Abstract
Aspergillus fumigatus is the main mold causing invasive fungal infection that shows high mortality rates. Therapeutic failure and the increase in drug resistance make it necessary to explore alternative treatments for this infection. We have evaluated the efficacy of amphotericin B at 0.8 mg/kg or 0.3 mg/kg of body weight combined with 40 mg/kg of posaconazole against three A. fumigatus isolates in a murine model of disseminated infection. The combination of the polyene and the azole led to a greater increase in survival and a significantly greater reduction in tissue burden than monotherapies.
INTRODUCTION
Aspergillus fumigatus is the most common mold causing invasive fungal infection (IFI) in immunocompromised patients (1), especially in those with hematological malignancies, with high mortality rates (2–4). Voriconazole (VRC) is the first-line therapy for the treatment of aspergillosis, but in patients with infections that are refractory to this drug, therapy options include other azoles such as itraconazole or posaconazole (PSC), lipid formulations of amphotericin B (LAMB), or echinocandins (5). Because of the relatively limited efficacy of the current antifungal treatments, an exploration of alternative strategies against this difficult-to-treat infection is crucial. Combinations of antifungal agents are not common therapies but might be good alternatives for infections by resistant organisms or when the standard treatments fail (6–11). Synergistic interactions of two drugs with different targets on the fungal cell can be more effective than each drug working alone. In addition, combined therapies can allow lower doses to be administered, with lower toxicity, faster cure, and probably lower costs. Since the efficacies of different antifungal combinations have been demonstrated by several studies in patients with aspergillosis (6, 7, 12), we were interested in evaluating the in vivo efficacy of the combination of amphotericin B (AMB) plus PSC against A. fumigatus. This combination had already been tested in a murine model of invasive aspergillosis caused by Aspergillus flavus (13), although no improvement over the PSC monotherapy was observed. Another study demonstrated the efficacy of suboptimal doses of VRC plus anidulafungin in a murine model of A. fumigatus infection (14), suggesting that combined therapies might have an important role as alternative treatments against systemic aspergillosis, allowing a reduction of the doses administered. One of the isolates tested in the present study (FMR 10528) had already been used in previous studies but showed a poor in vivo response to VRC when administered at 25 mg/kg of body weight despite having a low MIC (15, 16). The goals of this study were (i) to evaluate the efficacy of the combination of AMB plus PSC against isolates of A. fumigatus in a murine model of disseminated aspergillosis, comparing the results with those of the corresponding monotherapies and VRC, (ii) to investigate the presence of CYP51A gene mutations that might explain the poor in vivo response of such an isolate, and (iii) to perform adaptation experiments that can assess the ability of this isolate to develop azole resistance.
MATERIALS AND METHODS
Fungal isolates.
Two clinical isolates (FMR 10528 and FMR 13142) and one environmental isolate (FMR 7739) of A. fumigatus were used in this study. Fungi were grown on potato dextrose agar (PDA). The MICs were previously determined in triplicate following the CLSI guidelines (17). The MICs of AMB and PSC were 2 μg/ml and 0.5 μg/ml, respectively, for the strain FMR 7739 and 1 μg/ml and 0.25 μg/ml, respectively, for the strains FMR 10528 and FMR 13142. The MIC of VRC was 0.25 μg/ml for the three strains.
Inocula for both in vitro drug interaction testing and in vivo assays were prepared from 5-day-old cultures incubated at 37°C. Conidia were harvested with a sterile pipette by flooding the plates with sterile saline containing 0.025% Tween 20. The suspensions were adjusted to the desired concentrations by hemocytometer counting, and the viability was assessed by placing 10-fold dilutions on PDA plates.
In vitro antifungal interaction testing.
The interaction testing was carried out using a two-dimensional checkerboard microdilution method with 2-fold serial dilutions of AMB and PSC, ranging from 0.12 to 8 μg/ml and from 0.002 to 1 μg/ml, respectively. Readings were taken 48 h after incubation at 35°C using an inverted mirror and the MIC0 (100% growth inhibition) as the endpoint criterion. The fractional inhibitory concentration index (FICI) was used to classify drug interactions, which were defined as synergistic if the FICI was ≤0.5, antagonistic if the FICI was >4, and indifferent if the FICI was >0.5 but ≤4 (18). Tests were carried out in duplicate.
