Abstract
Treatment of Candida glabrata cystitis remains a therapeutic challenge, and an antifungal combination using flucytosine is one option. We describe two patients with refractory C. glabrata cystitis who failed flucytosine combined with caspofungin with early-acquired high-level resistance to flucytosine due to nonsense mutations in the FUR1 gene. Rapidly acquired flucytosine resistance with microbiological failure should discourage combination of caspofungin and flucytosine during urinary candidiasis.
TEXT
Candiduria is a therapeutic challenge. Current recommendations mostly rely on fluconazole, and options against intrinsically fluconazole-less-susceptible or -resistant species like Candida glabrata are limited (1). Whereas echinocandins exert a high fungicidal effect on Candida spp., their use in this setting remains unclear (2). They display poor glomerular filtration and tubular secretion, with only 1% of the drug excreted unchanged, whereas fluconazole and flucytosine achieve high urine concentrations (10 to 100 times the serum levels) (3). Limited observations have, however, reported successful echinocandin-based treatments during candiduria (4). Flucytosine also displays excellent activity against most Candida species, except C. krusei. High rates of acquired resistance during monotherapy are considered its major limitation and argue for systematic combination therapy, such as that with an azole, amphotericin B, or one echinocandin.
Here, we describe two patients with refractory C. glabrata cystitis who received caspofungin and flucytosine with early emergence of high-level flucytosine resistance caused by a stop codon in the FUR1 gene and microbiological failure.
Case 1.
A 66-year-old woman presented with symptomatic C. glabrata cystitis. She had undergone a kidney transplantation 1 month before but had no kidney abscess or urine retention. Blood cultures remained negative. The initial isolate was susceptible to caspofungin and flucytosine with the following MIC values (Etest; bioMérieux, Marcy l'Etoile, France): flucytosine, 0.023 mg/liter; amphotericin B, 0.19 mg/liter; fluconazole, 24 mg/liter; voriconazole, 0.5 mg/liter; and caspofungin, 0.125 mg/liter. Specifically, no colony evocative of a flucytosine-resistant subpopulation was evidenced in the inhibition ellipse of the Etest.
She received caspofungin (70 mg at day 1 and then 50 mg/day) for 3 weeks, and then flucytosine was added (50 mg/kg/day, according to creatinine clearance) for 3 subsequent weeks because of caspofungin monotherapy failure. The flucytosine serum peak concentration at day 10 was within the therapeutic range (49 mg/liter). Treatment was stopped because of persisting symptoms and microbiological failure. The C. glabrata isolate collected at day 6 of flucytosine treatment and at treatment completion was resistant to flucytosine (MIC of 32 mg/liter without an increase in the caspofungin MIC).
