be different among bacterial species if the compound affects growth, and perhaps virulence, of a particular pathogen at sub-MIC levels.
ACKNOWLEDGMENTS
We thank Lynn McCloskey for performing the susceptibility testing of the strains used in the study, Deborah Butler for fruitful discussions and data on the potent sub-MIC effect of PDF inhibitors, and Yaping Tu for his contribution to the PK calculations.
REFERENCES
- 1.Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG, Antimicrobial Availability Task Force of the Infectious Diseases Society of America. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Bassetti M, Merelli M, Temperoni C, Astilean A. 2013. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob 12:22. doi: 10.1186/1476-0711-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klevens RM, Edwards JR, Richards CL Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 122:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RR, Hota B, Ahmad I, Scott RD II, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. 2009. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 6.Pucci MJ, Bush K. 2013. Investigational antimicrobial agents of 2013. Clin Microbiol Rev 26:792–821. doi: 10.1128/CMR.00033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livermore DM. 2011. Discovery research: the scientific challenge of finding new antibiotics. J Antimicrob Chemother 66:1941–1944. doi: 10.1093/jac/dkr262. [DOI] [PubMed] [Google Scholar]
- 8.Adams JM. 1968. On the release of the formyl group from nascent protein. J Mol Biol 33:571–589. doi: 10.1016/0022-2836(68)90307-0. [DOI] [PubMed] [Google Scholar]
- 9.Ball LA, Kaesberg P. 1973. Cleavage of the N-terminal formylmethionine residue from a bacteriophage coat protein in vitro. J Mol Biol 79:531–537. doi: 10.1016/0022-2836(73)90404-X. [DOI] [PubMed] [Google Scholar]
- 10.Livingston DM, Leder P. 1969. Deformylation and protein biosynthesis. Biochemistry 8:435–443. doi: 10.1021/bi00829a059. [DOI] [PubMed] [Google Scholar]
- 11.O'Dwyer K, Hackel M, Hightower S, Hoban D, Bouchillon S, Qin D, Aubart K, Zalacain M, Butler D. 2013. Comparative analysis of the antibacterial activity of a novel peptide deformylase inhibitor, GSK1322322. Antimicrob Agents Chemother 57:2333–2342. doi: 10.1128/AAC.02566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naderer OJ, Dumont E, Zhu J, Kurtinecz M, Jones LS. 2013. Single-dose safety, tolerability, and pharmacokinetics of the antibiotic GSK1322322, a novel peptide deformylase inhibitor. Antimicrob Agents Chemother 57:2005–2009. doi: 10.1128/AAC.01779-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naderer OJ, Jones LS, Zhu J, Kurtinecz M, Dumont E. 2013. Safety, tolerability, and pharmacokinetics of oral and intravenous administration of GSK1322322, a peptide deformylase inhibitor. J Clin Pharmacol 53:1168–1176. [DOI] [PubMed] [Google Scholar]
- 14.Naderer OJ, Dumont E, Zhu J, Kurtinecz M, Jones LS. 2013. Safety, tolerability and pharmacokinetics of repeat dosing of the antibiotic GSK1322322, a peptide deformylase inhibitor: a randomized placebo-controlled study. J Antimicrob Chemother 68:1901–1909. doi: 10.1093/jac/dkt097. [DOI] [PubMed] [Google Scholar]
- 15.Corey R, Naderer OJ, O'Riordan WD, Dumont E, Jones LS, Kurtinecz M, Zhu JZ. 2014. Safety, tolerability, and efficacy of GSK1322322 in the treatment of acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 58:6518–6527. doi: 10.1128/AAC.03360-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aubart K, Zalacain M. 2006. Peptide deformylase inhibitors. Prog Med Chem 44:109–143. doi: 10.1016/S0079-6468(05)44403-3. [DOI] [PubMed] [Google Scholar]
- 17.Lofland D, Difuntorum S, Waller A, Clements JM, Weaver MK, Karlowsky JA, Johnson K. 2004. In vitro antibacterial activity of the peptide deformylase inhibitor BB-83698. J Antimicrob Chemother 53:664–668. doi: 10.1093/jac/dkh129. [DOI] [PubMed] [Google Scholar]