Infection.
For the in vivo studies, male OF-1 mice (Charles River, Criffa S.A., Barcelona, Spain) weighing approximately 30 g were used. Animals were housed under standard conditions with water and food ad libitum. All animal care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare and Ethics Committee.
One day prior to the infection, the animals were immunosuppressed by an intraperitoneal (i.p.) injection of 200 mg/kg of cyclophosphamide (Genoxal; Laboratories Funk S.A., Barcelona, Spain) and a single intravenous (i.v.) injection of 150 mg/kg of 5-fluorouracil (Fluorouracilo; Ferrer Farma S.A., Barcelona, Spain) (19).
Groups of 16 animals, 8 for survival and 8 for the fungal load study and for determining drug serum levels, randomly chosen, were challenged i.v. via the lateral vein with 1 × 103 CFU/animal of the strain FMR 13142 or 1 × 104 CFU of the strains FMR 7739 and FMR 10528. Inoculum sizes were adjusted for each strain in order to obtain a similar degree of infection in all the cases, causing 100% of the animals to die within 9 days. After challenge, the mice were checked daily for 30 days.
Treatments.
All treatments started 1 day after infection, and the animals were treated daily for 7 days. The treatments consisted of AMB at 0.8 mg/kg (amphotericin B deoxycholate; Xalabarder Pharmacy, Barcelona, Spain) administered i.v. (20), PSC (Noxafil; Schering-Plough Ltd., Hertfordshire, United Kingdom) at 20 mg/kg given orally by gavage (p.o.) twice a day (BID) (21), or VRC at 25 mg/kg (Vfend; Pfizer S.A., Madrid, Spain) administered p.o. (22). The combined therapies consisted of AMB plus PSC at the given doses, with the exception of animals infected with the strain FMR 7739, which received AMB 0.3 mg/kg in the combination due to the good efficacy obtained after the monotherapy with AMB at 0.8 mg/kg. From 2 days before the infection, animals receiving VRC were given 50% grapefruit juice instead of water. The control animals received no antifungal treatment. In order to prevent bacterial infections, mice received subcutaneous injections of 5 mg/kg/day of ceftazidime.
Tissue burden and bioassay.
The mice included in the tissue burden study (n = 8) were euthanized on day 6 postinfection, in order to compare the fungal load with that of the control group. Five animals from each group were also used to determine drug serum concentrations by bioassay. For the bioassay, 2 h after the 6th dose, the animals were anesthetized by inhalation of isoflurane, and approximately 1 ml of blood was extracted by cardiac puncture, the serum being obtained by blood centrifugation. The concentrations of PSC, VRC, and AMB from serum samples were determined by bioassay, using Candida parapsilosis ATCC 22019, as previously described (23). After blood extraction, animals were euthanized by cervical dislocation, and the kidneys and lungs were aseptically removed, weighed, and homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were placed on PDA plates and incubated for 48 h at 37°C to determine CFU per gram of tissue.
Amplification and sequencing of CYP51A.
Genomic DNA of each A. fumigatus isolate was extracted from 3-day-old cultures (24). PCRs were carried out in a 25-μl volume, containing 10.5 μl of water, 12.5 μl of Taq Kapa 2G Robust Ready (Kapa Biosystems Inc., Wilmington, MA, USA), 0.5 μl of each primer (10 μM), and 50 ng of genomic DNA. Table 1 lists the primers used for amplification and sequencing of the CYP51A gene. The amplification took place in a thermal cycler for 1 cycle of 5 min at 95°C, 35 cycles of 1 min at 95°C, 1 min at 58°C, and 2 min at 72°C, followed by 1 final cycle of 10 min at 72°C. The PCR products were analyzed by electrophoresis on 1% agarose gel.