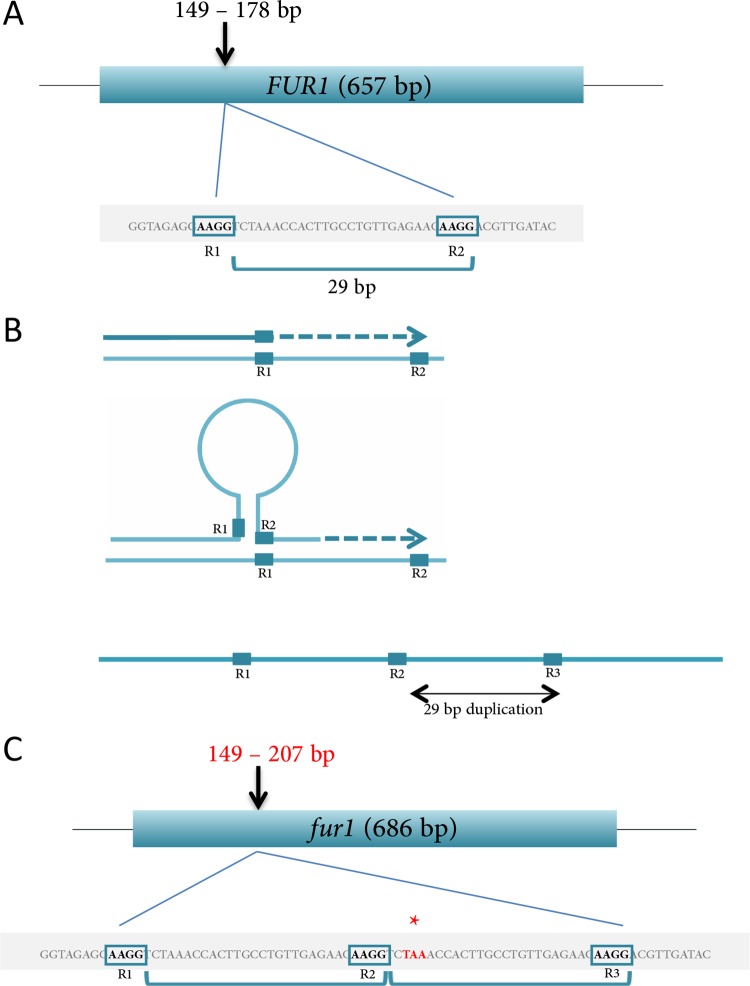
Strains collected before introduction of flucytosine and at day 6 of flucytosine treatment were sequenced for FCY1, FCY2, and FUR1, encoding, respectively, a cytosine permease, a cytosine deaminase, and a uracil phosphotransferase—three genes often exhibiting mutations leading to flucytosine resistance (5). Sequence analysis of the three genes in the initial wild isolate revealed the presence of a number of silent polymorphisms in FUR1 and FCY2 compared to the reference genome CBS138 (Table 1) (6). The same polymorphisms were found in the resistant strain that further displayed a 29-bp insertion within FUR1, creating a frameshift and a premature Stop codon, yielding a truncated protein (Fig. 1). This insertion is the result of a 29-bp duplication, probably by slippage between two short repeated sequences (Fig. 1) (7). Furthermore, isolates collected before and after flucytosine treatment showed identical multilocus sequence type (MLST) profiles (8).
TABLE 1.
Summary of polymorphisms and mutations in the FUR1 and FCY2 genes of flucytosine-sensitive and -resistant Candida glabrata clinical isolates
| Patient | Polymorphism or mutation in C. glabrata isolatea |
|||||||
|---|---|---|---|---|---|---|---|---|
| Flucytosine sensitive |
Flucytosine resistant |
|||||||
| Genotype |
Phenotype |
Genotype |
Phenotype |
|||||
| FUR1 | FCY2 | Fur1p | Fcy2p | FUR1 | FCY2 | Fur1p | Fcy2p | |
| 1 | A96G | G369C | R32R | V123V | A96G | G369C | R32R | V123V |
| T117C | T468C | S39S | C156C | T117C | T468C | S39S | C156C | |
| G178G | A915G | WT | L305L | Ins 178–207 | A915G | V61Stop | L305L | |
| T355C | L119L | T355C | L119L | |||||
| C370T | L124L | C370T | L124L | |||||
| T528C | D176D | T528C | D176D | |||||
| 2 | T355C | A693G | L119L | R231R | T355C | A693G | L119L | R232R |
| G368G | C813T | WT | A271A | G368T | C813T | E122Stop | A271A | |
| C370T | G1008A | L124L | V336V | C370T | G1008A | L124L | V336V | |
| T528C | C1425T | D176D | S475S | T528C | C1425T | D176D | S475S | |
| T1530A | P510P | T1530A | P510P | |||||
Underlined text indicates nucleic acid changes within FUR1 and FCY2 genes compared to the sequences of the reference strain CBS138. Boldface text indicates amino acid changes that occurred as a result of sequence insertion or nucleic acid mutation within the FUR1 gene. WT, wild type.
FIG 1.