- 18.Fritsche TR, Sader HS, Cleeland R, Jones RN. 2005. Comparative antimicrobial characterization of LBM415 (NVP PDF-713), a new peptide deformylase inhibitor of clinical importance. Antimicrob Agents Chemother 49:1468–1476. doi: 10.1128/AAC.49.4.1468-1476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolan P, Sun H, Macleod C, Bracken K, Evans TG. 2011. Pharmacokinetics and unexpected safety issues of LBM415, a novel oral peptide deformylase inhibitor. Clin Pharmacol Ther 90:256–262. doi: 10.1038/clpt.2011.101. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 9th ed Approved standard M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Butler D, Chen D, O'Dwyer K, Lewandowski T, Aubart K, Zalacain M. 2014. Potent sub-MIC effect of GSK1322322 and other peptide deformylase inhibitors on in vitro growth of Staphylococcus aureus. Antimicrob Agents Chemother 58:290–296. doi: 10.1128/AAC.01292-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19:261–268. doi: 10.1016/S0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 23.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 24.Owens RC Jr, Ambrose PG. 2007. Antimicrobial stewardship and the role of pharmacokinetics-pharmacodynamics in the modern antibiotic era. Diagn Microbiol Infect Dis 57:77S–83S. doi: 10.1016/j.diagmicrobio.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Mouton JW, Ambrose PG, Canton R, Drusano GL, Harbarth S, MacGowan A, Theuretzbacher U, Turnidge J. 2011. Conserving antibiotics for the future: new ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist Updat 14:107–117. doi: 10.1016/j.drup.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10; quiz, 11–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 27.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat Rev Microbiol 2:289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 28.Andes D, Craig WA. 2006. Pharmacodynamics of a new streptogramin, XRP 2868, in murine thigh and lung infection models. Antimicrob Agents Chemother 50:243–249. doi: 10.1128/AAC.50.1.243-249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig WA. 2014. Introduction to pharmacodynamics. In Da Vinks M. (ed), Fundamentals of antimicrobial pharmacokinetics and pharmacodynamics. Springer, New York, NY. [Google Scholar]
- 30.Yoshizawa S, Tateda K, Saga T, Ishii Y, Yamaguchi K. 2012. Virulence-suppressing effects of linezolid on methicillin-resistant Staphylococcus aureus: possible contribution to early defervescence. Antimicrob Agents Chemother 56:1744–1748. doi: 10.1128/AAC.05430-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gemmell CG, Ford CW. 2002. Virulence factor expression by Gram-positive cocci exposed to subinhibitory concentrations of linezolid. J Antimicrob Chemother 50:665–672. doi: 10.1093/jac/dkf192. [DOI] [PubMed] [Google Scholar]
- 32.Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, Hacker J. 1998. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 42:2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proctor RA, Olbrantz PJ, Mosher DF. 1983. Subinhibitory concentrations of antibiotics alter fibronectin binding to Staphylococcus aureus. Antimicrob Agents Chemother 24:823–826. doi: 10.1128/AAC.24.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasigade JP, Moulay A, Lhoste Y, Tristan A, Bes M, Vandenesch F, Etienne J, Lina G, Laurent F, Dumitrescu O. 2011. Impact of sub-inhibitory antibiotics on fibronectin-mediated host cell adhesion and invasion by Staphylococcus aureus. BMC Microbiol 11:263. doi: 10.1186/1471-2180-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drusano GL, Liu W, Kulawy R, Louie A. 2011. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob Agents Chemother 55:5300–5305. doi: 10.1128/AAC.00502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lodise TP, Drusano GL. 2014. Use of pharmacokinetic/pharmacodynamic systems analyses to inform dose selection of tedizolid phosphate. Clin Infect Dis 58(Suppl 1):S28–S34. doi: 10.1093/cid/cit615. [DOI] [PubMed] [Google Scholar]
- 37.Lee DG, Murakami Y, Andes DR, Craig WA. 2013. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 10(5) and 10(7) CFU injected into opposite thighs of neutropenic mice. Antimicrob Agents Chemother 57:1434–1441. doi: 10.1128/AAC.00362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]