TABLE 1.
Primers used for the amplification of CYP51A of A. fumigatus
| Primer | Sequence |
|---|---|
| Cyp51 AF F1 | 5′-CACCCTCCCTGTGTCTCCT-3′ |
| Cyp51 AF R1 | 5′-CCGATCACACCAAATCCTTT-3′ |
| Cyp51 AF S1 | 5′-CTCAGCCGTGAGTTTGGAAC-3′ |
| Cyp51 AF S2 | 5′-CCTCACAGCCAAAAGTCCTC-3′ |
| Cyp51 AF S3 | 5′-ATTGTCCCAATTCCAAGCTG-3′ |
| Cyp51 AF S4 | 5′-TCTCTGCACGCAAAGAA-3′ |
The CYP51A gene sequences of 10 known wild-type A. fumigatus isolates were used to generate a consensus sequence (data not shown). This sequence served as a negative control (CYP51A without mutations) and was used for comparison with the sequences of the three isolates tested. Lasergene SeqMan (DNAStar, Madison, WI, USA) was used for generating the consensus wild-type CYP51A sequence and for checking the quality of the sequences, and Mega 6 (25) was used for alignment and gene comparison.
In vitro and in vivo adaptation experiments.
An in vitro adaptation experiment (26, 27) was carried out to investigate the poor in vivo efficacy of azoles against the strains FMR 7739 and FMR 10528, despite their low MICs. Each strain was passaged 3 times at 1-week intervals on PDA containing PSC or VRC at a concentration corresponding to half of the MIC. The susceptibilities to PSC and VRC of both strains before antifungal exposure and of their 3 subcultures exposed to azoles were determined by a microdilution method (17). The assay was carried out in duplicate. In addition, A. fumigatus strains were recovered from the lungs and kidneys of those animals infected and treated for 6 days with the azole monotherapies, and the MICs were determined.
Statistical analysis.
The mean survival time (MST) was estimated by the Kaplan-Meier method and compared among groups using the log rank test. The tissue burdens from the control and treated groups were compared using the Mann-Whitney U test. All statistical analyses used GraphPad Prism 6.0 for Windows. P values of ≤0.05 were considered statistically significant.
Nucleotide sequence accession numbers.
The CYP51 sequences from FMR 7739, FMR 10528, and FMR 13142 have been deposited in the NCBI database and are available under GenBank accession numbers KT070084, KT070085, and KT070086, respectively.
RESULTS
In vitro interaction testing.
The effects of the in vitro interaction between AMB and PSC were indifferent for the three strains, with FICIs ranging from 0.56 to 0.73 (data not shown).
In vivo studies.
Untreated animals began to die on day 4 to 5 postinfection, and on day 10 no animals were alive. For the strain FMR 7739, AMB at 0.8 mg/kg significantly increased the survival of the animals compared to that of the controls (P < 0.0001) and of the other monotherapy groups (P ≤ 0.0085), all animals being alive at the end of the experiment (day 30 postinfection). Therefore, for the combined therapy, a suboptimal dose of AMB was tested, i.e., AMB at 0.3 mg/kg, which also prolonged the survival significantly with respect to that of the control group (P = 0.0384). In contrast, neither PSC nor VRC by itself was able to increase survival (P = 0.3926 and P = 0.227, respectively). The combination significantly increased survival compared to that of the animals treated with AMB at 0.3 mg/kg, PSC, or VRC (P ≤ 0.0313).
With the strain FMR 10528, an increase in survival was only observed with AMB at 0.8 mg/kg and the combination (P ≤ 0.029). In addition, the combined therapy worked better than the monotherapies (P ≤ 0.0418). In the case of the strain FMR 13142, all of the therapies, including the combination, significantly increased the survival of the animals (P ≤ 0.0269) (Fig. 1).
FIG 1.