Schematic representation of the transition between the FUR1 allele of the flucytosine-susceptible strain and the mutated version (fur1) of the flucytosine-resistant strain in patient 1. (A) Schematic representation of the region of C. glabrata FUR1 gene of the sensitive strain, which includes two 4-bp homologous sequences, R1 and R2 (AAGG). The DNA sequence between the 3′ ends of the two repeats is 29 bp long. (B) When transient denaturation occurs during DNA replication, the replicating strand sometimes anneals to another site (R2 on R1), forming a transient bubble. When replication starts again, the 25-bp segment located between the two repeated priming sites (R1 and R2) will be duplicated, as well as the proximal repeat, resulting in a third homologous sequence (R3) and a 29-bp duplication. (C) Schematic representation of the region of C. glabrata fur1 gene of the resistant strain, which includes a duplicated region of 29 bp. This insertion creates a frameshift and a premature Stop codon (*).
Case 2.
A 65-year-old man presented with C. glabrata cystitis. His history included retroperitoneal fibrosis with obstructive chronic kidney disease requiring a double-J catheter (creatinine clearance, 35 ml/min). He had no kidney/prostatic abscess or urine retention. Blood cultures were negative. The strain was susceptible to caspofungin and flucytosine, with MIC values (Etest) as follows: flucytosine, 0.047 mg/liter; amphotericin B, 0.25 mg/liter; fluconazole, 64 mg/liter; voriconazole, 1 mg/liter; and caspofungin, 0.06 mg/liter. Here again, no colony evocative of a flucytosine-resistant subpopulation was evidenced in the inhibition ellipse of the Etest. He received caspofungin (150 mg/day, in accordance to the dosage evaluated by Betts et al.) plus flucytosine (50 mg/kg/day according to creatinine clearance) for 4 weeks. The peak flucytosine serum concentration at day 4 was 57 mg/liter (9). Urine culture performed 10 days after antifungal initiation yielded C. glabrata with acquired resistance to flucytosine. The MIC values were as follows: flucytosine, 32 mg/liter; and caspofungin, 0.125 mg/liter. The strain collected before introduction of flucytosine was sequenced as described before and exhibited silent polymorphisms in FUR1 and FCY2 compared to the reference strain (Table 1). The resistant strain displayed the same polymorphisms but had one extra nonsense mutation within FUR1, creating a premature Stop codon and hence giving rise to a truncated protein (Table 1). As observed in case 1, both the strains collected prior to and after flucytosine treatment shared the same specific MLST profile.
Overall, the following important conclusions can be drawn from these observations.
(i) These two patients had no concomitant condition that might have explained treatment failure: there was no upper tract infection or parenchymal abscess, urine obstruction, or fungemia. Both received caspofungin/oral flucytosine at standard or even high doses, and both had flucytosine peak concentrations in the therapeutic range. Treatment failure highlights that caspofungin at a standard dose (50 mg/day) or high dose (150 mg/day) did not achieve the clearance of C. glabrata in the urine of patients with refractory fungal cystitis. This is in accordance with previous data evidencing reduced fungicidal effect of echinocandins toward C. glabrata (10).
(ii) Failure to eradicate infection was probably the consequence of the reported low urine excretion of caspofungin, although no urinary therapeutic drug monitoring was performed (2). Indeed, in both cases caspofungin could not prevent the acquisition of flucytosine resistance that is bound to occur under monotherapy, knowing that the initial and subsequent C. glabrata isolates shared the same MLST profile in each patient, suggesting they were isogenic (11).
(iii) In these two cases, high-level resistance to flucytosine is likely due to the acquisition of a Stop codon in the FUR1 gene, through two different mechanisms: duplication of sequence resulting in a frameshift (patient 1) and point mutation (patient 2). Previous reports suggest that high-level flucytosine resistance in clinical isolates is conferred by a wide array of point mutations conferring null phenotypes for Fcy1 or Fur1 (5, 12). Here we reported a new mechanism involving insertion of DNA fragment within the FUR1 gene, probably due to replication error through slippage between two short repeated sequences leading to a premature Stop codon. This mechanism led to the fast acquisition of flucytosine resistance in vivo. To our knowledge this had never been reported in the context of concomitant echinocandin prescription so far and never in such a small time interval: previous observations indeed reported acquired resistance 1 to 6 months after the treatment's initiation (12, 13, 14). Altogether, these two observations advocate against the use of caspofungin and flucytosine combinations for the treatment of C. glabrata cystitis.
ACKNOWLEDGMENTS
C.C., C.E.S., S.B.-B., G.Q., E.S., A.S., and C.L. declare they have no conflicts of interest. O.L. is a consultant for Gilead Sciences and member of the speaker's bureau of Astellas, Merck, and Pfizer.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Fisher JF, Sobel JD, Kauffman CA, Newman CA. 2011. Candida urinary tract infections—treatment. Clin Infect Dis 52(Suppl 6):S457–S466. doi: 10.1093/cid/cir112. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 3.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ. 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):S19–S37. [DOI] [PubMed] [Google Scholar]
- 4.Sobel JD, Bradshaw SK, Lipka CJ, Kartsonis NA. 2007. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis 44:e46–e49. doi: 10.1086/510432. [DOI] [PubMed] [Google Scholar]
- 5.Edlind TD, Katiyar SK. 2010. Mutational analysis of flucytosine resistance in Candida glabrata. Antimicrob Agents Chemother 54:4733–4738. doi: 10.1128/AAC.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inglis DO, Arnaud MB, Binkley J, Shah P, Skrzypek MS, Wymore F, Binkley G, Miyasato SR, Simison M, Sherlock G. 2012. The Candida genome database incorporates multiple Candida species: multispecies search and analysis tools with curated gene and protein information for Candida albicans and Candida glabrata. Nucleic Acids Res 40:D667–D674. doi: 10.1093/nar/gkr945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughn JN, Bennetzen JL. 2014. Natural insertions in rice commonly form tandem duplications indicative of patch-mediated double-strand break induction and repair. Proc Natl Acad Sci U S A 111:6684–6689. doi: 10.1073/pnas.1321854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodgson AR, Pujol C, Denning DW, Soll DR, Fox AJ. 2003. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J Clin Microbiol 41:5709–5717. doi: 10.1128/JCM.41.12.5709-5717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, Herbrecht R, Ruiz-Palacios G, Young JA, Baddley JW, Strohmaier KM, Tucker KA, Taylor AF, Kartsonis NA. 2009. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis 48:1676–1684. doi: 10.1086/598933. [DOI] [PubMed] [Google Scholar]
- 10.Howard SJ, Livermore J, Sharp A, Goodwin J, Gregson L, Alastruey-Izquierdo A, Perlin DS, Warn PA, Hope WW. 2011. Pharmacodynamics of echinocandins against Candida glabrata: requirement for dosage escalation to achieve maximal antifungal activity in neutropenic hosts. Antimicrob Agents Chemother 55:4880–4887. doi: 10.1128/AAC.00621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paladino JA, Crass RE. 1982. Amphotericin B and flucytosine in the treatment of candidal cystitis. Clin Pharm 1:349–352. [PubMed] [Google Scholar]
- 12.Chapeland-Leclerc F, Hennequin C, Papon N, Noel T, Girard A, Socie G, Ribaud P, Lacroix C. 2010. Acquisition of flucytosine, azole, and caspofungin resistance in Candida glabrata bloodstream isolates serially obtained from a hematopoietic stem cell transplant recipient. Antimicrob Agents Chemother 54:1360–1362. doi: 10.1128/AAC.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan RL, Goldberg MJ. 1972. C. albicans resistance to 5-fluorocytosine. Br Med J 3:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeprich PD, Ingraham JL, Kleker E, Winship MJ. 1974. Development of resistance to 5-fluorocytosine in Candida parapsilosis during therapy. J Infect Dis 130:112–118. doi: 10.1093/infdis/130.2.112. [DOI] [PubMed] [Google Scholar]