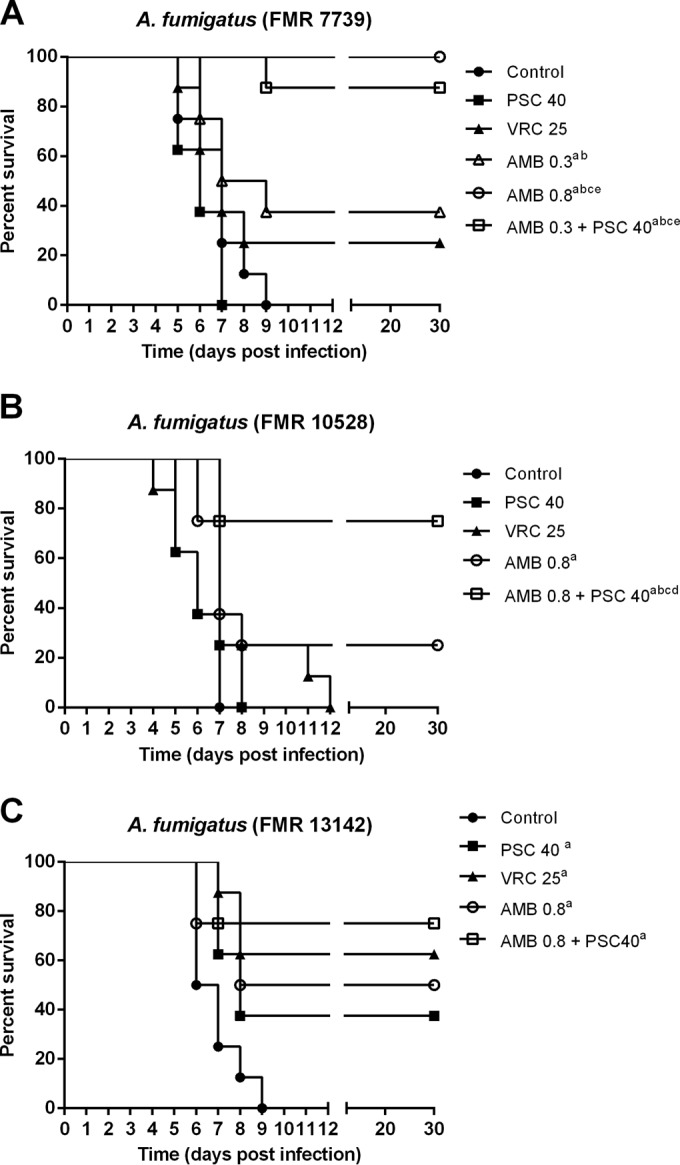
Cumulative mortality of immunosuppressed mice infected with A. fumigatus strains FMR 7739 (A), FMR 10528 (B), and FMR 13142 (C). AMB 0.8, amphotericin B at 0.8 mg/kg once a day (QD); PSC 40, posaconazole at 20 mg/kg BID; VRC 25, voriconazole at 25 mg/kg QD. aP ≤ 0.05 versus control; bP < 0.05 versus PSC 40; cP < 0.05 versus VRC 25; dP < 0.05 versus AMB 0.8; eP < 0.05 versus AMB 0.3.
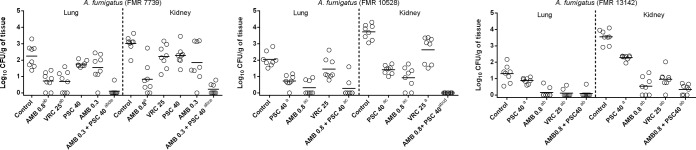
In the fungal load study, the combination of AMB plus PSC showed efficacy in reducing the numbers of CFU in the two organs and in all strains studied. This was even better than the monotherapies with either azole in all strains, with the exception of the lungs from the animals infected with the strain FMR 13142, where the combination equaled the efficacy of VRC. The combination also improved the efficacy of AMB 0.8 in the lungs of the animals infected with the strain FMR 7739 and in the kidneys of those infected with FMR 10528.
AMB at 0.8 mg/kg and VRC were able to reduce the tissue burdens in the two organs from animals infected with each of the three strains (P ≤ 0.0002 and P ≤ 0.0298, respectively), with the only exception being VRC against the strain FMR 10528 in lungs (P = 0.1044). PSC reduced the fungal burden of the kidneys of the animals infected with each of the three strains (P ≤ 0.0463) and in the lungs of the animals infected with the strain FMR 10528 (P = 0.0002) (Fig. 2).
FIG 2.
Effect of antifungal treatments on colony counts of A. fumigatus strains FMR 7739 (A), FMR 10528 (B), or FMR 13142 (C) in lungs and kidneys of immunosuppressed mice. AMB 0.8, amphotericin B at 0.8 mg/kg once a day (QD); PSC 40, posaconazole at 20 mg/kg BID; VRC 25, voriconazole at 25 mg/kg QD. Horizontal lines indicate median values. aP < 0.05 versus control; bP < 0.05 versus PSC 40; cP < 0.05 versus VRC 25; dP < 0.05 versus AMB 0.8; eP < 0.05 versus AMB 0.3.
The serum concentrations of AMB at 0.8 mg/kg, PSC at 40 mg/kg, and VRC at 25 mg/kg were higher than the MICs, with values of 4.28 ± 0.31, 6.34 ± 0.90, and 9.99 ± 0.71 μg/ml, respectively.
Amplification and sequencing of CYP51A.
No mutations were found in the CYP51A gene sequences of the three strains tested, such sequences being identical to that of the wild-type consensus sequence.
In vitro and in vivo adaptation experiments.
With two of the strains grown on PDA plates containing PSC or VRC, no increases in MICs were observed. Only the strain FMR 10528 showed the VRC MIC two dilutions higher than before drug exposure. However, important morphological changes were observed. The colony growth rate decreased in the three strains tested. There was also a noticeable reduction of sporulation and change in the pigmentation of the colonies of the strain FMR 10528, from green to pale green.
The MICs of VRC and PSC against the isolates recovered from treated animals were the same or one dilution higher than that obtained originally. These isolates also showed a reduction in the growth rate and sporulation and also a change in the colony color.
DISCUSSION
Due to the important increase in the azole resistance of Aspergillus and the associated therapeutic failure, finding alternatives to the current therapies is crucial. In the present study, we tested the combination of AMB plus PSC in a neutropenic model of disseminated aspergillosis, using three A. fumigatus strains. In previous studies conducted in animal models, VRC at 25 mg/kg demonstrated poor efficacy against systemic aspergillosis by one of the strains included in the present study, i.e., strain FMR 10528 (15, 16). Now, we can corroborate the lack of efficacy of VRC administered at 25 mg/kg, and, in addition, we have also found a therapeutic failure of PSC administered at 40 mg/kg against two of the three strains assayed. It is worth mentioning that no correlation was found between the two parameters of efficacy used, i.e., survival and fungal burden. Azoles were not able to improve survival in those animals infected with the strains FMR 7739 or FMR 10528, but there was a reduction in fungal burden in at least one organ.
PSC is known to show good efficacy against A. fumigatus infections, which decreases when the fungus harbors mutations in the CYP51A gene (28, 29); however, in the present study, no mutations in the CYP51A gene were found. In order to explain the lack of efficacy, azole adaptation assays were carried out to determine the ability of the strains to develop resistance to the drugs, as continuous contact with a compound is known to be able to result in tolerance and development of resistance to it, as seems to occur with A. fumigatus and azoles (30–32).
In a previous study, Salas et al. (15) demonstrated that A. fumigatus strains varied greatly in their in vivo responses to VRC, particularly those isolates with MIC values of ≥0.25 μg/ml. This has been observed in the present study for both VRC and PSC, suggesting a strain-to-strain variability. In the present study, all three strains show MICs for VRC of 0.25 μg/ml and for PSC of 0.25 to 0.5 μg/ml, all values being below the epidemiological cutoff values (ECVs) (33). Different studies suggest that MICs and ECVs are useful predictors of azole efficacy in vivo (15, 29). However, other studies suggest that the pharmacokinetics (PK) and particularly the determination of the area under the concentration-time curve (AUC)/MIC correlate better with efficacy than MICs, the AUC/MIC values being >25 and >100 for VRC and PSC, respectively, predictors of successful outcome (34, 35). Although the PK parameters have not been determined in the present study, previous studies have demonstrated the efficacy of VRC and PSC administered at 25 mg/kg and 40 mg/kg, respectively, against Aspergillus spp. in murine models (14, 15, 22, 28, 29, 36) and the fact that multiple dosing of VRC at 20 mg/kg in mice resulted in an AUC0–24 of 58.1 h · mg/liter, leading to efficacy against invasive aspergillosis by a strain with a MIC of 0.25 μg/ml (14). Other studies testing PSC and VRC doses similar to those used in our experiment have shown AUC values indicative of correct exposure (28, 37–39).
Little information is available on the in vitro interaction of AMB and PSC against A. fumigatus. Perkhofer et al. (40) reported in vitro indifference of this combination against 88% of the Aspergillus isolates studied. Although we found indifferences in the in vitro studies, such a combination has shown significant in vivo efficacy. In vitro results are not always found to be predictive of synergistic effects in animal models or in the clinical setting, which is why other authors have reviewed the categorization of drug interactions, i.e., synergy, indifference, or antagonism, based on the FICI. Meletiadis et al. found a better correlation between the FICI and the outcome if a FICI of <1 was considered indicative of synergy (41) instead of a FICI of ≤0.5. Our results show a good correlation between the in vitro and the in vivo data when we considered the synergistic effects at a FICI of <1; however, more studies are necessary to extrapolate the meaning of the FICI to the outcome.
This study has some limitations. In the combined therapy, AMB was administered at 0.8 mg/kg except against the infection by one strain (FMR 7739), for which AMB was used at 0.3 mg/kg due to the good efficacy it showed as a monotherapy at 0.8 mg/kg. Moreover, we used different inoculum sizes for each strain in order to obtain the same degree of infection.
Overall, our results demonstrate that the combination AMB plus PSC shows efficacy against A. fumigatus, improving the efficacy of the monotherapies with azoles and AMB in some cases, and might represent an alternative when the recommended treatment fails, with a possible reduction of the dose and, consequently, the toxicity and the cost. More studies testing more strains are needed to determine more accurately the role of such a combination in the treatment of invasive aspergillosis.
REFERENCES
- 1.Mayr A, Lass-Flörl C. 2011. Epidemiology and antifungal resistance in invasive aspergillosis according to primary disease: review of the literature. Eur J Med Res 16:153–157. doi: 10.1186/2047-783X-16-4-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 48:265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 3.Parody R, Martino R, Sánchez F, Subirá M, Hidalgo A, Sierra J. 2009. Predicting survival in adults with invasive aspergillosis during therapy for hematological malignancies or after hematopoietic stem cell transplantation: single-center analysis and validation of the Seattle, French, and Strasbourg prognostic indexes. Am J Hematol 84:571–578. doi: 10.1002/ajh.21488. [DOI] [PubMed] [Google Scholar]
- 4.Ramos ER, Jiang Y, Hachem R, Kassis C, Kontoyiannis DP, Raad I. 2011. Outcome analysis of invasive aspergillosis in hematologic malignancy and hematopoietic stem cell transplant patients: the role of novel antimold azoles. Oncologist 16:1049–1060. doi: 10.1634/theoncologist.2010-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik J-A, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 6.Maertens J, Glasmacher A, Herbrecht R, Thiebaut A, Cordonnier C, Segal BH, Killar J, Taylor A, Kartsonis N, Patterson TF. 2006. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer 107:2888–2897. doi: 10.1002/cncr.22348. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Limaye AP, Forrest G, Safdar N, Muñoz P, Pursell K, Houston S, Rosso F, Montoya JG, Patton P, Del Busto R, Aguado JM, Fisher RA, Klintmalm GB, Miller R, Wagener MM, Lewis RE, Kontoyiannis DP, Husain S. 2006. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation 81:320–326. doi: 10.1097/01.tp.0000202421.94822.f7. [DOI] [PubMed] [Google Scholar]
- 8.Liu P, Mould DR. 2014. Population pharmacokinetic-pharmacodynamic analysis of voriconazole and anidulafungin in adult patients with invasive aspergillosis. Antimicrob Agents Chemother 58:4727–4736. doi: 10.1128/AAC.02809-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raad II, Zakhem EA, Helou EG, Jiang Y, Kontoyiannis DP, Hachem R. 2015. Clinical experience of the use of voriconazole, caspofungin or the combination in primary and salvage therapy of invasive aspergillosis in haematological malignancies. Int J Antimicrob Agents 45:283–288. doi: 10.1016/j.ijantimicag.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ. 2015. Combination antifungal therapy for invasive aspergillosis. Ann Intern Med 162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa T, Matsumoto K, Tsujimoto K, Hishiya N, Yamada Y, Uno K, Kasahara K, Maeda K, Nario K, Mikasa K, Morita K. 2015. Chronic invasive sinus and intracerebral aspergillosis controlled by combination therapy with micafungin and a daily dose of 400 mg itraconazole oral solution. J Infect Chemother 21:134–137. doi: 10.1016/j.jiac.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Marr KA, Boeckh M, Carter RA, Kim HW, Corey L. 2004. Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis 39:797–802. doi: 10.1086/423380. [DOI] [PubMed] [Google Scholar]
- 13.Najvar LK, Cacciapuoti A, Hernandez S, Halpern J, Bocanegra R, Gurnani M, Menzel F, Loebenberg D, Graybill JR. 2004. Activity of posaconazole combined with amphotericin B against Aspergillus flavus infection in mice: comparative studies in two laboratories. Microbiology 48:758–764. doi: 10.1128/AAC.48.3.758-764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seyedmousavi S, Brüggemann RJM, Melchers WJG, Rijs AJMM, Verweij PE, Mouton JW. 2013. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. J Antimicrob Chemother 68:385–393. doi: 10.1093/jac/dks402. [DOI] [PubMed] [Google Scholar]
- 15.Salas V, Javier Pastor F, Calvo E, Sutton DA, Fothergill AW, Guarro J. 2013. Evaluation of the in vitro activity of voriconazole as predictive of in vivo outcome in a murine Aspergillus fumigatus infection model. Antimicrob Agents Chemother 57:1404–1408. doi: 10.1128/AAC.01331-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoval-Denis M, Pastor FJ, Capilla J, Guarro J. 2013. Efficacy of amphotericin B at suboptimal dose combined with voriconazole in a murine model of Aspergillus fumigatus infection with poor in vivo response to the azole. Antimicrob Agents Chemother 57:4540–4542. doi: 10.1128/AAC.00563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 19.Ortoneda M, Capilla J, Pastor FJ, Serena C, Guarro J. 2004. Interaction of granulocyte colony-stimulating factor and high doses of liposomal amphotericin B in the treatment of systemic murine scedosporiosis. Diagn Microbiol Infect Dis 50:247–251. doi: 10.1016/j.diagmicrobio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Graybill JR. 2000. The role of murine models in the development of antifungal therapy for systemic mycoses. Drug Resist Updat 3:364–383. doi: 10.1054/drup.2000.0171. [DOI] [PubMed] [Google Scholar]
- 21.Salas V, Pastor FJ, Rodríguez MM, Calvo E, Mayayo E, Guarro J. 2011. In vitro activity and in vivo efficacy of posaconazole in treatment of murine infections by different isolates of the Aspergillus terreus complex. Antimicrob Agents Chemother 55:676–679. doi: 10.1128/AAC.00736-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warn PA, Sharp A, Mosquera J, Spickermann J, Schmitt-Hoffmann A, Heep M, Denning DW. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J Antimicrob Chemother 58:1198–1207. doi: 10.1093/jac/dkl396. [DOI] [PubMed] [Google Scholar]
- 23.Sugar AM, Liu XP. 2001. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob Agents Chemother 45:601–604. doi: 10.1128/AAC.45.2.601-604.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lackner M, Najafzadeh MJ, Sun J, Lu Q, de Hoog GS. 2012. Rapid identification of Pseudallescheria and Scedosporium strains by using rolling circle amplification. Appl Environ Microbiol 78:126–133. doi: 10.1128/AEM.05280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faria-Ramos I, Farinha S, Neves-Maia J, Tavares P, Miranda IM, Estevinho LM, Pina-Vaz C, Rodrigues AG. 2014. Development of cross-resistance by Aspergillus fumigatus to clinical azoles following exposure to prochloraz, an agricultural azole. BMC Microbiol 14:155. doi: 10.1186/1471-2180-14-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hryncewicz-Gwóźdź A, Kalinowska K, Plomer-Niezgoda E, Bielecki J, Jagielski T. 2013. Increase in resistance to fluconazole and itraconazole in Trichophyton rubrum clinical isolates by sequential passages in vitro under drug pressure. Mycopathologia 176:49–55. doi: 10.1007/s11046-013-9655-y. [DOI] [PubMed] [Google Scholar]
- 28.Mavridou E, Brüggemann RJM, Melchers WJG, Mouton JW, Verweij PE. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob Agents Chemother 54:860–865. doi: 10.1128/AAC.00931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepak AJ, Marchillo K, VanHecker J, Andes DR. 2013. Posaconazole pharmacodynamic target determination against wild-type and Cyp51 mutant isolates of Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 57:579–585. doi: 10.1128/AAC.01279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snelders E, Huis In't Veld RAG, Rijs AJMM, Kema GHJ, Melchers WJG, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snelders E, Camps SMT, Karawajczyk A, Schaftenaar G, Kema GHJ, van der Lee HA, Klaassen CH, Melchers WJG, Verweij PE. 2012. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 7:e31801. doi: 10.1371/journal.pone.0031801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 33.Espinel-Ingroff A, Diekema DJ, Fothergill A, Johnson E, Pelaez T, Pfaller MA, Rinaldi MG, Canton E, Turnidge J. 2010. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). J Clin Microbiol 48:3251–3257. doi: 10.1128/JCM.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andes D, Marchillo K, Stamstad T, Conklin R. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob Agents Chemother 47:3165–3169. doi: 10.1128/AAC.47.10.3165-3169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andes D, Marchillo K, Conklin R, Krishna G, Ezzet F, Cacciapuoti A, Loebenberg D. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob Agents Chemother 48:137–142. doi: 10.1128/AAC.48.1.137-142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oakley KL, Morrissey G, Denning DW. 1997. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother 41:1504–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seyedmousavi S, Bruggemann RJM, Melchers WJG, Verweij PE, Mouton JW. 2014. Intrapulmonary posaconazole penetration at the infection site in an immunosuppressed murine model of invasive pulmonary aspergillosis receiving oral prophylactic regimens. Antimicrob Agents Chemother 58:2964–2967. doi: 10.1128/AAC.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petraitiene R, Petraitis V, Groll A, Sein HT, Piscitelli S, Candelario M, Field-Ridley A, Avila N, Bacher J, Walsh TJ. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob Agents Chemother 45:857–869. doi: 10.1128/AAC.45.3.857-869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard SJ, Lestner JM, Sharp A, Gregson L, Goodwin J, Slater J, Majithiya JB, Warn PA, Hope WW. 2011. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J Infect Dis 203:1324–1332. doi: 10.1093/infdis/jir023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkhofer S, Lugger H, Dierich MP, Lass-Flörl C. 2007. Posaconazole enhances the activity of amphotericin B against Aspergillus hyphae in vitro. Antimicrob Agents Chemother 51:791–793. doi: 10.1128/AAC.01024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meletiadis J, Pournaras S, Roilides E, Walsh TJ. 2010. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob Agents Chemother 54:602–609. doi: 10.1128/AAC.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]